The study identifies a sterol- and oxysterol binding protein (OSBP)-regulated phosphatidylinositol 4-kinase that regulates ceramide transport protein (CERT) activity and sphingomyelin (SM) synthesis. RNA interference silencing experiments identify PI4KIIα; as the mediator of Golgi recruitment of CERT, providing a potential mechanism for coordinating assembly of SM and cholesterol in the Golgi or more distal compartments.
Abstract
Cholesterol and sphingomyelin (SM) associate in raft domains and are metabolically coregulated. One aspect of coordinate regulation occurs in the Golgi apparatus where oxysterol binding protein (OSBP) mediates sterol-dependent activation of ceramide transport protein (CERT) activity and SM synthesis. Because CERT transfer activity is dependent on its phosphatidylinositol 4 phosphate [PtdIns(4)P]-specific pleckstrin homology domain, we investigated whether OSBP activation of CERT involved a Golgi-associated PtdIns 4-kinase (PI4K). Cell fractionation experiments revealed that Golgi/endosome-enriched membranes from 25-hydroxycholesterol-treated Chinese hamster ovary cells had increased activity of a sterol-sensitive PI4K that was blocked by small interfering RNA silencing of OSBP. Consistent with this sterol-requirement, OSBP silencing also reduced the cholesterol content of endosome/trans-Golgi network (TGN) fractions containing PI4KIIα. PI4KIIα, but not PI4KIIIβ, was required for oxysterol-activation of SM synthesis and recruitment of CERT to the Golgi apparatus. However, neither PI4KIIα nor PI4KIIIβ expression was required for 25-hydroxycholesterol–dependent translocation of OSBP to the Golgi apparatus. The presence of OSBP, CERT, and PI4KIIα in the TGN of oxysterol-stimulated cells suggests that OSBP couples sterol binding or transfer activity with regulation of PI4KIIα activity, leading to CERT recruitment to the TGN and increased SM synthesis.
INTRODUCTION
As a consequence of physical association in detergent-resistant membranes or raft domains, the metabolism of cholesterol and sphingomyelin (SM) is tightly coupled (Ridgway, 2000). The capacity of plasma membrane rafts to absorb cholesterol is dictated by SM content, which ultimately affects feedback regulation of de novo cholesterol synthesis and esterification in the endoplasmic reticulum (ER) (Porn and Slotte, 1990; Scheek et al., 1997; Leppimaki et al., 1998). Similarly, experimental and pathophysiological conditions that alter cellular cholesterol levels cause reciprocal changes in SM mass and synthesis (Ridgway, 2000). The Golgi apparatus is the site of assembly of cholesterol, SM, glycosphingolipids, and proteins into preraft membranes (Brown and Rose, 1992; Heino et al., 2000). De novo sphingolipid synthesis required for membrane assembly in late Golgi compartments is dependent on transfer proteins for delivery of ceramide and glucosylceramide (GlcCer) precursors from the ER (Hanada et al., 2003; D'Angelo et al., 2007; Halter et al., 2007). Cholesterol synthesized in the ER or released during lipoprotein degradation in the endosomal/lysosomal compartment is also delivered to the Golgi apparatus and plasma membrane by ill-defined nonvesicular mechanisms (reviewed in Ikonen, 2008). Collectively this points to an essential, integrative role for cholesterol and sphingolipid transfer proteins during the initial stages of raft domain assembly at the Golgi apparatus.
