Abstract
Recent genome-wide association studies have identified a genetic locus at human chromosome 8q24 as having minor alleles associated with lower levels of plasma triglyceride (TG) and LDL cholesterol (LDL-C), higher levels of HDL-C, as well as decreased risk for myocardial infarction. This locus contains only one annotated gene, tribbles homolog 1 (TRIB1), which has not previously been implicated in lipoprotein metabolism. Here we demonstrate a role for Trib1 as a regulator of lipoprotein metabolism in mice. Hepatic-specific overexpression of Trib1 reduced levels of plasma TG and cholesterol by reducing VLDL production; conversely, Trib1-knockout mice showed elevated levels of plasma TG and cholesterol due to increased VLDL production. Hepatic Trib1 expression was inversely associated with the expression of key lipogenic genes and measures of lipogenesis. Thus, we provide functional evidence for what we believe to be a novel gene regulating hepatic lipogenesis and VLDL production in mice that influences plasma lipids and risk for myocardial infarction in humans.
Introduction
Aberrant plasma lipoprotein concentrations of cholesterol, including LDL cholesterol (LDL-C) and HDL-C, and of triglycerides (TG) are heritable risk factors for atherosclerotic cardiovascular disease, the major cause of death in most of the world. Recent genome-wide association studies (GWASs) have identified novel genetic loci associated with plasma lipid traits (1–6). Many of these loci represent known Mendelian disorders of lipoprotein metabolism (e.g., LDLR, LPL, LIPC, APOB, ABCA1, CETP, and PCSK9). However, several novel loci contain genes not previously implicated in lipoprotein metabolism, and the mechanisms underlying these associations remain unknown. At present, there is active debate as to whether statistical associations at new GWAS loci can be harnessed to understand physiological mechanisms (7, 8).
One of the most compelling new loci associated with plasma lipids is on chromosome 8q24 (1, 3). In a GWAS involving nearly 100,000 individuals, SNPs at 8q24 were genome-wide significantly associated with TG, LDL-C, and HDL-C (6). Importantly, the minor alleles at these SNPs were associated with an atheroprotective lipid phenotype of lower TG, lower LDL-C, and higher HDL-C and were also associated with a significantly reduced risk of coronary heart disease (6).
The associated interval at 8q24 contains only one annotated gene, TRIB1 (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI44213DS1), encoding the protein tribbles homolog 1. TRIB1 is a member of the recently identified tribbles protein family, with proposed roles as adaptor or scaffold protein (9). However, no prior mechanistic connection to lipoprotein metabolism has been made, and the physiological functions and interactions of TRIB1 remain largely unknown. Here, we investigated whether TRIB1 is the gene responsible for the associations between 8q24 and plasma lipids and explored potential mechanisms by which TRIB1 may influence lipid metabolism.
Results and Discussion
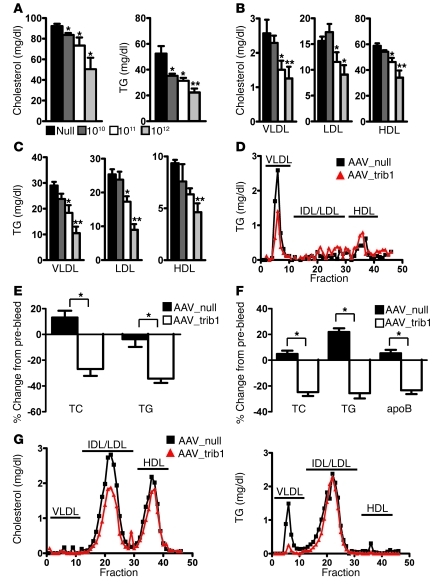
We first quantified TRIB1 mRNA levels in cDNA samples from 16 human tissues and found that it was expressed ubiquitously, with high expression in liver (Supplemental Figure 2). Since the liver is the major site for the formation, secretion, and clearance of circulating lipoproteins, we investigated the effect of hepatic Trib1 overexpression on plasma lipids in mice, by using an adeno-associated virus serotype 8 (AAV8) vector expressing murine Trib1 (AAV_trib1) under a liver-specific promoter. Injection of this vector in wild-type mice resulted in stable Trib1 expression and reduced cholesterol and TG in a dose-dependent manner, with maximum reductions of 45% and 57% for plasma cholesterol and TG, respectively (Figure 1A and Supplemental Figure 3A). Hepatotoxicity of the AAV8 injection was ruled out, since plasma alanine aminotransferase (ALT) levels were not elevated after AAV8 administration (Supplemental Figure 3B). We next examined which lipoprotein fractions were affected by Trib1 overexpression and found significantly reduced plasma levels of VLDL, LDL, and HDL cholesterol and TG (Figure 1, B and C). The difference in directionality of HDL changes in mice compared with humans is not surprising, since mice lack cholesterol ester transfer protein, which permits crosstalk between (V)LDL and HDL metabolism via the exchange of neutral lipids (10). Trib1 overexpression also led to a distinct reduction in the VLDL-TG peak, but no obvious shift in particle size, as assessed by FPLC (Figure 1D).
