Summary
The centrosome is the principal microtubule organizing center (MTOC) of animal cells [1]. Accurate centrosome duplication is fundamental for genome integrity and entails the formation of one procentriole next to each existing centriole, once per cell cycle. The procentriole then elongates to eventually reach the same size as that of the centriole. The mechanisms that govern elongation of the centriolar cylinder and their potential relevance for cell division are not known. Here, we show that the SAS-4-related protein CPAP [2] is required for centrosome duplication in cycling human cells. Furthermore, we demonstrate that CPAP overexpression results in the formation of abnormal long centrioles. This also promotes formation of more than one procentriole in the vicinity of such overly long centrioles, eventually resulting in the presence of supernumerary MTOCs. This in turn leads to multipolar spindle assembly and cytokinesis defects. Overall, our findings suggest that centriole length must be carefully regulated to restrict procentriole number and thus ensure accurate cell division.
Results and discussion
CPAP is required for centriole duplication in cycling human cells
The centrosome comprises the centrioles and the pericentriolar material (PCM). Microtubule nucleation occurs within the PCM, whereas centrioles are crucial for organizing the PCM [3, 4]. A typical centriole in human cells is a ∼200×500-nm cylinder whose walls are formed by nine stable microtubule blades [1]. These blades consist of microtubule triplets from the ‘proximal’ end of the centriole until approximately two thirds of its length and of microtubule doublets thereafter until the ‘distal’ end. Mature centrioles harbor electron-dense sub-distal and distal appendages that mediate microtubule anchorage in proliferating cells and attachment of the centriole to the plasma membrane during ciliogenesis, respectively [1].
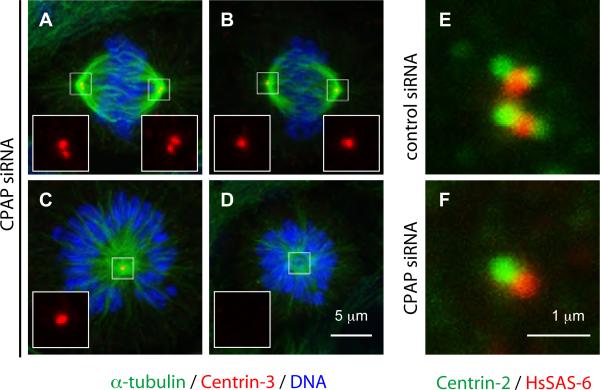
Centrosome duplication typically begins at the G1 to S transition when one procentriole forms next to the proximal end of each of the two centrioles [5, 6]. A handful of proteins has been identified initially in C. elegans as being essential for procentriole formation, including SAS-4, which promotes addition of centriolar microtubules [4, 7, 8]. siRNA-mediated depletion of the SAS-4-related protein CPAP interferes with centriolar amplification in S-phase arrested human cells [9]. Accordingly, we found that depletion of CPAP prevents centriole duplication in cycling human cells (Fig. 1A-D, Fig. S1A-F). Furthermore, partial CPAP depletion leads to asymmetric distribution of the PCM and the core centriolar protein Centrin-3 between the two spindle poles (Fig. S2A-B). Similar asymmetry and structurally defective centrioles were observed after partial depletion of SAS-4 in C. elegans [4], raising the possibility that the amount of PCM is proportional to centriole size also in human cells. Importantly, we found that the proximal procentriolar protein HsSAS-6 [9, 10] is recruited and maintained despite CPAP depletion (Fig. 1E-F, Fig. S2C-E). By contrast, the CPAP signal is confined to the parental centriole in cells depleted of HsSAS-6 (Fig. S2F-G). Together, these results indicate that CPAP acts downstream of HsSAS-6 during procentriole formation in cycling human cells, in line with findings in C. elegans [8, 11].
Figure 1. CPAP is required for centriole duplication in cycling cells.
(A-D) U2-OS cells transfected with CPAP siRNAs for 72 h and stained for α-tubulin (green), Centrin-3 (red). In this and other figure panels, DNA is viewed in blue and insets show higher magnification views of denoted regions of interest. Two representative bipolar (A-B) and monopolar (C-D) figures are shown. (E-F) U2-OS cells treated with the indicated siRNAs for 72 h, a synchronized by a thymidine block for the last 24 h and stained with antibodies against Centrin-2 (green) and HsSAS-6 (red). 73% of cells treated with control siRNAs displayed HsSAS-6 signals near Centrin-foci (N=226), compared to 76% of cells treated with CPAP siRNA with a single Centrin focus (N=54).
