Abstract
Necrotizing enterocolitis (NEC) is a devastating intestinal disease of neonates, and clinical studies suggest the beneficial effect of probiotics in NEC prevention. Recently, we have shown that administration of Bifidobacterium bifidum protects against NEC in a rat model. Intestinal apoptosis can be suppressed by activation of cyclooxygenase-2 (COX-2) and increased production of prostaglandin E2 (PGE2). The present study investigates the effect of B. bifidum on intestinal apoptosis in the rat NEC model and in an intestinal epithelial cell line (IEC-6), as a mechanism of protection against mucosal injury. Premature rats were divided into the following three groups: dam fed, hand fed with formula (NEC), or hand fed with formula supplemented with B. bifidum (NEC + B. bifidum). Intestinal Toll-like receptor-2 (TLR-2), COX-2, PGE2, and apoptotic regulators were measured. The effect of B. bifidum was verified in IEC-6 cells using a model of cytokine-induced apoptosis. Administration of B. bifidum increased expression of TLR-2, COX-2, and PGE2 and significantly reduced apoptosis in the intestinal epithelium of both in vivo and in vitro models. The Bax-to-Bcl-w ratio was shifted toward cell survival, and the number of cleaved caspase-3 positive cells was markedly decreased in B. bifidum-treated rats. Experiments in IEC-6 cells showed anti-apoptotic effect of B. bifidum. Inhibition of COX-2 signaling blocked the protective effect of B. bifidum treatment in both in vivo and in vitro models. In conclusion, oral administration of B. bifidum activates TLR-2 in the intestinal epithelium. B. bifidum increases expression of COX-2, which leads to higher production of PGE2 in the ileum and protects against intestinal apoptosis associated with NEC. This study indicates the ability of B. bifidum to downregulate apoptosis in the rat NEC model and in IEC-6 cells by a COX-2-dependent matter and suggests a molecular mechanism by which this probiotic reduces mucosal injury and preserves intestinal integrity.
Keywords: epithelial homeostasis, enteral nutrition, mucosal inflammation, probiotics
necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in prematurely born infants. The key risk factors for development of this disease are prematurity, the introduction of enteral feeding, and bacterial colonization (12, 25, 33). After birth, a sterile newborn's gut is colonized within a few days. Prematurely born infants frequently experience postponed colonization caused by intestinal immaturity and exposure to broad-spectrum antibiotics (9). Probiotic bacteria (Bifidobacterium or Lactobacillus) dominate the intestinal microbiota of breast-fed babies, whereas formula-fed babies have more diverse microbiota (13, 16).
Three clinical studies indicate the beneficial effect of probiotics in the prevention of NEC (1, 19, 29). However, inconsistencies in probiotic mixtures and feeding protocols used make it difficult to address the molecular mechanisms of this protection. A recent report from our laboratory shows that oral administration of B. bifidum protects the small intestine against NEC in the neonatal rat model (24). This protective effect is associated with reduction of inflammation in the ileum, regulation of the mucus layer formation, and improvement of intestinal integrity (24).
Toll-like receptors (TLRs) are pattern-recognition receptors expressed on the surface of immune and intestinal epithelial cells (3). The interaction between enteric bacteria and TLRs is crucial for maintainance of intestinal epithelial homeostasis and essential for mucosal protection against gut injury (38). Because the microbial ligands detected by TLRs originate from both commensal and pathogenic microbes, TLRs are also responsible for protective signals allowing the intestine to tolerate the beneficial microflora (39). TLR-4 has been the most frequently studied in NEC models, and it is suggested that activation of TLR-4 signaling leads to increased intestinal injury (22, 27, 30). In colitis models, TLR-2 protects intestinal mucosa against injury via regulation of epithelial apoptosis (3, 4, 36), and, recently, a role for TLR-2 in NEC pathogenesis was also suggested (30, 41). However, the role of TLR-2 in probiotics-mediated protection against NEC is not known.
Upregulation of cyclooxygenase-2 (COX-2) is known to suppress apoptosis through prostaglandin E2 (PGE2) production in the gut (15, 31). Whereas high levels of intestinal COX-2 are reported in both human (6) and experimental (6, 17) NEC, the role of COX-2 and PGE2 in NEC pathogenesis is still controversial and not fully understood (31).
Intestinal epithelial homeostasis is maintained by balancing the rate between cell proliferation and cell loss, and apoptosis accounts for the majority of cell loss in the gut lumen (37). An important class of molecules that regulate enterocyte apoptosis is the Bcl-2 family (26). Members of the Bcl-2 family are involved in signaling pathways regulating caspase-3 activity necessary for chromatin condensation and DNA fragmentation that characterize apoptosis. The balance of pro- and anti-apoptotic Bcl-2 proteins is critical for cell survival (42). We (8) and others (21) showed that an uncontrolled increase of intestinal epithelial apoptosis leads to severe NEC injury.
The aim of this study was to further explore the molecular mechanisms underlying the protective effect of B. bifidum against experimental NEC, specifically, to determine if oral administration of B. bifidum in a rat model of NEC activates TLR-2, and whether increased COX-2 and PGE2 production is involved in downregulation of intestinal epithelial apoptosis in the site of injury. This hypothesis was then verified in an in vitro model using IEC-6 cells.
