Abstract
Studies suggest motor deficit asymmetry may help predict the pattern of cognitive impairment in individuals with Parkinson disease (PD). We tested this hypothesis using a highly validated and sensitive spatial memory task, spatial delayed response (SDR), and clinical and neuroimaging measures of PD asymmetry. We predicted SDR performance would be more impaired by PD-related changes in the right side of the brain than in the left. PD (n = 35) and control (n = 28) participants performed the SDR task. PD participants either had worse motor deficits on the right (RPD) or left (LPD) side of the body. Some participants also had magnetic resonance imaging for measurement of their substantia nigra (SN) volumes. The LPD group performed worse on the SDR task than the RPD and control groups. Right SN volume accounted for a unique and significant portion of the variance in SDR error, with smaller volume predicting poorer performance. In conclusion, left motor dysfunction and smaller right SN volume are associated with poorer spatial memory.
Keywords: Parkinson disease, Working memory, Spatial, Laterality, Substantia nigra, Magnetic resonance imaging
1. Introduction
Cognitive dysfunction is well-established in non-demented persons with Parkinson disease (PD). Aspects of executive control, such as working memory (the maintenance and manipulation of information online to guide behavioral response), are particularly affected, but there is significant variability from patient to patient (for review, see Pillon, Boller, Levy, & Dubois, 2001). Several groups have hypothesized that the nigrostriatal dopamine depletion present in PD leads to dysfunction of the prefrontal cortex, a region critical for optimal working memory (D’Esposito et al., 1998; Pillon et al., 2001; Taylor & Saint-Cyr, 1995).
Because motor dysfunction may directly reflect damage to the nigrostriatal dopaminergic system, investigators have explored the relationship between dopamine deficiency and cognition in PD by correlating patterns of cognitive performance with motor deficits. Cognitive impairment is almost always related to increased overall motor severity (Green et al., 2002; Locascio, Corkin, & Growdon, 2003), but its association with more specific aspects of motor dysfunction such as asymmetry is less clear. The motor manifestations of PD typically begin and persist asymmetrically (Hoehn & Yahr, 1967; Lee et al., 1995), reflecting asymmetric dopaminergic degeneration in the substantia nigra (SN) (Kempster, Gibb, Stern, & Lees, 1989). This pattern of asymmetry makes PD a useful model in which to investigate the effects of subcortical degeneration on cognitive functions associated with each hemisphere. Cognitive deficits may in part depend on which hemisphere of the brain is more affected and how much asymmetry is present.
This possibility has been addressed in previous studies, but results have been mixed. A number of studies fully or partially support the expected pattern of lateralized cognitive deficits: PD participants with worse left-sided motor dysfunction perform more poorly on visuospatial (right hemisphere) tasks and those with worse right-sided motor dysfunction perform more poorly on verbally mediated (left-hemisphere) tasks (Amick, Grace, & Chou, 2006; Blonder, Gur, Gur, Saykin, & Hurtig, 1989; Huber, Miller, Bohaska, Christy, & Bornstein, 1992; Spicer, Roberts, & LeWitt, 1988; Starkstein, Leiguarda, Gershanik, & Berthier, 1987; Taylor, Saint-Cyr, & Lang, 1986). Others found widespread cognitive deficits in participants with worse left-sided dysfunction while participants with worse right-sided dysfunction were relatively cognitively spared (Direnfeld et al., 1984; Tomer, Levin, & Weiner, 1993). Still others found no cognitive differences in regard to motor asymmetry (Barber, Tomer, Sroka, & Myslobodsky, 1985; Huber, Freidenberg, Shuttleworth, Paulson, & Clapp, 1989; St. Clair, Borod, Sliwinski, Cote, & Stern, 1998) or suggest that type, rather than side, of predominant or initial motor manifestation is the most important factor (Riklan, Stellar, & Reynolds, 1990; Zetusky & Jankovic, 1985).
Some of this work was limited by use of non-specific or poorly validated tasks, small sample sizes or participants in varied stages of disease progression. Additionally, researchers used different scales for measuring motor deficits and based group inclusion criteria on different aspects of asymmetry, which may contribute to the controversy. For example, some investigators chose to categorize participants according to initial side of symptom onset (Amick et al., 2006; Katzen, Levin, & Weiner, 2006; Tomer et al., 1993) while others used current ratings of absolute motor asymmetry (Barber et al., 1985; Blonder et al., 1989; Riklan et al., 1990); relatively little attention has been paid to the degree of motor asymmetry at the time of cognitive testing (Huber et al., 1992; Tomer et al., 1993).
The purpose of this study was to determine whether PD asymmetry affects short term spatial memory performance. To help clarify previous disparate findings, we chose to measure cognitive deficits in PD with a sensitive memory paradigm—the spatial delayed response (SDR) task—which has been validated extensively in animal and human studies as reflecting dorsolateral prefrontal cortex and dopaminergic system functioning (Funahashi, Bruce, & Goldman-Rakic, 1989, 1993; Gibbs & D’Esposito, 2005; Goldman-Rakic, Muly, & Williams, 2000; Leung, Gore, & Goldman-Rakic, 2002; Luciana, Depue, Arbisi, & Leon, 1992; McCarthy et al., 1996; Müller, von Cramon, & Pollmann, 1998; Williams & Goldman-Rakic, 1995). The dorsolateral prefrontal cortex is a target of the striatofrontal circuitry that is disrupted by dopamine loss in PD (Alexander, DeLong, & Strick, 1986), and SDR tasks have been shown to be affected by PD (Postle, Jonides, Smith, Corkin, & Growdon, 1997). To reduce extraneous variables, we included only mildly affected PD participants with consistent and clear motor asymmetry since onset and considered absolute side of symptom onset as well as the current degree of motor asymmetry in our analyses. Also unique to our study is the use of in vivo measurement of SN volumes as a possible additional indicator of disease severity or asymmetry.
We hypothesized that SDR performance would be more impaired by PD-related changes in the right side of the brain compared to the left due to the right hemisphere’s preference for handling spatial material. Therefore, we predicted that participants with worse left-sided motor dysfunction would perform worse on the SDR task than those with worse right-sided motor dysfunction, and we speculated that poorer SDR performance would be accompanied by smaller right SN volumes.
