Use of patterned surfaces, reverse genetics, and time-controlled photoinactivation showed that β1 but not β3 integrins are required for invadosome formation, self-assembly, and stabilization into a ring structure. The activation state of β1 as well as its phosphorylation by protein kinase C on Ser785 control these process and link to the degradative function.
Abstract
Invadosomes are adhesion structures involved in tissue invasion that are characterized by an intense actin polymerization–depolymerization associated with β1 and β3 integrins and coupled to extracellular matrix (ECM) degradation activity. We induced the formation of invadosomes by expressing the constitutive active form of Src, SrcYF, in different cell types. Use of ECM surfaces micropatterned at the subcellular scale clearly showed that in mesenchymal cells, integrin signaling controls invadosome activity. Using β1−/− or β3−/− cells, it seemed that β1A but not β3 integrins are essential for initiation of invadosome formation. Protein kinase C activity was shown to regulate autoassembly of invadosomes into a ring-like metastructure (rosette), probably by phosphorylation of Ser785 on the β1A tail. Moreover, our study clearly showed that β1A links actin dynamics and ECM degradation in invadosomes. Finally, a new strategy based on fusion of the photosensitizer KillerRed to the β1A cytoplasmic domain allowed specific and immediate loss of function of β1A, resulting in disorganization and disassembly of invadosomes and formation of focal adhesions.
INTRODUCTION
Invadosome is a general term for structures implicated in tissue invasion processes that share similarities in organization, composition, dynamics, or function with podosomes, present in nontransformed cells (such as macrophages, osteoclasts, dendritic cells, endothelial cells, and smooth muscle cells), or invadopodia, present in cancer cells. This class of structure can be simply defined as an adhesion structure centered on an actin column, where intense actin activity takes place, and is linked with localized extracellular matrix (ECM) degradation activity. Invadosomes can form isolated dot-like structures centered on a rapidly polymerizing actin column associated with actin regulators (such as cortactin, Wiskott-Aldrich syndrome protein, Rho GTPases, and fascin), adhesion molecules (such as paxillin, talin, and integrins), regulators of membrane dynamics (such as Tsk5, IQ motif containing GTPase activating protein 1, and vesicle-associated membrane protein 7), metalloproteases, and regulatory kinases (such as focal adhesion kinase [FAK]/Pyk2, p21-activated kinase, and Src) (Linder and Aepfelbacher, 2003; Weaver, 2006; Ory et al., 2008; Vignjevic and Montagnac, 2008; Albiges-Rizo et al., 2009; Poincloux et al., 2009). Moreover, like podosomes, invadosomes have the capacity to self-assemble into round metastructures known as rosettes or rings that can expand in diameter and fuse with each other. This treadmilling behavior is based on the coordinated assembly of new, individual actin columns, connected to each other by a “cloud” of F-actin at the outer rim, with disassembly of the original structure occurring at the inner rim of the rosette (Destaing et al., 2003, 2005; Jurdic et al., 2006; Badowski et al., 2008).
Invadosomes were originally described in cells expressing the oncogene v-Src and have been reported in many subsequent studies (Marchisio et al., 1984; Tarone et al., 1985; Mueller et al., 1992; Linder and Aepfelbacher, 2003; Bowden et al., 2006). The essential role of the nonreceptor tyrosine kinase c-Src in podosomes has been shown in Src−/− osteoclasts, where reduced bone resorption is associated with poorly functioning podosomes (Yoneda et al., 1993; Hall et al., 1994). The action of c-Src in podosomes is complex and essential for initiation of podosome assembly, intensity of the actin flux, and architecture and disassembly of the structure (Luxenburg et al., 2006; Ayala et al., 2008; Destaing et al., 2008). These adhesion structures also can be induced by activation of protein kinase Cs (PKCs) by phorbol ester treatment, suggesting a synergistic activity of this pathway with the tyrosine kinase Src (Hai et al., 2002; Tatin et al., 2006).
Thus, it seems that both invadosome formation and self-assembly involve an inside-out signaling process. The question of which specific stimuli from the external environment (those participating in outside-in signaling) are able to induce invadosome formation remains unanswered. This led us to explore the functions of the integrin receptor families in the regulation of invadosome activity because these receptors integrate the composition, concentration, and compliance of the ECM. Integrins are heterodimers that oscillate between conformations having low and high affinity for the ECM. This switch corresponds to integrin activation and is favored by the disruption of the salt bridge between the α and β subunits induced by talin interaction with the β cytosolic domain of the integrin molecules (Shattil et al., 2010). Moreover, this class of proteins has a strong link with ECM degradation, interacting directly with the membrane-associated metalloprotease MT1-MMP (also known as MMP14; Galvez et al., 2002). Although numerous studies have localized different integrins in podosomes and invadosomes, the specific functions of integrins in invadosomes have been poorly described. Indeed, αvβ3 is found in osteoclast podosomes and in invadosomes and invadopodia in many cancer cells (Zambonin-Zallone et al., 1989): α3β1 in 804G carcinoma cells (Spinardi et al., 2004), α4β1 in monocyte podosomes and β2 integrins in macrophage podosomes (Duong and Rodan, 2000), and β1 integrins in osteoclast podosomes (Helfrich et al., 1996). Due to the role of podosomes in bone resorption by osteoclasts, a major role was assigned to αvβ3 integrin in this process based on the correlation between the strong increase in the expression of αvβ3 and the formation of podosomes during osteoclast differentiation (Mimura et al., 1994; Pfaff and Jurdic, 2001; Destaing et al., 2003; Jurdic et al., 2006). Moreover, perturbation of αvβ3 integrin by disintegrin modulates osteoclast migration and podosome formation (Nakamura et al., 1999; Blair et al., 2009). However, β3−/− mice show only a slight increase in bone mass in comparison with Src−/− osteoclasts, which are only associated with a disorganization of podosomes belts (McHugh et al., 2000; Faccio et al., 2003). In macrophages, Rho B inactivation results in decreased surface expression of integrins β2 and β3, whereas β1 surface expression remains constant. However, modification of this integrin pattern does not affect podosome formation (Wheeler and Ridley, 2007). In contrast, the importance of β1 in invadopodia was suggested by activation of these integrins by soluble antibody, which leads to an increase in ECM degradation (Nakahara et al., 1998).
To clarify the role of integrins in invadosome formation, autoassembly, and organization, we have induced them by the expression of a constitutively active Src mutant, SrcYF, in cells in which either the β1 or β3 gene has been deleted, and we performed reverse genetic analysis by expression of exogenous wild-type or mutant human integrin chains. Surprisingly, invadosome initiation and autoassembly into rings were found to be strictly dependent on ECM signaling, through β1 and not β3 integrins, despite the high concentration of β3 in these structures. Invadosome autoassembly probably involves the phosphorylation of Ser785 on the β1A cytosolic tail by PKC activity because PKC stimulation can be mimicked by the phosphomimetic mutants S785D or S785E. In addition, the modulation of β1A affinity by expression of β1A mutant in a genetic null background uncouples actin dynamics and the ECM-degradative activity of invadosomes. Finally, a new method to induce quick loss of function of β1A by photoinactivation revealed that this integrin also plays a key role in invadosome metastructure stabilization. Thus, it seems that β1A is a key modulator of invadosome function in invasion processes.
MATERIALS AND METHODS
Antibodies and Reagents
Antibodies for immunoblotting and immunofluorescence were obtained from the following commercial sources: rabbit anti-phospho-Tyr 418 Src and anti-phospho-Tyr397 FAK (Invitrogen, Carlsbad, CA), mouse anti-Src (GD11; Millipore, Billerica, MA), mouse anti-actin (Sigma-Aldrich, St. Louis, MO), mouse anti-paxillin (BD Biosciences, Franklin Lakes, NJ), mouse anti-human β1 integrin (clone 4B7R; Becton Dickinson, le Pont de Claix, France), rat anti-mouse β1 integrin (MB1.2; Millipore), and rat anti-mouse β3 integrin (clone LucA5; Emfret Analytics, Würzburg, Germany).