MATERIALS AND METHODS
Animal Model
This protocol was approved by the Animal Care and Use Committee of the University of Arizona (A-324801–95081). Seventy-six neonatal Sprague-Dawley rats (Charles River Labs, Pontage, MI) were collected by caesarian section 24 h before their scheduled birth, and the first feeding started 2 h after delivery. Animals were hand fed six times daily with a total volume of 850 μl of rat milk substitute/day (23). Experimental NEC was induced by asphyxia (breathing 100% nitrogen gas for 60 s) and cold stress (4°C for 10 min) twice daily (11). Neonatal rats were divided into the following experimental groups: hand fed with formula (NEC; n = 30); hand fed with formula containing 5 × 106 colony-forming units (CFU) of B. bifidum OLB6378 in two feedings per day (NEC + B. bifidum; n = 30); and dam fed littermates fed by surrogate mothers as a baseline control (DF, n = 16).
After 96 h, all surviving animals were terminated via decapitation. Animals that developed signs of distress or imminent death before 96 h were terminated and included in the study. Pathological changes in the intestinal architecture were evaluated using our previously described NEC scoring system (24).
B. Bifidum Culture
B. bifidum OLB6378 was incubated in MRS broth with 0.1% of sodium bicarbonate (Fisher, Wilmington, DE) and 0.1% of l-cysteine hydrochloride monohydrate (Sigma) for 48 h in anaerobic conditions at 37°C. Absorbance at 600 nm was measured to determine the number of CFU per 1 ml. B. bifidum was pelleted from the broth (10,000 rpm; 5 min) and washed with PBS before use.
Cell Culture
A rat intestinal epithelial cell line, IEC-6 (ATCC, Manassas, VA), was cultured in DMEM containing 5% FBS, 10 mg/l insulin, and 40 mg/ml gentamicin at a density of 1 × 106 cells/ml and allowed to adhere overnight. Cells were then rinsed with PBS, and DMEM media containing 0.5% FBS only was used for the 15-min period of B. bifidum pretreatment (1 × 105 CFU/ml). Cells were washed with PBS and incubated in DMEM media (containing 5% FBS, 10 mg/l insulin, and 40 mg/ml gentamicin) with tumor necrosis factor (TNF)-α and interferon (IFN)-γ at concentrations of 400 ng/ml each for an additional 4 h.
In Vivo and In Vitro Experiments with COX-2 Inhibitor
In vivo study.
Neonatal rats were divided into the following groups in addition to previously described experimental groups: hand fed with formula and injected intraperitoneally one time a day with 5 mg/kg of the COX-2 inhibitor Celecoxib (Sigma, St. Louis, MO) (NEC + C; n = 8); or hand fed with formula containing 5 × 106 CFU of B. bifidum OLB6378 in two feedings per day and injected intraperitoneally once a day with 5 mg/kg the COX-2 inhibitor Celecoxib (NEC + C + B. bifidum; n = 8).
In vitro study.
IEC-6 cells were treated with Celecoxib (1 μM) for 1 h before B. bifidum pretreatment and exposure to the cytokine mixture as described above.
Flow Cytometry
The annexin V-FITC apoptosis detection kit (Calbiochem, Gibbstown, NJ) was used to identify cell membrane alterations that accompany programmed cell death by labeling cells with annexin V-FITC and propidium iodide (PI). The procedure was carried out according to the manufacturer's instructions. The percentage of apoptotic cells was determined using two-color flow cytometric analysis with a FACScan flow cytometer (BD Biosciences, San Jose, CA). Appropriate electronic compensation was adjusted by acquiring cell populations stained with each dye individually, as well as with an unstained control (AZCC/ARL-Division of Biotechnology Cytometry Core Facility http://cytometry.arl.arizona.edu).
Immunohistology
Tissue staining.
A 2-cm section of distal ileum was collected from each animal and fixed overnight in 70% ethanol, paraffin-embedded, and sectioned at 4–6 μm. Serial sections were stained for TLR-2, COX-2, or cleaved caspase-3 (CC-3).
cox-2 and cc-3.
After deparaffinization and rehydration, sections were blocked with 1.5% rabbit serum (Vector Laboratories, Burlingame, CA) in PBS for 30 min and then incubated with either rabbit polyclonal CC-3 (Cell Signaling, Danvers, MA) or COX-2 (Cayman Chemical, Ann Arbor, MI) antibody for 1 h, washed with PBS, and incubated with goat anti-rabbit biotinylated secondary antibody (Vector Laboratories) for 30 min. Vectastain Elite ABC reagent (Vector Laboratories) was then applied, followed by diaminobenzidine as a substrate. Sections were counterstained with hematoxylin, dehydrated, and covered with a cover slip.
tlr-2.
After deparaffinization and rehydration, sections were blocked in 5% BSA to prevent nonspecific staining and incubated with goat anti-TLR-2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by incubation with Alexa Fluor 594-conjugated anti-goat secondary antibody (Molecular Probes, Eugene, OR) for 1 h and covered with a cover slip with Prolong gold anti-fade reagent with 4′,6-diamidino-2-phenylindole (DAPI).
Cell staining.
IEC-6 cells were fixed with 3% formaldehyde for 30 min, rinsed with PBS, covered with methanol for 6 min, allowed to dry, and, after a PBS wash, were incubated with 1.5% rabbit serum (Vector Laboratories) in PBS for 1 h. Cells were then stained with CC-3 Alexa Fluor 488-conjugated primary antibody (Cell Signaling) at 4°C overnight, washed with PBS, and covered with cover slip with Prolong gold anti-fade reagent with DAPI. Goat anti-TLR-2 polyclonal primary antibody (Santa Cruz Biotechnology) was used (incubated at 4°C overnight), followed by incubation with Alexa Fluor 594-labeled secondary antibody for 1 h, and covered with a cover slip with Prolong gold anti-fade reagent with DAPI.
