Abstract
Fas/CD95-induced apoptosis of hepatocytes in vivo proceeds through the so-called type II pathway, requiring the pro-apoptotic BH3-only Bcl-2 family member Bid for mitochondrial death signaling. Consequently, Bid-deficient mice are protected from anti-Fas antibody injection induced fatal hepatitis. Here we report the unexpected finding that freshly isolated mouse hepatocytes, cultured on collagen or Matrigel™, become independent of Bid for Fas-induced apoptosis, thereby switching death signaling from type II to type I. In such in vitro cultures, FasL activates caspase-3 without Bid cleavage, Bax/Bak activation or cytochrome c release, and neither Bid ablation nor Bcl-2 overexpression is protective. The type II to type I switch depends on extracellular matrix adhesion, as primary hepatocytes in suspension die in a Bid-dependent manner. Moreover, the switch is specific for FasL-induced apoptosis as collagen-plated Bid-deficient hepatocytes are protected from TNFα/ActD-induced apoptosis.
Conclusion
Our data suggest a selective crosstalk between extracellular matrix and Fas-mediated signaling which favours mitochondria-independent type I apoptosis induction.
Keywords: Apoptosis, collagen, death receptor signaling, liver cells, extracellular matrix
The death ligand FasL/CD95L is a member of the TNF cytokine family that plays a crucial role in regulating cell death for controlling T as well as B cell homeostasis, the cytolysis of virally infected cells and the resolution of immune responses1. The highest constitutive expression of Fas, the receptor for FasL, is found in hepatocytes2,3. Mice injected with a lethal dose of the agonistic monoclonal anti-Fas antibody (Jo2) exhibit massive apoptosis of liver cells and die within a few hours from fulminant hepatitis3–5. In addition, liver cells are thought to die, at least in part, through Fas-mediated apoptosis during viral and autoimmune hepatitis, alcoholic liver disease as well as endotoxin- or ischemia/reperfusion-induced liver damage2,6,7. Perplexingly, Fas is also critical for liver regeneration after partial hepatectomy8 when liver damage activates anti-apoptotic signaling pathways that switch Fas-mediated signals from primarily apoptotic to hepatocyte growth inducing ones9. These findings indicate that, as for the related TNFα/TNF-R1 cytokine/receptor signaling pathway, the outcome of FasL/Fas-induced signaling is the result of a crosstalk between different signaling pathways dependent on cell type, cell state and environmental factors9,10.
After binding, FasL causes Fas receptor multimerization and then recruitment of the adaptor FADD/MORT1 and the pro-form of caspase-8 to the cytoplasmic death domain of the receptor, leading to formation of the so-called death inducing signaling complex (DISC)11. The dimerization of caspase-8 at the DISC provokes its activation through conformational change, which is followed by autoproteolytic processing and release into the cytosol. The ensuing apoptotic program can kill cells via two different pathways, type I or type II12. In type I cells, caspase-8 directly processes and activates the effector caspases -3 and -7, and this suffices to execute cell demolition. In type II cells, apoptosis induction also requires that caspase-8 cleaves the pro-apoptotic BH3-only Bcl-2 family member Bid, forming tBid, which translocates to mitochondria to activate the multi-BH domain pro-apoptotic Bcl-2 family members Bax and Bak by so far unknown mechanisms4,5,13. Bax/Bak-mediated mitochondrial membrane permeabilization results in the release of cytochrome c from the intermembrane space14. Once in the cytosol, cytochrome c binds to Apaf-1 and thereby recruits pro-caspase-9 into an apoptosomal complex, which processes and activates effector caspases -3 and -715. The reasons for the differences between type I and type II cells are still unclear12, although differences in the expression of inhibitors of the death receptor signaling cascade, such as c-FLIP or XIAP, have been proposed to be responsible. While FLIP is an inhibitory caspase-8 analog, acting at the DISC16, XIAP blocks the enzymatic activity of caspases -9. -3 and -715,17. The caspase inhibitory effect of XIAP can be overcome by apoptogenic proteins, such as Smac/DIABLO and Htr2A/Omi, which are released from mitochondria together with cytochrome c18–20. They bind to XIAP and neutralize its caspase binding activity and/or target it for proteasomal degradation. It has therefore been postulated that cells with high levels of XIAP would require tBid/mitochondrial-mediated amplification of the caspase cascade to overcome the caspase-inhibitory function of XIAP11.