Alexa 546-phalloidin, as well as Alexa 488, gelatin-Oregon green, and Alexa 488-, 546-, and 633-conjugated secondary antibodies were from Invitrogen.
Plasmids
pEGFP-actin vector was obtained from Clontech (Palo Alto, CA). pBabe green fluorescent protein (GFP)-paxillin was provided by Dr. M. Hiraishi (Department of Molecular Biology, Osaka Bioscience Institute, Suita, Osaka, Japan), pBabe red fluorescent protein (RFP)-cortactin was engineered from the initial constructs, pDsRed-N1-cortactin was from Dr. P. Jurdic (ENS Lyon, France), and pcDNA3-mRFP was from Addgen (Cambridge, MA).
Plasmids pFB-Neo-human β1-GFP, vasodilator-stimulated phosphoprotein-RFP, and pBabe-Src Y527F were the generous gifts of Dr. M. Humphries (University of Manchester, Manchester, United Kingdom), Prof. Gertler (The David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA), and Dr. H. Gil-Henn (Yale University, New Haven, CT), respectively.
For the pTRFP or KillerRed-tagged human β1 construct, the pTRFP or KillerRed coding sequences were polymerase chain reaction (PCR) amplified from the original pTagRFP-N or pKillerRed-N vectors (Evrogen, Moscow, Russia) and used to replace GFP of pFB-Neo-human β1-GFP to generate an in-frame C-terminal fusion. pCLMGF-human β1 wild-type (WT), pCLMGF-human β1 L747R, pCLMGF-human β1 D759A, pCLMGF-human β1 S785A, and pCLMGF-human β1 S785E constructs were made using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) using the following primer pairs: D759A: forward, 5′-gcttttaatgataattcatGCCagaagggagtttgc-3′ and reverse, 5′-gcaaactcccttctGGCatgaattatcattaaaagc-3′; S785D: forward, 5′-cgggtgaaaatcctatttataagGATgccgtaacaactgtgg-3′ and reverse, 5′-ccacagttgttacggcATCcttataaataggattttcacccg-3′; S785E: forward, 5′-cgggtgaaaatcctatttataagGAGgccgtaacaactgtgg-3′ and reverse, 5′-ccacagttgttacggcCTCcttataaataggattttcacccg-3′; and S785A: forward, 5′-cgggtgaaaatcctatttataagGCTgccgtaacaactgtgg-3′ and reverse, 5′-ccacagttgttacggcAGCcttataaataggattttcacccg-3′.
Cell Culture and Infection
MEF β3+/+ and β3−/− cells were the generous gift of Dr. Richard Hynes (The David H. Koch Institute for Integrative Cancer Research). Mouse embryonic fibroblasts (MEFs) isolated from β1loxP/loxP mice between embryonic day 12 and postnatal day 1, as described in Ferguson et al. (2009), were the generous gift of Prof. Reinhard Fässler (Max Planck Institute of Biochemistry, Martinsreid, Germany).
A population of primary mouse osteoblast-enriched cells was isolated from newborn mouse calvaria by using a mixture of 0.3 mg/ml collagenase type I (Sigma-Aldrich) and 0.25% trypsin (Invitrogen), as described previously (Bellows et al., 1986; Bouvard et al., 2007). Cells were grown in α-minimal essential medium containing 10% fetal calf serum (FCS). Primary osteoblasts (passage 2) were immortalized by transduction with a retrovirus expressing the large simian virus 40 T antigen (Fassler et al., 1995); cloned; and tested for their ability to express alkaline phosphatase upon differentiation (Mansukhani et al., 2000), as described previously (Bouvard et al., 2007). At least five clones from floxed mice were isolated. Rescue of β1A integrin expression in cells was performed via retroviral infection using the pCLMFG-β1 vectors, as described previously (Bouvard et al., 2007; Millon-Fremillon et al., 2008). Cells were grown in DMEM containing glutamine and supplemented with 10% FCS and 1% penicillin/streptomycin. Cre adenovirus was purchased from the University of Iowa Gene Transfer Vector Core (Iowa City, IA). Maximal β1 depletion was achieved within 4 d. Cells were generally used for experiments between 6 and 9 d after Cre recombinase delivery. In most experiments, cDNAs were delivered via retroviral transduction after packaging in Phoenix-Eco or Phoenix-Ampho cells (American Type Culture Collection, Manassas, VA). Supernatant containing viral particles from transduced cells was harvested; filtered; and after addition of 8 μg/ml polybrene (Sigma-Aldrich), it was used to infect fibroblasts. For rescue experiments, cells were infected with exogenous β1 constructs, before addition of the CRE recombinase, to remove endogenous integrin. Before use, the cells were either serum starved for 14 h to have SrcYF activity as the main signaling pathway in the cells or treated for 1 h with 2 μM phorbol myristyl acetate (PMA). Either treatment allowed the development of many invadosomes and rosettes.
Micropatterning and Functionalization
Patterned protein glass coverslips were performed according to Guillou et al. (2008), with slight modifications. Glass coverslips (22 × 22 mm) were washed in a solution of sulfuric acid and hydrogen peroxide (7:3, vol/vol) for 30 min, dried, and then dipped for 1 h in a solution of octadecyltrimethoxysilane and aminopropyltrimethoxy silane (3:1, mol:mol; Sigma-Aldrich) in toluene. Positive photoresist resin (Shipley, S1805, Rhom & Haas Electronic Materials, Villeurbane, France) was spin coated and cured according to the manufacturer's protocol to form a uniform, UV-sensitive film 0.5 μm in thickness. The coated coverslips were then insolated with UV light using a Karl Süss aligner (MJB3, SUSS MicroTec, Saint-Jeoire, France) at 436 nm and 15 mJ/cm2 through a chromium mask. The irradiated pattern was revealed with microposit developer concentrate in deionized water (1:1, vol/vol; Shipley, MF CD-26, Rhom & Haas Electronic Materials). The patterned coverslips were incubated for 1 h at 37°C in a solution of gelatin-rhodamine isothiocyanate (RITC) and 10 μg/ml vitronectin in phosphate-buffered saline (PBS). Substrates were rinsed in PBS and then in absolute ethanol in an ultrasonic water bath to dissolve the photoresist resin. Finally, either antiadhesive triblock copolymer Pluronic F127 (Sigma-Aldrich) at a concentration of 4% in water for 1 h 30 min at 37°C, or a solution of FN7–10-FITC (fibronectin type III domains 7–10 conjugated to fluorescein isothiocyanate [FITC]) at 5–15 μg/ml in PBS was adsorbed to the complementary pattern revealed after resin dissolution by ethanol for 1 h at 37°C. After a last rinse in PBS, cells (155 cells/mm2) were seeded and incubated overnight, before fixation and staining.
Degradation Assays
Coverslips were coated with 1 mg/ml gelatin-Oregon green, fixed with 4% (wt/vol) paraformaldehyde/0.5% glutaraldehyde for 30 min at 4°C, washed with 30 mg/ml sodium borohydride in PBS, sterilized with 70% ethanol, and rinsed once with PBS. Cells were seeded on the coverslips in culture medium for 2 h before being imaged for 14 h with an Axiovert 200M microscope (Carl Zeiss Microimaging, Gottingen, Germany) equipped with a MicroMax 5-MHz, 10× (numerical aperture [NA] 0.25) LDplan objective.
Microscopy and Photoinactivation
For immunofluorescence analysis, cells were fixed with 4% paraformaldehyde in PBS, pH 7.4, and imaged with an Axiovert 200M microscope equipped with CoolSNAP HQ2, 63× (NA 1.4) Plan Apochromat and 100× (NA 1.4) Plan Apochromat objectives and a filter set to specifically detect Alexa 488/GFP or Alexa 546/pTRFP/KillerRed. At least 700 podosome structures were analyzed for each condition; representative data from to three to five independent experiments are presented.