An Olympus IX-70 inverted fluorescent microscope equipped with a ×40 oil immersion objective was used to evaluate CC-3-positive cells and TLR-2 staining.
Western Blot Analysis
Individual frozen ileal samples were homogenized with a hand-held homogenizer (Pellet Pestle; Kimble/Kontes, Vineland, NJ) in a ×5 volume of ice-cold homogenization buffer (50 mM Tris·HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 0.1% SDS; 1% sodium deoxycholic acid; 1% Triton X-100; 50 mM dithiothreitol; 50 μg/ml aprotinin; 50 μg/ml leupeptin; and 5 mM phenylmethylsulfonyl fluoride). The homogenates were centrifuged at 10,000 rpm for 5 min at 4°C, and the supernatant was collected. Cell lysates from IEC-6 cells were prepared after a wash with PBS using solubilization buffer (20 mM Tris, pH 8.0; 2 mM EDTA; 150 mM NaCl; 1% Triton X-100; and 10 mM SDS).
Total protein concentration was quantified using the Bradford protein assay. For protein analysis, 40 μg (tissue) or 15 μg (cells) of protein were added to an equal volume of ×2 Laemmli sample buffer and boiled for 5 min. The samples were run on 10% polyacrylamide gels at 110 V for 1.5 h. Protein was transferred to Immuno-Blot polyvinylidene difluoride membranes (Bio-Rad) at 100 mA for 1.5 h. Membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma) for 1 h at room temperature and then incubated overnight at 4°C with one of the following rabbit polyclonal antibodies: anti-Bax (1:500; BD Pharmigen, San Diego, CA), anti-Bcl-w, (1:100; Stressgen, Ann Arbor, MI), anti-COX-2 (1:200; Abcam, Cambridge, MA), or a goat polyclonal antibody anti-TLR-2 (1:50; Santa Cruz Biotechnology). After extensive washing, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-rabbit or anti-goat IgG (Santa Cruz Biotechnology). Proteins were visualized with a chemiluminescent system (Pierce, Rockford, IL) and exposed to X-ray film.
PGE2 Enzyme Immunoassay
The high-sensitivity PGE2 enzyme immunoassay kit (Assay Designs, Ann Arbor, MI) was used to determine the concentration of PGE2 in serum and in ileal tissue homogenates. Trunk blood was collected and centrifuged for 5 min at 5,000 rpm. Serum was collected and stored at −80°C until the assay was performed.
Individual frozen ileal samples were processed in the same manner as for Western blot analysis. Total protein concentration was quantified using the Bradford protein assay. Samples were diluted 1:10 with assay buffer before analysis. Results were calculated per 1 μg of protein.
RNA Preparation, RT, and Real-Time PCR
Total RNA was isolated from IEC-6 cells using the RNeasy Plus Mini Kit (Qiagen, Santa Clarita, CA) as described in the manufacturer's protocol. RNA concentrations were quantified at 260 nm, and the purity and integrity were determined using a NanoDrop (Thermo Fisher Scientific, Wilmington, DE).
RT and real-time PCR assays were performed to quantify steady-state mRNA levels of COX-2. cDNA was synthetized from 0.2 μg of total RNA. Predeveloped TaqMan primer and probe were used for the detection of COX-2 (Applied Biosystems, Foster City, CA). Reporter dye emission was detected by an automated sequence detector combined with ABI Prism 7700 Sequence Detection System software (Applied Biosystems). Real-time PCR quantification was performed with TaqMan 18S controls.
Statistics
Statistical analyses between experimental groups were performed using ANOVA followed by Fisher protected least-significant difference test. The χ2-test was used to analyze the difference in incidence of disease. All numerical data are expressed as means ± SE.
RESULTS
Oral Administration of B. bifidum Reduces the Severity and Incidence of NEC
As shown previously, ileal damage in rats administered B. bifidum was significantly reduced (P ≤ 0.01) to a median histological score of 1.0 compared with 2.0 in the NEC group, and the incidence of NEC was markedly decreased to 17% in the NEC + B. bifidum group compared with the NEC group with an incidence of 57%. In DF rats, the median histological score was 0.5 and incidence of NEC 0% (24).
B. Bifidum Increases Intestinal Expression of TLR-2 in a Rat Model of NEC
In the intestine, cross talk between enteric bacteria and the host is mediated in part by TLRs. Appropriate bacterial colonization and the activation of TLRs play a key role in the pathogenesis of NEC (30, 41), and administration of gram-positive probiotic bacteria increases the expression of TLR-2 in the intestinal epithelium (10). Thus we hypothesized that B. bifidum treatment of experimental NEC will stimulate expression of TLR-2 in the ileal epithelium.
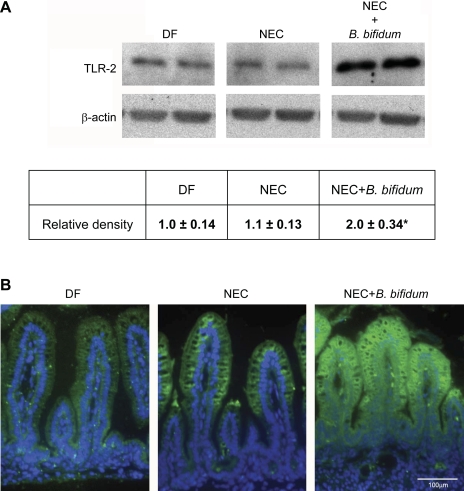
Western blot analysis showed significantly higher levels of TLR-2 in the B. bifidum group compared with the DF and NEC groups (Fig. 1A). The intensity of TLR-2 staining was markedly elevated in ileal tissue of rats receiving B. bifidum. Animals in the NEC group expressed very low intensity of the staining, and, interestingly, staining in the DF group was barely detectable (Fig. 1B). These data suggest the response of the intestine to the presence of B. bifidum in the diet.