CPAP overexpression induces threads with stable microtubules and centriolar markers
To gain insights into the mechanisms by which CPAP promotes centriole formation, we provided cells with excess CPAP. Strikingly, overexpression of untagged, GFP-tagged or mCherry-tagged CPAP results in the appearance of CPAP-containing threads in the vicinity of the nucleus in U2-OS cells (Fig. 2A-B and data not shown). Such threads are also observed in other cell lines (Fig. S3D-O), as well as in primary human umbilical vein endothelial cells (HUVECs) (Fig. S3P-S).
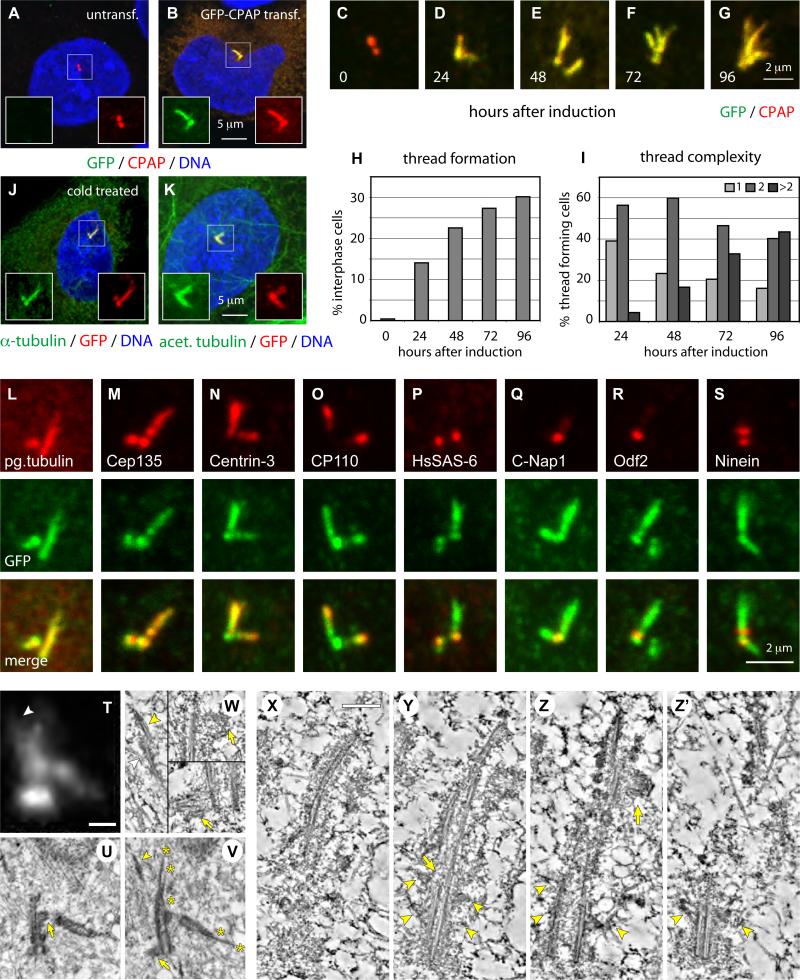
Figure 2. CPAP overexpression induces the formation of abnormal elongated centrioles.
(A-B) U2-OS cells not transfected (A) or transiently transfected with GFP-CPAP for 72 h (B) and stained with antibodies against GFP (green) and CPAP (red).
(C-I) i-GFP-CPAP cells induced with doxycycline for 0, 24, 48, 72 or 96 h prior to fixation and stained with antibodies against GFP (green) and CPAP (red). Representative thread configurations are shown in (C-G). Panel (H) shows the frequency of threads (N>250 at each time point), panel (I) their complexity, as determined by the number of free ends per thread.
(J-K) U2-OS cells transiently transfected with GFP-CPAP for 72 h, incubated for 1 h at 4°C and stained with antibodies against α-tubulin (green) and GFP (red) (J), or with antibodies against acetylated tubulin (green) and GFP (red).