2. Methods
2.1. Participants
This study was approved by the institutional review board at Washington University School of Medicine (WUSM), and all participants gave written informed consent. Study participants included 35 PD and 28 healthy control volunteers who performed cognitive testing. A subset of these participants (19 PD, 15 control) also underwent magnetic resonance imaging (MRI) on the same day as cognitive testing. Participants with self-reported psychiatric diagnoses or current significant psychiatric symptoms, head injury, neurosurgery or other neurological conditions were excluded.
PD participants were diagnosed with clinically definite idiopathic PD by a neurologist in the Movement Disorders Clinic at WUSM. All had Hoehn and Yahr stage I or II (Hoehn et al., 1967), indicating relatively mild predominantly unilateral signs of disease. PD participants were classified as having symptoms that started on the right (RPD) or left (LPD) side of the body and remained more severe on that side of the body. This was determined by detailed clinical chart review and corroborated by patient report. The Unified Parkinson’s Disease Rating Scale Motor subscale was used as a measure of current motor severity (UPDRSm) (Fahn, Elton, & Members of the UPDRS Development Committee, 1987). Each PD participant was rated while off PD medications overnight.
2.2. Materials
2.2.1. SDR task
The SDR task was administered to each participant to assess short term spatial memory. Participants focused on a central fixation cross that appeared on a computer screen placed approximately 60 cm away from them. While fixated, a cue (open dot 1 cm in diameter) appeared for 150 ms in any of 32 possible unmarked locations at an 11.43 cm radius from the central fixation. Cues were evenly distributed between left and right sides of the screen. A delay period (5 or 15 s) was then imposed. During the delay, participants performed a continuous performance task in which a series of geometric shapes (triangle, square, and diamond) appeared in place of the fixation cross. Participants pressed the spacebar whenever the diamond shape appeared. This task engaged the participants and reduced their ability to rehearse information during the delay. After the delay, the fixation cue returned and the participant touched the computer screen where s/he remembered seeing the cue. Responses were then coded by the experimenter while the participant’s finger was still on the screen. Responses were measured in X and Y coordinates and compared with the actual location of the cue. Delay trials and trials with no mnemonic load (cue-present trials) were presented in random order. On the cue-present trials, the cue was present during the response phase. This set of trials gave an indication of participants’ pointing and raters’ coding accuracies, accounting for error associated with motor deficits and measurement. Mean error in pixels (distance between recall and actual cue) was calculated for each participant for each type of trial. There were four practice trials that could be repeated if necessary and 24 experimental trials (eight trials at each delay and eight cue-present trials).
2.2.2. FAS
Verbal fluency was tested using the FAS (Lezak, 1995). Participants were asked to say as many words they could recall that started with each of those letters (F, A, and S) in three separate 60 s trials, excluding proper nouns, numbers and the same word with different suffixes. The score is the sum of all acceptable words produced in the three one-minute trials. Comparisons were made after controlling for age and premorbid ability (WRAT-3R, see below).
2.2.3. Wide range achievement test III, reading subtest (WRAT-3R)
The WRAT-3R measures oral reading ability and is an accurate predictor for overall verbal intelligence, especially within the range of average intelligence, in normal and neurological populations (Griffin, Mindt, Rankin, Ritchie, & Scott, 2002; Johnstone, Callahan, Kapila, & Bouman, 1996). Because oral reading ability is thought to be a fairly stable skill, we used this test as an estimate of premorbid intellectual functioning. We administered the test according to standard instructions and computed the age-corrected standard score for each participant (Wilkinson, 1993).
2.3. Degree of asymmetry of motor dysfunction
Degree of asymmetry of motor dysfunction for each PD participant was determined by calculating a motor asymmetry score according to the following formula, which divides the difference between right and left UPDRSm scores by the average of those scores:
UPDRSm right and left scores were calculated by summing each side’s ratings for rigidity, tremor and bradykinesia (finger tapping, foot tapping, hand agility, and pronation–supination movement). This formula yields a score with absolute values ranging from 0 (symmetric) to 2 (exclusively unilateral symptoms), with negative scores indicating more severe deficits on the left side of the body and positive scores indicating more severe deficits on the right side of the body.
2.4. Anatomy
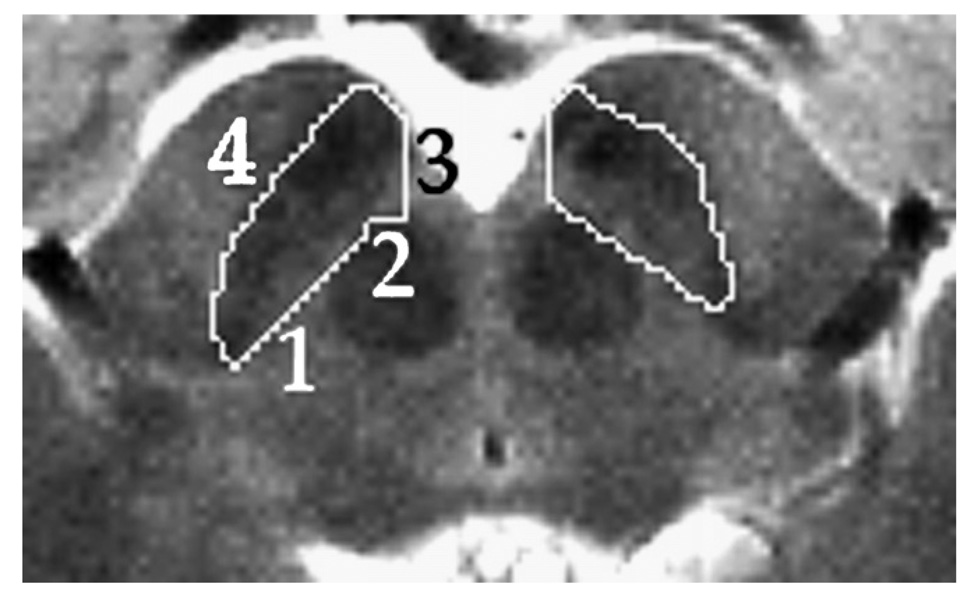
Since histologically-defined SN is not discernible with current MR technology, the volume we measured was defined as a practical compromise between the desired region (i.e. anatomic SN) and reliable landmarks visible on the MR scans. One can calculate from the data of Damier, Hirsch, Agid, and Graybiel (1999b) that more than 80% of the dopaminergic neurons that degenerate in PD are located in the region described as follows. Only that part of SN that appears on the same slices on which the red nucleus appears was included. On each transverse slice the SN region boundary is defined by a simple closed curve with four segments (see Fig. 1). Segment 1 is formed by a line tangent to the anterior border of the red nucleus and to the posterior border of SN pars reticulata (SNr). Segment 3 is formed by the sagittal line tangent to the medial border of the SNr. Segments 2 and 4 are the curved anterior border of red nucleus and SNr respectively, connecting the endpoints of Segments 1 and 3.