Fig. 1.
Effect of oral administration of Bifidobacterium bifidum on expression and localization of Toll-like receptor-2 (TLR-2) in the ileum. A: representative TLR-2 (90-kDa) bands from Western blot analyses are shown for dam-fed littermates fed by surrogate mothers as a baseline control (DF, n = 5), littermates hand fed with formula (NEC, n = 5), and littermates hand fed with formula containing 5 × 106 colony-forming units (CFU) of B. bifidum OLB6378 in two feedings per day (NEC + B. bifidum, n = 5). All samples were analyzed on the same gel. Mean relative density to β-actin was calculated for all groups. *P ≤ 0.05 vs. NEC and DF. B: representative slides from DF, NEC, and NEC + B. bifidum groups (n = 6 animals/experimental group). Magnification: ×400.
B. Bifidum Stimulates COX-2 and PGE2 Production in the Ileum
COX-2 is a rate-limiting enzyme for PGE2 biosynthesis. It is known that overproduction of PGE2 can suppress intestinal epithelial apoptosis (15). Therefore, we evaluated the expression of COX-2 protein in the ileum by immunohistochemistry. In addition, PGE2 production was evaluated in ileal homogenates and in serum using a high-sensitivity PGE2 immunoassay.
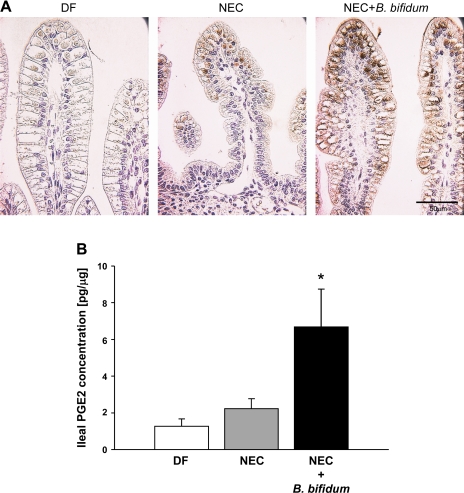
Staining for COX-2 revealed more intense staining in the ileum of animals receiving B. bifidum treatment compared with the NEC or DF groups (Fig. 2A). The signal for COX-2 in the NEC and NEC + B. bifidum groups was localized in epithelial cells at the top of the villi. In the DF group, the intensity of COX-2 staining was very low or not detected (Fig. 2A).
Fig. 2.
Effect of B. bifidum treatment on cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production in the ileal tissue. A: ileal tissue stained for COX-2; representative slides from DF, NEC, and NEC + B. bifidum groups are shown (n = 6 animals/experimental group). Magnification: ×400. B: PGE2 concentration (pg/ml) in ileal homogenates of DF (n = 7), NEC (n = 7), and B. bifidum-treated animals (n = 7) calculated per 1 μg of protein. *P ≤ 0.05 vs. NEC and DF.
The ileal luminal concentration of PGE2 was significantly elevated in the B. bifidum-treated group compared with the DF and NEC groups (P ≤ 0.05 vs. DF or NEC; Fig. 2B). In contrast, the concentration of PGE2 in the serum did not show any significant changes between experimental groups (data not shown). These results suggest activation of COX-2 by B. bifidum administration, which results in increased production of PGE2 in the terminal ileum, the site of injury.
B. Bifidum Blocks Apoptosis in the Ileum of Neonatal Rats
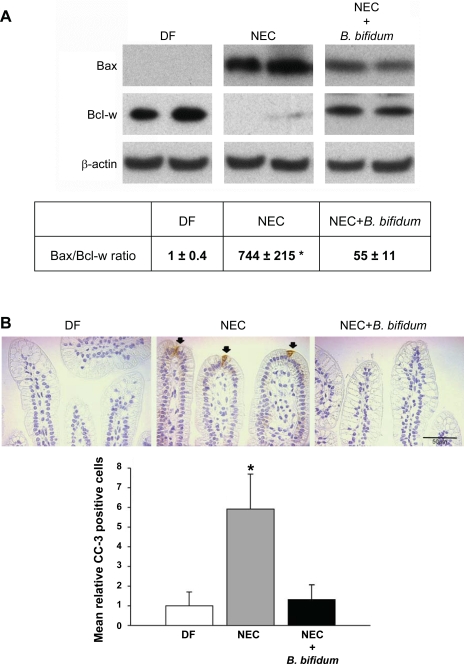
Although severe NEC is associated with extensive bowel necrosis, accelerated apoptosis of the intestinal epithelium is observed in NEC patients (14) and in experimental NEC models (8, 21). To determine if B. bifidum treatment of NEC alters intestinal epithelial cell apoptosis, we evaluated protein levels of the pro-apototic protein Bax and the anti-apoptotic protein Bcl-w in the ileum of DF, NEC, and NEC + B. bifidum rats using Western blot analysis (Fig. 3A).
Fig. 3.