In vivo and in vitro, many Fas-sensitive cells, including lymphocytes, die via the type I pathway, demonstrated by the findings that neither loss of Bid nor Bcl-2 over-expression protects these cells from Fas-induced apoptosis5,21. In contrast, hepatocytes require the type II amplification loop for cell killing since Bid-deficient mice are resistant to agonistic anti-Fas antibody (Jo2) induced hepatocyte apoptosis and fatal hepatitis4,5.
To understand the detailed mechanisms of hepatocyte killing, regeneration and differentiation, it is necessary to develop in vitro culture models. The standard protocol has been to plate hepatocytes, after collagenase assisted isolation from the liver, on collagen I or certain other extracellular matrices, such as Matrigel™, and to use the cells within 2–3 days for cellular or biochemical analysis22–24. By applying this protocol, we found that Fas-induced cell death signaling is switched from a type II to I pathway.
Experimental Procedures
Collagen and Matrigel coating
6 well plates or 12 mm glass coverslips were incubated with 1 mL of collagen I (4.5 mg/mL) (diluted 1:10 in PBS) per well for 30 min at 37 °C. Subsequently, the collagen solution was removed before seeding the cells. The coating with Matrigel™ Matrix was performed as described by the manufacturer (BD Biosciences). At room temperature, Matrigel™ matrix polymerizes to produce biologically active matrix material, resembling the mammalian cellular basement membrane.
Isolation and cultivation of primary mouse hepatocytes
Primary mouse hepatocytes were isolated from 6–12 week old wt, bid−/−, bak−/−(Jackson Laboratories), fas−/− (from M. Simon, Freiburg) or xiap−/− (from J. Silke, Melbourne) mice (all kept on a C57BL/6 genetic background) based on the collagenase perfusion technique24, with the following modifications. The isolated hepatocytes were plated on collagen I- or Matrigel™-coated plates containing Williams' medium E supplemented with 10% FCS, 100 nM dexamethasone, 2 mM L-glutamine and 1% penicillin/streptomycin solution (complete WME, Biochrom). After 4 h of adherence, the cells were washed twice in PBS and incubated overnight in complete WME but without dexamethasone in a 5% CO2 incubator at 37 °C before treatment with FasL.
Hepatocytes in suspension were used for apoptosis assays directly after isolation without plating on dishes. For this purpose, the cells were resuspended in complete WME without dexamethasone for cell counting, treated with FasL or left untreated and carefully shaken in a 5% CO2 incubator at 37 °C for different time points.
Results
In contrast to in vivo conditions, FasL-induced caspase-3 activation and apoptosis of collagen-plated hepatocytes does not depend on Bid
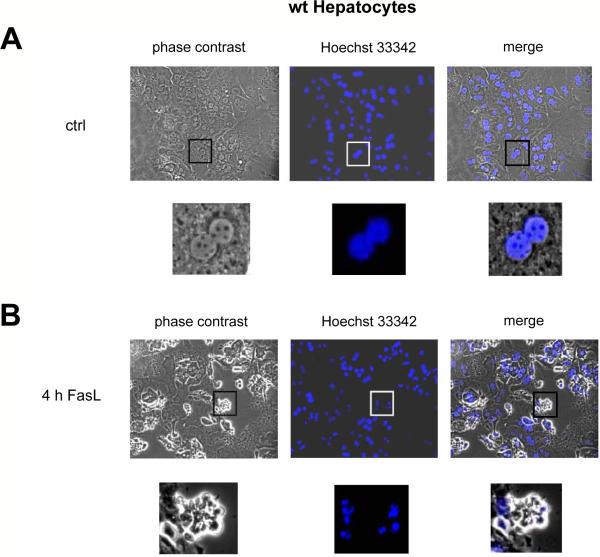
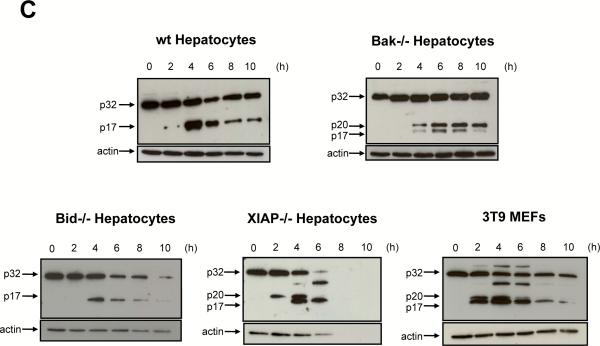