Fig. 1.
Horizontal slice from a single subject’s T2-weighted spin echo image cropped to show midbrain area. Numbers indicate boundaries used to define the SN region. See Section 2: Anatomy for further details.
We decided to include part of SNr because small fronds of SN pars compacta (SNc) extend into SNr and include a substantial number of dopaminergic neurons (Damier, Hirsch, Agid, & Graybiel, 1999a). This interdigitating boundary between SNc and SNr cannot be reliably visualized at the MRI resolution available. The region we define also excludes a small portion of SN pars dorsalis, but this excluded portion represents only about 10% of all dopaminergic cells in SN and only about 7% of the dopaminergic cells that die in PD (Damier et al., 1999b).
2.5. Volumetry
Cavalieri’s theorem (Cavalieri, 1653; Gundersen & Jensen, 1987) demonstrates that an unbiased estimate of the volume of a structure is produced by summing cross-sectional areas on equally spaced parallel planes and multiplying the sum by the distance between adjacent planes. For this measurement to be unbiased, the position of the first slice must be uniformly randomly distributed along the axis perpendicular to the planes (Gundersen & Jensen, 1987; Mayhew & Olsen, 1991). Also, stereologic volume measurement is optimized by “slicing” the object to be measured in the same orientation relative to each participant’s anatomy (Gundersen, 1992).
The left and right SN were considered independently. The intersection area on each plane was determined by one rater who traced the SN on each slice using software developed in our laboratory, according to the anatomical rules described above. The rater was blind to participant diagnosis and age at the time of the tracing. Image intensity was scaled linearly for each participant to minimize across-subject variability in visual edge-finding. The modal intensity of within-brain voxels on the most inferior slice on which red nucleus appeared was determined and multiplied by 2.25. This product was chosen as the upper threshold for the grayscale display, with zero as the lower threshold.
2.6. Image acquisition
Magnetic resonance images were acquired with the Siemens Allegra 3T head-only scanner using online correction for anatomical distortion introduced by the short coils. High-resolution T1-weighted images (3D sagittal MP-RAGE) were acquired first and used to determine the acquisition plane for the primary images used in this study. Specifically, for comparison with a published autopsy study, we determined from the MP-RAGE the plane of section used by Damier and colleagues (1999a) in their autopsy study of the midbrain, i.e. the plane perpendicular to the midsagittal plane that passes through the ponsmedulla junction anteriorly in the midline, and the inferior edge of the rostral two bodies of the corpora quadrigemina posteriorly. A T2-weighted spin echo image was acquired parallel to this plane (TR = 5000 ms, TE = 96 ms, flip angle = 180°, effective voxel size = 0.47 × 0.47 × 2.0 mm3, 2 acquisitions, acquisition time = 10 min 46 s).
This T2-weighted image was used to define the SN volume of interest. To produce an unbiased volume measure, the slice position along the neuraxis was randomized. The dorsal–rostral position of the center of the slab was randomly chosen at 0.1 mm intervals between 0.0 and 1.9 mm dorsal to the anatomical plane described in the preceding paragraph. In other words, relative to each participant’s brainstem anatomy, the slice orientation was nearly identical across participants, but the slice position along the rostral–caudal axis was chosen randomly to the nearest 0.1 mm for each participant.
2.7. Analysis
Statistical analyses were carried out using SPSS for Windows version 12.0. Demographic and clinical characteristics were compared across participant subgroups. Mean values of continuous variables were compared using ANOVAs (PD and control groups) and unpaired t-tests (PD groups only), and χ2 tests were used for categorical variables. Separate general linear models with length of SDR delay (cue-present, 5 and 15 s) and SN side (right, left) as the repeated measure were used to determine the effect of subject group on SDR performance and SN volumes, respectively. To explore asymmetry predictors of SDR performance, the measures of motor (UPDRSm) and brain (SN volumes) asymmetry were evaluated against SDR 15 s delay error in a series of simple bivariate correlations (Pearson r). Significant variables were then used as predictors in separate hierarchical regression models with SDR 15 s delay error as the dependent variable and age, WRAT-3R score, whole brain volume, disease duration, and UPDRSm score forced-entered as known influential variables. The proportion of additional variance explained (change in R2) by each measure of asymmetry was tested for significance to determine its unique contribution to SDR performance. All tests were 2-tailed. A p-value of <0.05 was considered significant.
3. Results
3.1. Participant characteristics
Demographic and clinical characteristics of the sample are in Table 1. There were no significant differences between the participant subgroups for any of these variables (p > 0.19). Of the PD participants, 26 were chronically treated with medication and 18 of these were on medications at the time of testing. Of the 26 treated PD participants, 15 were receiving carbidopa–levodopa exclusively (RPD = 8, LPD = 7), 5 were receiving a dopamine agonist exclusively (i.e. pramipexole, pergolide; RPD = 3; LPD = 2) and 6 were receiving carbidopa–levodopa with a COMT inhibitor (i.e. entacapone) or dopamine agonist, or both (RPD = 3; LPD = 3). χ2 and Fisher’s exact tests indicated that the PD subgroups were not significantly different in the number of participants treated with medications vs. medication naïve and in the number of participants on vs. off medications during cognitive testing (p > 0.34). The PD subgroups were also equivalent in total UPDRSm, tremor, rigidity and bradykinesia scores (p > 0.21). There were no significant differences in verbal fluency performance between LPD, RPD and controls after accounting for age and WRAT-3R score for the entire group as well as for the MRI subset (p > 0.46). These effects remained the same after comparing only the LPD and RPD groups and additionally covarying disease duration and UPDRSm score (p > 0.89).
Table 1.