Evaluation of apoptotic markers in the ileum after B. bifidum treatment of NEC. A: representative Bax (26 kDa) and Bcl-w (21 kDa) bands from Western blot analyses of ileal tissue from DF (n = 5), NEC (n = 5), and NEC + B. bifidum (n = 5) groups and the ratio between pro-apoptotic Bax and anti-apoptotic Bcl-w. All samples were analyzed on the same gel. *P ≤ 0.05 vs. NEC + B. bifidum and DF. B: representative slides from DF, NEC, and NEC + B. bifidum groups stained for cleaved caspase-3 (CC-3). Arrows indicate CC-3 positively stained cells. Magnification: ×400. Mean relative number of CC-3 positive cells in the site of NEC injury (n = 5 animals/experimental group). Data are expressed as mean CC-3 positive cells/100 epithelial cells. *P ≤ 0.05 vs. NEC + B. bifidum and DF.
In the ileum of B. bifidum-treated rats, protein levels of pro-apoptotic Bax were significantly decreased compared with the NEC group, whereas levels of anti-apoptotic Bcl-w were increased to levels found in the DF group (Fig. 3A). The Bax-to-Bcl-w ratio was shifted toward cell survival in the NEC + B. bifidum group (P ≤ 0.05; Fig. 3A).
Cleavage of procaspases to active caspases is a hallmark of most apoptotic systems, and the detection of CC-3 is a sensitive indicator of apotosis (18). To confirm changes in apoptosis, CC-3 staining of ileal tissue was performed and evaluated (Fig. 3B). The number of CC-3-positive cells was significantly decreased in the NEC + B. bifidum group compared with the NEC group, similar to values seen in the healthy DF group (P < 0.05). There were no positively labeled cells in the crypts.
Proliferation was also evaluated in the ileal sections by proliferating cell nuclear antigen staining. There were no statistically significant changes observed between the groups (data not shown). Thus alteration of intestinal epithelial proliferation can be excluded as a potential mechanism by which B. bifidum treatment protects intestinal mucosa against NEC.
Inhibition of COX-2 in the Rat Model of NEC Suppresses the Effect of B. Bifidum
Administration of COX-2 inhibitor to animals treated with B. bifidum (NEC + C + B. bifidum) resulted in increased incidence (75%) and severity of NEC (median histological score of 2) compared with NEC animals (57%) and animals treated with B. bifidum (17%). There was no difference in the incidence or severity of injury between animals in the NEC + C + B. bifidum group and the NEC + C group (75%, median histological score of 2).
To determine if inhibition of COX-2 affects changes in apoptosis seen in the ileum of neonatal rats treated with or without B. bifidum, CC-3 staining was performed and evaluated. The mean relative number of CC-3 positive cells was significantly increased in the NEC + C + B. bifidum (11.5 ± 3.0) and NEC + C (11.6 ± 3.6) groups compared with the NEC + B. bifidum group (1.3 ± 0.7) (P < 0.01). There was not a statistically significant difference between the NEC group and the NEC + C or NEC + C + B. bifidum group. Positively labeled cells were found only at the top of the villi and none in the crypts.
These results suggest not only an important role of COX-2 in NEC pathogenesis as previously shown (17) but also a possible mechanism by which B. bifidum protects against development of NEC through a decrease of apoptosis in the ileum.
B. Bifidum Induces the Expression of TLR-2 in IEC-6 cells
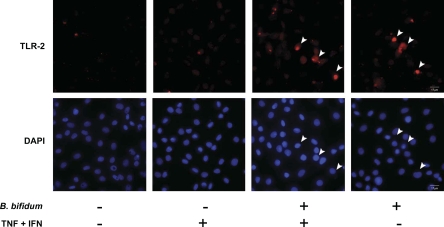
To verify the anti-apoptotic effect of B. bifidum on intestinal epithelium observed in the rat NEC model, we used a cytokine-induced apoptotic model in IEC-6 cells. This model mimics conditions in experimental NEC because TNF-α and IFN-γ are key regulators of inflammation in this disease. The presence of TLR-2 was detected only in cells pretreated with B. bifidum (Fig. 4). Control cells and cells exposed to the cytokine mixture only did not exhibit any detectable level of COX-2 signal.
Fig. 4.
TLR-2 expression in IEC-6 cells evaluated by fluorescent microscopy. Cells were treated with or without tumor necrosis factor (TNF)-α and interferon (IFN)-γ (400 ng/ml each) for 4 h and with or without a 15-min pretreatment with B. bifidum. Arrowheads indicate representative TLR-2-expressing cells and their nuclei [stained with 4′,6-diamidino-2-phenylindole (DAPI)].
B. Bifidum Activates COX-2 in IEC-6 Cells
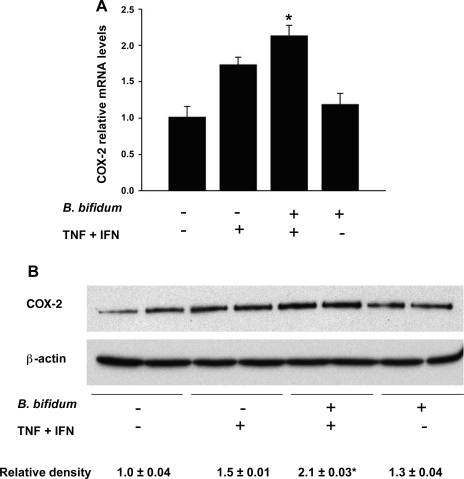
To clarify the role of COX-2 in intestinal epithelium, we used Real-Time PCR and Western blot analysis to measure mRNA and protein levels of this enzyme in IEC-6 cells. Gene expression of COX-2 was significantly increased in IEC-6 cells pretreated with B. bifidum and then exposed to the cytokine mixture compared with control or B. bifidum groups (P ≤ 0.05; Fig. 5A). Quantification of COX-2 protein using Western blot analysis confirmed the gene expression results. In IEC-6 cells pretreated with B. bifidum followed by the exposure to the cytokine mixture, COX-2 protein levels were significantly increased compared with all other groups (P ≤ 0.05; Fig. 5B). These data show that the combination of proinflammatory cytokines and B. bifidum leads to increased levels of COX-2 in IEC-6 cells, which is similar to what was observed in the animal model.