For live imaging, cells were seeded at subconfluent densities in 35-mm serum-coated glass-bottomed dishes (1.5 mm in thickness; MatTek, Ashland, MA) and allowed to grow for 12–48 h before imaging. DMEM was replaced by a CO2-independent medium (Invitrogen), placed on a heated 37°C stage (Carl Zeiss Microimaging), and imaged with the same Axiovert 200M microscope setup described above. Total internal reflection fluorescence (TIRF) microscopy was carried out with the same setup as described above and equipped with the TIRF 1 slider (Carl Zeiss Microimaging).
For photoinactivation experiments, an Axiovert 200M microscope equipped with a 63× (NA 1.4) Plan Apochromat objective and triple-filter set 25HE (excitation, TBP 405 + 495 + 575 [HE]; beam splitter, TFT 435 + 510 + 600 [HE], and emission, TBP 460 + 530 + 625 [HE]) (Carl Zeiss Microimaging) was used for illumination. Cells were imaged as described for live imaging, followed by a pause to allow illumination of the whole field of observation for 40–50 s (average, 45 s) with a 100-W HBO mercury lamp at 100% power.
For fluorescence recovery after photobleaching experiments, an LSM510 ConfoCor microscope equipped with a 40× (NA 1.2) Plan apochromat objective (Carl Zeiss Microimaging) was used. The fluorescence recovery after bleaching time (3.2 s) was observed primarily in actin spots that persisted throughout the entire recovery time.
Imaging Series 7.0 software (Molecular Devices, Sunnyvale, CA) was used to mount .avi movies from image stacks. Images extracted from stacks were processed with Photoshop CS2 (Adobe Systems, San Jose, CA) and ImageJ (http://rsb.info.nih.gov/ij/). Significance of the differences between standard deviations was analyzed in Excel (Microsoft, Redmond, WA) with an F-test.
RESULTS
The ECM Controls the Assembly of Invadosomes
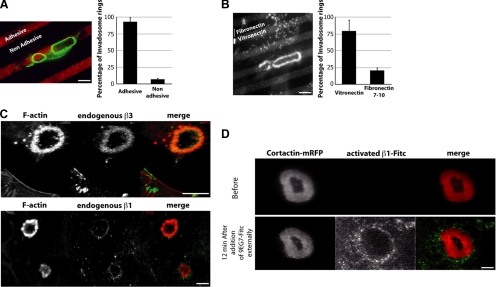
To determine the role of ECM signaling on invadosome activity, we plated MEFs transformed with constitutively active SrcYF on a layer of ECM micropatterned at the subcellular scale. Cells were seeded on alternate stripes of adhesive (gelatin-RITC mixed with vitronectin; Figure 1A, red areas) and nonadhesive areas (Pluronic F127; Figure 1A, black areas), 5 and 10 μm in width, respectively. The invadosome rosettes were present on the adhesive stripes 95% of the time and continued to expand along the axis of these stripes (Figure 1A). These rosette formations suggested that the initiation of invadosome assembly is promoted by ECM signaling. Because integrins are the major actors in matrix sensing, we wanted to precisely define the roles played by specific subclasses of this receptor family. Therefore, we plated MEF-SrcYF onto patterned surfaces composed of alternating 5-μm stripes of RITC-vitronectin, which preferentially binds β3 integrins, and unlabeled fibronectin, which preferentially binds β1 integrins. Rosettes were more abundant on vitronectin, although the cells spread on both adhesive surfaces. In contrast, individual podosomes were seen on both surfaces (Figure 1B). However, on glass coverslips homogenously coated with fibronectin or vitronectin, MEF-SrcYF generated similar individual invadosomes and rosettes (data not shown). This result was reproduced with double patterned coverslips with either laminin and vitronectin, or collagen and vitronectin (Supplemental Figure S1, B and C). These data indicated that invadosomes have a preference for vitronectin-coated areas, suggesting an essential role of β3 integrin in invadosome activity (Figure 1B). However, most of the time, rosettes were initiated at the boundary of fibronectin and vitronectin areas (Supplemental Figure S1A). This peculiar location suggested that despite their location on vitronectin, some outside-in signaling from fibronectin, possibly through β1 integrins, was necessary to initiate invadosome self-organization.
Figure 1.
Extracellular matrix sensing by β1 and β3 integrins controls invadosome formation and localization. (A) Most SrcYF-expressing cells formed invadosome rosettes (visualized by F-actin staining, in green) only on the adhesive surface (gelatin-tetramethylrhodamine B isothiocyanate [TRITC], in red, mixed with vitronectin) and not on antiadhesive areas (Pluronic F127, black areas). (B) Invadosome rosettes stained by phalloidin-TRITC show higher affinity for vitronectin-FITC (light gray bands), sensed by members of the β3 integrin family, than for fibronectin (black bands), sensed by members of both the β1 and β3 integrin families. (C) The β1 and β3 integrins show distinct patterns of localization, observed in MEF-SrcYF cells. β3 (in green in merge) highly colocalized with F-actin, whereas β1 staining is limited to the rosette periphery. (D) 9EG7 antibody β1 staining is specific of the activated form of the integrin. Antibody against activated form of β1 directly conjugated to FITC (9EG7-FITC) was added externally and localized around the invadosome visualized by cortactin-mRFP (in red in merge) expressed in live MEF-SrcYF cells. Bars, 5 μm (A and B), 4 μm (C), and 2 μm (D).
To determine the importance of β1 and β3 integrins in invadosome activity, we analyzed the locations of these molecules and compared them with those of β1 integrins after spreading of the cells on endogenous matrix (48-h culture on glass coverslips). Because F-actin was found to be a reliable marker of nascent and mature invadosomes (Badowski et al., 2008), double staining with either β1 and paxillin, or β3 antibodies and phalloidin, was carried out. Confocal scanning microscopy showed colocalization of β3 and F-actin, whereas β1 integrins were mostly excluded from the rosettes and accumulated at the outer rim, suggesting a possible function in newly formed invadosomes (Figure 1C). The early assembled invadosomes contain mostly the actin core, including cortactin. Detection of the active conformation of β1A was carried out after external addition in the medium of 9FG7 antibody directly conjugated to FITC and visualized by TIRF microscopy on living cells expressing cortactin-monomeric(m)RFP. This analysis revealed that β1 integrins did not colocalize with cortactin; rather, they were in proximity to the rim of the invadosome rosette (Figure 1D). Although β1 integrins accumulate around rosettes, our results did not support a structural role for them, in contrast to β3 integrins, which mediate adhesion and are a component of the mature invadosome.
β1 Integrins Are Key Regulators of Invadosome Formation
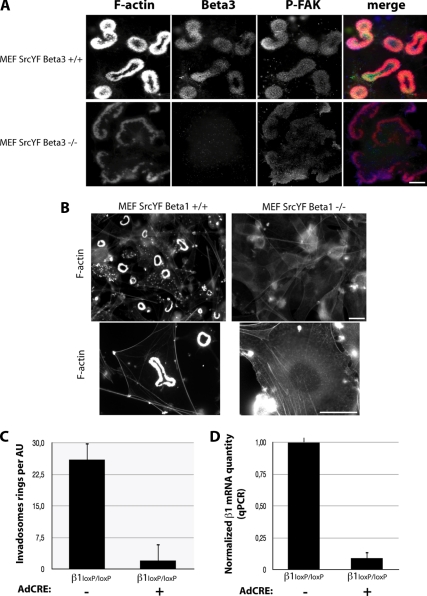
To investigate the involvement of β3 integrins in invadosome assembly, we induced these structures in β3+/+ and β3−/− MEFs by expressing the constitutively active form of Src. Surprisingly, MEF-SrcYF β3−/− cells displayed invadosome rosettes, although these structures were narrower and exhibited less intense actin staining than those observed in MEF-SrcYF β3+/+ cells (Figure 2A). In line with these findings, MEF-YFSrc established invadosomes rosettes on the β1-specific substrates laminin111 and collagen (data not shown). Conversely, β1 depletion in MEF-SrcYF β1loxP/loxP cells, generated by adenoviral delivery of Cre recombinase, resulted in invadosome formation in <5% of cells (Figure 2B, top; and C). The invadosomes observed in this small percentage of cells were probably due to a lack of Cre expression in some uninfected cells, as shown by quantitative PCR (Figure 2D). β1 depletion in MEF-SrcYF β1loxP/loxP was characterized by an increase in cell spreading and reorganization of F-actin into more abundant stress fibers (Figure 2B, bottom).