Characteristics of each participant subgroup
| Whole group (N = 63) | MRI subset (N = 34) | |||||
|---|---|---|---|---|---|---|
| C | RPD | LPD | C | RPD | LPD | |
| n | 28 | 19 | 16 | 15 | 11 | 8 |
| Age in years | 54.1 (14.0) | 59.3 (12.0) | 57.5 (11.0) | 57.7 (10.2) | 56.5 (10.6) | 55.3 (11.5) |
| Male/female ratio | 13/15 | 8/11 | 10/6 | 5/10 | 3/8 | 5/3 |
| Education level in years | 14.8 (2.8) | 15.5 (2.7) | 14.8 (2.8) | 14.3 (2.7) | 15.8 (2.9) | 14.0 (3.0) |
| WRAT-3R | 107.0 (6.8) | 107.4 (6.9) | 101.4 (13.1) | 104.7 (5.8) | 107.3 (7.9) | 99.5 (14.1) |
| Verbal fluency | 42.8 (13.8) | 39.8 (16.6) | 42.6 (15.2) | 46.0 (13.8) | 45.3 (15.4) | 47.5 (16.8) |
| Medication status | ||||||
| Treated/naïve | — | 13/6 | 13/3 | — | 10/1 | 8/0 |
| On/off (at testing) | — | 10/9 | 8/8 | — | 7/4 | 7/1 |
| Duration of disease in years | — | 4.5 (3.6) | 4.1 (4.4) | — | 5.9 (4.1) | 6.4 (5.3) |
| UPDRSm | — | 22.1 (10.9) | 20.3 (8.9) | — | 24.6 (12.9) | 23.7 (8.0) |
| Symptom score | ||||||
| Tremor | — | 3.8 (2.9) | 2.2 (2.1) | — | 4.5 (3.3) | 3.1 (2.5) |
| Rigidity | — | 4.4 (2.7) | 4.7 (2.8) | — | 4.4 (2.8) | 4.5 (3.2) |
| Bradykinesia | — | 8.3 (4.7) | 7.6 (3.3) | — | 9.4 (5.4) | 9.0 (3.3) |
| Motor asymmetry score | — | 0.67 (0.54) | −0.78 (0.45) | — | 0.66 (0.48) | −0.61 (0.15) |
| SN volume (cm3) | ||||||
| Right SN | — | — | — | 0.53 (0.08) | 0.51 (0.08) | 0.45 (0.09) |
| Left SN | — | — | — | 0.53 (0.09) | 0.53 (0.05) | 0.47 (0.10) |
| SN asymmetry score | — | — | — | 0.01 (0.18) | −0.04 (0.07) | −0.03 (0.13) |
Numbers represent means (standard deviation) or number of participants.
C, control; RPD, PD participants with worse right-sided symptoms; LPD, PD participants with worse left-sided symptoms; WRAT-3R, wide range achievement test III reading task standard score; UPDRSm, unified Parkinson’s disease rating scale, motor subscore; SN, substantia nigra.
3.2. Group comparisons
3.2.1. SDR performance
SDR performance comparisons between LPD, RPD and controls were done after controlling for age and WRAT-3R score. When comparing only the LPD and RPD groups, we also controlled for disease duration and total UPDRSm score.
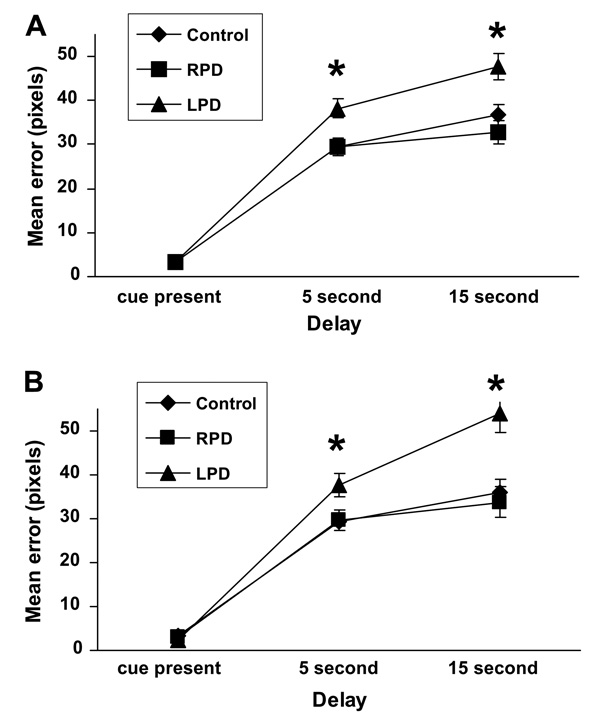
There was a significant within-subjects effect of SDR delay length on performance, F(2, 116) = 6.02, p = 0.004. As expected, mean error increased as the length of time between cue presentation and retrieval increased. This is consistent with the design of the task, whereby longer delays are hypothesized to be more demanding cognitively and thus produce higher error rate. More interesting was the interaction effect between SDR delay length and worse side of symptoms, F(4, 116) = 4.18, p = 0.005), and the between-subjects effect of worse side of symptoms on SDR performance, F(2, 58) = 8.46, p = 0.001. Follow-up testing showed that the between-subjects effect was significant in both delay conditions but not in the cue-present condition (5 s: F(2, 60) = 5.82, p = 0.005; 15 s: F(2, 60) = 7.29, p = 0.001; cue present: F(2, 60) = 0.24, p = 0.79), which indicates there was no fundamental difference in pointing accuracy among the groups. Post hoc tests showed that the LPD group had significantly higher error rate in the delay conditions than the RPD and control groups, which did not differ from each other (Fig. 2A). All of these effects remained the same when comparing the LPD and RPD groups only.
Fig. 2.
Group differences in SDR performance error (mean ± SEM) across delay conditions for (A) the entire group of participants (control, n = 28; RPD, n = 19; LPD, n = 16), and (B) the MRI subset (control, n = 15; RPD, n = 11; LPD, n = 8) after controlling for age and WRAT-3R score. * LPD>RPD and control, p < 0.01.
Results were similar for the group of participants who had an MRI on the same day as cognitive testing. There was no significant within-subjects effect of SDR delay length on performance, F(2, 58) = 1.23, p = 0.30; however, there was a trend toward higher error rate with increasing delay length. The interaction between delay and more affected motor side and the between-subjects effect of worse side of motor deficits on SDR performance remained significant (interaction: F(4, 58) = 5.20, p = 0.003; main effect of group: F(2, 29) = 6.68, p = 0.004) again for both delay conditions but not for the cue-present condition (5 s: F(2, 31) = 3.73, p = 0.03 ; 15 s: F(2,31) = 7.80, p = 0.002; cue present: F(2, 31) = 1.97, p = 0.16). Post hoc tests showed that the LPD group had significantly higher error rate in the delay conditions than the RPD and control groups, which did not differ from each other (Fig. 2B). As with the larger group, these effects remained the same when comparing the LPD and RPD groups only.