Fig. 5.
Evaluation of COX-2 in IEC-6 cells. Cells were treated with or without TNF-α and IFN-γ (400 ng/ml each) for 4 h with or without pretreatment for 15 min with B. bifidum. A: COX-2 mRNA levels evaluated using real-time PCR. The mean steady-state mRNA level for the control group (no treatment) was assigned a value of 1.0, and mean mRNA levels for all other groups were determined relative to this number. *P ≤ 0.05 vs. control (no treatment) and B. bifidum (+) TNF + INF (−). B: representative COX-2 (70-kDa) bands from Western blot analyses of IEC-6 cell lysates. Mean relative density to β-actin is shown for all groups. *P ≤ 0.05 vs. control (no treatment); B. bifidum (−) TNF + INF (+); and B. bifidum (+) TNF + INF (−).
B. Bifidum Reduces Apoptosis in IEC-6 cells
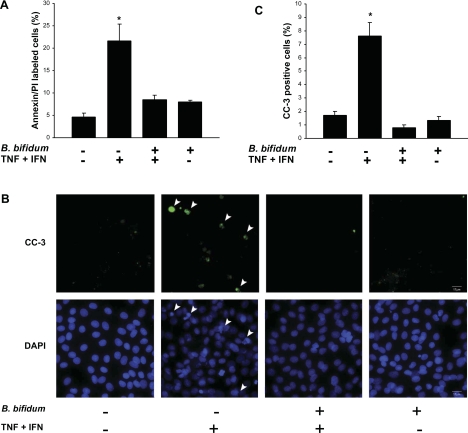
To test the hypothesis that B. bifidum reduces epithelial apoptosis, we labeled IEC-6 cells in all experimental groups with annexin V-FITC and PI and analyzed these by flow cytometry. The results revealed a significant decrease of apoptotic cells in IEC-6 cells pretreated with B. bifidum and exposed to the cytokine mixture in contrast to cells exposed to the cytokine mixture only (Fig. 6A). Cells pretreated with B. bifidum that were not exposed to cytokines did not show any differences from control cells.
Fig. 6.
Apoptosis in IEC-6 cells and the effect of B. bifidum pretreatment. Cells were treated with or without TNF-α and IFN-γ (400 ng/ml each) for 4 h and with or without a 15-min pretreatment with B. bifidum. A: percentage of annexin/propidium iodide (PI)-labeled cells analyzed by flow cytometry. *P ≤ 0.05 vs. control (no treatment); B. bifidum (+) TNF + INF (+); and B. bifidum (+) TNF + INF (−). B: CC-3-stained cells evaluated by fluorescent microscopy. Arrowheads indicate representative apoptotic cells/nuclei. Magnification: ×400. C: the percentage of CC-3-positive cells. *P ≤ 0.05 vs. control (no treatment); B. bifidum (+) TNF + INF (+); and B. bifidum (+) TNF + INF (−).
Immunofluorescent staining for activated CC-3 was used to determine apoptotic changes in IEC-6 cells exposed to B. bifidum treatment (Fig. 6B). Positive cells were counted in 10 different fields, and the ratio of positive cells per total number of cells was plotted (Fig. 6C). Pretreatment of IEC-6 cells with B. bifidum before exposure to the cytokine mixture resulted in a significant decrease of CC-3 positive cells (P < 0.05) vs. cells treated with the cytokine mixture alone. The results showed a statistically significant increase in the number of CC-3 positive cells when treated with the cytokine mixture compared with control (P < 0.05). Thus B. bifidum pretreatment blocks cytokine-induced apoptosis in IEC-6 cells.
Inhibition of COX-2 Eliminates the Effect of B. Bifidum on Apoptosis in IEC-6 Cells
To further clarify the importance of COX-2 in regulation of apoptosis by B. bifidum, the COX-2 inhibitor Celecoxib was used to pretreat IEC-6 cells, which were labeled with annexin/PI and analyzed by flow cytometry.
The number of apoptotic cells was significantly increased in the group pretreated with Celecoxib, treated with B. bifidum, and exposed to the cytokine mixture (31.2 ± 5.2) compared with the control group pretreated with Celecoxib only (7.4 ± 3.5). There was no statistically significant difference between the following groups: Celecoxib + cytokine mixture (18.0 ± 0.5), Celecoxib + B. bifidum + cytokine mixture, Celecoxib + B. bifidum (28.6 ± 2.8).
These results confirm the importance of COX-2 in the protection of intestinal epithelium against apoptosis by a B. bifidum treatment.