(L-S) Representative high magnification images of threads from i-GFP-CPAP cells at different stages of the cell cycle induced with doxycycline for 48 h and stained with antibodies against GFP (green) and polyglutamylated (pg.) tubulin (L), Cep135 (M), Centrin-3 (N), CP110 (O), HsSAS-6 (P), C-Nap1 (Q), Odf2 (R) or Ninein (S), respectively (each in red).
(T) i-GFP-CPAP cell 72 hours after induction with doxycycline. Maximal-intensity projection of a through-focus series. Size bar is 500 nm.
(U-V) Two sequential 100-nm thin EM sections through the central part of the centrosome in the same cell. Both mother and daughter centrioles are elongated and their distal ends are distorted (asterisks in V mark distal ends of individual microtubule blades). Two procentrioles (arrows) associate with the mother centriole, one near the proximal end, one near the distal end. There is also an electron-dense thread located ∼600 nm away from the distal end of the mother centriole (arrowhead in V), which corresponds to a lower-intensity CPAP-GFP signal (arrowhead in T).
(W) Individual 2.6-nm slices from the tomogram of the section presented in (V). Tomography reveals that the electron-dense thread denoted with an arrowhead in (V) corresponds to two parallel microtubule doublets (arrowheads in 1) not connected to the centriole. Both procentrioles are poorly organized and contain only individual microtubules (arrows in 2 and 3).
(X-Z’) 15-nm tomography slices illustrating the structure of an elongated mother centriole (see Fig. S4 for complete series of 100-nm thin sections; see also Movie S1). The proximal part of the centriole is cylindrical (Y-Z’), while the distal part is severely distorted. The blades are formed by microtubule doublets and some of the blades are discontinuous (arrow in Y). The entire length of this centriole is embedded in prominent electron-opaque pericentriolar material (PCM). Several sub-distal appendages are present on one side of the centriole (arrowheads in Y-Z’, see also Fig. S4E). In addition to the regular procentriole (see Fig. S4I-J), an ectopic procentriole is present near the distal end of this centriole (arrow in Z). Size bar is 250 nm.
To further analyze the threads, we generated a U2-OS cell line dubbed i-GFP-CPAP that stably maintains a plasmid allowing doxycycline inducible GFP-CPAP expression. We found that the frequency of i-GFP-CPAP cells harboring threads steadily increases upon induction with doxycycline (Fig. 2H). Moreover, threads tend to be longer and more complex at later time points (Fig. 2C-G, I). Threads contain acetylated tubulin, a hallmark of stable microtubules (Fig. 2K) [12]. Accordingly, threads are resistant to microtubule depolymerization (Fig. 2J).
These observations prompted us to investigate whether threads are related to centrioles. We found that threads contain polyglutamylated tubulin, Cep135 and Centrin-3 (Fig. 2L-N), which are all characteristic of centriolar cylinders [13-15]. CP110, which normally localizes to the distal part of procentrioles and centrioles [16], is present at the end of threads (Fig. 2O) or along their length (data not shown). In contrast, HsSAS-6 and C-Nap1, which marks the base of the centriole [17], are present at one end of the threads but never along their length (Fig. 2P-Q). Odf2 and Ninein, which mark the appendages of the mother centriole [18, 19], also associate with the threads but do not extend along their length (Fig. 2R-S). Overall, we conclude that threads formed upon CPAP overexpression bear proteins that are characteristic of centriolar cylinders.
Threads are abnormal elongated centrioles
To characterize threads at the ultrastructural level, we employed same-cell correlative light microscopy/serial-section electron-microscopy (EM) on twelve i-GFP-CPAP cells with prominent threads 72 hr after doxycycline induction (Fig. 2T-Z’, Fig. S4; Movie S1). For two of these cells, select sections were further investigated by dual-axis EM tomography. These analyses revealed that threads correspond to abnormal centrioles that reach up to 3.5 μm in length (Fig. 2U-V, 2X-Z; Fig. S4E-F), seven times the length of normal centrioles (Fig. S4I, centriole 3). Nine cells contained overly long centrioles as well as centrioles of normal length (see Fig. S4), whereas in the remaining three cells all centrioles were overly long (see Fig. 2U-V). The structural organization of the proximal end of overly long centrioles appears largely normal (Fig. 2Y-Z’), although sometimes microtubule doublets are present instead of triplets (Movie S1). By contrast, distal ends of overly long centrioles are distorted, with microtubule blades terminating at different distances from the proximal end (Fig. 2V, stars).