We performed subgroup analyses to explore the possible effects of medication status on SDR performance. Within the entire group of PD participants and within the LPD and RPD groups, there were no differences in SDR performance between participants on and off medications (p > 0.56). Within the on and off medications sub groups, the differences between the LPD and RPD groups were consistent with those described above: LPD participants had significantly higher error rate in the delay conditions of the SDR task than RPD participants (p < 0.04).
3.2.2. Motor asymmetry
Motor asymmetry scores were significantly different between the RPD and LPD groups for the whole sample as well as for the MRI subset (p < 0.001; see Table 1). The groups differed in the expected direction such that RPD participants’ scores were positive and LPD participants’ scores were negative, indicating our initial dichotomization for worse side of motor symptoms was congruent with current motor dysfunction asymmetry scores. We then compared the absolute values of group motor asymmetry scores to investigate possible differences in degree of motor asymmetry between the PD subgroups. For the whole sample and for the MRI subset, there were no significant differences in degree of motor asymmetry between the RPD and LPD groups (p > 0.51). Thus, although we had two distinct groups of PD participants separable by absolute side of worse motor function, the groups were equivalent in the degree of asymmetry of their motor signs.
3.2.3. SN volume
Mean SN volumes for each of the participant groups in the MRI subset are displayed in Table 1. There were no significant differences between subject groups in right or left SN volumes after controlling for whole brain volume, F(2,30) = 2.85, p = 0.07. There was no within-subjects effect of SN side (left vs. right) on SN volume (p = 0.55) nor was there an interaction effect between SN side and subject group on SN volume (p = 0.66). This pattern remained the same when comparing the LPD and RPD groups only.
We calculated an SN volume asymmetry score for each participant according to the formula used to calculate the motor asymmetry score (see above). This calculation yields a score with absolute values ranging from 0 (symmetric) to 2 (exclusively unilateral SN degeneration), with negative scores indicating more right SN degeneration and positive scores indicating more left SN degeneration. There were no significant differences between groups in SN volume asymmetry (p = 0.63) or in degree of SN volume asymmetry (i.e. absolute value of SN volume asymmetry score; p = 0.44), nor did SN volume asymmetry score correlate significantly with motor asymmetry score (r = −0.05, p = 0.83). The inverse correlation between total SN volume and total UPDRSm score was not significant (r = −0.36, p = 0.10).
3.3. Asymmetry predictors of SDR performance
3.3.1. Motor asymmetry
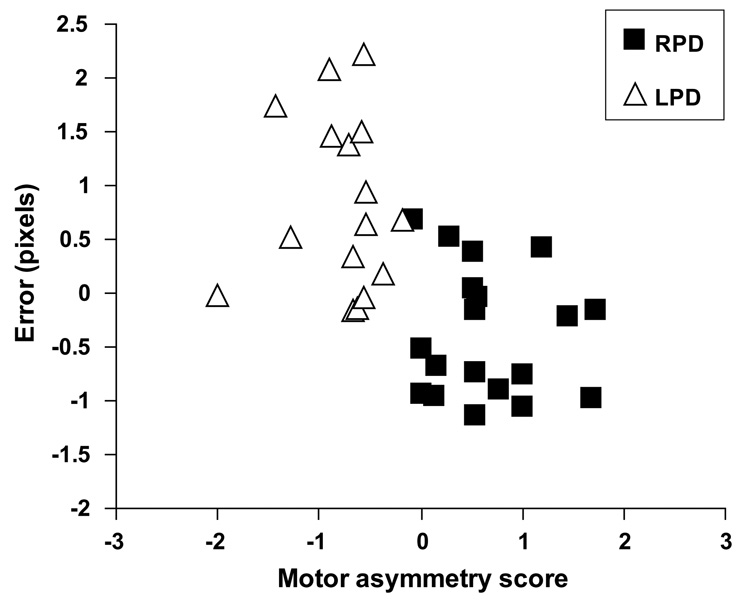
Across all of the PD participants, SDR 15 s delay error was significantly correlated with motor symptom asymmetry score (r = −0.49, p = 0.02) such that more negative motor asymmetry scores were associated with higher error rate. In a linear regression, motor asymmetry score accounted for a unique and significant portion of the variance in SDR 15 s delay performance after controlling for age, WRAT-3R, duration of disease and UPDRSm (R2 change = 0.12; F change (1, 29) = 5.20, p = 0.03), and the overall model was significant (R2 = 0.33; F(5, 29) = 2.90, p = 0.03). However, when the correlation between motor asymmetry and SDR 15 s delay error was examined within the RPD and LPD subgroups separately, the correlation coefficients were markedly reduced and not significant (RPD: r = −0.20, p = 0.41; LPD: r = 0.11, p = 0.69). Furthermore, visual inspection of the data revealed distinct clusters corresponding to the two groups, which indicated a bimodal rather than linear association between motor symptom asymmetry and SDR performance (Fig. 3). Total, right and left UPDRSm scores did not correlate with SDR performance across or within the PD subgroups (p > 0.14). There were no significant correlations between the severity of specific motor signs (i.e. tremor, rigidity and bradykinesia scores) and SDR performance across or within the PD subgroups (p > 0.21).
Fig. 3.
Relationship between motor asymmetry score and SDR 15 s delay error after accounting for age, WRAT-3R score, disease duration and UPDRSm for the PD participants (RPD, n = 19; LPD, n = 16).