We first confirmed in newly generated Bid−/− mice5 that anti-Fas-induced caspase-3 and -7 activation and hepatocyte apoptosis in vivo required Bid and hence the mitochondrial type II pathway (Suppl. Mat, Fig. S1). We then isolated hepatocytes from C57BL/6 mice and plated them on collagen I. 95% of these cells exhibited a binuclear morphology, typical of hepatocytes (Fig. 1A), and they survived for up to 3 days (data not shown). After 24 h of culture the cells were treated with 50 ng/mL FasL from the supernatant of Neuro2A cells secreting a bioactive form of membrane-bound FasL25 (quantitation and controls, Figs. S2/S3). Alternatively, the cells were exposed to recombinant Fc-FasL26, which produced identical results (Fig. S2). As shown in Fig. 1B, FasL-treated primary hepatocytes exhibited typical signs of apoptosis, such as cell shrinkage, plasma membrane blebbing and nuclear condensation and fragmentation. To quantify apoptosis, we performed FACS analysis after staining cells with GFP-annexin-V plus propidium iodide (PI), counting the GFP-annexin-V/PI-double negative cells as “surviving cells”. The number of surviving cells gradually decreased upon FasL treatment, reaching 20% after 10 h (Fig. 1E). Consistent with apoptosis induction, the activity of the effector caspases-3 and -7 (Fig. 2A) and the processing of the p32 proform of caspase-3 to its active p20/p17 fragments (Fig. 2C) increased progressively over time. Immunofluorescence staining confirmed the intracellular activation of caspase-3 in FasL-treated hepatocytes (Fig. 3D). Unexpectedly, the kinetics of apoptosis, as assessed by morphological examination (Fig. 1C/D), by GFP-annexin-V/PI staining (Fig. 1E) and the processing and activation of caspase-3, measured both in vitro (Fig. 2A/C) and inside the cells (Fig. 3E) were indistinguishable between Bid−/− and wild-type primary hepatocytes at various FasL concentrations (10 – 100 ng/mL) (Fig. S4A/B). This finding indicated that freshly isolated hepatocytes plated on collagen I switch Fas-induced death signaling from a Bid-dependent type II to a Bid-independent type I pathway.
Figure 1. Apoptotic morphology and annexin-V/PI staining of FasL-treated primary hepatocytes plated on collagen.
(A–D) Phase contrast and DNA fluorescence (Hoechst 33342) microscopy of untreated (A,C) and N2A FasL-treated (4 h, 50 ng/ml) (B,D) wt (A,B) and Bid−/− (C,D) primary mouse hepatocytes cultured on collagen. Magnifications are 200 × and 800 ×, respectively. (E) FACS analysis of GFP-annexin-V/PI stained wt, Bid−/−, Bak−/−, XIAP−/− primary mouse hepatocytes and wt 3T9 MEFs treated with 50 ng/mL N2A FasL for 0–10 h. The percentages of GFP-annexin-V/PI double negative cells are depicted. The values represent the means of 3 independent experiments ± s.d.
Figure 2. Kinetics of caspase-3 processing and activation are similar between wt, Bid−/− and Bak−/− hepatocytes but are accelerated in XIAP−/− cultured hepatocytes and 3T9 MEFs.
(A,B) Fluorogenic caspase-3/-7 activity assay (relative fluorescence units/RFU) of wt, Bid−/− and Bak−/− hepatocytes (A) and XIAP−/− hepatocytes and 3T9 MEFs (B). The graphs are shown separately because of the 10-fold difference in the caspase activities. (C) Western blot analysis for inactive p32 and active p20/p17 caspase-3 in the cytosols of the indicated cells. Probing for actin is shown as a loading control. The values in (A,B) represent the means of 3 independent experiments ± s.d.
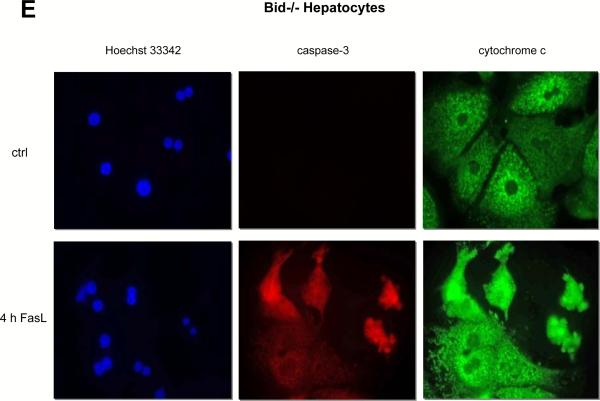
Figure 3. Lack of Bid cleavage, cytochrome c and Smac release and caspase-9 processing in FasL-treated primary hepatocytes.