Figure 1. Hepatic Trib1 overexpression reduces plasma lipid levels in mice.
C57BL/6 mice (n = 5 per group) were injected with 1012 genome copies (gc) control (AAV_null) or 1010, 1011, or 1012 gc AAV_trib1. (A) Plasma cholesterol and TG levels were measured 2 weeks after injection. Plasma lipoproteins were isolated by sequential ultracentrifugation, and cholesterol (B) and TG (C) levels were determined in the VLDL, LDL, and HDL fractions. (D) Pooled plasma samples were fractionated by FPLC, and TG levels were determined in collected fractions. IDL, intermediate-density lipoprotein. (E) Effects of AAV_trib1 injection (1012 gc) on plasma total cholesterol (TC) and TG in Ldlr–/– mice and (F) LAhB-H mice (n = 6 per group). Human plasma apoB was determined in LAhB-H mice and was reduced after AAV_trib1 injection. (G) Distribution of cholesterol and TG in FPLC-fractionated plasma of AAV-injected LAhB-H mice. *P < 0.05, **P < 0.005, AAV_null versus AAV_trib1 groups.
To investigate a potential role for the LDL receptor (LDLR) in mediating the effects of Trib1 overexpression, we turned to Ldlr-deficient (Ldlr–/–) mice. In this hypercholesterolemic mouse model, Trib1 overexpression also led to significant reductions in cholesterol (27%) and TG (34%) (Figure 1E), suggesting that the LDLR does not significantly contribute to the effect of Trib1 on plasma lipids. We then studied a “humanized” Apobec1-knockout mouse expressing human apolipoprotein B-100 (apoB) on the background of LDLR haploinsufficiency (LAhB-H), which have a lipoprotein profile remarkably similar to that of humans (Supplemental Table 1). Trib1 overexpression in these mice significantly reduced plasma cholesterol, TG, and apoB by 25%, 26%, and 23%, respectively (Figure 1F). Further, the reduction in plasma apoB concentration was associated with reduced cholesterol and TG in VLDL and LDL, consistent with an overall reduction of apoB-containing lipoproteins (Figure 1G).