Ceramide transfer protein (CERT; Hanada et al., 2003) and the GlcCer transfer protein FAPP2 (D'Angelo et al., 2007; Halter et al., 2007) functionally link metabolic pathways by delivering ER-derived precursors to the Golgi apparatus for sphingolipid synthesis. In contrast, the final product of the mevalonate pathway, cholesterol, must be exported from the ER against a concentration gradient (Lange, 1991). Cholesterol transport from the ER is energy and temperature dependent but does not involve a vesicular mechanism (Urbani and Simoni, 1990; Heino et al., 2000), indirectly supporting a role for transport proteins in cholesterol delivery from the ER to the plasma membrane or trans Golgi/trans-Golgi network (TGN). Numerous proteins with sterol-binding folds have been identified that could fulfill this transport function; however, only two are reported to specifically interact with the ER and Golgi apparatus; oxysterol binding protein (OSBP) and OSBP-related protein 9 (ORP9) (Ridgway et al., 1992; Wyles and Ridgway, 2004). Both belong to the 12-member OSBP gene family that is characterized by a C-terminal cholesterol/oxysterol binding domain (Olkkonen and Levine, 2004). OSBP and ORP9L also contain two phenylalanines in an acid tract (FFAT) motifs that binds vesicle-associated membrane protein-associated protein (VAP) in the ER (Wyles et al., 2002; Wyles and Ridgway, 2004), and phosphatidylinositol 4 phosphate [PtdIns(4)P]-specific pleckstrin homology (PH) domains that interact with the Golgi apparatus (Lagace et al., 1997; Levine and Munro, 2002; Wyles and Ridgway, 2004). The yeast OSBP homologue Osh4p (Raychaudhuri et al., 2006), OSBP, and ORP9L (Ngo and Ridgway, 2009) catalyze in vitro phosphatidylinositol (PtdIns)-phosphate–dependent sterol transfer activity, indicating that the in vivo function of this family could be transfer of cholesterol, oxysterols, or both between membranes. The cholesterol transfer activity of ORP9L has been implicated in ER–Golgi protein trafficking and maintenance of endosomal cholesterol content (Ngo and Ridgway, 2009). In contrast, OSBP mediates sterol-dependent recruitment of CERT to the Golgi apparatus, resulting in increased ceramide transfer and SM synthesis (Perry and Ridgway, 2006), and negatively regulates ABCA1 expression and cholesterol efflux in a post-Golgi compartment by an SM-independent mechanism (Bowden and Ridgway, 2008). Because both of these functions required the sterol binding activity of OSBP, which also displays cholesterol transfer activity in vitro (Ngo and Ridgway, 2009), a possible mechanism could involve modulation of the sterol content of target membranes.
Recruitment of CERT to the Golgi apparatus by OSBP seems to involve a secondary mediator and not a direct physical interaction (Perry and Ridgway, 2006). Because CERT interaction with the Golgi apparatus is dependent on its PtdIns(4)P-specific PH domain (Hanada et al., 2003), we tested whether 25-hydroxycholesterol and OSBP activated a PtdIns 4-kinase (PI4K) that mediated CERT recruitment to the Golgi apparatus. In vitro analysis of PI4K activity in a Golgi/endosome-enriched fraction from control and oxysterol-treated Chinese hamster ovary (CHO) cells, coupled with small interfering RNA (siRNA) experiments, revealed that PI4KIIα was required for oxysterol activation of SM synthesis and CERT recruitment to the Golgi. The reported dependence of PI4KIIα activity on cholesterol suggests that OSBP could regulate kinase activity by altering its membrane environment.
MATERIALS AND METHODS
Materials
pEGFP-CERT and pEGFP-PI4KIIα were described previously (Perry and Ridgway, 2006; Waugh et al., 2006). Alexa Fluor488-conjugated and unconjugated giantin antibodies were purchased from Covance (Emeryville, CA). Antibodies against γadaptin and PI4KIIIβ were from BD Biosciences Transduction Laboratories (Mississauga, ON, Canada). An antibody against early endosomal antigen 1 (EEA1) was from Affinity Bioreagents (Golden, CO). Sheep anti-human TGN46 and rabbit anti-mouse TGN38 antibodies were from AbD Serotec (Raleigh, NC) and Novus Biologicals (Littleton, CO), respectively. Antibodies against OSBP were described previously (Ridgway et al., 1998). Anti-CERT immunoglobulin (Ig)Y, horseradish peroxidase-conjugated rabbit anti-IgY, and Opti-Prep density gradient media were purchased from Sigma-Aldrich (Oakville, ON, Canada). Mouse monoclonal antibody (mAb) 1C4 was generated according to standard methods using recombinant rat PI4KIIα expressed in Sf9 cells as immunogen. 1C4 specifically recognizes the PI4KIIα isoform from human, rat, and hamster species. The 1C4 hybridoma line was cultured in RPMI 1640 medium containing 2.5% ultralow IgG fetal calf serum (FCS) (Invitrogen, Paisley, United Kingdom) and supplemented with 5% Doma-Drive (Immune Systems, Bristol, United Kingdom). Culture supernatants were used in indirect immunofluorescence (1:2 dilution) and Western immunoblotting (1:4 dilution). We purchased [3H]serine and [32P]γ-ATP from PerkinElmer-Cetus (Woodbridge, ON, Canada). PtdIns(4)P, PtdIns, SM, and ceramide were from Sigma-Aldrich or Echelon (Salt Lake City, UT).