Western blot analysis of Bid (A) cytochrome c (B) Smac/DIABLO (C) and caspase-9 (F) in the cytosolic and mitochondrial fractions of wt and Bid−/− primary hepatocytes and 3T9 MEFs treated with 50 ng/mL N2A FasL for up to 10 h. 3T9 TE: Total extract of 3T9 MEFs as control. Probing for actin (cytosol) or the F1 subunit of the mitochondrial ATP synthase (ATP-S) is shown as a loading control. (D,E) Hoechst 33342 staining (blue) and activated caspase-3 (red) and cytochrome c (green) immunofluorescence analysis of untreated and N2A FasL-treated wt and Bid−/− hepatocytes. Magnification is 630 ×.
Lack of Bid cleavage and activation of mitochondrial effectors during FasL-induced apoptosis of collagen-plated hepatocytes
To further substantiate our findings, we performed a detailed biochemical analysis of the components of type I and type II signaling in FasL-treated primary hepatocytes in culture. As a positive control, we used primary as well as transformed (3T9) mouse embryo fibroblasts (MEFs), which showed a typical type II signaling behaviour. In these fibroblasts, FasL caused the formation and mitochondrial translocation of p15 tBid (Fig. 3A), the release of cytochrome c (Fig. 3B) and Smac (Fig. 3C) and caspase-3 activation (Fig. 2B/C). Moreover, apoptosis occurred even faster than in hepatocytes and was largely inhibited by loss of Bid (Fig. S5). In contrast, in cultured wt as well as Bid−/− hepatocytes, neither p15 tBid formation/mitochondrial translocation (Fig. 3A) nor the release of cytochrome c (Fig. 3B) could be detected by Western blotting. Remarkably, Smac was abundant in the cytosol of hepatocytes for unknown reasons, but its level was not affected by the presence or absence of Bid or by FasL treatment (Fig. 3C). Immunofluorescence staining confirmed that, upon FasL treatment, both wt and Bid−/− hepatocytes retained cytochrome c in punctuate structures, although the overall fluorescence intensity increased due to cell shrinkage (Fig. 3D/E). Consistent with this notion, we did not observe activation of the downstream target of cytochrome c, caspase-9, in FasL-treated hepatocytes in culture (Fig. 3F). Although low amounts of the partially processed forms of caspase-9 were detected between 2 – 6 h of treatment, this was also seen in Bid−/− hepatocytes. By contrast, FasL-treated MEFs displayed a clear processing of caspase-9 to the p37/p35 fragments (Fig. 3F).
Mitochondrial outer membrane permeabilization depends on the multi-BH domain pro-apoptotic Bcl-2 family members Bax and Bak27. While Bak is constitutively localized to mitochondria, Bax is mostly found in the cytosol of healthy cells28. Indeed, we found that wt hepatocytes contained all Bax in the cytosol (Fig. S6A). In response to treatment with FasL, we did not observe translocation of Bax to mitochondria (Fig. S6A), suggesting that Bax was not activated in FasL-treated hepatocytes. This was not due to a technical problem to detect Bax translocation, as Bax migration to mitochondria was readily detectable in FasL-treated MEFs (Fig. S6A). To examine the role of Bak in FasL-induced apoptosis, we isolated hepatocytes from Bak-deficient mice. Upon treatment with FasL, these cells showed similar kinetics of apoptosis (Fig. 1E) and caspase-3 activation (Fig. 2A) and processing (Fig. 2C) and a failure to translocate Bax (Fig. S6A) as wt cells. Moreover, over-expression of Bcl-2 could not protect primary hepatocytes from FasL-induced apoptosis (Suppl. Mat, Fig. S6B), consistent with the notion that cell killing occurred through the type I pathway12,20.