EM revealed additional abnormalities in the organization of elongated centrioles. Sub-distal appendages, which are normally organized in a tight ring around the mother centriole, are positioned more randomly along overly long mothers, with several sub-distal appendages sometimes present on one side (arrowheads in Fig. 2Y-Z’, Fig. S4). We also consistently found microtubule doublets decorated with electron-opaque material that are not directly connected to overly elongated centrioles (Fig. 2V-W, arrowheads).
Excess elongation of the procentriole or the centriole can initiate in G2
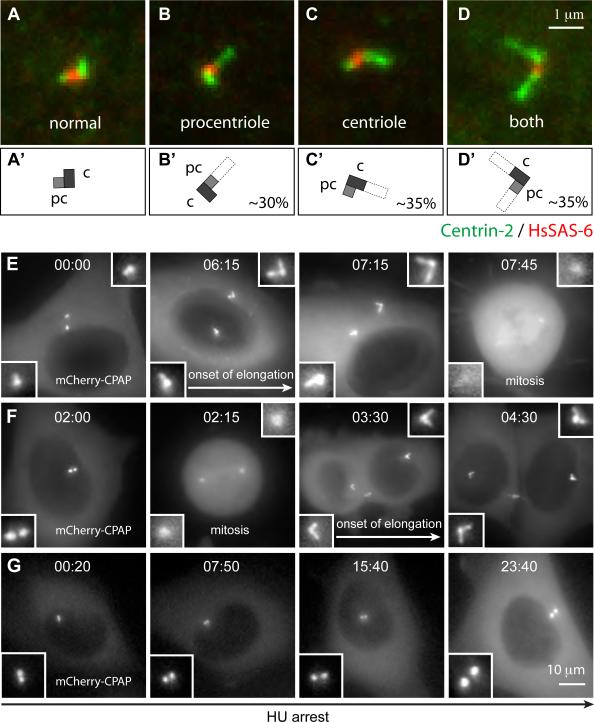
We addressed whether there is excess elongation strictly of the centriole or also of the procentriole. Since threads persist during mitosis (Fig. S3A-C) and thus might be inherited from previous cell cycles, we examined prophase i-GFP-CPAP cells 18 h after induction to ensure analysis within the first cell cycle after overexpression. Using antibodies against HsSAS-6 to mark the proximal part of the procentriole and against Centrin-2 [20] to label the distal part of procentrioles and centrioles, as well as threads, we assessed which centriolar cylinder underwent excess elongation. This analysis revealed excess elongation of the procentriole in ∼30% of cases, of the centriole in ∼35% of cases, and of both centriolar cylinders in the remaining ∼35% (Fig. 3A-D, N=34). As expected for elongation stemming also from the centriole, depletion of HsSAS-6 does not prevent thread formation in cells overexpressing CPAP (data not shown). We conclude that excess CPAP can result in abnormal elongation of both procentriole and centriole.
Figure 3. Elongation of the procentriole or the centriole occurs in G2 or shortly thereafter.
(A-D) i-GFP-CPAP cells induced with doxycycline for 18 h and stained with antibodies against Centrin-2 (green) and HsSAS-6 (red). Centrioles (c) and procentrioles (pc) are drawn schematically, and overly long centriolar cylinders are indicated in white (A’-D’). Percentages represent fraction of each sort among centrosomes with threads. In 16/50 thread-bearing centrosomes, the single thread could not be unambiguously assigned to either procentriole or centriole, resulting in a slight underestimate of the frequency of single elongation events. Note also that 6% of uninduced prophase cells exhibited threads, all stemming from the centriole.
(E-F) Live imaging of cycling i-mCherry-CPAP cells. In this and other live imaging panels, time is denoted in hours:minutes, with 00:00 corresponding to the onset of the time-lapse recording. Threads are first detected at 06:15 in (E), before mitosis, and at 03:30 in (F), after mitosis. See also Movies S2 and S3.
(G) Live imaging of i-mCherry-CPAP cell held in 2 mM hydroxyurea (HU) for 24 hours prior to incubation with both HU and doxycycline for 24 hours and onset of filming. Note that threads do not form during the entire duration of the recording, which also indicates that thread formation in cycling cells is not simply due to continued exposure to elevated CPAP levels. See also Movie S4. This results was observed in 4/5 cells held in HU, whereas 1/5 cell formed threads as CPAP levels became extremely elevated before cell death. Related results were obtained by immunofluorescence analysis of cells held in S phase: 22% of cycling i-GFP-CPAP U2-OS cells (N=154) harbored threads 48 hours after induction by doxycyclin, compared to 3% (N=125) when held in thymidine.