3.3.2. SN volume asymmetry
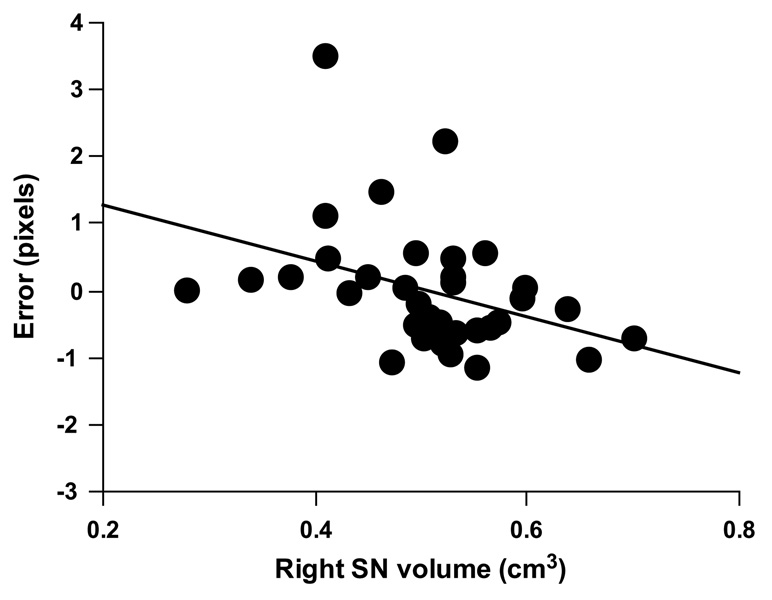
Across the MRI subset of PD and control participants, SDR 15 s delay error was significantly correlated with right SN volume (r = −0.42, p = 0.01) while the correlation with left SN volume did not reach significance (r = −0.31, p = 0.07). Smaller SN volumes were associated with higher error rate. In a linear regression, right SN volume accounted for a unique and significant portion of the variance in SDR 15 s delay performance after controlling for age, WRAT-3R and whole brain volume (R2 change = 0.16, F change (1, 29) = 5.75, p = 0.02, Fig. 4). The overall model did not reach significance (R2 = 0.22, F(4, 29) = 2.04, p = .11). The additional proportion of variance accounted for by left SN volume was not significant (R2 change = 0.07; F change (1, 29) = 2.36, p = 0.14). SN volume asymmetry score did not correlate with SDR performance (r = −0.26, p = 0.25).
Fig. 4.
Relationship between right SN volume and SDR 15 s delay error after accounting for age, WRAT-3R score and whole brain volume for the MRI subset of participants (N = 34; R2 change = 0.16, p = 0.02).
4. Discussion
Performance on spatial working memory relates to asymmetry of motor function and MR measurements of SN volume in people with PD. PD participants with left predominant motor dysfunction performed significantly worse on a spatial delayed response task than PD participants with right predominant motor dysfunction, who performed similarly to controls. In addition, poorer spatial delayed response performance was related to smaller right SN volumes. These effects were independent of other contributors to cognition in normal aging and in PD (age, premorbid intelligence, duration of disease, medication status and motor severity). They were also independent of type of motor dysfunction, as our groups had similar degrees of tremor, rigidity and bradykinesia and the severity of these motor signs was not related to spatial delayed response performance. Thus, our data support the hypothesis that worse right-brain disease severity is related to worse spatial working memory performance.
Our motor asymmetry findings are consistent with a number of studies where LPD participants performed more poorly than RPD participants on visuospatial tasks (Amick et al., 2006; Blonder et al., 1989; Katzen et al., 2006; Tomer et al., 1993), or where RPD participants’ performance was comparable to that of controls (Direnfeld et al., 1984; Katzen et al., 2006). However, only a few researchers looked at visuospatial memory specifically (Amick et al., 2006; Blonder et al., 1989; St. Clair et al., 1998; Starkstein et al., 1987; Tomer et al., 1993) and none of them used tasks validated to measure short term spatial memory. Rather, previous tasks involved memory for objects or may have been susceptible to the employment of verbal strategies, such as rehearsal or mnemonics, to mediate task performance. This perceptual mixing could have attenuated LPD and RPD differences in previous studies, which may explain why we found such marked differences in performance with a relatively small sample in the early stages of disease progression. The use of non-specific tasks confounds interpretation of cognitive results in terms of specific brain networks and functions.
Tasks that assess single cognitive processes and isolated neural systems are preferred to address questions about specific brain-behavior relationships. The SDR task used in this study is modeled specifically after the oculo-motor delayed response task (OMDR) (Funahashi et al., 1989) and requires the participant to maintain purely spatial information over a delay. Single-cell recording and lesion studies in non-human primates show that performance on the OMDR task relies on the principal sulcus region—the area analogous to the dorsolateral prefrontal cortex in humans (Funahashi et al., 1989, 1993). Human neuroimaging studies using the SDR task consistently demonstrate involvement of the right dorsolateral prefrontal cortex as well (D’Esposito et al., 1998; Jonides et al., 1993; Leung et al., 2002; McCarthy et al., 1996). Additionally, task-related neuronal activity in the dorsolateral prefrontal cortex and, consequently, task performance are modulated by dopamine and gamma-aminobutyric acid (GABA) in monkeys and in humans (Gibbs et al., 2005; Goldman-Rakic et al., 2000; Lewis & Moghaddam, 2006; Luciana et al., 1992; Müller et al., 1998; Rao, Williams, & Goldman-Rakic, 2000).
Right-sided PD neuropathology may disrupt performance on the SDR task by a number of possible pathways. Traditional hypotheses would posit that dopaminergic degeneration in the right SNc leads to decreased dopamine in the right caudate nucleus, which disrupts right prefrontal dorsolateral cortex function. This would account for our association between motor dysfunction and spatial memory, as dopamine cell loss in the SNc is also the putative mechanism for motor impairment. Another possibility is that right SNr degeneration disrupts the spatial tuning effects of GABAergic transmission in the right dorsolateral prefrontal cortex and impairs the maintenance of spatial information (Rao et al., 2000). This hypothesis is supported by monkey studies showing that SNr output indirectly targets the dorsolateral prefrontal cortex and that neurons in the SNr demonstrate activity corresponding to the activity observed in the dorsolateral prefrontal cortex during spatial memory tasks (Middleton & Strick, 2000). There is evidence that the SNr begins to degenerate early in PD (Anik, Iseri, Demirci, Komsuoglu, & Inan, 2007). Our volumetric measurements include a portion of the SNr along with the SNc, which may help to explain why they are more related to SDR than to motor performance.
Support for our SN asymmetry hypothesis was mixed. In support of our hypothesis, smaller right SN was predictive of worse SDR performance. In addition, there was an inverse relationship between SN volume asymmetry score and SDR performance such that more degeneration on the right compared to the left side of the brain (i.e. more negative SN volume asymmetry score) tended to be associated with worse performance (i.e. higher error rate). However, we cannot conclude from our data that the effect is driven entirely by asymmetric degeneration rather than by severity of overall nigral degeneration since left SN volume was moderately correlated with SDR performance and the relationship between SN volume asymmetry score and SDR performance was not statistically significant. Nonetheless, the strong correlation between smaller right SN volumes and poorer SDR performance suggests there is a lateralized relationship.
