Abstract
Due to the many similarities in mechanisms of action, targets and effects, progesterone, estrogen and neurotrophins have been implicated in synaptic plasticity as well as in neuroprotection and neurodegeneration. In this study, we examined the interactions between 17-β-estradiol (E2) and progesterone (P4) and BDNF on both plasticity and excitotoxicity in rat cultured hippocampal slices. First, we evaluated the neuroprotective effects of E2 and P4 against NMDA toxicity in cultured rat hippocampal slices. As previously reported, pretreatment with 10 nM E2 (24 h) was neuroprotective against NMDA toxicity. However, progesterone (10 nM) added 20 h after E2 treatment for 4 h, reversed its protective effect. In addition, the same E2 treatment resulted in an increase in BDNF protein levels as well as in activation of its receptor, TrkB, while addition of P4 attenuated E2-mediated increase in BDNF and TrkB levels. Furthermore, E2-mediated neuroprotection was eliminated by a BDNF scavenger, TrkB-Fc. Our results indicate that E2 neuroprotective effects are mediated through the BDNF pathway, and that, under certain conditions, P4 antagonizes the protective effect of estrogen.
Keywords: Hormones, Excitotoxicity, Neurodegeneration, Hippocampus, Rat
INTRODUCTION
There is substantial evidence that the actions of steroid hormones extend well beyond reproductive organs. Multiple extra-hypothalamic regions within the central nervous system have been identified as targets for gonadal steroid hormones such as progesterone and estrogen, including hippocampus and cortex (Dohanich et al. 1997; Weiland et al. 1999; Akwa et al. 1996). Depletion of these steroid hormones in post-menopausal women has been established as a risk factor for the development of Alzheimer's disease (AD). A wealth of data suggests a neuroprotective role for 17β-estradiol, a main form of estrogen, in various animal models of neurodegeneration. Emerging data also suggest that progesterone (P4) has potent neuroprotective effects. For example, several studies have demonstrated that progesterone is neuroprotective against glutamate-induced cell death (Kaur et al. 2007; Nilsen and Brinton 2002; Nilsen and Brinton 2003) and traumatic brain injury (Robertson et al. 2006; Roof et al. 1997). Like 17β-estradiol (E2), P4 likely stimulates several different mechanisms of neuroprotection, including activation of neurotrophins. In addition, reduced metabolites of progesterone, such as 3-α-hydroxy-5α-pregnan-20-one (THP, allopregnanolone) have been shown to mediate, at least in part, the neuroprotective effects of progesterone (Ciriza et al. 2004). Interactions between estrogen and progesterone have also been documented in brain, as estrogen stimulates progesterone receptor expression in CA1 region of hippocampus and in medial preoptic nucleus (Alves et al. 2000; Chung et al. 2006). However, the possible regulation by P4 of E2-mediated neuroprotective effects has not been extensively investigated.
Several studies have indicated a similar pattern of expression and functional interactions for brain-derived neurotrophic factor (BDNF) and estrogen. BDNF and its receptor TrkB are present in cortex, hippocampus, midbrain, spinal cord, and hypothalamus and in principal neurons as well as in glial cells (Scharfman and MacLusky 2006; Sohrabji and Lewis 2006). In forebrain, BDNF-synthesizing neurons also express estrogen receptors, and the BDNF gene contains an ERE element, suggesting possible interactions between estradiol and BDNF regulation (Miranda et al. 1993). The relationships between progesterone and BDNF appear to be more complex, as progesterone can stimulate BDNF expression in motoneurons, while BDNF has been shown to inhibit progesterone synthesis in the periphery (Gonzalez et al. 2005; Jensen and Johnson 2001). Moreover, progesterone has been shown to decrease BDNF expression in hippocampus and increase it in cortex (Frye and Rhodes 2005; Kaur et al. 2007). The inconsistencies in these interactions remain to be elucidated. Whether E2 and BDNF act synergistically or in parallel to exert neuroprotective effects in the CNS has not been investigated. The present study was therefore directed at testing interactions between estrogen and progesterone in an in-vitro model of excitotoxicity, and at evaluating the role of BDNF in mediating estradiol-induced neuroprotection using NMDA treatment of cultured hippocampal slices to elicit neuronal death.
MATERIALS AND METHODS
Animals
Animals were treated in accordance with the principles and procedures of the National Institutes of Health Guide for the Care and Use of Laboratory Animals; all protocols were approved by the Institutional Animal Care and Use Committee of the University of Southern California. Timed pregnant Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA) and kept in the vivarium in a temperature- and light-controlled environment with a 12 h light/dark cycle. On the day of experimentation, rats were removed from their home cage and anesthetized using halothane.