We sought to determine when excess elongation initiates during the cell cycle. We generated a doxycycline-inducible U2-OS cell line dubbed i-mCherry-CPAP that proved well suited for long-term live imaging due to lower phototoxicity compared to i-GFP-CPAP. Individual cells were imaged by combinational 3-D fluorescence/DIC time-lapse microscopy starting 12−20 h after induction. We analyzed sixteen cells in which centrosomes initially seemed normal but then underwent abnormal elongation. In eight cells, the first signs of excess elongation are observed before mitosis, during late G2 (Fig. 3E), with additional elongation during the ensuing G1 (Movie S2). The other eight cells enter mitosis with apparently normal centrosomes, but exhibit elongated structures immediately thereafter (Fig. 3F, Movie S3). The limited resolution of light microscopy does not allow us to rule out that excess elongation always initiates during late G2 but is not always detected. We found also that threads usually do not form in cells arrested in S phase (Fig. 3G, Movie S4). It is noteworthy that in cycling cells the levels of endogenous CPAP increase as cells progress through G2 (Fig. S5), when elongation of procentrioles normally takes place. Overall, we conclude that CPAP overexpression results in excess elongation of centriolar cylinders starting in G2.
CPAP overexpression results in supernumerary procentrioles and disorganized centrosomes
Given that regular centrioles recruit the PCM [3, 4], we reasoned that overly long centrioles could also recruit PCM. Accordingly, we found that the PCM components γ-tubulin [21], pericentrin [22], NEDD1 [23] and Cep192 [24, 25] almost invariably decorate the threads formed upon GFP-CPAP overexpression in U2-OS cells, even at early time points after induction (Fig. 4A-D; Fig. S3D-O and data not shown). As anticipated from these observations, depolymerization/regrowth experiments established that microtubules can nucleate from threads (Fig. 4D, arrows). Excess PCM facilitates procentriole formation in S-phase arrested Chinese Hamster Ovary (CHO) cells [26], raising the possibility that the enlarged PCM surrounding threads upon CPAP overexpression could likewise promote procentriole formation. Intriguingly, we observed that six of the twelve cells examined by serial-section EM contain more than the maximal number of two centrioles and two procentrioles normally present in a cell. In three cases, in addition to the procentriole at the expected position near the proximal end of the centriole (Fig. 2V, arrow), there is an ectopic procentriole in the vicinity of the overly elongated segment (Fig. 2W, Z, arrows). Furthermore, serial-section EM revealed that all procentrioles contain microtubule singlets (Fig. 2W, arrows) or doublets (data not shown) and that they reside inside clouds of PCM (see Fig. S4F). The presence of supernumerary procentrioles was confirmed by immunofluorescence analysis, with ectopic procentrioles emanating from the side of threads being marked at their proximal end by HsSAS-6 (Fig. 4E, arrow 3). We found that such additional HsSAS-6 foci are present in 16% of cells with threads 48 hours after induction (N=74) and in 36% of them 72 hours after induction (N=107). Overall, our analyses indicate that abnormal elongated centrioles recruit PCM and promote the formation of increasingly more supernumerary procentrioles, thus eventually resulting in centrosomes with severely disorganized architecture.