Figure 2.
β1A, and not β3, integrin is essential for invadosome formation. (A) β3 is not essential for invadosome formation and self-assembly into rosettes, visualized by F-actin (in red in merge) and phospho-Y397-FAK (in blue in merge) staining, which occurs in both MEF-SrcYF β3 +/+ or −/− cells. (B) In contrast, β1 depletion in MEF-SrcYF β1LoxP/LoxP expressing the CRE recombinase for 96 h resulted in the disappearance of isolated invadosomes or in rosettes probed by phalloidin staining. (C) Quantification of the percentage of cells forming invadosomes reveals that almost 95% of MEF-SrcYF β1LoxP/LoxP treated with CRE recombinase did not form this structure 4 d after infection (n = 650 counted cells/condition). (D) Quantification by qPCR shows an average decrease of 95% in the level of β1 mRNA 96 h post-CRE treatment. Bars, 3 μm (A) and 10 μm (B).
To confirm this unexpected and dramatic effect of removal of β1 and generalize this finding to other cell types, we applied the same strategy of inducing invadosomes through SrcYF expression in selected preosteoblastic pOBL β1loxP/loxP cells purified after Cre treatment. As for MEFs, the expression of SrcYF induced the formation of individual invadosomes and rosettes in β1+/+ preosteoblasts but not in their β1−/− counterparts. This loss of β1 was also accompanied by an increase in stress fibers within the cell bodies (Figure 3, A and D). These modifications suggested some impairment of Src activity. However, Western analysis of cell lysates did not reveal significant changes in either Y416 phosphorylation, which characterizes Src activation, or in total Src expression (Figure 3B). Thus, our data suggested that β1 integrins have an essential role in the initiation of invadosome assembly.
Figure 3.
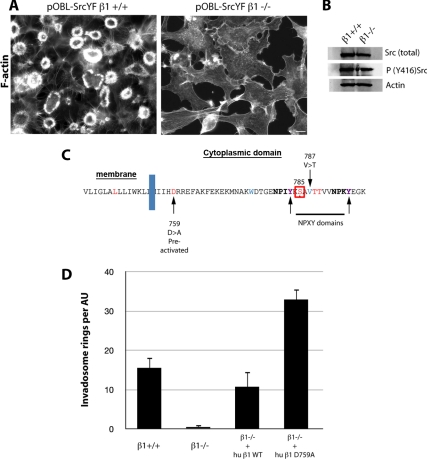
Activation of β1 stimulates invadosome autoassembly. (A) β1 depletion in pOBL-SrcYF induced the disappearance of invadosomes, shown by phalloidin staining of F-actin. Bar, 5 μm. (B) β1 depletion does not affect Src activation. Lysates of pOBL-SrcYF β1+/+ and −/− cells were probed by Western blotting for phospho-SrcY416, a marker of Src activation, total Src, and actin. (C) Amino-acyl sequence of the cytoplasmic domain of β1A integrin and the location of the main mutation that activates this integrin. (D) Expression of the preactivated mutants of β1 (β1 D759A) in pOBL-SrcYF β1−/− cells dramatically increases the number of invadosome rosettes per airy unit, whereas β1WT mutant rescue the rosette number up to the control level.
Activation of β1 Integrins Stimulates Invadosome Autoassembly into Rosettes
Most of the β1 integrins around the rosettes were stained with the 9EG7 monoclonal antibody, indicating that these receptors were bound to their ECM ligands and therefore probably in their high-affinity state (Figure 1D). To test the role of integrin activation in invadopodia dynamics, we carried out a structure–function study and re-expressed exogenous human β1 chains bearing the point mutation D759A, which is known to modulate β1 activation state, in β1−/− pOBL-SrcYF cells (Figure 3C). This mutation promotes the integrin high-affinity state by breaking the saline bridge to the nearby arginine residue on the α chain (Shattil et al., 2010). This mutant showed a dramatic increase in rosette number compared with wild-type cells (Figure 3D). In conclusion, in addition to the involvement of β1A in the initiation of assembly of individual podosomes, the activation of β1A integrins seems to potentiate invadosome autoassembly.
PKC Directly Targets β1A Integrin to Control Invadosome Autoassembly
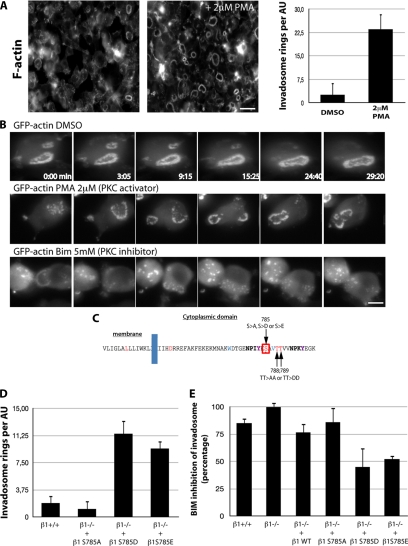
Previous studies in the literature show that in addition to Src signaling, the activation of conventional PKCs promotes invadosome formation in a variety of cell types (Hai et al., 2002; Tatin et al., 2006). Indeed, under standard cell culture conditions, PMA treatment stimulated invadosome autoassembly into rosettes in a manner similar to conditions of serum starvation (Figure 4A). Based on the treadmilling process in invadosome turnover (Badowski et al., 2008), the increase in invadosome autoassembly could be due to an increase in either rosette stability or dynamics. To address this question, live-cell imaging was carried out on pOBL-SrcYF cells stably expressing GFP-actin, and cells were grown in serum-supplemented DMEM in the presence or absence of PMA. In the absence of PMA, the few rosettes that could be detected were quite stable over 30 min. Conversely, activation of PKCs largely decreased invadosome life span to <10 min, resulting in a dramatic increase in rosette expansion and turnover (Figure 4B). In contrast, inhibition of PKCs by the general inhibitor bisindolylmaleimide I (BIM) resulted in rapid dissociation of rosettes, whereas the formation of individual invadosomes was still observed (Figure 4B). In the cytosolic domain of the β1A integrin subunit, many serine and threonine residues have been shown to be potential PKC phosphorylation sites modulating integrin function (Stroeken et al., 2000; Mulrooney et al., 2001; Figure 4C). Among those residues, Ser785 was shown previously to be strongly phosphorylated upon Src overexpression (Sakai et al., 2001). To investigate the importance of the PKC-dependent signaling pathway on β1A in the invadosome autoassembly process, we expressed the β1 integrin mutants S785A, or S785D or S785E (nonphosphorylable or phosphomimetic forms, respectively), in a null genetic background and modulated PKC activity with an activator (PMA) or inhibitor (BIM). Consistent with the view that Ser785 is a direct target of PKC, only the phosphomimetic mutants S785D and S785E were able to stimulate invadosome autoassembly into rosettes in the absence of PMA (Figure 4D). The same phosphomimetic mutants were partly resistant to BIM inhibition compared with wild type and the nonphosphorylatable S785A mutant (Figure 4E). In addition, the S785A mutant poorly rescued rosette assembly after PMA treatment (Supplemental Figure S2). Together, these data support the view that Ser785 on the β1 subunit is one of the primary targets of PKC in the regulatory pathway of invadosome rosette autoassembly in cells.
Figure 4.