Preparation of cultured hippocampal slices
Organotypic hippocampal slice cultures were prepared from postnatal day 8–10 Sprague-Dawley rat pups according to previously described methods (Stoppini et al. 1991). Briefly, following decapitation, brains were removed from the cranium and placed into chilled cutting medium (Earle's MEM, 25 mM HEPES, 10 mM Tris-base, 10 mM D-glucose, 3 mM MgCl2, pH 7.2). After isolation of hippocampi, 400 μm thick transversal slices were cut with a McIlwain tissue chopper. Slices were placed into chilled cutting medium, inspected and separated under a microscope. Six slices from the midsection of the hippocampus were then placed onto a 0.4 μm culture plate insert (Millipore, Billerica, MA) in 6-well flat bottom tissue culture plates (BD Falcon, San Jose, CA). Cultures were maintained in 1 mL of steroid-deficient maintenance medium containing 20% heat inactivated charcoal stripped horse serum (Sigma, St. Louis, MO), Earle's balanced salt solution, Basal Medium Eagle (Sigma), 20 mM NaCl, 0.2 mM CaCl2, 1.7 mM MgSO4, 2.7 mM L-glutamine, 27 mM HEPES, 5 mM NaHCO3, 48 mM D-glucose, 0.5 mM ascorbic acid, 0.5% penicillin/streptomycin and 0.01 % insulin (Sigma); pH 7.4. Slices were kept at 35 °C with 5% CO2 and maintained for 14 days with complete medium exchange every three days prior to experiments.
Treatment of cultured hippocampal slices
Cultured hippocampal slices were maintained in vitro for two weeks prior to treatments to allow recovery from sectioning damage and completion of the trisynaptic circuitry (see Nakagami et al. 1997). At 14 DIV, cultures were supplemented with the appropriate treatments (hormones, vehicle, NMDA) in serum-free medium for the indicated periods of time. Controls received vehicle (DMSO) treatments in similar hormone-deprived conditions to account for procedural or vehicle-related effects.
17ß-estradiol (E2) was diluted in dimethylsulfoxide (DMSO) to a stock solution of 10 μM and was further diluted in culture medium to a final concentration of 10 nM and applied for 24 h. We have previously shown that at this concentration, E2 is neuroprotective against NMDA toxicity (Bi et al., 2000). We devised a treatment protocol that could parallel the ratio of P4 to E2 treatment previously used in in-vivo experiments (Bimonte-Nelson et al., 2004) and assumed to represent physiological levels of hormones. The temporal pattern of P4 exposure was selected to investigate the minimum exposure time required to antagonize estrogen's effects in a 24 h time period. Ten nM P4 was diluted in DMSO and applied to the culture medium for ½ h, 1 h, 2 h, 4 h, 12 h, 20 h and 24 h during continuous 24 h E2 exposure. P4 was diluted in the same manner as E2 and applied to the culture medium at a final concentration of 10 nM for 4 h alone or following 20 h of E2 treatment. NMDA (50 μM) was applied for 3 h following hormone washout. TrkB Fc (0.5 μg/mL) was co-administered with E2 for 24 h. Vehicle controls were performed in parallel and received 0.1% DMSO in serum-free medium.
Cell viability assessment
Slices used for protein analysis were collected at the end of treatments. Slices used for neuroprotection assays were washed with serum-free medium and underwent a 24 h recovery period in serum-free media containing propidium iodide (PI; 4 μM, Calbiochem). After recovery, the medium was collected to measure the amount of lactate dehydrogenase (LDH) released using a spectrophotometric assay. Slices were imaged with an epifluorescent microscope using an inverted Leica DMIRB with a 550LP filter and fitted with a 5x phase contrast objective and a Spot RT slider color CCD camera (Roper Scientific, Tucson, AZ). These images were captured using Photoshop CS2, converted to monochrome and then pseudo-colored only to illustrate regional intensity. Quantification of raw images was done on Photoshop CS2 by manually selecting regions of interest (ROI) corresponding to CA1, CA3 and dentate gyrus (DG) of hippocampus based on the rat atlas of hippocampus and morphological markers. Pixel intensity values were then recorded on Excel (Microsoft). Absolute intensity was calculated using total pixel intensity in the analyzed regions. Values were recorded as percentage of the values found in slices treated with NMDA (50 μM) for 24 h to induce maximal cell death (NMDAmax).