DISCUSSION
Recently, we reported that B. bifidum OLB6378 reduces intestinal injury and improves intestinal integrity in the rat model of NEC (24). In the present study, we demonstrate that oral administration of B. bifidum to NEC rats stimulates TLR-2 expression in the ileal epithelium, enhances epithelial expression of COX-2, and increases intestinal production of PGE2. B. bifidum treatment of experimental NEC also results in a strong anti-apoptotic response in the site of intestinal injury. These results are confirmed in studies with intestinal epithelial cells, which mimic the point of first contact between bacteria and host. Indeed, pretreatment of IEC-6 cells with B. bifidum stimulates TLR-2 and COX-2 expression and blocks cytokine-induced apoptosis in these cells. These data suggest that the downregulation of intestinal apoptosis is a molecular mechanism by which B. bifidum reduces mucosal injury and preserves intestinal integrity.
Bacterial colonization of the gastrointestinal tract begins immediately after birth and is important for the development of immune and digestive functions (12). In contrast to pathogenical organisms such as Bacteroides, Clostridia, and Staphylococcus, probiotics such as Bifidobacterium and Lactobacillus are considered beneficial for the developing intestine (9). Because intestinal colonization can be altered nutritionally, oral administration of probiotic bacteria is a logical approach for establishing healthy intestinal microbiota in neonates. Indeed, clinical studies showed beneficial effects of probiotics in the prevention of NEC, but the molecular and cellular mechanisms were not studied in these trials (1, 19, 29).
The pathogenesis of NEC is unknown, but a breach in the intestinal mucosal barrier may allow bacterial translocation across the epithelium, resulting in an inflammatory response that leads to NEC (9). Previously, we have reported that the intestinal epithelial barrier is compromised in the rat model of NEC (7) and B. bifidum treatment improves formation of epithelial tight junctions (TJ) in the ileum of NEC rats (24). A link between activation of TLR-2 signaling and preservation of intestinal TJ integrity was previously shown in other models of intestinal inflammation. For example, treatment of dextran sodium sulfate-induced colitis with TLR-2 agonist protects TJ integrity and decreases intestinal permeability (3, 4), and mucosal repair is delayed when colitis is induced in TLR-2-deficient mice (3, 38). Results from our study show increased expression of TLR-2 in rats treated with B. bifidum. Thus we speculate that elevated TLR-2 in B. bifidum-treated animals is responsible for protecting the intestine against acute injury through the formation of functional TJs, resulting in the improvement of intestinal integrity.
Upregulation of COX-2 can lead to suppression of intestinal epithelial apoptosis through production of PGE2 (5, 15). However, the role of COX-2 in NEC pathogenesis is still not clear. High levels of intestinal COX-2 are reported in animal models of NEC, suggesting its pathogenic effects during intestinal inflammation (6, 17). Conversely, administration of a COX-2 inhibitor results in a higher degree of intestinal inflammation in the rat NEC model (17), and studies with COX-2 knockout mice describe increased intestinal damage compared with wild-type controls (40). There have been only a few studies exploring the effect of different probiotics on COX-2 expression in intestinal cells or rat models of colitis. Both in vivo and in vitro studies show decreased COX-2 with probiotic treatment (28, 34, 35) except for Lactobacillus acidophilus, which significantly increased COX-2 expression in intestinal cells (34). This demonstrates how the effects of probiotics vary, and the protective mechanisms depend on each strain. Our results show increased expression of COX-2 in the villus epithelium in animals treated with B. bifidum compared with animals with NEC and an increased luminal concentration of PGE2. Inhibition of COX-2 resulted in loss of B. bifidum protective effect against NEC injury in the neonatal rats. We conclude that an increase of COX-2 and consequently higher production of PGE2 in the ileum plays an important role in protection against NEC. Because PGE2 is a known anti-apoptotic regulator in epithelial cells (2, 43), we evaluated expression of major apoptotic regulators in the ileum.
Uncontrolled apoptosis may lead to a massive loss of cells in NEC (8, 21). The protective effect of Lactobacillus strains against NEC and their ability to regulate intestinal apoptosis was reported (20, 29). Several in vitro studies described the anti-apoptotic effect of lactobacilli and proteins produced by Lactobacillus GG in intestinal epithelial cells (20, 44, 45). Furthermore, the probiotic mixture VSL#3 prevented apoptosis in a murine model of colitis (32). Our results are the first to demonstrate that B. bifidum regulates apoptosis in the ileum of neonatal rats in favor of cell survival. The Bax-to-Bcl-w ratio clearly shows a decrease of apoptosis in the ileal tissue of the B. bifidum group, which is further confirmed with a reduced number of CC-3 positive cells. Taken together, these results indicate that B. bifidum-mediated reduction of NEC is associated with a reduction of epithelial apoptosis in the site of injury. Data from studies with COX-2 inhibitor clearly show that COX-2 is involved in apoptosis regulation in the ileum of neonatal rats subjected to the NEC protocol, and we can conclude that B. bifidum decreases apoptosis by a COX-2-dependent manner.
Cytokines are key regulators of inflammation in NEC, and TNF-α and IFN-γ are the major cytokines associated with intestinal inflammation. We used these cytokines to induce apoptosis in IEC-6 cells as a relevant condition to NEC and to confirm that B. bifidum prevents apoptosis in intestinal epithelial cells in an environment lacking additional stress factors that might be involved in the animal model. Indeed, our results show that TLR-2 was detected only in IEC-6 cells exposed to pretreatment with B. bifidum. These findings confirm activation of epithelial TLR-2 in the presence of the gram-positive bacteria. Pretreatment with B. bifidum leads to upregulation of COX-2 and reduction of apoptosis in IEC-6 cells. COX-2 inhibition results in an increase of apoptosis in cells treated with B. bifidum, confirming the role of COX-2 in the anti-apoptotic mechanism and supporting our findings in the rat NEC model.