PKC regulates invadosome autoassembly by phosphorylating Ser785 of β1A integrins. (A) PKC activation by a 60-min treatment of pOBL-SrcYF cells with 2 μM PMA induces a massive increase in invadosome rosette autoassembly, visualized by phalloidin staining. (B) Extracted images from time series (in minutes) from representative observations of pOBL-SrcYF cells expressing GFP-actin and treated with either dimethyl sulfoxide (DMSO; control), the PKC activator PMA (2 μM), and the PKC inhibitor BIM (5 mM). PKC activity regulates the dynamics and maintenance of the invadosome autoassembly state. (C) Amino-acyl sequence of the cytoplasmic domain of β1A integrin and the location of the main PKC targets. (D) Quantification of invadosomes per Airy unit (AU) shows that a mutation mimicking a constitutive phosphorylated form of Ser785 (Asp or Glu) dramatically increases the formation of rosettes in pOBL-SrcYF β1−/− cells. The nonphosphorylatable mutant of β1 at this site (S785A) has no effect on rosette formation. (E) The number of rosettes/AU was quantified in pOBL-SrcYF cells treated with 2 μM PMA or simultaneously with PMA and 5 mM BIM for 60 min. The inhibitory effect of BIM was determined by calculating the percent inhibition of invadosome rosette formation. Mutants mimicking a constitutively phosphorylated Ser785 (S785D and S785E) show only 50% inhibition after BIM treatment, indicating that this residue on β1 is a major target of PKC in the regulation of invadosome autoassembly. Bars, 20 μm (A) and 5 μm (B).
β1 Integrin Downstream Signaling Links Invadosome Formation and ECM Degradation Properties
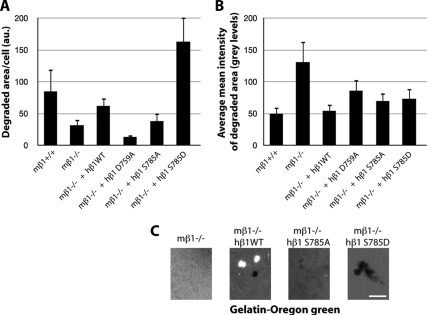
Because invadosomes are major sites of ECM degradation, we investigated the involvement of β1 integrins in this crucial invadosome function by quantifying gelatin-Oregon green surface degradation, normalized per cell, over time. Even in the absence of invadosomes, pOBL-SrcYF β1−/− cells showed very low, but significant, ECM degradation activity 4 h after plating on gelatin (Figure 5, A and C). Quantification of the average fluorescence intensity of the digested area is an efficient way to determine the extent or the depth of digestion. It was clearly shown that surfaces degraded by pOBL-SrcYF β1−/− cells were poorly digested (Figure 5B) compared with rescued cells. The same results were obtained for MEF-SrcYF cells (data not shown). As shown previously, this structure–function study allowed exploration of the function of β1A in the ECM degradative activity of invadosomes. Re-expression of wild-type human β1A integrin rescued the degradation phenotype of pOBL-SrcYF β1−/− cells in terms of extent and depth of the ECM digestion (Figure 5, A and B). In contrast, the rescue of pOBL-SrcYF β1−/− cells with human β1A D759A, a mutant that preactivates the integrin, led to the formation of numerous invadosomes, but which poorly digested ECM. Because wild-type integrin cycles between low- and high-affinity states, this loss of function when the high-affinity state is promoted suggests the importance of the cycle of β1 activation–inactivation in this process. Thus, modulation of the β1A integrin activation state allowed, for the first time, the uncoupling of invadosome formation and ECM degradation activity. Moreover, regulation of invadosomes by PKC through phosphorylation of Ser785 was shown to be an essential step in activating invadosome ECM degradation properties. Indeed, the nonphosphorylatable mutant S785A of β1A strongly reduced the average surface area digested per cell, without affecting the depth of the degradation (Figure 5B). Conversely, the phosphomimetic β1A mutant S785D dramatically increased invadosome ECM degradation activity. Thus, not only the affinity state of β1 but also signaling pathways downstream of β1AS785 phosphorylation (probably by PKC) control the coupling of invadosome assembly and ECM degradation activity.
Figure 5.
β1 activity and signaling control invadosome ECM degradation activity. (A) Quantification of the degraded surface of gelatin-Oregon green per cell reveals that β1 has an essential function in this invadosome function. Surprisingly, expression of the activated mutant of β1 (D759A) induces numerous invadosome rosettes associated with poor degradation activity. Moreover, constitutive activation of the signaling pathway downstream of phosphorylation of Ser785 strongly stimulates ECM degradation. (B) β1 also controls the quality of the degradation, as revealed by quantification of the average intensity of the resorbed areas. The few areas degraded in the pOBL-SrcYF β1−/− cells are poorly digested because they are close to the maximal fluorescence intensity of a nondigested surface (255) in comparison with pOBL-SrcYF β1+/+ cells (values closer to 0 indicate a totally black, completely digested area). Between 600 and 1050 cells were counted per condition. (C) Representative images extracted from the time series of pOBL-SrcYF β1−/− cells expressing or not expressing either human β1 WT, D759A, S785A, or S785D spread on a layer of degradable gelatin-Oregon green. Bar, 10 μm.
β1 Integrins Stabilizes Both Rosettes and Individual Invadosomes
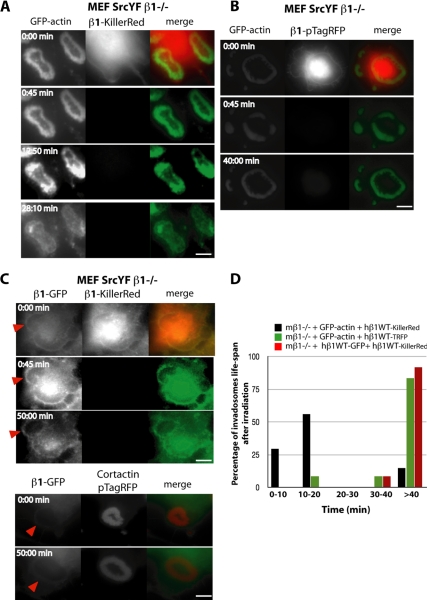
Having established that β1A integrins are important in initiating formation of individual invadosomes in the autoassembly of rosettes, and in invadosome degradative function, we wanted to determine whether signaling from β1A integrins located at the rosette periphery was required to simply initiate or to maintain rosette structure and dynamics. To address this question, we developed an improved photoinactivation strategy based on the use of the photosensitizer KillerRed to achieve inducible loss of the β1A integrin chain. In brief, we fused KillerRed to β1A in place of GFP and expressed it in MEF-SrcYF β1loxP/loxP cells. The endogenous β1 gene was deleted in almost 100% of the cells 7 d post-Cre expression, as monitored by quantitative (q)PCR analysis of mouse β1 mRNA (data not shown). In this genetic null background, the β1-KillerRed was functional because it allowed the assembly of rosettes and was correctly localized at their periphery. GFP-actin dynamics was followed after 45-s irradiation with red light in the KillerRed excitation spectrum (585–615 nm). KillerRed locally produces reactive oxygen species (ROS), resulting in the rapid inactivation of the protein to which it is fused. Indeed, photoinactivation of β1-KillerRed resulted in a 95% decrease in red fluorescence, which was not recovered over the time of the observation (a maximum of 40 min after irradiation). This loss of β1A function led to the massive disorganization of the rosettes in <20 min (Figure 6, A and D).
Figure 6.
Photoinactivation revealed role of β1 in maintaining invadosome self-assembly. (A) Representative images extracted from time series of MEF-SrcYF β1−/− cells expressing human β1-KillerRed and GFP-actin. Exogenous human β1-KillerRed is functional in rescuing invadosome formation and its proper localization at the periphery. Exposure of KillerRed to light for 45 s is followed by fluctuations in intensity and disorganization of GFP-actin in the invadosome rosette. (B) Representative images extracted from time series of MEF-SrcYF β1−/− cells expressing human β1-pTagRFP, which has the same excitation and emission spectrum but is much more photostable than KillerRed, and GFP-actin. Light irradiation without ROS production is not sufficient to dissociate invadosomes. (C) Representative images extracted from time series of MEF-SrcYF β1−/− cells expressing human β1-KillerRed and human β1-GFP. These two proteins colocalized, but ROS production at this level of β1-KillerRed has no effect on either β1-GFP stability or on any other important proteins for invadosome integrity because invadosomes are still present when β1-KillerRed is photoinactivated. Moreover, β1-KillerRed depletion did not affect β1-GFP behavior in comparison to β1-GFP present in untreated invadosome visualized by cortactin-pTRFP. (D) Photoinactivation of β1-KillerRed leads to rapid and specific disorganization of invadosomes. Histograms, show the distribution of the percentage of cells where invadosomes are disorganized at various times after light irradiation. The x-axis shows time in minutes. Twelve to 39cells per condition were monitored. Bars, 3 μm (A–C).