Western blot analysis
At the end of experimental treatments, slices were collected and homogenized by sonication, and an aliquot of the homogenate was taken to determine protein concentration by the Bradford method (BioRad, Hercules, CA). Samples were prepared for immunoblotting by dilution 1:1 in Laemmli buffer with 5% ß-mercaptoethanol (BioRad). Twenty to fifty μg of proteins were loaded onto 15% SDS-PAGE gels or 4-20% gradient gels (BioRad) along with Precision unstained molecular weight markers to approximate protein molecular weights (BioRad). Following electrophoresis, proteins were electroblotted onto NitroPure nitrocellulose membranes (Osmonics, Minnetonka, MN). Western blot membranes were washed with TBS-Tween (0.05%) and blocked with 5% non-fat milk.
Antibodies
Immunodetection of proteins was performed using 1:200 anti-BDNF (Cat. # sc-546, Santa Cruz Biotech, Santa Cruz, CA), 1:1,000 anti-phospho TrkA/TrkB and 1:1,000 TrkB (Cell Signaling Technology, Danvers, MA), and 1:10,000 anti-β-Actin antibodies, along with peroxidase-labeled secondary antibodies, 1:2,000 anti-rabbit for BDNF, pTRK and TrkB and 1:10,000 anti-mouse for β-Actin (Jackson ImmunoResearch, Westgrove, PA). BDNF signal was optimized by using 40 ug of protein, a 1:200 dilution of the antibody and blocking in 5% milk for 1 h. Initial studies using a BDNF standard showed a strong band with the predicted 14 kDa apparent Mr and a faint band around 25 kDa. Likewise, pTrk and TrkB antibody specificity was corroborated with activation of the TrkB receptor by BDNF and reversal of activation by the addition of K252a in HEK-TrkB cells (data not shown). Unlabeled standards were tagged using peroxidase-labeled StrepTactin (BioRad). Immunoblots were visualized autoradiographically using enhanced chemiluminescence (Pierce).
Densitometric analyses
To quantify protein levels, films were scanned (CanonScan N656U scanner; Canon, Lake Success, NY) at 300 dpi and analyzed. Band intensities were calculated using Image J software. Immunoblot ratios were normalized to protein and were reported as a percentage of vehicle control.
Statistical analysis
One-way analyses of variance (ANOVA) followed by Tukey's Multiple comparison post-hoc tests were used for pair-wise comparisons between experimental treatments. Data were analyzed using GraphPad Prism 4 software (San Diego, CA) and significance level was set at 0.05. Results were expressed as means ± S.E.M. for the indicated number of experiments.
RESULTS
P4 reverses E2-mediated neuroprotection against NMDA-induced neurotoxicity
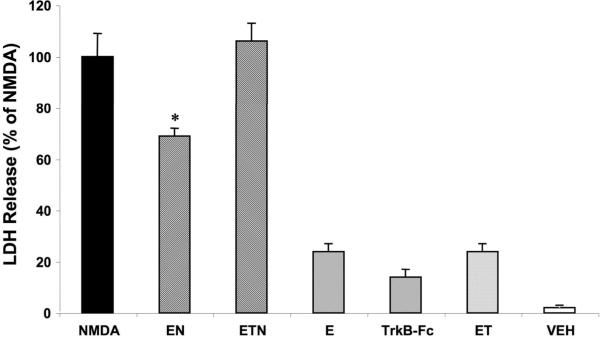
To determine whether P4 interferes with the neuroprotective effects of E2, cultured hippocampal slices were treated with E2 for 24 h and P4 was added to the medium during the final 4 h of the 24 h treatment period. Hormones were washed out by 2 successive medium changes prior to NMDA treatment (50 μM NMDA, 3 h). As previously shown (Bi et al., 2001), E2 (10 nM) significantly reduced the amount of NMDA-induced LDH release (Fig. 1). Addition of P4 (10 nM) 20 h after initiating E2 pretreatment (E2 + P4 + NMDA) reversed the reduction in LDH release to levels identical to those found in NMDA treated slices (Fig. 1) (One way ANOVA F (7,193) = 43.45, p < 0.0001). Treatment with E2, P4 or E2+P4 alone did not increase LDH release as compared to that measured in control slices.
Figure 1. P4-mediated reversal of E2-induced neuroprotection against NMDA toxicity in cultured hippocampal slices.
A. Treatment protocol timeline for hormones and NMDA. Treatment with P4 (10 nM), E2 (10 nM), or NMDA (50 μM) was performed as indicated in the diagram. Vehicle was administered for the length of the experiment (not shown).