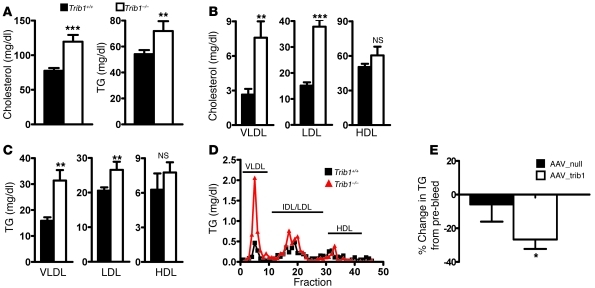
Next, we used Trib1-deficient (Trib1–/–) mice (11) to study the consequences of Trib1 loss of function on lipid metabolism in vivo. We found that Trib1 deficiency increased plasma cholesterol and TG levels by 54% and 33%, respectively (Figure 2A). The increase in plasma lipids was predominantly mediated by significantly elevated VLDL-C and LDL-C levels (Figure 2B) and significantly elevated VLDL-TG and LDL-TG levels (Figure 2C). This finding was further corroborated by plasma FPLC analysis, demonstrating an increased VLDL-TG peak in Trib1–/– mice (Figure 2D). Hence, our data demonstrate that hepatic Trib1 overexpression and Trib1 deficiency in mice have opposite effects on plasma cholesterol and TG. To lend further support to the concept that the plasma lipid phenotype of Trib1–/– mice is due specifically to the lack of hepatic Trib1, we reconstituted hepatic Trib1 expression in Trib1–/– mice using the AAV_Trib1 vector and demonstrated a 27% reduction in plasma TG, sufficient to effectively normalize plasma TG in Trib1–/– mice (Figure 2E).
Figure 2. Trib1 deficiency increases plasma lipid levels in mice.
Chow-fed Trib1–/– mice (n = 8) and littermate controls (Trib1+/+, n = 13) were phenotyped at 8 weeks of age (*P < 0.05, **P < 0.01, ***P < 0.001). (A) Plasma cholesterol and TG were elevated in Trib1–/– mice. VLDL, LDL, and HDL were isolated by sequential ultracentrifugation, and significant increases in cholesterol (B) and TG (C) were observed in VLDL and LDL of Trib1–/– mice. (D) Pooled plasma samples were separated by FPLC, and TG was determined in collected fractions. (E) Trib1–/– mice (n = 4) were injected with 1011 gc AAV_trib1 to reconstitute hepatic expression of Trib1. Plasma TG was measured 2 weeks after injection and the percentage change compared with that in control mice receiving AAV_null.
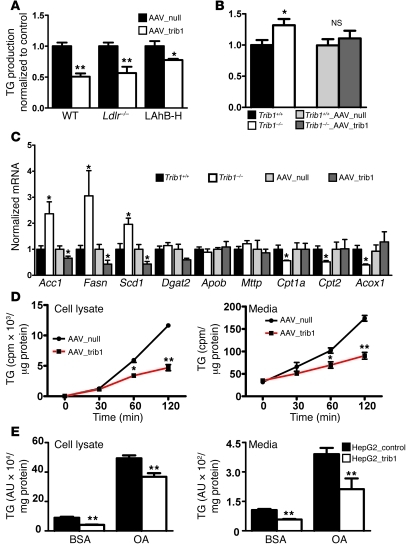
Since Trib1 expression affected levels of apoB-containing lipoproteins VLDL and LDL and reduced plasma apoB when overexpressed in LahB-H mice, we reasoned that hepatic Trib1 might influence VLDL production. To test this hypothesis, we assessed in vivo VLDL-TG production in Trib1-overexpressing and -deficient mice. Hepatic Trib1 overexpression in each of 3 different genetic backgrounds studied (wild-type, Ldlr–/–, and LAhB-H) significantly reduced VLDL-TG production (Figure 3A). In contrast, we observed significantly increased VLDL-TG production in Trib1–/– mice compared with controls (Figure 3B). When we reconstituted hepatic Trib1 expression in Trib1–/– mice by AAV_trib1 injection, VLDL-TG production decreased to levels of controls (Figure 3B). Further, NMR analysis was performed on pooled plasma samples from the VLDL-TG secretion study with LAhB-H mice. Trib1 overexpression reduced the concentration of newly synthesized VLDL particles (Supplemental Figure 3C) but did not change VLDL particle size (Supplemental Figure 3D). These data suggest that Trib1 affects overall apoB particle concentration without significantly altering particle composition.
Figure 3. Effects of Trib1 on hepatic VLDL production, gene expression, and lipogenesis.