XIAP is not degraded in FasL-treated hepatocytes in culture and its deletion sensitizes the cells to caspase-3 activation and apoptosis
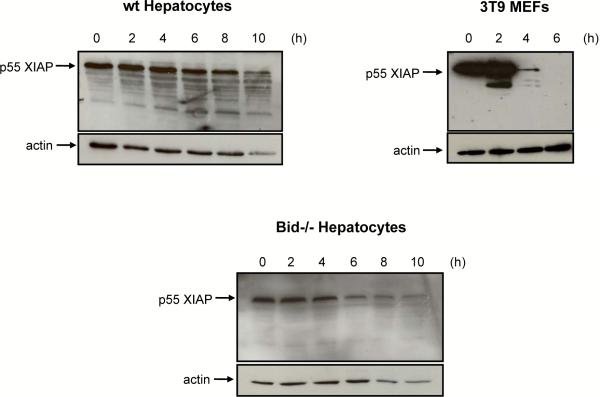
XIAP is an endogenous inhibitor of caspases-3, -7 and -917. In cells undergoing mitochondria dependent apoptosis, XIAP is neutralized and/or degraded by the proteasome18–20. We examined the expression and fate of XIAP in FasL-treated 3T9 MEFs and primary hepatocytes. Healthy MEFs expressed high levels of XIAP, but this was rapidly degraded in response to FasL due to activation of the type II pathway (Fig. 4). By contrast, in healthy, primary hepatocytes plated on collagen (from both wt and bid−/− mice), XIAP levels were much lower than in MEFs and they remained low for up to 8 h of FasL treatment (Fig. 4). Thereafter, XIAP levels started to diminish, but at that time 70–80% of the hepatocytes had already undergone apoptosis (Fig. 1E), indicating that XIAP degradation was a consequence and not a cause of cell killing. Nevertheless, some caspase-3 in hepatocytes was held in check by XIAP, since XIAP−/− cells displayed a faster and ~10 times higher caspase-3 activation (Fig. 2B/C) and accelerated apoptosis (Fig. 1E) in response to FasL treatment than wt hepatocytes.
Figure 4. Treatment with FasL triggers degradation of XIAP in MEFs but not in hepatocytes.
Western blot analysis of XIAP in cytosolic fractions of wt and Bid−/− hepatocytes and wt 3T9 MEFs treated with 50 ng/mL N2A FasL for up to 10 h. Probing for actin is shown as a loading control.
The type II to type I switch is specific for FasL and does not occur with TNF/actinomycinD (ActD)-induced apoptosis
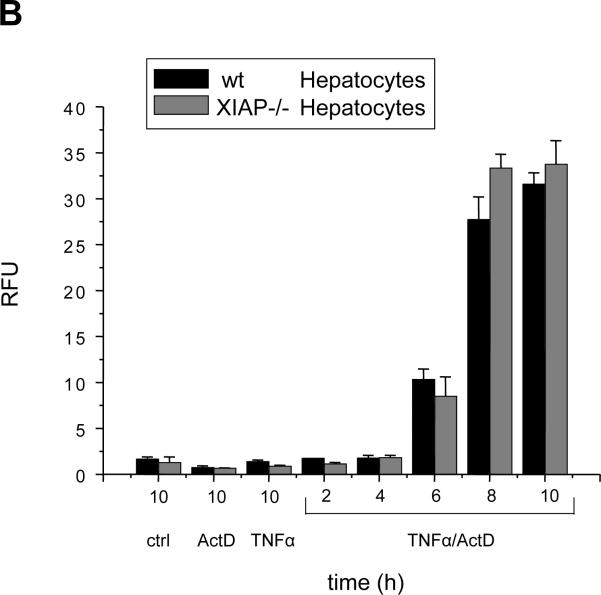
Type I and II apoptosis pathways were also reported for TNFα/TNF-R1 signaling29. To investigate whether collagen plating affected components, which are common to TNFα/TNF-R1 and FasL/Fas signaling, we treated wt and Bid−/−primary hepatocytes with TNFα plus ActD. The presence of actinomycin D was necessary to block NFκB-transduced survival signaling, which is normally triggered by TNFα-TNF-R1 stimulation29. Importantly, TNFα/ActD (and also FasL/ActD) induced classical apoptosis of primary hepatocytes because cell death was effectively inhibited by the broad spectrum caspase inhibitor Q-VD-OPH (Fig. S7A/B). Strikingly, while wt hepatocytes showed high caspase-3 activity between 6–8 h of TNFα/ActD treatment, only little caspase-3 activation was detected in the Bid−/− cells (Fig. 5A). This demonstrates that collagen plated hepatocytes retained mitochondria-mediated type II signaling in response TNFα/ActD. This finding was independent of the dose of TNFα used (Fig. S4C). Consistent with type II signaling, TNFα/ActD caused rapid degradation of XIAP (data not shown), and apoptosis sensitivity was similar between wt and XIAP−/− hepatocytes (Fig. 5B).
Figure 5. Caspase-3 activation requires Bid when primary hepatocytes are treated with TNFα/ActD instead of FasL (± ActD).
(A,B) Fluorogenic caspase-3/-7 activity assay on cytosolic extracts of wt and Bid−/− (A) or wt and XIAP−/− (B) primary mouse hepatocytes treated for the indicated times with 25 ng/mL TNFα plus 0.4 μ-g/mL ActD, 50 ng/mL N2A FasL plus 0.4 μ-g/mL ActD or TNFα and ActD alone. The values represent the means of three independent experiments ± s.d.