B. LDH release in the medium measured 24 h after NDMA treatment. Medium was collected 24 h after initiation of NMDA treatment, and LDH activity was determined as described under Material and Methods. Results are means ± S.E.M of 7 experiments and are expressed as percent of release in NMDA-treated slices. Statistical significance was analyzed by ANOVA followed by Tukey's test for individual comparisons. *p < 0.001 vs. NMDA; † p < 0.001 vs. E2 + NMDA (EN); NS: not significant.
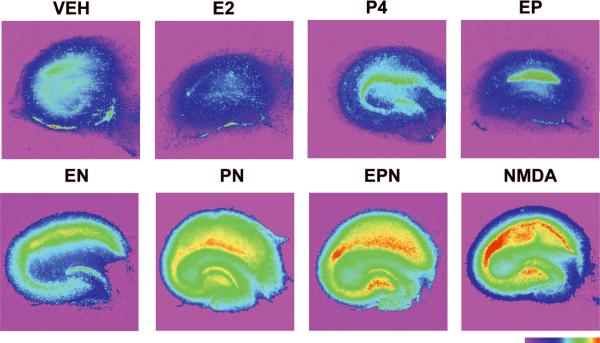
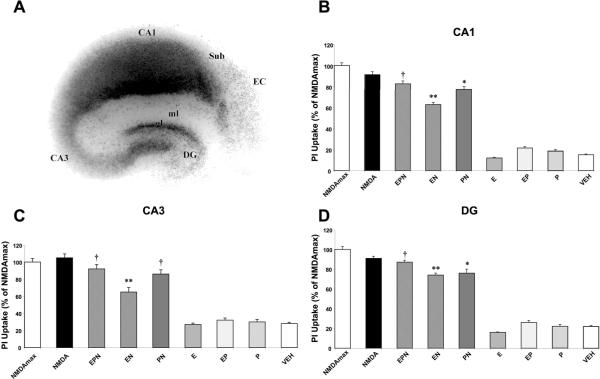
Similarly, analysis of propidium iodide (PI) uptake in slices treated under various conditions illustrated a marked difference between NMDA-treated slices in the absence or presence of hormones. Fluorescent images of slices treated were pseudo-colored only to illustrate regional intensity of cellular damage. Visual analysis of cultured hippocampal slices treated with vehicle, E2, P4 or E2 in combination with P4 indicated that none of these treatments produced a significant increase in PI uptake (Fig. 2, top). In contrast, cultured slices treated with NMDA (50 μM) for 3 h or 24 h exhibited increased PI uptake throughout the hippocampus, with the highest increase in CA1 (Fig. 2, bottom). Quantification of unmodified images indicated that maximal cell death was obtained in slices treated with NMDA for 24 h (Fig. 3b–d). In agreement with our previous results, pretreatment for 24 h with E2 (10 nM) prior to 3 h of NMDA exposure significantly reduced fluorescence intensity in CA1, CA3 and DG regions of hippocampus (p < 0.001; Fig. 3b–d). This reduction was reversed in all three regions by the addition of P4 to E2 and NMDA-treated slices (EPN) to levels that were not significantly different from those found in slices treated with NMDA alone. Interestingly, addition of P4 for 4 h alone resulted in significant protection from NMDA-mediated neurotoxicity in CA1 and DG, but not in CA3 (one way ANOVA for CA1: F (8, 267) = 349.4, p < 0.0001; ANOVA for CA3: F (8, 298) = 87.02, p < 0.0001; ANOVA for DG: F (8, 303) = 303.5, p < 0.0001; Fig. 3b–d).
Figure 2. Effects of E2 and P4 on NMDA-induced neuronal damage in cultured hippocampal slices, assessed with PI uptake.
Top Row: Representative images of PI uptake in slices treated with vehicle (VEH), E2 (10 nM), P4 (10 nM) or E2 (10 nM) + P4 (10 nM) (EP).
Bottom row: Representative images of PI uptake in slices treated with E2 (10 nM) + NMDA (50 μM) (EN), P4 (10 nM) + NMDA (PN), E2 + P4 + NMDA (EPN) and NMDA alone. NMDA treatment resulted in increased levels of fluorescence intensity, and estrogen attenuated this increase. P4 reversed the protective effects of E2. Color bar represents fluorescence intensity scale, with red illustrating highest intensity.
Figure 3. Quantitative analysis of PI uptake in cultured hippocampal slices.