ROS production in the cells could potentially have multiple indirect effects. To clearly show the specificity of this strategy, we first confirmed that the 45-s excitation with red light that resulted in KillerRed inactivation had no effect on invadosome structure, organization, and dynamics in cells expressing KillerRed alone in the cytosol (data not shown). Thus, nonlocalized ROS production after KillerRed irradiation had no effect on invadosomes or focal adhesions. To evaluate the potential toxicity of the 45-s red light irradiation and adsorption, KillerRed was replaced in the β1A fusion protein by TagRFP, another photostable fluorescent molecule with an excitation/emission spectra similar to that of KillerRed (Supplemental Movie 2). Indeed, 45-s red light excitation of β1A-TagRFP had no effect on invadosomes, which remained stable for more than 40 min after irradiation (Figure 6, B and D). Finally, the nonspecific effects of ROS production on proteins and membranes in the vicinity of β1 after KillerRed excitation were investigated by simultaneous expression of β1A-GFP and β1A-KillerRed in a β1−/− background, because the two proteins were probably located in proximity to each other (Supplemental Movie 3). In this environment, photoinactivation of β1-KillerRed had no effect on β1A-GFP, which still allowed the formation of characteristic invadosome rings, as seen in nontreated invadosome visualized by β1A-GFP and cortactin-TagRFP (Figure 6C, bottom; and Supplemental Movie 8). These data clearly show that ROS produced by photoinactivation of KillerRed act only on the KillerRed-tagged protein and not on surrounding macromolecules (Figure 6, C and D).
This photoinactivation strategy allowed us to monitor the specific effects of loss of β1A on pre-existing rosettes on a time scale of minutes. It is noteworthy that despite its specific localization around invadosome rings and its role in their initiation, inducible β1A depletion did not lead to progressive dissociation of the actin cytoskeleton from the inside to the outside of the rosettes, as would be expected because new actin structures are formed at the outer rim of the ring, whereas older parts of the structure are disassembled at its inner rim) (Figure 6A). Soon after photoinactivation, the polymerization of GFP-actin was not blocked but rather formed unstable waves inside the rosette before the disorganization of these structures (Supplemental Movies 1 and 7). Instability in the actin polymerization domains within the rosette was followed by the complete collapse of the structure and increased cell spreading associated with the rapid formation of stress fibers (Supplemental Movie 1). Occasionally, GFP-actin signals reminiscent of the localization of invadosomes remained (Figure 6A).
Photoinactivation of β1A Integrins Reveals the Integrin Functions in Invadosome Organization
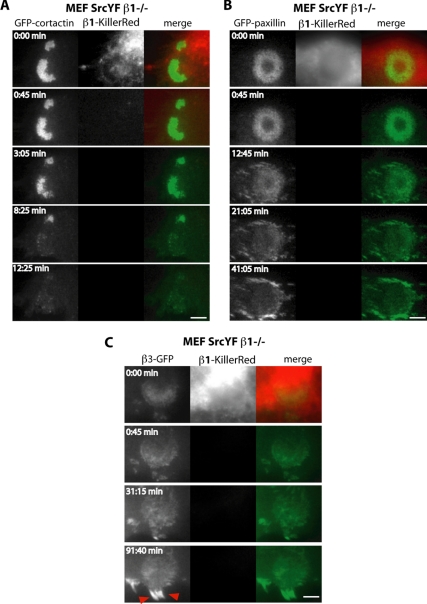
To understand the mechanism underlying the GFP-actin polymerization defect seen after loss of β1A function, the dynamics of the activity of the actin regulator cortactin fused to GFP was monitored (Supplemental Movie 4). The β1 photoinactivation was rapidly followed by the dissociation of cortactin from invadosomes, explaining the perturbation of actin dynamics (Figure 7A). Similarly, the dynamics of adhesion molecules such as paxillin and β3-GFP integrins was analyzed (Supplemental Movies 5 and 6). In particular, the induction of β1 loss of function led to paxillin disorganization in invadosomes, although a fraction of this GFP-tagged protein remained localized at the previous site in the rosette (Figure 7B). This was also the case for β3-GFP, but with this specific marker, it was clearer that invadosome disorganization was associated with massive new formation or growth of focal adhesions (Figure 7C, red arrows). This rapid reorganization of the cellular adhesive structures was consistent with our observations of the formation of multiple stress fibers in response to β1 photoinactivation (Supplemental Movie 1). In conclusion, despite its peripheral localization in the rosette, β1A integrin is a master regulator of invadosome assembly, organization, and stability.
Figure 7.
β1 photoinactivation leads to the loss of cortactin, disorganization of adhesion molecules within the invadosome metastructures, and induction of large, β3-rich focal adhesions. (A) Representative images extracted from time series of MEF-SrcYF β1−/− cells expressing human β1-KillerRed and GFP-cortactin. Photoinactivation of β1-KillerRed is followed by a slow decrease in GFP-cortactin fluorescence and its disappearance. (B) Representative images extracted from time series of MEF-SrcYF β1−/− cells expressing both human β1-KillerRed and GFP-paxillin. β1 photoinactivation leads to GFP-paxillin disorganization and decrease in intensity, but in contrast to what is observed with GFP-cortactin, GFP-paxillin remains associated with the invadosome. (C) Representative images extracted from time series of MEF-SrcYF β1−/− cells expressing human β1-KillerRed and β3-GFP. β1 photoinactivation leads to slow dissociation of invadosomes and the massive formation of β3-rich focal adhesions (red arrows). Bars, 3 μm (A–C).
DISCUSSION
Based on their high content and variety of integrins, invadosomes are integrin-dependent adhesive structures associated with intense actin dynamics and responsible for local ECM degradation. The role of a specific integrin type in invadosome regulation is unclear, due to transdominant activities among the integrin classes and the lack of specificity of the tools used such as specific inhibitors or antibodies. Herein, we used the power of genetics to understand the role of two major integrins classes (those pairing with β1A and β3 chains) in the formation and dynamics of invadosomes. This strategy is based on the induction of invadosomes by SrcYF expression in a β1 or β3 null genetic background. Thus, we are coupling the historical experiment by Tarone et al. (1985) with the re-expression of wild-type or mutant β chains, allowing a straightforward reverse genetic analysis of β1A or β3 integrins functions in invadosomes.
Although the rosette assembly associated with the colocalization of β3 and F-actin on patterned surfaces has clearly shown preferential invadosome localization on vitronectin, MEF SrcYF β3−/− cells form invadosome rosettes, indicating that other integrin types can compensate the loss of β3. This finding confirms previous data showing that β3−/− osteoclasts are able to form podosomes (Faccio et al., 2003).