Our volumetric analysis of SN is less clear regarding the relationship between brain and motor asymmetry. SN volume measurements were not congruent with our group categorization according to predominant side of motor dysfunction (RPD vs. LPD), nor did they discriminate PD participants as a whole from controls. These results are consistent with previous MR studies that have not detected SN volume loss in PD (Geng, Li, & Zee, 2006; Oikawa, Sasaki, Tamakawa, Ehara, & Tohyama, 2002), although others have shown the opposite (Sohmiya, Tanaka, Aihara, & Okamoto, 2004). From autopsy studies, it is clear that there is substantial neuronal loss in SN which correlates with contralateral motor dysfunction (Kempster et al., 1989); however, neuronal loss may not necessarily translate to measurable volume loss.
Our data do not support the notion put forth by Huber and colleagues (1992) that the degree of motor asymmetry should parallel the degree of cognitive impairment. They found a linear relationship between motor asymmetry and verbal cognition, whereby increasing right-sided motor asymmetry was associated with decreasing performance on verbal tasks. In the present study, degree of left-sided asymmetry appeared to predict degree of spatial memory impairment, but closer examination revealed that this association was driven by qualitative differences in performance between the groups rather than by a linear effect of relative asymmetry. This finding, along with the observations that motor signs tend to become more bilateral and cognition tends to worsen as the disease progresses, suggests that categorizing participants according to side of initial clinical predominance is sufficient when exploring these concepts.
One methodological limitation of our study is the lack of a congruent “left-hemisphere” working memory task with which to demonstrate a true divergence of cognitive profiles between the LPD and RPD groups. This restricts our ability to exclude the possibility that degeneration of right subcortical structures relates to widespread cognitive deficits across many domains, rather than to circumscribed right hemisphere memory processes. The former has been proposed (Direnfeld et al., 1984; Tomer et al., 1993), as the right hemisphere may mediate overall activation and attentional control, thus forming the foundation for cognitive processing (Mesulam, 1981). However, our verbal fluency results show that the LPD group was not globally cognitively impaired relative to the control and RPD groups. Other studies have demonstrated worse performance by RPD groups on a variety of cognitive tasks and specifically those that rely on verbal abilities (e.g. Blonder et al., 1989; Spicer et al., 1988; Williams et al., 2007). These points reduce the likelihood of widespread impairment in individuals with predominantly left-body motor dysfunction.
Other limitations include the relatively small sample size and the possibility that PD medications could have influenced behavior. The relationship between dopaminergic medication and cognition is complex (for review, see Cools, 2006) and a recent study has shown that dopaminergic medication may interact with asymmetry to influence cognitive function in PD (Tomer, Aharon-Peretz, & Tsitrinbaum, 2007). Nevertheless, our cognitive findings did not differ according to medication status. SDR performance was similar between participants on and off medications and was impaired in LPD relative to RPD participants regardless of their medication status. Future studies of this type will need to manipulate medication status explicitly.
In conclusion, we demonstrated that PD participants with worse left-sided motor dysfunction are impaired in spatial memory compared to those with worse right-sided motor dysfunction, whose spatial memory is equivalent to that of controls. Moreover, poorer spatial memory is related to right SN volume loss. These findings indicate that disease asymmetry should be considered when interpreting patterns of cognitive performance in persons with PD. Overlooking this factor in studies of cognition—especially those with unbalanced samples or with tasks that employ hemispherically lateralized cognitive functions—can lead to inconsistent results and faulty interpretations regarding the nature of cognitive impairment in PD and its neurological basis. By using the SDR task, which has been validated carefully across animal and human studies to measure the maintenance of spatial information and the integrity of the right dorsolateral prefrontal cortex, we are better able to infer the effects of right nigral degeneration on spatial working memory in PD. The use of MRI-based volumetry to investigate the association between asymmetrical subcortical degeneration, motor symptoms and cognitive performance warrants further exploration.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Reviews in Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amick MM, Grace J, Chou KL. Body side of motor symptom onset in Parkinson’s disease is associated with memory performance. Journal of the International Neuropsychological Society. 2006;12:736–740. doi: 10.1017/S1355617706060875. [DOI] [PubMed] [Google Scholar]
- Anik Y, Iseri P, Demirci A, Komsuoglu S, Inan N. Magnetization transfer ratio in early period of Parkinson disease. Academic Radiology. 2007;14:189–192. doi: 10.1016/j.acra.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Barber J, Tomer R, Sroka H, Myslobodsky MS. Does unilateral dopamine deficit contribute to depression? Psychiatry Research. 1985;15:17–24. doi: 10.1016/0165-1781(85)90035-6. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Gur RE, Gur RC, Saykin AJ, Hurtig HI. Neuropsychological functioning in hemiparkinsonism. Brain and Cognition. 1989;9:244–257. doi: 10.1016/0278-2626(89)90034-1. [DOI] [PubMed] [Google Scholar]
- Cavalieri B. Geometria Indivisibilibus Continuorum Nova quadam ratione promota. Bologna, Italy: Duciis; 1653. [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for l-DOPA treatment in Parkinson’s disease. Neuroscience and Biobehavioral Reviews. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999a;122(Pt. 8):1421–1436. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999b;122(Pt. 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Direnfeld LK, Albert ML, Volicer L, Langlais PJ, Marquis J, Kaplan E. Parkinson’s disease. The possible relationship of laterality to dementia and neurochemical findings. Archives of Neurology. 1984;41:935–941. doi: 10.1001/archneur.1984.04050200041016. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. New York: Macmillan; 1987. pp. 153–163. [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. Journal of Neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: Evidence for mnemonic “scotomas”. Journal of Neuroscience. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng DY, Li YX, Zee CS. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson’s disease. Neurosurgery. 2006;58:256–262. doi: 10.1227/01.NEU.0000194845.19462.7B. [DOI] [PubMed] [Google Scholar]