VLDL-TG production was determined over 4 hours after Pluronic-407 injection in (A) AAV_null– or AAV_trib1–treated C57BL/6 (WT), Ldlr–/–, and LAhB-H mice (n = 6 per group) and in (B) Trib1+/+, Trib1–/– (n = 6 per group), and AAV_null– or AAV_trib1–treated Trib1+/+ and Trib1–/– mice (n = 4 per group). Values are expressed as relative changes compared with respective controls. (C) Quantitative RT-PCR: Control values (Trib1+/+ and AAV_null) were defined as 1, and changes in Trib1–/– and AAV_trib1 groups expressed as relative amounts compared with controls (n = 6–8 per group). (D and E) Ex vivo TG synthesis and secretion in primary hepatocytes and HepG2 cells overexpressing TRIB1 (HepG2_trib1) or control vector (HepG2_control). Hepatocytes of AAV_trib1– and AAV_null–treated mice and HepG2_trib1 and HepG2_control cells (experiments were performed in triplicate) were radiolabeled with [3H]1,2,3-glycerol. (D) [3H]TG was determined in media and cellular lysates after 30, 60, and 120 minutes in primary hepatocytes. (E) HepG2 cells were labeled in the presence of either BSA or BSA plus oleic acid (OA, 0.4 mM) for 4 hours before measurement of [3H]TG. (*P < 0.05, **P < 0.01).
To gain further insight into the mechanism by which Trib1 modulates VLDL production, we examined mRNA expression of various genes associated with hepatic lipid metabolism in livers from Trib1-overexpressing and -deficient mice (Figure 3C). While transcript levels of diacylglycerol O-acyltransferase 2 (Dgat2), microsomal triglyceride transfer protein (Mttp), and apolipoprotein B (Apob) were unchanged, a significant decrease in the expression of genes involved in fatty acid oxidation (Cpt1a, Cpt2, and Acox1) was observed in Trib1–/– mice. Moreover, we consistently noted significant downregulation of the key lipogenic genes acetyl-CoA carboxylase 1 (Acc1), fatty acid synthase (Fasn), and stearoyl-coenzyme A desaturase1 (Scd1) in mice overexpressing Trib1 and upregulation of the same genes in Trib1–/– mice (Figure 3C). Previous studies in mice demonstrated that expression changes of these genes can have profound effects on VLDL secretion and plasma cholesterol and TG levels (12–14). Despite the potential of these lipogenic genes to promote or retard hepatic TG storage and steatosis, we detected no changes in hepatic lipid content in mice with Trib1 overexpression or deficiency (Supplemental Figure 4).
To investigate whether the expression differences of lipogenic genes directly correlated with the amount of newly synthesized TG, we harvested primary hepatocytes from LAhB-H Trib1-overexpressing mice. We observed a significant reduction in incorporation of 3H-labeled glycerol into cellular TG as well as secretion of newly synthesized TG into the media (Figure 3D). Identical results were obtained in studies where we used human HepG2 hepatoma cells with TRIB1 overexpression as an independent model to demonstrate the effect of TRIB1 on lipogenesis (Figure 3E). Further, we performed metabolic labeling experiments with 35S-methionine in HepG2 cells to investigate the effect of TRIB1 overexpression on apoB secretion. With TRIB1 overexpression we observed a significant decrease in labeled apoB secretion (Supplemental Figure 5). Reduced availability or synthesis of lipids for apoB lipidation leads to cotranslational targeting of apoB for ER-associated degradation (ERAD) (15). Based on our in vitro findings and in vivo data on apoB from LahB-H mice, it is tempting to speculate that TRIB1-mediated regulation of hepatic lipid availability might alter the secretion of apoB particles via a mechanism involving ERAD.
Questions remain about the precise molecular mechanisms by which Trib1 modulates the transcription of lipogenic genes in liver cells. Using immunofluorescence imaging in stably transfected HepG2 cells, we found that the tribbles-1 protein localized to the nucleus of hepatocytes (Supplemental Figure 6). Tribbles proteins do not have a DNA-binding domain, but do have the ability to interact with transcription factors (e.g., members of the c/EBP family) in the nucleus and promote their degradation (11, 16). Future studies will need to identify the nuclear interactions of Trib1 in hepatocytes and elucidate their functional consequences with regard to lipogenesis and VLDL production.