Cell Culture and Transfections
CHO cells were cultured in DMEM containing 5% FCS and proline (34 μg/ml) (medium A). In some experiments, cells were cultured in DMEM containing 5% lipoprotein deficient serum (LPDS). In CHO cells, siRNAs against OSBP (siOSBP2) (Perry and Ridgway, 2006), PI4KIIα (5′-GAGACGAGCCCGCUAGUGUUU-3′), and PI4KIIIβ (5′-GCAAG GAGCCUGUGUUCAUUU-3′) (Dharmacon RNA Technologies, Lafayette, CO) were used at a concentration of 30, 60, and 30 nM, respectively, to silence expression of the respective genes by using TransIT TKO transfection reagent (Mirus, Madison, WI). An exact sequence match between siRNAs and the hamster PI4KIIα and PI4KIIIβ mRNAs was confirmed by polymerase chain reaction (PCR) amplification and sequencing of the respective cDNAs. HeLa cells cultured in DMEM with 5% FCS were transfected with siRNAs against human PI4KIIIα (siRNA1, 5′-AGAAAGGGAGUGAGGACUAUU-3′; siRNA2, 5′-GUUUAACCGCAUCGGGCUAUU-3′) and PI4KIIIβ (siRNA1, 5′-GGGAUGACCUUCGGCAAGAUU-3′; siRNA2, 5′-GCUGAUUGCCGCUCGGAAAUU-3′) (Dharmacon RNA Technologies) by using TransIT TKO transfection reagent. Controls consisted of an identical concentration of two different nontargeting siRNA (siNT) with at least four mismatches with known human and mouse genes that were used interchangeably with similar results.
CHO cells stably expressing green fluorescent protein (GFP)-CERT were prepared by transfection with pEGFP-CERT by using Lipofectamine 2000 and selection in medium A containing G418 (600 μg/ml) for 14 d. Cells stably expressing the fusion protein were maintained in medium A with 300 μg G418/ml. OSBP was silenced in CHO cells by stable expression of shOSBP (Perry and Ridgway, 2006) by using a lentiviral vector (Block-iT Lentiviral shRNA Expression; Invitrogen) and selection in blasticidin (10 μg/ml) for 2 wk. Control cells stably expressing shOSBP containing three silent mutations to prevent activity (Perry and Ridgway, 2006) were prepared in an identical manner.
Subcellular Fractionation of CHO Cells
Golgi-enriched membranes were isolated on discontinuous sucrose gradients (Balch et al., 1984). In brief, CHO cells were scraped from 100-mm dishes; resuspended in 10 mM Tris-HCl, pH 7.4, 0.25 M sucrose 2 mM EDTA, 2 mM EGTA, 1 mM sodium pyrophosphate, 1 mM NaF, and 200 nM okadaic acid and protease inhibitors (Roche Diagnostics, Mannheim, Germany); and disrupted by 20 strokes in a Dounce homogenizer. The cell homogenate (0.9 ml) was mixed with 0.9 ml of 2.3 M sucrose in a 5-ml centrifuge tube, overlaid with 2.1 ml of 1.2 M sucrose followed by 1.2 ml of 0.8 M sucrose. The gradients were subject to centrifugation for 4 h at 110,000 × g by using an SW55 Ti rotor (Beckman Coulter, Fullerton, CA). Fractions were collected corresponding to the 0.8 M sucrose layer (fraction I), the 0.8 M/1.2 M interface (fraction II), the 1.2 M sucrose layer (fraction II), the 1.2 M/1.3 M interface (fraction IV), and the 1.3 M layer (fraction V). Fraction II is enriched 25- to 40-fold in the activity of SM synthase, a Golgi enzyme, but also contains endosomes (Lagace et al., 1999).
β-Octylglucoside (β-OG)/deoxycholate (DOC) insoluble membranes were fractionated and assayed for PI4K activity as described previously (Waugh et al., 2003). In brief, CHO cells were solubilized in 2 ml of 10 mM β-OG/4 mM DOC, 100 mM Na2CO3, 10 mM EDTA, and 10 mM EGTA, pH 11.0, by sonication (6 5-s bursts) on ice. The resulting homogenate was mixed with an equal volume of 90% (wt/vol) sucrose/20 mM Tris-HCl, pH 7.4, in a 12-ml centrifuge tube and overlaid with 4 ml of 35% sucrose (wt/vol) and 5% sucrose (wt/vol). The gradient was subject to centrifugation at 175,000 × g for 16 h by using an SW41 Ti rotor (Beckman Coulter), and fractions (1.3 ml) were collected and assayed for PI4K activity as described below.