Images of slices treated as in Figure 2 were analyzed as described under Materials and Methods. Regions analyzed (CA1, CA3 and DG) are indicated in A. Results are expressed in percent of fluorescent intensity measured in slices treated with NMDA (50 μM) for 24 h, and are means ± S.E.M. of 12–14 images obtained in 6–8 slices. Statistical significance was analyzed by ANOVA followed by Tukey's test for individual comparisons. ** p < 0.001 as compared to NMDA treatment alone; * p < 0.05 as compared to NMDA treatment alone; † p < 0.05 as compared to treatment with E2 + NMDA (EN).
Antagonistic effects of P4 on E2-induced neuroprotection are not mediated by its metabolites
Allopregnanolone (ALLO), a metabolite of progesterone, has been shown be a potent GABAA receptor modulator. To investigate whether the effects observed after applying P4 for 4 h were due to its conversion to ALLO, we co-treated slices with both P4 and finasteride for 4 h after 20 h of E2 treatment. Finasteride inhibits the synthesis of 5α-reduced neurosteroids and has been shown to significantly attenuate the formation of ALLO both in-vitro and in-vivo (Izumi et al. 2007; Rhodes and Frye 2005). Furthermore the concentration of finasteride used in the present study has been used previously in hippocampal slices and neurons (Izumi et al. 2007; Mostallino et al. 2006). Co-treatment with P4 and finasteride did not significantly modify the results obtained using P4 alone‥ PI uptake was similar in slices treated with E2, P4 and NMDA and those treated with E2, P4, Finasteride and NMDA in CA1, CA3 and DG regions of hippocampus (data not shown).
E2-mediated neuroprotection is mediated by BDNF
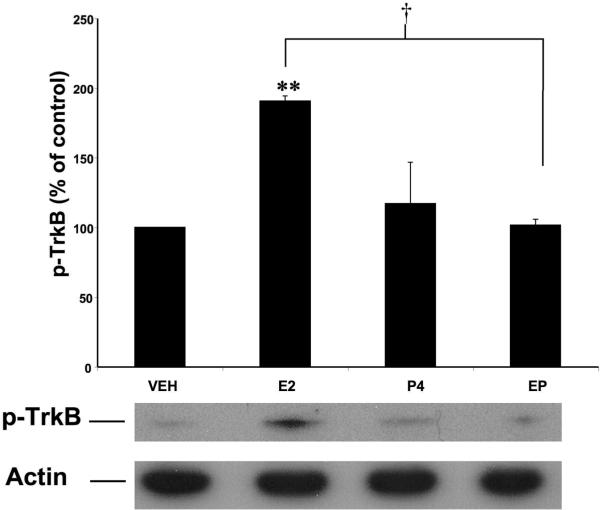
To further investigate the mechanisms underlying E2 neuroprotection, we utilized the BDNF scavenger, TrkB-Fc, to determine whether BDNF was involved in mediating E2 neuroprotective effects. Addition of 0.5 μg/mL TrkB Fc throughout the course of E2 pretreatment resulted in a significant attenuation of E2-mediated neuroprotection against NMDA (50 μM) toxicity (p < 0.001; Fig. 4). Addition of the BDNF scavenger alone had no significant effect on LDH release. We further tested BDNF involvement in E2 neuroprotection by assessing the state of phosphorylation/activation of the BDNF receptor, TrkB (one way ANOVA F (7, 14) = 16.16, p < 0.0001; Fig. 5). E2 treatment resulted in a significant increase in TrkB phosphorylation, suggesting that E2-mediated neuroprotection involves increased BDNF release followed by activation of the TrkB receptor.
Figure 4. BDNF mediates E2 neuroprotective effects against NMDA toxicity in cultured hippocampal slices.
Cultured hippocampal slices were treated as shown in Figure 1 and TrkB-Fc (0.5 μg/mL) was present during the duration of E2 treatment. Medium was collected 24 h after NMDA treatment and LDH assayed as indicated under Materials and Methods. Results are expressed as percent of LDH release measured in medium of slices treated with NMDA for 3 h and are means ± S.E.M. of 5–6 experiments. Statistical significance was analyzed by ANOVA followed by Tukey's test for individual comparisons. * p < 0.001 as compared to NMDA treated slices.
Figure 5. Effects of E2 and P4 on TrkB activation/phosphorylation in cultured hippocampal slices.
Cultured hippocampal slices were treated with E2 (10 nM), P4 (10 nM) or E2 + P4 as described in Figure 1. At the end of treatment, slices were collected and processed for western blot analysis of p-TrkB (bottom). Levels of p-TrkB were corrected with those of β-actin and results were expressed as percent of values found in vehicle-treated slices; they are means ± S.E.M. of 4–5 experiments. Statistical significance was analyzed by ANOVA followed by Tukey's test for individual comparisons. ** p < 0.001 as compared to vehicle-treated slices; † p < 0.001 as compared to E2 treated slices.