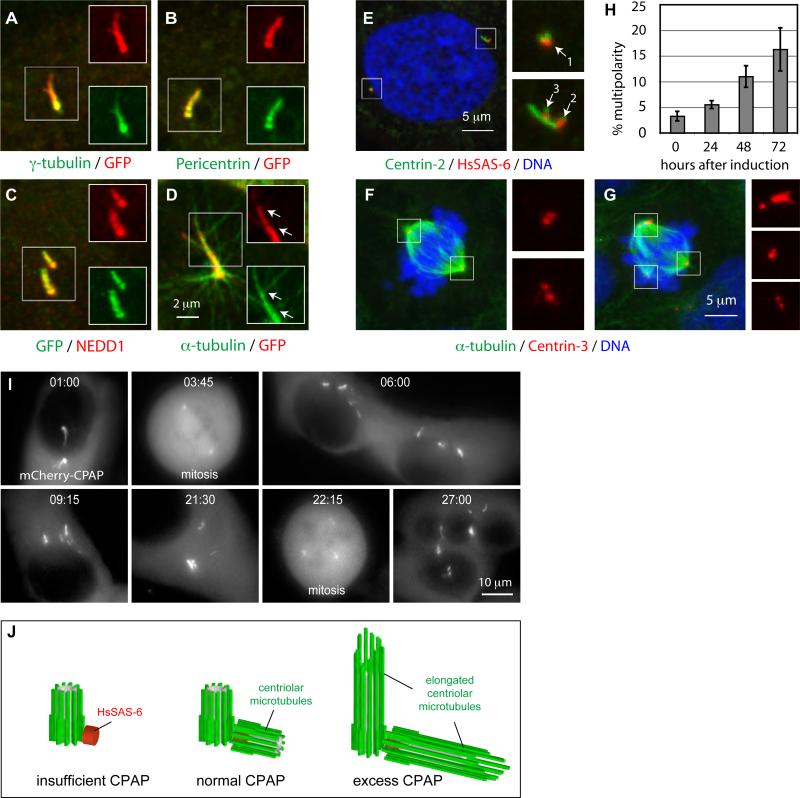
Figure 4. CPAP overexpression results in excess PCM recruitment, supernumerary procentrioles and defective cell division.
(A-C) Representative high magnification images from i-GFP-CPAP cells induced with doxycycline for 48 h and stained with antibodies against GFP (red) and γ-tubulin (A), pericentrin (B) or NEDD1 (C) (each in green).
(D) U2-OS cell transiently transfected with GFP-CPAP for 72 h, subjected to cold-induced depolymerization for 1 h and warmed up to 37°C for 4 min prior to staining with antibodies against α-tubulin (green) and GFP (red). Arrows point to microtubules emanating from threads.
(E) i-GFP-CPAP cells induced with doxycycline for 72 h and stained with antibodies against Centrin-2 (green) and HsSAS-6 (red). Arrows point to procentrioles (1 and 2 correspond to regular procentrioles and 3 to an ectopic procentriole formed in the vicinity of the overly long centriole).
(F-G) i-GFP-CPAP cells induced with doxycycline for 72 h and stained with antibodies against α-tubulin (green), Centrin-3 (red). Note that some cells with threads assemble a bipolar spindle (see Fig. S3B), and that some cells that do not harbor a thread assembled a multipolar spindle (data not shown).
(H) Fraction of multipolar spindles among i-GFP-CPAP cells induced with doxycycline for 0, 24, 48, or 72 h prior to fixation and staining with antibodies against α-tubulin. Data from two independent experiments were pooled (n>100 for each).
(I) Selected fluorescence (maximal-intensity projections) frames from time-lapse recordings of i-mCherry-CPAP cell. Note bipolar spindle assembly at 03:45, presence of multiple individual threads in the ensuing G1 (06:00) and multipolar spindle assembly at the next cell division (22:15). See also Movie S5, where corresponding DIC frames are shown in addition.
(J) CPAP is critical for centriole elongation. Whereas CPAP depletion (left) results in a procentriole that harbors HsSAS-6 but not more distal centriolar proteins, and presumably no microtubules, excess CPAP results in overly long centriolar cylinders (right). Note that HsSAS-6 is present in all three cases, but is less visible inside the centriole cylinder in the two rightmost panels.
CPAP overexpression results in multipolar spindle assembly
We next addressed the consequences of such abnormal figures for cell division. Fixed cell analysis established that ∼16% of cells assemble a multipolar spindle 72 h after induction of GFP-CPAP expression, in contrast to ∼2.5% in control cells (Fig. 4F-H, N>100 in each case). Accordingly, live cell imaging revealed that mitosis in cells overexpressing mCherry-CPAP and harboring threads were often multipolar (15/57 cells versus 2/35 in control cells). Furthermore, three cells with abnormal centrosomes did not complete mitosis and died (data not shown). Finally, although a bipolar spindle was assembled in the remaining 39 cells, cytokinesis failed in three of these (Fig. S6). Thus, overall, mitosis is defective in ∼35% of cells with elongated centrioles.