In conclusion, oral administration of B. bifidum activates TLR-2, increases expression of COX-2, and leads to higher production of PGE2 in the ileum of rat pups. B. bifidum treatment reduces the number of CC-3 positive cells and shifts the ratio between the pro-apoptotic protein Bax and the anti-apoptotic Bcl-w in favor of cell survival, and therefore provides a protection against intestinal apoptosis associated with NEC. Results from our experiments in IEC-6 cells confirm the protective effect of B. bifidum against cytokine-induced apoptosis. However, the definitive evidence of a direct link between TLR-2 activation and the upregulation of COX-2 in the intestinal epithelium has yet to be confirmed.
This study shows the ability of B. bifidum to downregulate apoptosis in both in vivo and in vitro models of NEC by a COX-2-dependent matter and suggests a molecular mechanism by which this probiotic reduces mucosal injury and preserves intestinal integrity.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-039657 (to B. Dvorak) and a gift from Meiji Dairies Corporation.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We acknowledge The Cytometry Core Facility, operated and administered as a partnership between Arizona Research Laboratories, Division of Biotechnology and the Arizona Cancer Center.
REFERENCES
- 1.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147: 192–196, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bos J, Smithee L, McClane B, Distefano RF, Uzal F, Songer JG, Mallonee S, Crutcher JM. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin Infect Dis 40: e78–e83, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127: 224–238, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol 66: 1465–1477, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Chung DH, Ethridge RT, Kim S, Owens-Stovall S, Hernandez A, Kelly DR, Evers BM. Molecular mechanisms contributing to necrotizing enterocolitis. Ann Surg 233: 835–842, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288: G755–G762, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Claud EC, Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol 42, Suppl 2: S46–S52, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Dogi CA, Galdeano CM, Perdigon G. Gut immune stimulation by non pathogenic Gram(+) and Gram(−) bacteria. Comparison with a probiotic strain. Cytokine 41: 223–231, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Emami CN, Petrosyan M, Giuliani S, Williams M, Hunter C, Prasadarao NV, Ford HR. Role of the host defense system and intestinal microbial flora in the pathogenesis of necrotizing enterocolitis. Surg Infect (Larchmt) 10: 407–417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 91: 48–55, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg 32: 275–282, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 131: 862–877, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed 80: F167–F173, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grishin AV, Wang J, Potoka DA, Hackam DJ, Upperman JS, Boyle P, Zamora R, Ford HR. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol 176: 580–588, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Holubec H, Payne CM, Bernstein H, Dvorakova K, Bernstein C, Waltmire CN, Warneke JA, Garewal H. Assessment of apoptosis by immunohistochemical markers compared with cellular morphology in ex vivo-stressed colonic mucosa. J Histochem Cytochem 53: 229–235, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Inter J Infect Dis 3: 197–202, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Hunter CJ, Williams M, Petrosyan M, Guner Y, Mittal R, Mock D, Upperman JS, Ford HR, Prasadarao NV. Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis. Infect Immun 77: 1031–1043, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental model of neonatal necrotizing enterocolitis. Pediatr Res 55: 622–629, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanno T, Koyanagi N, Katoku Y, Yonekubo A, Yajima T, Kuwata T, Kitagawa H, Harada E. Simplified preparation of a refined milk formula comparable to rat's milk: influence of the formula on development of the gut and brain in artificially reared rat pups. J Pediatr Gastroenter Nutr 24: 242–252, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G940–G949, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kliegman RM, Willoughby RE. Prevention of necrotizing enterocolitis with probiotics. Pediatrics 115: 171–172, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Knott AW, Juno RJ, Jarboe MD, Zhang Y, Profitt SA, Thoerner JC, Erwin CR, Warner BW. EGF receptor signaling affects bcl-2 family gene expression and apoptosis after massive small bowel resection. J Pediatr Surg 38: 875–880, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Lee B, Lee JH, Lee HS, Bae EA, Huh CS, Ahn YT, Kim DH. Glycosaminoglycan degradation-inhibitory lactic acid bacteria ameliorate 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. J Microbiol Biotechnol 19: 616–621, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, Tsao LY, Chen CH, Su BH. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122: 693–700, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G442–G450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lugo B, Ford HR, Grishin A. Molecular signaling in necrotizing enterocolitis: regulation of intestinal COX-2 expression. J Pediatr Surg 42: 1165–1171, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296: G1140–G1149, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics 125: 777–785, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Otte JM, Mahjurian-Namari R, Brand S, Werner I, Schmidt WE, Schmitz F. Probiotics regulate the expression of COX-2 in intestinal epithelial cells. Nutr Cancer 61: 103–113, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, Zarzuelo A, Galvez J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol 103: 836–844, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137: 209–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potten CS. Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol Gastrointest Liver Physiol 273: G253–G257, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Sansonetti P. Host-pathogen interactions: the seduction of molecular cross talk. Gut 50, Suppl 3: III2–III8, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigthorsson G, Simpson RJ, Walley M, Anthony A, Foster R, Hotz-Behoftsitz C, Palizban A, Pombo J, Watts J, Morham SG, Bjarnason I. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 122: 1913–1923, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like-receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 138: 185–196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern LE, Falcone RA, Jr, Kemp CJ, Stuart LA, Erwin CR, Warner BW. Effect of massive small bowel resection on the Bax/Bcl-w ratio and enterocyte apoptosis. J Gastrointest Surg 4: 93–100, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest 114: 1676–1685, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132: 562–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277: 50959–50965, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]