On the contrary, the specific role of the β1 chain in initiating invadosome assembly is highlighted by the fact that the loss of β1 resulted in the disappearance of both individual and self-assembled invadopodia in primary cells and cell lines. These data do not fit with an earlier study reporting the presence of invadosome rosettes in the β1 null cell lines Gβ11 and GD25 transformed with SrcYF. In that case, however, the re-expression of the β1A double mutant (Y783F, Y795F) that poorly responds to Src transformation (Sakai et al., 2001) clearly showed a trans dominant-negative effect on invadosome formation, pointing to a major role of this integrin chain in invadosome formation (Huveneers et al., 2008). Because these studies were using immortalized cells lines from knockout embryonic cells, one may assume that other compensatory mechanisms may have been at work. Indeed, in our hands, isolated clones of pOBL SrcYF β1−/− cells that normally do not form invadosomes, can form few invadosome rosettes after numerous passages in culture, suggesting that these immortalized cells lines start to set up compensatory mechanisms that rescue the formation of these adhesion structures (data not shown). At a first glance, and despite its localization around the rosette, the importance of β1 integrin is surprising because this integrin class appeared in wild-type cells to be excluded from invadosomes, which contain mostly β3 integrin chains. However, immunostaining revealed an increased concentration of β1 at the outer rim of the rosettes. Because no colocalization with cortactin, a marker of nascent and mature invadopodia and invadosomes (Artym et al., 2006; Badowski et al., 2008) was observed, it is likely that β1integrins do not belong to the F-actin architecture composing invadosomes but rather form a signaling platform to initiate invadosome assembly. To test this hypothesis, we developed a new approach to inactivate integrins at the minute time scale. The specific photoinactivation of β1-KillerRed showed that this integrin loss induced a rapid collapse of the whole structure, indicating that the β1 chain is required not only to initiate invadosome assembly but also to control the stability of the structure during the entire life span of the rosette. This β1 functional ablation allowed us also to observe the rosette collapse from the outside to the inside. Thus, it seems that β1A integrins can signal at distance to control the disassembly processes occurring at the inner rim of invadosomes (Badowski et al., 2008). Moreover, some residual F-actin, not associated with GFP-cortactin, forms an imprint after F-actin disassembly after photoinactivation corresponding to the initial rosette. This strongly suggests the presence of two distinct actin network in the rosette: the actin cores with high cortactin content that depend on β1 signaling, and a more stable actin cloud around individual podosomes that is reminiscent of the radial actin arrays observed in macrophage or in the sealing zone of osteoclasts (Evans et al., 2003; Luxenburg et al., 2007). Finally, photoinactivation of β1A-KillerRed is followed by the formation of focal adhesions, providing another example of the well described competition between focal adhesions and invadosomes.
To explore the β1A-dependent control mechanisms of invadosomes, we focus our attention on PKC that in addition to Src is a major inducer of invadosomes or podosomes (Hai et al., 2002; Tatin et al., 2006). Indeed, under standard cell culture conditions, PKC activity induced by PMA treatment greatly stimulated invadosome and rosette formation. The precise role of PKC activity in invadosomes is unclear. PKCα and PKCδ were shown to allow β1 integrin activation (Brenner et al., 2008). This latter finding could be indirect because another PKC, PKCθ, was shown to regulate the integrin activator Rap1 (Letschka et al., 2008). The other possibility is that PKC directly phosphorylates integrins. Indeed, Ser785 and threonine 788 and 789 on the cytoplasmic tail of this integrin are potential targets of PKC. Because it was shown previously that Ser785 phosphorylation alters cell spreading (Mulrooney et al., 2001) and that this particular residue was overphosphorylated upon Src overexpression (Sakai et al., 2001), phosphomimetic and the nonphosphorylable β1A mutants S785E and S785D or S785A, respectively, were expressed in β1A−/− SrcYF MEF and preosteoblasts cells. Phosphomimetic mutant expression strongly stimulated invadosome self-assembly in the absence of PMA and the mutant became partially resistant to the general PKC inhibitor BIM. These results strongly suggest that the direct phosphorylation of β1A on Ser785 by PKC is an important event in the regulation of in invadosome assembly. However, the partial inhibition of phosphomimetic β1A mutants by BIM treatment also suggests that in addition to the direct phosphorylation of the β1A chain, PKC stimulation by PMA acts at other different levels of invadosome assembly. This view is also confirmed by the fact that BIM treatment does not fully mimic the collapse of the rosette after β1-KillerRed photoinactivation but rather leads to the dissociation of the rosette into individual invadosomes.
The characterization of the ECM degradation by the multiple cell lines that we generated allowed us to determine the involvement of β1 integrins in this process and in invasion. This integrin is essential for proteolytic function of invadosomes. We observed a dramatic decrease in ECM degradation in pOBL β1−/− SrcYF cells. This result was expected because despite the transformation of the cells by SrcYF, the loss of the integrin chain was accompanied by an almost complete loss of invadosomes. More surprisingly, the level of ECM degradation did not correlate with the increase in rosette assembly. Indeed, the expression of the preactivated β1A mutant D759A in a β1 null genetic background resulted in a two- to threefold increase in the rosette number, which was associated with a significant decrease in the ECM degradation. To our knowledge, this is the first report of the uncoupling of invadosome formation and matrix degradation. This could be due to the property of β1A integrin to interact with the plasma membrane-associated metalloprotease MT1-MMP (also called MMP14), which localized to invadosomes and is essential for the ECM degradation (Galvez et al., 2002; Steffen et al., 2008). Interference with MT1–MMP trafficking at the plasma membrane revealed that MT1–MMP activation and cell invasiveness are tightly coupled (Uekita et al., 2001; Steffen et al., 2008). Several lines of evidence have now established that in addition to being endosomal passengers, an important function of integrins is to direct the trafficking of other receptors and cargos (for review, see Caswell et al., 2009). One can hypothesize that the affinity state of β1A integrin could modulate MT1–MMP trafficking at the plasma membrane and therefore impair its activity at the cell surface. This hypothesis is supported by the fact that PKCα increases integrin trafficking (Ng et al., 1999) and that expression of β1A S785D or S785E, which mimic PKC activation, results in increased ECM degradation.
In conclusion, our results support a central role of β1 integrin-dependent outside-in signaling pathways in the regulation of the multiple cell compartments involved in invadosome functions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Manaa Wafa for technical assistance and interest Drs. Richard Hynes and R. Fässler for providing β1−/− MEFs and β1 knockout conditional mice, respectively. This work was supported in part by the NanoFab microfabrication facility of Louis Neel Institute (Centre National de la Recherche Scientifique Unité Propre de Recherche 5051), the Association pour la Recherche contre le Cancer, the Ligne Nationale Contre le Cancer (équipe labelisée), the Agence Nationale pour la Recherche program PIRIBIO, and the fond d'intervention du Pôle Chimie Sciences du Vivant of Université J. Fourier.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-07-0580) on October 6, 2010.
