Abstract
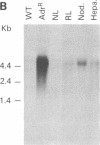
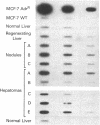
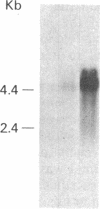
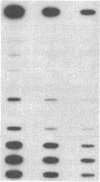
We have previously reported the isolation of a human breast cancer cell line resistant to doxorubicin (adriamycin; AdrR MCF-7 cells) that has also developed the phenotype of multidrug resistance (MDR). MDR in this cell line is associated with increased expression of mdr (P glycoprotein) gene sequences. The development of MDR in AdrR MCF-7 cells is also associated with changes in the expression of several phase I and phase II drug-detoxifying enzymes. These changes are remarkably similar to those associated with development of xenobiotic resistance in rat hyperplastic liver nodules, a well-studied model system of chemical carcinogenesis. Using an mdr-encoded cDNA sequence isolated from AdrR MCF-7 cells, we have examined the expression of mdr sequences in rat livers under a variety of experimental conditions. The expression of mdr increased 3-fold in regenerating liver. It was also elevated (3- to 12-fold) in several different samples of rat hyperplastic nodules and in four of five hepatomas that developed in this system. This suggests that overexpression of mdr, a gene previously associated with resistance to antineoplastic agents, may also be involved in the development of resistance to xenobiotics in rat hyperplastic nodules. In addition, although the acute administration of 2-acetylaminofluorene induced an 8-fold increase in hepatic mdr-encoded RNA, performance of a partial hepatectomy either before or after administration of 2-acetylaminofluorene resulted in a greater than 80-fold increase in mdr gene expression over that in normal untreated livers. This represents an important in vivo model system in which to study the acute regulation of this drug resistance gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Fojo A., Hanover J. A., Pastan I., Gottesman M. M. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985 Mar;11(2):117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- Aström A., DePierre J. W., Eriksson L. Characterization of drug-metabolizing systems in hyperplastic nodules from the livers of rats receiving 2-acetylaminofluorene in their diet. Carcinogenesis. 1983;4(5):577–581. doi: 10.1093/carcin/4.5.577. [DOI] [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Bock K. W., Lilienblum W., Pfeil H., Eriksson L. C. Increased uridine diphosphate-glucuronyltransferase activity in preneoplastic liver nodules and Morris hepatomas. Cancer Res. 1982 Sep;42(9):3747–3752. [PubMed] [Google Scholar]
- Cameron R., Sweeney G. D., Jones K., Lee G., Farber E. A relative deficiency of cytochrome P-450 and aryl hydrocarbon [benzo(a)pyrene] hydroxylase in hyperplastic nodules induced by 2-acetylaminofluorene in rat liver. Cancer Res. 1976 Nov;36(11 Pt 1):3888–3893. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Gottesman M. M., Pastan I. H. Increased vinblastine binding to membrane vesicles from multidrug-resistant KB cells. J Biol Chem. 1986 Jun 15;261(17):7921–7928. [PubMed] [Google Scholar]
- Cornwell M. M., Safa A. R., Felsted R. L., Gottesman M. M., Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984 Dec;44(12 Pt 1):5463–5474. [PubMed] [Google Scholar]
- Farber E., Parker S., Gruenstein M. The resistance of putative premalignant liver cell populations, hyperplastic nodules, to the acute cytotoxic effects of some hepatocarcinogens. Cancer Res. 1976 Nov;36(11 Pt 1):3879–3887. [PubMed] [Google Scholar]
- Farber E., Sarma D. S. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 1987 Jan;56(1):4–22. [PubMed] [Google Scholar]
- Farber E. The biochemistry of preneoplastic liver: a common metabolic pattern in hepatocyte nodules. Can J Biochem Cell Biol. 1984 Jun;62(6):486–494. doi: 10.1139/o84-066. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. E., Beckman C. A., Cowan K. H. 5' Nucleotide sequences influence serum-modulated expression of a human dihydrofolate reductase minigene. Mol Cell Biol. 1986 Mar;6(3):878–886. doi: 10.1128/mcb.6.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Roninson I., Varshavsky A., Housman D. E. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):337–341. doi: 10.1073/pnas.83.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman C. A., Engel L., Lowy D. R., Howley P. M. Virus-specific transcription in bovine papillomavirus-transformed mouse cells. Virology. 1982 May;119(1):22–34. doi: 10.1016/0042-6822(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Judah D. J., Legg R. F., Neal G. E. Development of resistance to cytotoxicity during aflatoxin carcinogenesis. Nature. 1977 Jan 27;265(5592):343–345. doi: 10.1038/265343b0. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kitahara A., Satoh K., Nishimura K., Ishikawa T., Ruike K., Sato K., Tsuda H., Ito N. Changes in molecular forms of rat hepatic glutathione S-transferase during chemical hepatocarcinogenesis. Cancer Res. 1984 Jun;44(6):2698–2703. [PubMed] [Google Scholar]
- Kodate C., Fukushi A., Narita T., Kudo H., Soma Y., Sato K. Human placental form of glutathione S-transferase (GST-pi) as a new immunohistochemical marker for human colonic carcinoma. Jpn J Cancer Res. 1986 Mar;77(3):226–229. [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Medline A., Farber E. Sequential analysis of hepatic carcinogenesis: the comparative architecture of preneoplastic, malignant, prenatal, postnatal and regenerating liver. Br J Cancer. 1979 Nov;40(5):782–790. doi: 10.1038/bjc.1979.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. H., Meyers M. B., Spengler B. A., Biedler J. L. Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res. 1983 Jan;43(1):222–228. [PubMed] [Google Scholar]
- Roninson I. B., Abelson H. T., Housman D. E., Howell N., Varshavsky A. Amplification of specific DNA sequences correlates with multi-drug resistance in Chinese hamster cells. Nature. 1984 Jun 14;309(5969):626–628. doi: 10.1038/309626a0. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Chin J. E., Choi K. G., Gros P., Housman D. E., Fojo A., Shen D. W., Gottesman M. M., Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomi M. W., Ho R. K., Sarma D. S., Farber E. A common biochemical pattern in preneoplastic hepatocyte nodules generated in four different models in the rat. Cancer Res. 1985 Feb;45(2):564–571. [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Scotto K. W., Biedler J. L., Melera P. W. Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science. 1986 May 9;232(4751):751–755. doi: 10.1126/science.2421411. [DOI] [PubMed] [Google Scholar]
- Solt D. B., Cayama E., Tsuda H., Enomoto K., Lee G., Farber E. Promotion of liver cancer development by brief exposure to dietary 2-acetylaminofluorene plus partial hepatectomy or carbon tetrachloride. Cancer Res. 1983 Jan;43(1):188–191. [PubMed] [Google Scholar]
- Spiewak Rinaudo J. A., Farber E. The pattern of metabolism of 2-acetylaminofluorene in carcinogen-induced hepatocyte nodules in comparison to normal liver. Carcinogenesis. 1986 Apr;7(4):523–528. doi: 10.1093/carcin/7.4.523. [DOI] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M., Nagamine Y., Farber E. Redifferentiation as a basis for remodeling of carcinogen-induced hepatocyte nodules to normal appearing liver. Cancer Res. 1983 Nov;43(11):5049–5058. [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Lee G., Farber E. Induction of resistant hepatocytes as a new principle for a possible short-term in vivo test for carcinogens. Cancer Res. 1980 Apr;40(4):1157–1164. [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]