To gain further insight into the cause of these mitotic abnormalities, we followed the progeny of single cells for at least two consecutive cell cycles. In seven of ten such lineages, the first mitosis was bipolar even when both centrosomes comprised long and complex threads, presumably because they remain in two discrete complexes (Fig. 4I, 03:45; Movie S5). However, during the ensuing G1, threads were released from the common complexes, with each thread or fragment thereof behaving as an individual unit (Fig. 4I, 06:00). These individualized units assembled a multipolar spindle during the subsequent mitosis (Fig. 4I, 22:15). Since the overall levels of mCherry-CPAP are similar during bipolar and multipolar spindle assembly in such cells (Movie S5), multipolar spindle assembly does not appear to result merely from excess CPAP. Furthermore, since the preceding mitosis was bipolar despite threads, they alone cannot be responsible for multipolarity. Instead, our analysis indicates that accumulation of supernumerary MTOCs, presumably due to the presence of ectopic procentrioles and the fragmentation of overly long centrioles, is at the root of multipolar spindle assembly upon CPAP overexpression.
On the control of centriole length
The importance of centriole length has received little attention thus far. Whereas centriole elongation can occur within minutes in rapidly dividing embryonic systems [27, 28], this process occurs in somatic cells at a rate that is at least an order of magnitude slower than that of spontaneous tubulin polymerization, suggesting that it is not driven merely by microtubule polymerization. Here, we demonstrate that overexpression of the SAS-4-related protein CPAP results in formation of overly long centrioles in human cells. We also uncover that this is accompanied by PCM recruitment along overly long centrioles and formation of supernumerary procentrioles, ultimately leading to multipolar spindle assembly and defective cell division.
How CPAP overexpression results in excess elongation of the centriolar cylinder is not yet clear. In C. elegans, electron-tomography indicates that SAS-4 is required for procentriole formation after SAS-6 recruitment and before centriolar microtubules are added [8]. Our findings that CPAP is needed for procentriole formation after HsSAS-6 recruitment and that excess elongation occurs following CPAP overexpression are compatible with the notion that this protein somehow promotes incorporation of centriolar microtubules (Fig. 4J).
Formation of threads within the centrosome has been reported in U2-OS cells following depletion of CP110 or of the CP110-associated protein Cep97 and was interpreted as corresponding to cilia-like structures [16]. We noticed several similarities between the threads forming in U2-OS cells in the absence of CP110 and upon overexpression of CPAP. For example, in both cases, threads contained Centrin, a centriolar protein not reported to be present in cilia. Therefore, we reinvestigated the phenotype of U2-OS cells depleted of CP110 using serial-section EM, and found abnormal elongated centrioles and microtubule doublets not connected to them, as upon CPAP overexpression (Fig. S4M-P). This suggests that CP110 also modulates centriole length in cycling U2-OS cells, which is compatible with the study by Nigg and colleagues in this issue of Current Biology reporting that CPAP and CP110 have antagonistic roles in regulating centriole length. Elongated structures that harbor centriolar proteins also form upon overexpression of the centriolar protein POC1, although they have not been characterized at the ultrastructural level [29]. In conclusion, our work suggests that centriole length must be restricted to prevent disruption of centriole architecture and faulty cell division. Therefore, proper regulation of proteins such as CPAP, CP110 or POC1 that set centriole length contributes to ensuring genome integrity.
Supplementary Material
Acknowledgements
We are grateful to Tang Tang for CPAP constructs, to Petr Strnad for advice in generating the cell lines, to Natsuko Imaizumi and Gaetana Restivo for help with primary cultures, to Michel Bornens, Bernard Eddé, Hiroaki Ishikawa, Ryoko Kuriyama, Jens Lüders, Erich Nigg, Laurence Pelletier, Jeff Salisbury and Viesturs Simanis for their gift of antibodies, to Erich Nigg and Tang Tang for discussing results prior to publication, as well as to Virginie Hachet, Daiju Kitagawa and Petr Strnad for useful comments on the manuscript. We acknowledge use of Wadsworth Center's EM Core facility and the technical assistance of Yimin Dong. Supported by grants from Oncosuisse (OCS KLS 02024-02-2007, to PG), from the NIH (GM59363, to AK and GM06627 to B.F.M) and the BBSRC (BB/D012201/1, to MM).