P4 reverses E2-induced increase in BDNF levels and TrkB activation
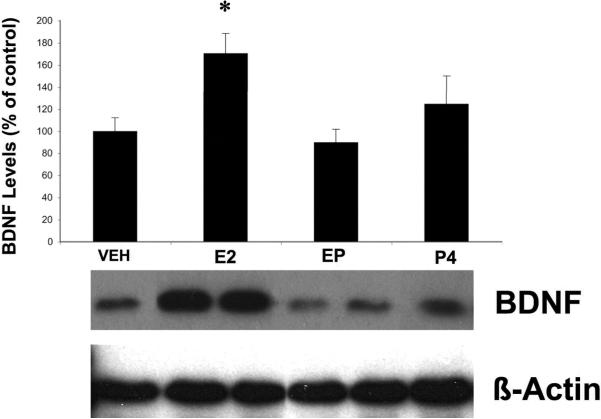
E2 has previously been shown to increase BDNF expression in various brain regions, including hippocampus. For instance, ovariectomy results in a significant decrease in hippocampal BDNF mRNA, and E2 replacement partially restores BDNF levels (Singh et al., 1995). We therefore measured BDNF levels in cultured hippocampal slices following 24 h treatment with E2 (10 nM). Western blot analysis indicated that this treatment resulted in a 75% increase in BDNF levels (Fig. 6). Since this rise in BDNF expression was temporally associated with the neuroprotective effects seen at the end of E2 treatment, we examined whether the addition of P4 (10 nM) had any effects on E2-induced BDNF increase. Interestingly, addition of P4 during the final 4 h of E2 treatment significantly reversed E2-induced increase in BDNF levels. Furthermore, P4 treatment alone did not significantly increase BDNF protein expression after 4 h of treatment (one way ANOVA F (3, 75) = 5.644, p = 0.0015; Fig. 6). In addition, P4 treatment also eliminated E2-mediated TrkB phosphorylation/activation and did not induce receptor activation on its own (one way ANOVA F (3, 9) = 15.09, p = 0.0007; Fig. 5).
Figure 6. Effects of E2 and P4 on BDNF levels in cultured hippocampal slices.
Cultured hippocampal slices were treated with E2 (10 nM), P4 (10 nM) or their combination according to the protocol shown in Fig. 1A. Control slices were treated with vehicle. Slices were collected at the end of treatment, and processed for determination of BDNF levels with western blots as described under Material and Methods. Results were normalized to levels of β-actin and were expressed as percent of control; they are means ± S.E.M. of 7 experiments. Statistical significance was analyzed by ANOVA followed by Tukey's test for individual comparisons. * p < 0.05 as compared to control to E2 + P4-treated samples.
DISCUSSION
A substantial body of evidence indicates that estrogen, the biologically most potent and prevalent female hormone is neuroprotective under a variety of conditions. However, the paucity of studies exploring the effects of progesterone may account for some of the inconsistencies reported in the literature. It is now clearly established that menopause, as well as ovariectomy, results in a depletion of both estrogen and progesterone. Thus, the decline in progesterone as well as in estrogen may play a significant role in the various deficits, including cognitive deficits, which follow menopause and should be closely taken into account when trying to relate age-related hormonal and cognitive changes. In addition, accumulating evidence suggests that estrogen and progesterone differentially affect several neurobiological processes and that it is their interactions on these processes that influence brain systems involved in learning and memory. For example, while progesterone has no effect on spine density on its own, it prevents estradiol-induced increases in spine density when co-administered (Murphy and Segal 2000). Similarly, although E2 treatment results in neuroprotection against glutamate toxicity, the combination of E2 and progestin either enhances or attenuates neuroprotection, depending on the type of progestin (Nilsen and Brinton 2002). Furthermore, in vivo results also show a detrimental effect of co-treating aged OVX rats with E2 and P4. While E2 increased neurotrophin levels in entorhinal cortex, concomitant treatment with P4 attenuated this increase (Bimonte-Nelson et al. 2004). As with many other studies, these results do not take into account the temporal relationships between hormone administration, which may explain some of the discrepancies in the current literature. As of yet, there are still conflicting data regarding the complex interactions between various gonadal hormones and their differential effects on various brain systems.