REFERENCES
- Albiges-Rizo C., Destaing O., Fourcade B., Planus E., Block M. R. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J. Cell Sci. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym V. V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K. M., Mueller S. C. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- Ayala I., Baldassarre M., Giacchetti G., Caldieri G., Tete S., Luini A., Buccione R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J. Cell Sci. 2008;121:369–378. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- Badowski C., Pawlak G., Grichine A., Chabadel A., Oddou C., Jurdic P., Pfaff M., Albiges-Rizo C., Block M. R. Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization. Mol. Biol. Cell. 2008;19:633–645. doi: 10.1091/mbc.E06-01-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows C. G., Sodek J., Yao K. L., Aubin J. E. Phenotypic differences in subclones and long-term cultures of clonally derived rat bone cell lines. J. Cell. Biochem. 1986;31:153–169. doi: 10.1002/jcb.240310207. [DOI] [PubMed] [Google Scholar]
- Blair H. C., Yaroslavskiy B. B., Robinson L. J., Mapara M. Y., Pangrazio A., Guo L., Chen K., Vezzoni P., Tolar J., Orchard P. J. Osteopetrosis with micro-lacunar resorption because of defective integrin organization. Lab. Invest. 2009;89:1007–1017. doi: 10.1038/labinvest.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D., Aszodi A., Kostka G., Block M. R., Albiges-Rizo C., Fassler R. Defective osteoblast function in ICAP-1-deficient mice. Development. 2007;134:2615–2625. doi: 10.1242/dev.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden E. T., Onikoyi E., Slack R., Myoui A., Yoneda T., Yamada K. M., Mueller S. C. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp. Cell Res. 2006;312:1240–1253. doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Brenner W., Greber I., Gudejko-Thiel J., Beitz S., Schneider E., Walenta S., Peters K., Unger R., Thuroff J. W. Migration of renal carcinoma cells is dependent on protein kinase Cdelta via beta1 integrin and focal adhesion kinase. Int. J. Oncol. 2008;32:1125–1131. [PubMed] [Google Scholar]
- Caswell P. T., Vadrevu S., Norman J. C. Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Geminard J. C., Jurdic P., Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Gilquin B., Chabadel A., Khochbin S., Ory S., Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 2005;118:2901–2911. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- Destaing O., Sanjay A., Itzstein C., Horne W. C., Toomre D., De Camilli P., Baron R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L. T., Rodan G. A. PYK2 is an adhesion kinase in macrophages, localized in podosomes and activated by beta(2)-integrin ligation. Cell Motil. Cytoskeleton. 2000;47:174–188. doi: 10.1002/1097-0169(200011)47:3<174::AID-CM2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Evans J. G., Correia I., Krasavina O., Watson N., Matsudaira P. Macrophage podosomes assemble at the leading lamella by growth and fragmentation. J. Cell Biol. 2003;161:697–705. doi: 10.1083/jcb.200212037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccio R., Takeshita S., Zallone A., Ross F. P., Teitelbaum S. L. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J. Clin. Invest. 2003;111:749–758. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R., Pfaff M., Murphy J., Noegel A. A., Johansson S., Timpl R., Albrecht R. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J. Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. M., et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez B. G., Matias-Roman S., Yanez-Mo M., Sanchez-Madrid F., Arroyo A. G. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J. Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou H., Depraz-Depland A., Planus E., Vianay B., Chaussy J., Grichine A., Albiges-Rizo C., Block M. R. Lamellipodia nucleation by filopodia depends on integrin occupancy and downstream Rac1 signaling. Exp. Cell Res. 2008;314:478–488. doi: 10.1016/j.yexcr.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Hai C. M., Hahne P., Harrington E. O., Gimona M. Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells. Exp. Cell Res. 2002;280:64–74. doi: 10.1006/excr.2002.5592. [DOI] [PubMed] [Google Scholar]
- Hall T. J., Schaeublin M., Missbach M. Evidence that c-src is involved in the process of osteoclastic bone resorption. Biochem. Biophys. Res. Commun. 1994;199:1237–1244. doi: 10.1006/bbrc.1994.1363. [DOI] [PubMed] [Google Scholar]
- Helfrich M. H., Nesbitt S. A., Lakkakorpi P. T., Barnes M. J., Bodary S. C., Shankar G., Mason W. T., Mendrick D. L., Vaananen H. K., Horton M. A. Beta 1 integrins and osteoclast function: involvement in collagen recognition and bone resorption. Bone. 1996;19:317–328. doi: 10.1016/s8756-3282(96)00223-2. [DOI] [PubMed] [Google Scholar]
- Huveneers S., Arslan S., van de Water B., Sonnenberg A., Danen E. H. Integrins uncouple Src-induced morphological and oncogenic transformation. J. Biol. Chem. 2008;283:13243–13251. doi: 10.1074/jbc.M800927200. [DOI] [PubMed] [Google Scholar]
- Jurdic P., Saltel F., Chabadel A., Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur. J. Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Letschka T., Kollmann V., Pfeifhofer-Obermair C., Lutz-Nicoladoni C., Obermair G. J., Fresser F., Leitges M., Hermann-Kleiter N., Kaminski S., Baier G. PKC-theta selectively controls the adhesion-stimulating molecule Rap1. Blood. 2008;112:4617–4627. doi: 10.1182/blood-2007-11-121111. [DOI] [PubMed] [Google Scholar]
- Linder S., Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Luxenburg C., Geblinger D., Klein E., Anderson K., Hanein D., Geiger B., Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C., Parsons J. T., Addadi L., Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J. Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- Mansukhani A., Bellosta P., Sahni M., Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J. Cell Biol. 2000;149:1297–1308. doi: 10.1083/jcb.149.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Naldini L., Primavera M. V., Teti A., Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J. Cell Biol. 1984;99:1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh K. P., Hodivala-Dilke K., Zheng M. H., Namba N., Lam J., Novack D., Feng X., Ross F. P., Hynes R. O., Teitelbaum S. L. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millon-Fremillon A., Bouvard D., Grichine A., Manet-Dupe S., Block M. R., Albiges-Rizo C. Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent beta1-integrin affinity. J. Cell Biol. 2008;180:427–441. doi: 10.1083/jcb.200707142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura H., Cao X., Ross F. P., Chiba M., Teitelbaum S. L. 1,25-Dihydroxyvitamin D3 transcriptionally activates the beta 3-integrin subunit gene in avian osteoclast precursors. Endocrinology. 1994;134:1061–1066. doi: 10.1210/endo.134.3.8119143. [DOI] [PubMed] [Google Scholar]
- Mueller S. C., Yeh Y., Chen W. T. Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J. Cell Biol. 1992;119:1309–1325. doi: 10.1083/jcb.119.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney J. P., Hong T., Grabel L. B. Serine 785 phosphorylation of the beta1 cytoplasmic domain modulates beta1A-integrin-dependent functions. J. Cell Sci. 2001;114:2525–2533. doi: 10.1242/jcs.114.13.2525. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Mueller S. C., Nomizu M., Yamada Y., Yeh Y., Chen W. T. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J. Biol. Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- Nakamura I., Pilkington M. F., Lakkakorpi P. T., Lipfert L., Sims S. M., Dixon S. J., Rodan G. A., Duong L. T. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci. 1999;112:3985–3993. doi: 10.1242/jcs.112.22.3985. [DOI] [PubMed] [Google Scholar]
- Ng T., Shima D., Squire A., Bastiaens P. I., Gschmeissner S., Humphries M. J., Parker P. J. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory S., Brazier H., Pawlak G., Blangy A. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur. J. Cell Biol. 2008;87:469–477. doi: 10.1016/j.ejcb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Pfaff M., Jurdic P. Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin alphaVbeta3. J. Cell Sci. 2001;114:2775–2786. doi: 10.1242/jcs.114.15.2775. [DOI] [PubMed] [Google Scholar]
- Poincloux R., Lizarraga F., Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- Sakai T., Jove R., Fassler R., Mosher D. F. Role of the cytoplasmic tyrosines of beta 1A integrins in transformation by v-src. Proc. Natl. Acad. Sci. USA. 2001;98:3808–3813. doi: 10.1073/pnas.240456398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Kim C., Ginsberg M. H. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinardi L., Rietdorf J., Nitsch L., Bono M., Tacchetti C., Way M., Marchisio P. C. A dynamic podosome-like structure of epithelial cells. Exp. Cell Res. 2004;295:360–374. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Steffen A., Le Dez G., Poincloux R., Recchi C., Nassoy P., Rottner K., Galli T., Chavrier P. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr. Biol. 2008;18:926–931. doi: 10.1016/j.cub.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Stroeken P. J., van Rijthoven E. A., Boer E., Geerts D., Roos E. Cytoplasmic domain mutants of beta1 integrin, expressed in beta 1-knockout lymphoma cells, have distinct effects on adhesion, invasion and metastasis. Oncogene. 2000;19:1232–1238. doi: 10.1038/sj.onc.1203423. [DOI] [PubMed] [Google Scholar]
- Tarone G., Cirillo D., Giancotti F. G., Comoglio P. M., Marchisio P. C. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Tatin F., Varon C., Genot E., Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J. Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- Uekita T., Itoh Y., Yana I., Ohno H., Seiki M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J. Cell Biol. 2001;155:1345–1356. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignjevic D., Montagnac G. Reorganisation of the dendritic actin network during cancer cell migration and invasion. Semin. Cancer Biol. 2008;18:12–22. doi: 10.1016/j.semcancer.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Weaver A. M. Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- Wheeler A. P., Ridley A. J. RhoB affects macrophage adhesion, integrin expression and migration. Exp. Cell Res. 2007;313:3505–3516. doi: 10.1016/j.yexcr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Lowe C., Lee C. H., Gutierrez G., Niewolna M., Williams P. J., Izbicka E., Uehara Y., Mundy G. R. Herbimycin A, a pp60c-src tyrosine kinase inhibitor, inhibits osteoclastic bone resorption in vitro and hypercalcemia in vivo. J. Clin Invest. 1993;91:2791–2795. doi: 10.1172/JCI116521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambonin-Zallone A., Teti A., Grano M., Rubinacci A., Abbadini M., Gaboli M., Marchisio P. C. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a beta 3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp. Cell Res. 1989;182:645–652. doi: 10.1016/0014-4827(89)90266-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.