The type II to type I switch occurs upon plating hepatocytes on Matrigel™ but not if the cells are kept in suspension
As the FasL type II to type I apoptosis signaling switch was seen with cell culturing on collagen, we wondered whether it also occurred when freshly isolated hepatocytes were plated on other extracellular matrices, such as Matrigel™. This matrix is a solubilized basement membrane preparation extracted from EHS mouse sarcoma, a tumor rich in ECM proteins. Its major component is laminin, followed by collagen IV, heparan sulfate proteoglycans, and entactin. When seeded on Matrigel™, primary hepatocytes showed typical features of type I apoptotic signaling in response to FasL treatment (Suppl. Mat., Fig. S8), indicating that other extracellular matrix components were as potent as collagen to induce the type II to I switch of apoptosis signaling in freshly isolated hepatocytes.
We then examined the possibility that the differences in FasL signaling in hepatocytes between in vivo and in vitro might be due to the fact that the cells were forced to re-attach to an extracellular matrix after detachment from their natural environment. For this purpose, we kept primary hepatocytes in suspension cultures and treated them with FasL directly after isolation. (This protocol had to be employed since it was reported that hepatocytes in suspension survive only for 1–2 days22.) As shown in Fig. 6A, hepatocytes in suspension cultures were rapidly killed by FasL (within 4 h) and showed fast and efficient caspase-3 processing (Fig. 6C) and activation (Fig. 6B). Interestingly, we clearly detected FasL-induced release of cytochrome c into the cytosol (Fig. 6D) and the processing of caspase-9 into its p37 form (Fig. 6C). Remarkably, cytochrome c release (Fig. 6D), caspase-9 processing and caspase-3 activation/processing (Fig. 6B/C) as well as apoptosis induction (Fig. 6A) were all significantly inhibited by loss of Bid. These results indicate that hepatocytes retain the mitochondria-mediated type II FasL signaling pathway for apoptosis when they are maintained in single-cell suspension.
Figure 6. Primary hepatocytes kept in suspension require Bid for FasL-induced cytochrome c release, caspase-9/-3 activation and apoptosis.
(A) MTT assay, (B) fluorogenic caspase-3/-7 activity assay (on cytosolic extracts, relative fluorescence units/RFU) (C) caspase-3 and caspase-9 Western blot analysis (on the same blot) and (D) cytochrome c subcellular localization by Western blot analysis of wt and Bid−/− hepatocytes cultured in suspension and treated with 50 ng/mL N2A FasL for up to 4 h. The values in (A,B) represent the means of three independent experiments ± s.d. In (C,D) probing for actin is shown as a loading control.
Discussion
Here we report that FasL/Fas signaling is switched from the Bid/mitochondria-dependent type II to the Bid/mitochondria-independent type I pathway when freshly isolated mouse hepatocytes are plated on collagen or Matrigel™. Consistently, the switch does not occur when hepatocytes are kept in single-cell suspension after isolation. These results indicate that extracellular matrix components trigger signaling cascades that crosstalk with FasL/Fas-signaling to abrogate its dependence on the Bid-mediated mitochondrial amplification loop for apoptosis induction.
Given that death receptors use common downstream signaling components, it was surprising that we did not observe a type II to type I apoptosis signaling switch when hepatocytes were treated with TNFα/ActD. One might argue that the presence of ActD may have prevented this switch. However, this appears unlikely, as FasL/ActD-induced caspase activation was also Bid-independent (type I) in these cells (Figs. 5/S4B). How might then extracellular matrix components specifically affect FasL/Fas signaling? Possibly, these components could activate intracellular signaling pathways via integrins that intercept the FasL/Fas pathway at discrete signaling modules. Integrins are known to associate on their cytoplasmic side with integrin-linked kinase (ILK) and focal adhesion kinase (FAK), which activate downstream signaling through the PI3K/AKT/GSK-3, MEK/MAPK and JNK pathways30,31. Any of these kinases or kinases activated further downstream might phosphorylate and thereby change the activity and/or stability of Fas signaling components, such as FADD, caspase-8, Bid, the caspase-8 inhibitor FLIP or the caspase-3/-9 inhibitor XIAP. For example, phosphorylation of Bid by casein kinases I and II has been shown to prevent its cleavage by caspase-832,33. Moreover, FLIP phosphorylation by JNK marks the protein for proteasomal degradation via the E3 ubiquitin ligase Itch34. Preliminary results from our lab revealed, however, that pharmacological inhibition of PI3K, MAPK, casein kinase I and II, JNK or GSK-3 did not affect the collagen plating-induced signaling switch of hepatocytes from type II to type I (Fig. S9). Moreover, cytosolic and membrane-associated levels of caspase-8 were unchanged (Fig. S10) and FLIP protein was undetectable (data not shown) in extracts from either collagen-plated or suspension hepatocytes. This indicates that the type II to I signaling switch was not caused by alterations in the levels of FLIP or caspase-8, in contrast to a previous report12.