In summary, GWAS identified SNPs near TRIB1 to be associated with plasma TG, LDL-C, and HDL-C levels and risk for myocardial infarction. Prior to our study, these associations had not been functionally validated, and potential mechanisms were unknown. Our studies in mice establish that hepatic expression of Trib1 regulates plasma VLDL and LDL cholesterol and TG levels. Further, we provide functional evidence for the underlying mechanism, namely, that hepatic Trib1 influences expression of lipogenic genes and lipogenesis, with subsequent effects on VLDL production and plasma lipids. Our studies are among the first to illustrate a strategy for how genotype-phenotype associations from GWASs can be validated using functional genetic approaches in mice and tissue culture to gain mechanistic insights into the action of novel disease genes. Since variants at the TRIB1 locus are directly associated with a reduced risk for myocardial infarction in humans, our results raise the prospect that TRIB1-modulating agents may represent a novel approach for the treatment of dyslipidemia and prevention of myocardial infarction.
Methods
An expanded Methods section is provided in Supplemental Methods.
Animals.
Male C57BL/6 and Ldlr-deficient mice (B6.129S7-Ldlrtm1Her/J) were obtained from The Jackson Laboratory. Apobec1–/–;APOB-Tg;Ldlr+/– (LAhB-H) mice were generated in our laboratory. The generation of Trib1–/– mice was previously described (11). All studies were performed in male mice, and littermates were used as controls. Animal experiments were reviewed and approved by the Institutional Animal Care and Use Committees of the Rockefeller University and University of Pennsylvania.
Trib1 liver overexpression.
Murine Trib1 cDNA was subcloned into a specialized vector for use by the University of Pennsylvania’s Vector Core for production of AAV8 particles expressing Trib1 (AAV_trib1), as described previously (17). Empty AAV8 particles (AAV_null) were used as control.
Blood parameters.
Plasma was obtained from fasted mice. Cholesterol, TG, and ALT levels were determined using colorimetric assays (Roche Diagnostics, Thermo Scientific). Plasma lipoproteins were isolated by sequential ultracentrifugation. FPLC was carried out as described previously (18).
In vivo VLDL secretion assay.
VLDL-TG secretion rates were determined in fasted mice over a 4-hour time period after injection of Pluronic-407 (1 mg/g body weight) (18). NMR lipoprotein analysis of pooled plasma samples was performed by LipoScience.
Real-time PCR analysis.
Human MTC Multiple Tissue cDNA Panels I and II (Clontech) were used for TRIB1 expression analysis in 16 human tissues. In mouse studies, cDNA was generated from total RNA extracted from frozen livers using standard protocols. Quantitative RT-PCR was carried out on an ABI PRISM 7900 Sequence Detector (Applied Biosystems). Relative expression differences were calculated using the comparative Ct (ΔΔCt) method and β-actin as housekeeping gene.
Generation of HepG2 cells with stable TRIB1 overexpression.
HepG2 cells overexpressing human TRIB1 with a C-terminal FLAG-tag (HepG2_trib1) or cell-surface marker ΔLNGFR (HepG2_control) were generated using recombinant retroviruses as described previously (19, 20).
Preparation of primary mouse hepatocytes.
Hepatocytes were isolated from LAhB-H mice 21 days after injection of either AAV_trib1 or AAV_null particles as described (21).
TG synthesis in mouse primary hepatocytes and HepG2 cells.
Primary hepatocytes of AAV_trib1– and AAV_null–treated mice and HepG2_trib1 and HepG2_control cells were cultured in the presence of 0.4 mM oleic acid conjugated with BSA or BSA only (HepG2 cells) and 5 μCi [3H]-1,2,3 glycerol (American Radiolabeled Chemicals) for up to 4 hours. Cellular lipids were extracted with hexane:isopropanol (3:2, vol/vol), and lipids from collected media were extracted according to the method of Bligh and Dyer (22). After separation of lipid fractions by TLC, the TG band was excised and radioactivity measured by scintillation counting. In experiments using HepG2 cells, TLC plates were exposed on storage phosphor screens. The TG bands were analyzed by densitometry, and values were expressed as arbitrary units (AU). Data were normalized to total cellular protein.