In our study, we utilized organotypic hippocampal slices prepared from P9 pups of both genders and cultured for two weeks with media supplemented with charcoal-stripped horse serum. This method has been shown to reduce estrogen levels to less than 10−12 M in culture media containing 10% heat-inactivated FCS (Sigma Product information sheet), and dextrancoated charcoal has been utilized to separate free from antibody-bound progesterone (Jensen and Johnson 2001). Preliminary results did not show a sex effect, thus results from hippocampal slices of both sexes were combined for all experiments. Consequently, we chose Western Blot as a protein detection method instead of ELISA. BDNF ELISA kit (Promega, WI) does not distinguish between the mature and pro-BDNF forms; therefore, we utilized the BDNF antibodies (Santa Cruz Biotech, Santa Cruz, CA) in Western Blotting techniques to detect the mature form of BDNF migrating at 14 kDa. However, this antibody reveals a distinct pattern of expression compared with other antibodies, which may be due to differential binding to specific conformations of BDNF (Scharfman and MacLusky 2006). Similarly, the pTrkB antibody used does not readily distinguish pTrkB from pTrkA, since the binding sequences in the two receptors are identical. Nonetheless, we believe our data suggests a strong relationship between E2-mediated upregulation of BDNF protein and neuroprotection.
Our results indicate that P4, under certain conditions, produces effects that counteract E2's neuroprotective effects in hippocampus. In agreement with our previous findings, E2 was neuroprotective against NMDA-induced neurotoxicity in organotypic hippocampal slices (Bi et al. 2000). In the present study, 10 nM P4 applied for 4 h following a 20 h E2 pretreatment prevented E2-mediated neuroprotection against NMDA-elicited increases in both LDH release and PI uptake. Addition of the 5α - reductase inhibitor finasteride to E2 + P4-treated samples did not modify P4-induced reversal of E2-mediated protection. This was not unexpected, as most effects of allopregnanolone, the major metabolite of P4, require micromolar concentrations (Lockhart et al. 2002; Xilouri and Papazafiri 2006). Since we used 10 nM P4 in our experiments, it is likely that the concentration of allopregnanolone generated would be less than 10 nM.
E2 treatment increased BDNF levels and phosphorylation of its receptor, TrkB, suggesting that E2 can trigger both an increased expression of BDNF as well as its release, followed by activation of the TrkB receptor. Addition of the BNDF scavenger, TrkB-Fc, to the culture medium attenuated E2-mediated neuroprotection, further strengthening the idea that BDNF release plays a critical role in E2-mediated neuroprotection. On the other hand, P4 treatment did not result in increased BDNF levels or phosphorylation of TrkB, although it exhibited neuroprotective effects, at least in CA1 and DG. Our results are therefore in agreement with previously reported data indicating that P4 can be neuroprotective against glutamate neurotoxicity (Kaur et al. 2007; Nilsen and Brinton, 2002).
A recent review provides evidence for TrkB signaling as a mediator of estrogen effects in hippocampus (Spencer et al. 2008) TrkB immunoreactivity was found to fluctuate during the estrous cycle, with the highest intensity in CA3 and dentate gyrus during proestrus, when both estrogen and BDNF protein levels are also highest. Although it is not yet clear whether E2 directly or indirectly activates the TrkB receptor, our results indicate that activation of TrkB plays a critical role in E2-induced neuroprotection from NMDA toxicity under these conditions. Our results also suggest that P4 neuroprotective effects are probably not mediated through BDNF signaling. In fact, P4 treatment prevented E2-mediated increased BDNF expression and TrkB phosphorylation. This effect is likely to account for the prevention by P4 of E2 neuroprotective effects. However, it should be noted that there are discrepancies in the literature regarding the relationship between P4 and BDNF. P4 was shown to reduce BDNF levels in hippocampus, while increasing mRNA and protein levels in cortical explants (Frye and Rhodes 2005; Kaur et al. 2007). A recent study also reported prevention by P4 of E2-mediated neuroprotection against kainate excitotoxicity, although P4 alone did not exhibit neuroprotective effects (Rosario et al. 2006). The mechanisms underlying the antagonist effect of P4 on E2-mediated increased BDNF expression and signaling are not yet known, but the short time-course of P4 effects suggests that the mechanism does not involve traditional genomic pathways. An attractive hypothesis that could account for our observed results has been suggested by our colleagues, who have discovered that P4 treatment results in the rapid down-regulation of E2 receptors (Jayaraman and Pike, in press).