An attractive possibility for integrin signals to interfere with the FasL/Fas signaling pathway, without the need for modifying downstream signaling components, would be via a direct interaction between integrins and Fas. Such a mechanism has been reported for the HGF receptor c-Met. In healthy hepatocytes c-Met was found to physically interact with Fas, preventing its activation35,36. In response to high levels of HGF or FasL, c-Met is released from Fas, allowing FADD and caspase-8 recruitment and apoptosis induction35,36. Interestingly, a recent report showed that high doses of HGF could bypass the mitochondrial requirement for Fas-induced apoptosis in Jurkat cells and hepatocytes, leading to a type I signalling phenotype37. Similarly, integrins could modulate the Fas, but not the TNF-R1 DISC to engage type I instead of type II signaling. It is also conceivable that integrin activation may modify lipid rafts on the plasma membrane. These specialized cholesterol-rich micro-domains have been reported to recruit or exclude Fas and their associated signaling molecules and thereby change intracellular signaling behaviour38.39.
As XIAP needs to be inactivated and/or removed by mitochondrial type II signaling for effective apoptosis to occur, it was suggested that it determines the type of apoptotic signaling in response to FasL11,18–20. Interestingly, while MEFs express high levels of XIAP, which is rapidly degraded in response to FasL treatment, hepatocytes express only little XIAP that allows most caspase-3 to be directly activated by caspase-8. It is, however, unclear why this direct pathway is not used upon treatment with TNFα/ActD in the very same hepatocytes. XIAP−/− mice were found to be more susceptible to anti-Fas antibody induced hepatitis than wild-type mice (T.K., J. Silke, C.B. and A.S., in preparation) although we expected that removal of XIAP would not further sensitize type II cells in vivo. Thus, while XIAP may determine the sensitivity of cells towards death ligand induced apoptosis, additional mechanisms must exist to regulate the switch from type I to II or vice versa. We envisaged the possibility that other IAPs, such as cIAP1 and cIAP2, could be involved in the conversion. As shown in Figure S11A, whereas MEFs express cIAP1 in a stable form, no cIAP1 protein could be detected in healthy or FasL-treated hepatocytes, irrespective of whether death signalling employed the type I (suspension) or type II (on collagen) pathway. By contrast, cIAP2 appears to be transcriptionally upregulated in response to FasL treatment in collagen plated (type I) hepatocytes, but not in type II MEFs, indicating that this IAP may play a role in the apoptosis pathway signaling switch.
The question remains why hepatocytes in suspension (at least in part) resemble the in vivo situation despite the fact that they are not in their natural environment with respect to extra-cellular matrix, neighbouring cells (stellar cells, Kupffer cells, etc.) and cytokines/growth factors. We speculate that collagen I and Matrigel™ may not provide the optimal (correct) extracellular matrix components that maintain the in vivo function of hepatocytes with respect to apoptosis sensitivity. We are in the process of screening arrays of different extracellular mixtures to identify the cocktail of components that retain type II FasL-induced apoptosis signalling in hepatocytes in vitro. This will be important for future studies with primary hepatocytes in vitro as the findings obtained from these cultures should reflect in vivo situations as close as possible.
Supplementary Material
Acknowledgements
We thank Markus Simon, MPI, Freiburg, for the Fas−/− mice, John Silke, La Trobe University, Melbourne, for the XIAP−/− mice and the mouse cIAP1 antibody, David Huang, WEHI, Parkville, for the anti-Bid antibody, Ron Jemmerson, University of Minnesota, Minneapolis, for the antibodies to cytochrome c, Adriano Fontana, Zürich, for the Neuro2A-FasL and -Neo cell lines and Pascal Schneider, Lausanne, for the Fc-FasL. This work was supported by the HepatoSys programme of the BMBF (CB, DW, RP, KS, IM, FvW) and the Spemann Graduate School of Biology and Medicine of the Deutsche Forschungsgemeinschaft (GSC-4) (CB, SV). AS and TK were supported by grants (program #257502) and fellowships from the NHMRC (Canberra), the NCI (NIH, USA; #CA 80188, #CA 43540), the Leukemia and Lymphoma Society of America (SCOR grant; #7015), the JDRF/NHMRC, the Swiss National Science Foundation, the Roche Research Foundation and the Novartis Foundation (TK).