References
- 1.Azimzadeh J, Bornens M. Structure and duplication of the centrosome. Journal of Cell Science. 2007;120:2139–2142. doi: 10.1242/jcs.005231. [DOI] [PubMed] [Google Scholar]
- 2.Hung LY, Tang CJ, Tang TK. Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex. Mol Cell Biol. 2000;20:7813–7825. doi: 10.1128/mcb.20.20.7813-7825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Eddé B, Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkham M, Müller-Reichert T, Oegema K, Grill S, Hyman A. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell. 2003;112:575–587. doi: 10.1016/s0092-8674(03)00117-x. [DOI] [PubMed] [Google Scholar]
- 5.Strnad P, Gönczy P. Mechanisms of procentriole formation. Trends in Cell Biology. 2008 doi: 10.1016/j.tcb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- 7.Leidel S, Gönczy P. SAS-4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Developmental Cell. 2003;4:431–439. doi: 10.1016/s1534-5807(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 8.Pelletier L, O'Toole E, Schwager A, Hyman A, Müller-Reichert T. Centriole assembly in Caenorhabditis elegans. Nature. 2006;444:619–623. doi: 10.1038/nature05318. [DOI] [PubMed] [Google Scholar]
- 9.Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Developmental Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gönczy P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Developmental Cell. 2007;13:203–213. doi: 10.1016/j.devcel.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delattre M, Canard C, Gönczy P. Sequential protein recruitment in C. elegans centriole formation. Curr Biol. 2006;16:1844–1849. doi: 10.1016/j.cub.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 12.Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff A, de Nechaud B, Chillet D, Mazarguil H, Desbruyeres E, Audebert S, Edde B, Gros F, Denoulet P. Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol. 1992;59:425–432. [PubMed] [Google Scholar]
- 14.Middendorp S, Küntziger T, Abraham Y, Holmes S, Bordes N, Paintrand M, Paoletti A, Bornens M. A role for centrin 3 in centrosome reproduction. J Cell Biol. 2000;148:405–416. doi: 10.1083/jcb.148.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta T, Essner R, Ryu JH, Palazzo RE, Uetake Y, Kuriyama R. Characterization of Cep135, a novel coiled-coil centrosomal protein involved in microtubule organization in mammalian cells. J Cell Biol. 2002;156:87–99. doi: 10.1083/jcb.200108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and CP110 suppress a cilia assembly program. Cell. 2007;130:678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol. 1998;141:1563–1574. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa H, Kubo A, Tsukita S, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7:517–524. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- 19.Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. Journal of Cell Science. 2000;113(Pt 17):3013–3023. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- 20.Sanders MA, Salisbury JL. Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol. 1994;124:795–805. doi: 10.1083/jcb.124.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. Journal of Cell Science. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- 22.Dictenberg JB, Zimmerman W, Sparks CA, Young A, Vidair C, Zheng Y, Carrington W, Fay FS, Doxsey SJ. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lüders J, Patel U, Stearns T. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat Cell Biol. 2006;8:137–147. doi: 10.1038/ncb1349. [DOI] [PubMed] [Google Scholar]
- 24.Zhu F, Lawo S, Bird A, Pinchev D, Ralph A, Richter C, Müller-Reichert T, Kittler R, Hyman AA, Pelletier L. The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr Biol. 2008;18:136–141. doi: 10.1016/j.cub.2007.12.055. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Ferreria MA, Rath U, Buster DW, Chanda SK, Caldwell JS, Rines DR, Sharp DJ. Human Cep192 is required for mitotic centrosome and spindle assembly. Curr Biol. 2007;17:1960–1966. doi: 10.1016/j.cub.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Loncarek J, Hergert P, Magidson V, Khodjakov A. Control of daughter centriole formation by the pericentriolar material. Nat Cell Biol. 2008;10:322–328. doi: 10.1038/ncb1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palazzo RE, Vaisberg E, Cole RW, Rieder CL. Centriole duplication in lysates of Spisula solidissima oocytes. Science. 1992;256:219–221. doi: 10.1126/science.1566068. [DOI] [PubMed] [Google Scholar]
- 28.Pelletier L. Centrioles: duplicating precariously. Curr Biol. 2007;17:R770–773. doi: 10.1016/j.cub.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Keller LC, Geimer S, Romijn E, Yates J, 3rd, Zamora I, Marshall WF. Molecular Architecture of the Centriole Proteome: The Conserved WD40 Domain Protein POC1 Is Required for Centriole Duplication and Length Control. Mol Biol Cell. 2008 doi: 10.1091/mbc.E08-06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.