CHO cells stably expressing shOSBP or a nontargeting control were homogenized in 0.25 M sucrose, 1 mM EDTA, and 10 mM Tris-HCl, pH 7.4, by 15 passages through a 25-gauge needle and centrifuged for 10 min at 2000 × g. The postnuclear supernatant was then fractionated on 6–26% Opti-Prep density gradients (1.059–1.156 g/ml) by centrifugation at 200,000 × g for 3 h in an SW55 Ti rotor (Urano et al., 2008). Fractions were collected by aspiration and assayed for unesterified cholesterol content by the Amplex Red method according to the manufacturer's instructions (Invitrogen). The distribution of cellular organelles in the gradient was determined by immunoblotting for VAP A (ER), NPC1 (late endosomes), TGN38 (TGN), and caveolin (endosomes/plasma membrane).
Measurement of PI4K Activity
PI4K activity was assayed as described, with minor changes (Waugh et al., 1998). In brief, PI4K activity in CHO membrane fractions (2–5 μg of protein) was assayed in 25 mM HEPES, pH 7.4, 20 mM MgCl2, and 2 μg of phosphatidylinositol (from a 2 mg/ml stock in 0.2% Triton X-100) in a final volume of 100 μl. The reaction was initiated by the addition of 40 μM [32P]γ-ATP (0.25 μCi/nmol), incubated at 37°C for 5 min, and terminated by the addition of 0.5 M HCl. Lipids were extracted, separated by thin layer chromatography (TLC) in a solvent system of CHCl3:acetone:MeOH:acetic acid:H2O (40:15:18:12:10, vol/vol), and 32P-labeled lipids were visualized by autoradiography. Bands corresponding to [32P]PtdIns(4)P (determined with an authentic standard) were scraped and quantified by scintillation counting.
Immunofluorescence Microscopy
CHO cells were fixed in 4% (wt/vol) paraformaldehyde for 15 min at 20°C and permeabilized in 0.05% (vol/vol) Triton X-100 for 10 min at 4°C (Perry and Ridgway, 2006). Cells were treated with phosphate-buffered saline (PBS) containing 1% (wt/vol) bovine serum albumin, and incubated with primary and Alexa Fluor-conjugated secondary antibodies as indicated in figure legends. Cells were washed twice with PBS and mounted on microscope slides with Mowiol 4-88 (Calbiochem, San Diego, CA). To disperse the Golgi apparatus, cells were treated with nocodazole (2 μg/ml) for 30 min before the fixation step. Fluorescence images were captured using an LSM510/AxioVert 100M inverted microscope (Carl Zeiss, Thornwood, NY) equipped with 63× and 100× oil immersion objectives (numerical aperture [NA] 1.4).
Cholesterol was visualized by incubating cells with filipin (50 μg/ml in PBS) for 30 min followed by mounting in Mowiol 4-88. Images were captured at identical exposure times using an Axiovert 200M fluorescence microscope (Carl Zeiss) equipped with an axioCam HRm charge-coupled device camera, 4,6-diamidino-2-phenylindole filter package, and 63× oil immersion objective (NA 1.4).
GFP-CERT has a complex pattern of cellular localization but translocates to multiple perinuclear Golgi structures in the presence of 25-hydroxycholesterol. To quantify the change in GFP-CERT localization, images of control and oxysterol-treated CHO cells were captured under identical conditions, and ImageJ software (National Institutes of Health, Bethesda, MD) was used to set pixel thresholds for images of solvent-treated control cells. The change in pixel area above threshold values (normalized to cell number) was measured in oxysterol-activated cells to determine the relative increase in GFP-CERT translocation to perinuclear Golgi structures.
Metabolic Labeling of Sphingolipids
CHO or HeLa cells were transiently transfected with PI4KIIIβ or PIKIIα siRNAs for 48 or 72 h and treated with 25-hydroxycholesterol (2 μg/ml) for 6 h, and then sphingolipids were labeled with [3H]serine (10 μCi/ml) for the final 2 h of the oxysterol treatment. Radiolabeled SM, GlcCer, and ceramide were extracted with CHCl3:MeOH, separated by TLC, and quantified by scintillation counting (Perry and Ridgway, 2006).