Statistics.
All data represent mean ± SEM. Results were analyzed by 2-tailed Student’s t test or ANOVA with Dunnett’s post-test, when multiple comparisons to control group were made. Statistical significance was defined as P < 0.05.
Supplementary Material
Acknowledgments
We thank Jeffrey Billheimer, Deborah Cromley, Edwige Eduoard, Hui Li, Dawn Marchadier, John Millar, Valeska Redon, Alanna Strong, Mao-Sen Sun, Aisha Wilson, and Sonja Stadler for assistance and suggestions. We acknowledge the National Heart, Lung and Blood Institute Gene Therapy Resource Program for providing support for viral vector production as well as the Vector Core laboratory of the University of Pennsylvania for producing the vectors. This work was supported in part by P01-HL059407 and RC2-HL101864 from the NIH and a “Freedom to Discover” Unrestricted Cardiovascular Research Grant from Bristol-Myers Squibb (to D.J. Rader). R. Burkhardt was supported by a fellowship of the Deutsche Forschungsgemeinschaft (BU2263/1-1), V.D. Fedorov by NIH MSTP grant GM07739, and S. Toh by an American Heart Association fellowship (0315294T) and the Institute for Translational Medicine and Therapeutics, University of Pennsylvania.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(12):4410–4414. doi:10.1172/JCI44213.
References
- 1.Kathiresan S, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40(2):189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kathiresan S, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41(1):56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willer CJ, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aulchenko YS, et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41(1):47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabatti C, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41(1):35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couzin-Frankel J. Major heart disease genes prove elusive. Science. 2010;328(5983):1220–1221. doi: 10.1126/science.328.5983.1220. [DOI] [PubMed] [Google Scholar]
- 8.McClellan J, King MC. Genetic heterogeneity in human disease. Cell. 2010;141(2):210–217. doi: 10.1016/j.cell.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Kiss-Toth E, et al. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J Biol Chem. 2004;279(41):42703–42708. doi: 10.1074/jbc.M407732200. [DOI] [PubMed] [Google Scholar]
- 10.Kawano K, Qin SC, Lin M, Tall AR, Jiang XC. Cholesteryl ester transfer protein and phospholipid transfer protein have nonoverlapping functions in vivo. J Biol Chem. 2000;275(38):29477–29481. doi: 10.1074/jbc.M003523200. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, et al. Enhanced TLR-mediated NF-IL6 dependent gene expression by Trib1 deficiency. J Exp Med. 2007;204(9):2233–2239. doi: 10.1084/jem.20070183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen P, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297(5579):240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 13.Mao J, et al. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 2006;103(22):8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarthy MV, et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1(5):309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res. 2009;50 suppl:S162–S166. doi: 10.1194/jlr.R800090-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dedhia PH, et al. Differential ability of Tribbles family members to promote degradation of C/EBPalpha and induce acute myelogenous leukemia. Blood. 2010;116(8):1321–1328. doi: 10.1182/blood-2009-07-229450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitajima K, Marchadier DH, Miller GC, Gao GP, Wilson JM, Rader DJ. Complete prevention of atherosclerosis in apoE-deficient mice by hepatic human apoE gene transfer with adeno-associated virus serotypes 7 and 8. Arterioscler Thromb Vasc Biol. 2006;26(8):1852–1857. doi: 10.1161/01.ATV.0000231520.26490.54. [DOI] [PubMed] [Google Scholar]
- 18.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005;46(9):2023–2028. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Gallardo HF, Tan C, Ory D, Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90(3):952–957. [PubMed] [Google Scholar]
- 20.Stephan MT, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13(12):1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 21.Millar JS, et al. Short-term overexpression of DGAT1 or DGAT2 increases hepatic triglyceride but not VLDL triglyceride or apoB production. J Lipid Res. 2006;47(10):2297–2305. doi: 10.1194/jlr.M600213-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.