References
- 1.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 2.Kanzler S, Galle PR. Apoptosis and the liver. Semin Canc Biol. 2000;10:173–184. doi: 10.1006/scbi.2000.0318. [DOI] [PubMed] [Google Scholar]
- 3.Osagawara J, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 2003;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 4.Yin XM, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann T, et al. The BH3-only protein Bid is dispensable for DNA damage-and replicative stress-induced apoptosis or cell-cycle arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Galle PR, Krammer PH. CD95-induced apoptosis in human liver disease. Semin Liver Dis. 1998;18:141–151. doi: 10.1055/s-2007-1007150. [DOI] [PubMed] [Google Scholar]
- 7.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 8.Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med. 2000;6:920–923. doi: 10.1038/78688. [DOI] [PubMed] [Google Scholar]
- 9.Peter ME, et al. The CD95 receptor: Apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160–171. doi: 10.1034/j.1600-0528.2002.017411.x. [DOI] [PubMed] [Google Scholar]
- 11.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 12.Scaffidi C, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross A, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while Bcl-xL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 14.Youle RJ, Strasser A. The Bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 15.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 16.Hyer ML, Samuel T, Reed JC. The FLIP-side of Fas signaling. Clin Cancer Res. 2006;12:5929–5931. doi: 10.1158/1078-0432.CCR-06-2098. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi R, et al. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 18.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Li S, et al. Relief of extrinsic pathway inhibition by Bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. J Biol Chem. 2002;277:26912–26920. doi: 10.1074/jbc.M200726200. [DOI] [PubMed] [Google Scholar]
- 20.Sun XM, Bratton SB, Butterworth M, MacFarlane M, Cohen GM. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis-protein. J Biol Chem. 2002;277:11345–11351. doi: 10.1074/jbc.M109893200. [DOI] [PubMed] [Google Scholar]
- 21.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block GD, et al. Population expansion, clonal growth, and specific differentitation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGFα in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewitt NJ, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- 24.Klingmüller U, et al. Primary mouse hepatocytes for systems biology approaches: a standardized in vitro system for modelling of signal transduction pathways. Syst Biol (Stevenage) 2006;152:193–200. doi: 10.1049/ip-syb:20050067. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu M, Fontana A, Takeda Y, Yagita H, Yoshimoto T, Matsuzawa A. Induction of antitumor immunity with Fas/APO-1 ligand (CD95L)-transfected neuroblastoma Neuro-2a cells. J Immunol. 1999;162:7350–7357. [PubMed] [Google Scholar]
- 26.Holler N, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–277. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 29.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 30.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 31.Hehlgans S, Haase M, Cordes N. Signaling via integrins: implications for cells survival and anticancer strategies. Biochem Biophys Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Degli Esposti M, Ferry G, Masdehors P, Boutin JA, Hickman JA, Dive C. Post-translational modification of Bid has differential effects on its susceptibility to cleavage by caspase 8 or 3. J Biol Chem. 2003;278:15749–15757. doi: 10.1074/jbc.M209208200. [DOI] [PubMed] [Google Scholar]
- 33.Desagher S, et al. Phosphorlyation of Bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 34.Chang L, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, et al. A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol Cell. 2002;9:411–421. doi: 10.1016/s1097-2765(02)00439-2. [DOI] [PubMed] [Google Scholar]
- 36.Smyth LA, Brady HJ. cMet and Fas receptor interaction inhibits death-inducing signaling complex formation in endothelial cells. Hypertension. 2005;46:100–106. doi: 10.1161/01.HYP.0000167991.82153.16. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Difrancesca D, Wang X, Zarnegar R, Michalopoulos GK, Yin X-M. Promotion of Fas-mediated apoptosis in type II cells by high doses of hepatocyte growth factor bypasses the mitochondrial requirement. J Cell Physiol. 2007;213:556–463. doi: 10.1002/jcp.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hueber AO, Bernard AM, Herincs Z, Couzinet A, He HT. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep. 2002;3:190–196. doi: 10.1093/embo-reports/kvf022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Reilly LA, et al. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell Death Differ. 2004;11:724–736. doi: 10.1038/sj.cdd.4401408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.