In the hippocampus of the adult rat, BDNF mRNA is present in dentate gyrus granule cells and hippocampal pyramidal cells (Scharfman and MacLusky 2006). Both of the classical progesterone receptors isoforms (PRA and PRB) are also expressed in all regions of the hippocampus (Brinton et al. 2008). Finally both mRNA and protein expression of ERα and ERβ are found in numerous cell types (Scharfman and MacLusky 2006; Simerly et al. 1990), with some co-localization with BDNF-expressing cells (Blurton-Jones et al. 2004). Although the relative distribution of these receptors is similar in hippocampus, the functional role of each receptor remains to be elucidated.
It is becoming increasingly clear that understanding the interactions between E2 and P4 is paramount to understanding the complexities underlying age-related cognitive deficits and diseases like Alzheimer's disease (AD). In a mouse model of AD, P4 treatment did not affect Aβ accumulation or behavioral performance in a Y-maze, although it did reduce tau hyperphosphorylation. When P4 was combined with E2 treatment, the neuroprotective effects of E2 against Aβ accumulation were attenuated (Carroll et al. 2007). Together with our results, recent studies present strong evidence that although E2 and P4 may exert similar actions, their combination and the potential effects of the temporal order of treatment on neurotrophin regulation represent factors that need to be taken into account to understand the role of these hormones as neuroprotective agents and potential treatments for neurodegenerative disorders.
ACKNOWLEDGEMENTS
Contract grant sponsor: National Institutes of Health; Contract grant number 1P01AG026572, Contract training grant number T32 AG000093-25.
The authors thank Anna Knize for technical assistance. This study was supported by NIA training grant T32 AG000093-25 to CCA and NIH grant 1P01AG026572 to MB.
ABBREVIATIONS
- E2
17ß-estradiol
- P4
progesterone
- BDNF
brain-derived nerve growth factor
- LDH
lactate dehydrogenase
- PI
propidium iodide
REFERENCES
- Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER alpha) gene-disrupted mice. J Comp Neurol. 2000;427(2):185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci U S A. 2000;97(7):3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15(17):2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Kuan PN, Tuszynski MH. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol. 2004;468(3):347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29(2):313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27(48):13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, Pak TR, Weiser MJ, Hinds LR, Andersen ME, Handa RJ. Progestin receptor expression in the developing rat brain depends upon activation of estrogen receptor alpha and not estrogen receptor beta. Brain Res. 2006;1082(1):50–60. doi: 10.1016/j.brainres.2006.01.109. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16(1):58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Estrogen-priming can enhance progesterone's anti-seizure effects in part by increasing hippocampal levels of allopregnanolone. Pharmacol Biochem Behav. 2005;81(4):907–916. doi: 10.1016/j.pbb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Labombarda F, Deniselle MC, Mougel A, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol. 2005;94(1–3):143–149. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Murayama K, Tokuda K, Krishnan K, Covey DF, Zorumski CF. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. Eur J Neurosci. 2007;26(7):1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Jensen T, Johnson AL. Expression and function of brain-derived neurotrophin factor and its receptor, TrkB, in ovarian follicles from the domestic hen (Gallus gallus domesticus) J Exp Biol. 2001;204(Pt 12):2087–2095. doi: 10.1242/jeb.204.12.2087. [DOI] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85(11):2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart EM, Warner DS, Pearlstein RD, Penning DH, Mehrabani S, Boustany RM. Allopregnanolone attenuates N-methyl-D-aspartate-induced excitotoxicity and apoptosis in the human NT2 cell line in culture. Neurosci Lett. 2002;328(1):33–36. doi: 10.1016/s0304-3940(02)00448-2. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci U S A. 1993;90(14):6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G. Changes in expression of the delta subunit of the GABA (A) receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem. 2006;99(1):321–332. doi: 10.1111/j.1471-4159.2006.04055.x. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Progesterone prevents estradiol-induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology. 2000;72(3):133–143. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- Nakagami Y, Saito H, Matsuki N. Optical recording of trisynaptic pathway in rat hippocampal slices with a voltage-sensitive dye. Neuroscience. 1997;81(1):1–8. doi: 10.1016/s0306-4522(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143(1):205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100(18):10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Actions at GABA(A) receptors in the hippocampus may mediate some antiseizure effects of progestins. Epilepsy Behav. 2005;6(3):320–327. doi: 10.1016/j.yebeh.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197(1):235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31(1):1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099(1):206–210. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27(4):415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27(4):404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Xilouri M, Papazafiri P. Anti-apoptotic effects of allopregnanolone on P19 neurons. Eur J Neurosci. 2006;23(1):43–54. doi: 10.1111/j.1460-9568.2005.04548.x. [DOI] [PubMed] [Google Scholar]