- Gibbs SE, D’Esposito M. Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:212–221. doi: 10.3758/cabn.5.2.212. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, III, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Research: Brain Research Reviews. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Green J, McDonald WM, Vitek JL, Evatt M, Freeman A, Haber M, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- Griffin SL, Mindt MR, Rankin EJ, Ritchie AJ, Scott JG. Estimating premorbid intelligence: Comparison of traditional and contemporary methods across the intelligence continuum. Archives of Clinical Neuropsychology. 2002;17:497–507. [PubMed] [Google Scholar]
- Gundersen HJG. Stereology: The fast lane between neuroanatomy and brain function—or still only a tightrope? Acta Neurologica Scandinavica Supplementum. 1992;137:8–13. doi: 10.1111/j.1600-0404.1992.tb05032.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. Journal of Microscience. 1987;147(Pt. 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Huber SJ, Freidenberg DL, Shuttleworth EC, Paulson GW, Clapp LE. Neuropsychological similarities in lateralized parkinsonism. Cortex. 1989;25:461–470. doi: 10.1016/s0010-9452(89)80059-0. [DOI] [PubMed] [Google Scholar]
- Huber SJ, Miller H, Bohaska L, Christy JA, Bornstein RA. Asymmetrical cognitive differences associated with hemiparkinsonism. Archives of Clinical Neuropsychology. 1992;7:471–480. [PubMed] [Google Scholar]
- Johnstone B, Callahan CD, Kapila CJ, Bouman DE. The comparability of the WRAT-R reading test and NAART as estimates of premorbid intelligence in neurologically impaired patients. Archives of Clinical Neuropsychology. 1996;11:513–519. [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun M. Letters to Nature: Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Katzen HL, Levin BE, Weiner W. Side and type of motor symptom influence cognition in Parkinson’s disease. Movement Disorders. 2006;21:1947–1953. doi: 10.1002/mds.21105. [DOI] [PubMed] [Google Scholar]
- Kempster PA, Gibb WR, Stern GM, Lees AJ. Asymmetry of substantia nigra neuronal loss in Parkinson’s disease and its relevance to the mechanism of levodopa related motor fluctuations. Journal of Neurology, Neurosurgery, and Psychiatry. 1989;52:72–76. doi: 10.1136/jnnp.52.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Schulzer M, Mak E, Hammerstad JP, Calne S, Calne DB. Patterns of asymmetry do not change over the course of idiopathic parkinsonism: Implications for pathogenesis. Neurology. 1995;45:435–439. doi: 10.1212/wnl.45.3.435. [DOI] [PubMed] [Google Scholar]
- Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. Journal of Cognitive Neuroscience. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: Convergence of gamma-aminobutyric acid and glutamate alterations. Archives of Neurology. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3rd ed. Oxford: Oxford University Press; 1995. [Google Scholar]
- Locascio JJ, Corkin S, Growdon JH. Relation between clinical characteristics of Parkinson’s disease and cognitive decline. Journal of Clinical and Experimental Neuropsychology. 2003;25:94–109. doi: 10.1076/jcen.25.1.94.13624. [DOI] [PubMed] [Google Scholar]
- Luciana M, Depue RA, Arbisi P, Leon A. Facilitation of working memory in humans by a D2 dopamine receptor agonist. Journal of Cognitive Neuroscience. 1992;4:58–68. doi: 10.1162/jocn.1992.4.1.58. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Olsen DR. Magnetic resonance imaging (MRI) and model-free estimates of brain volume determined using the Cavalieri principle. Journal of Anatomy. 1991;178:133–144. [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cerebral Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain and Cognition. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Müller U, von Cramon DY, Pollmann S. D1- versus D2- receptor modulation of visuospatial working memory in humans. Journal of Neuroscience. 1998;18:2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa H, Sasaki M, Tamakawa Y, Ehara S, Tohyama K. The substantia nigra in Parkinson disease: Proton density-weighted spin-echo and fast short inversion time inversion-recovery MR findings. American Journal of Neuroradiology. 2002;23:1747–1756. [PMC free article] [PubMed] [Google Scholar]
- Pillon B, Boller F, Levy R, Dubois B. Cognitive deficits and dementia in Parkinson’s disease. In: Boller F, Cappa SF, editors. Handbook of neuropsychology. 2nd ed. Amsterdam: Elsevier; 2001. pp. 311–371. [Google Scholar]
- Postle BR, Jonides J, Smith EE, Corkin S, Growdon JH. Spatial, but not object, delayed response is impaired in early Parkinson’s disease. Neuropsychology. 1997;11:171–179. doi: 10.1037//0894-4105.11.2.171. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. Journal of Neuroscience. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riklan M, Stellar S, Reynolds C. The relationship of memory and cognition in Parkinson’s disease to lateralisation of motor symptoms. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53:359–360. doi: 10.1136/jnnp.53.4.359-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohmiya M, Tanaka M, Aihara Y, Okamoto K. Structural changes in the midbrain with aging and Parkinson’s disease: An MRI study. Neurobiology of Aging. 2004;25:449–453. doi: 10.1016/S0197-4580(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Spicer KB, Roberts RJ, LeWitt PA. Neuropsychological performance in lateralized parkinsonism. Archives of Neurology. 1988;45:429–432. doi: 10.1001/archneur.1988.00520280079019. [DOI] [PubMed] [Google Scholar]
- St. Clair J, Borod JC, Sliwinski M, Cote LJ, Stern Y. Cognitive and affective functioning in Parkinson’s disease patients with lateralized motor signs. Journal of Clinical and Experimental Neuropsychology. 1998;20:320–327. doi: 10.1076/jcen.20.3.320.820. [DOI] [PubMed] [Google Scholar]
- Starkstein S, Leiguarda R, Gershanik O, Berthier M. Neuropsychological disturbances in hemiparkinson’s disease. Neurology. 1987;37:1762–1764. doi: 10.1212/wnl.37.11.1762. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA. The neuropsychology of Parkinson’s disease. Brain and Cognition. 1995;28:281–296. doi: 10.1006/brcg.1995.1258. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain. 1986;109(Pt. 5):845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Tomer R, Aharon-Peretz J, Tsitrinbaum Z. Dopamine asymmetry interacts with medication to affect cognition in Parkinson’s disease. Neuropsychologia. 2007;45:357–367. doi: 10.1016/j.neuropsychologia.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Tomer R, Levin BE, Weiner WJ. Side of onset of motor symptoms influences cognition in Parkinson’s disease. Annals of Neurology. 1993;34:579–584. doi: 10.1002/ana.410340412. [DOI] [PubMed] [Google Scholar]
- Wilkinson GW. WRAT-3: Wide range achievement test administration manual. 3rd ed. Wilmington, DE: Western psychological services; 1993. [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Williams LN, Seignourel P, Crucian GP, Okun MS, Rodriguez RL, Skidmore FM, et al. Laterality, region, and type of motor dysfunction correlate with cognitive impairment in Parkinson’s disease. Movement Disorders. 2007;22:141–145. doi: 10.1002/mds.21220. [DOI] [PubMed] [Google Scholar]
- Zetusky WJ, Jankovic J. Laterality and symptom association in Parkinson’s disease. Archives of Neurology. 1985;42:1132–1133. doi: 10.1001/archneur.1985.04060110010001. [DOI] [PubMed] [Google Scholar]