RESULTS
A Golgi/Endosome-associated PI4K Activity Is Activated by 25-Hydroxycholesterol and OSBP
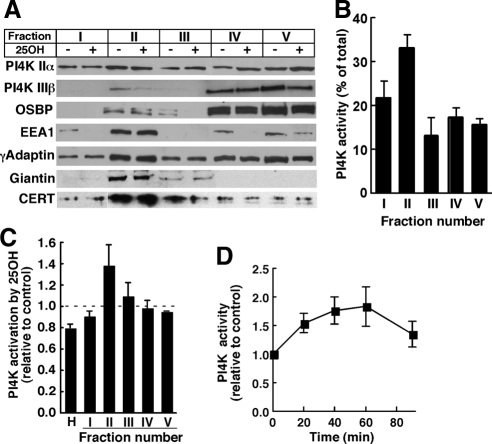
Localization of OSBP, CERT, and FAPP2 to the Golgi apparatus (Lagace et al., 1997; Levine and Munro, 2002; Hanada et al., 2003; Wyles and Ridgway, 2004) involves interaction of their PH domains with PtdIns(4)P synthesized by the Golgi-resident kinases PI4KIIα and/or PI4KIIIβ (Balla et al., 2005; Toth et al., 2006; D'Angelo et al., 2007). Because OSBP promotes sterol-dependent CERT translocation and increased SM synthesis in the Golgi apparatus, we reasoned that this could involve OSBP-dependent activation of a PI4K in the Golgi apparatus. To test this, homogenates of control and 25-hydroxycholesterol-treated CHO cells were fractionated on discontinuous sucrose gradients and analyzed for PI4K expression and activity (Figure 1). This protocol separates Golgi-enriched buoyant membranes (fraction II) from denser ER, mitochondrial, and nuclear membranes (fractions III–V) (Balch et al., 1984). Equivalent amounts (based on volume) of each fraction were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted for PI4KIIα, PI4KIIIβ, OSBP, γadaptin, EEA1, giantin, and CERT (Figure 1A). Giantin, a Golgi resident protein, was primarily detected in fraction II. The γadaptin, a component of the activator protein-1 complex involved in clathrin assembly at the TGN (Bonifacino and Traub, 2003), also was enriched in fraction II but was detected in other fraction as well. Although fraction II was enriched in Golgi markers, it also contained endosomes as indicate by enrichment in EEA1 (Stenmark et al., 1996). PI4KIIα in control CHO homogenates was distributed throughout the gradient but was enriched in fraction II. PI4KIIIβ was detected in fraction II, but the majority was in fractions IV and V. In contrast to OSBP, CERT was enriched in fraction II and relatively absent from dense fractions. Oxysterol treatment did not reproducibly affect the distribution of PI4KIIα, PI4KIIIβ, or CERT in any of the sucrose gradient fractions. Some fractions had minor and inconsistent changes in OSBP and EEA1 distribution in response to 25-hydroxycholesterol treatments. Neither OSBP nor PI4KIIIβ was enriched in fraction II of control or oxysterol-treated cells, even though both displayed prominent Golgi staining (see Figure 9), indicating dissociation from membranes during isolation. Fraction II was not particularly enriched in PI4KIIα or PI4KIIIβ (Figure 1A) but contained >30% of total PI4K activity, indicating it represents a highly active fraction (Figure 1B; Waugh et al., 2003). PI4K activity in fraction II was not affected by treatment with 10 μM wortmannin (data not shown), confirming the relative absence of PI4K type III isoforms (Nakanishi et al., 1995).
Figure 1.
Analysis of an oxysterol-activated PI4K in the Golgi and endosomes. (A) Homogenates of CHO cells, cultured in the absence (−) or presence (+) of 25-hydroxycholesterol (25OH; 2.5 μg/ml) for 1 h, were fractionated on discontinuous sucrose gradients. Equivalent volumes of fractions I–V were resolved by SDS-PAGE and immunoblotted for the indicated proteins as described in Materials and Methods. (B) Sucrose gradient fractions of untreated CHO cell homogenates were assayed for PI4K activity and expressed as a percentage of total. Results are the mean and SEM of three experiments. (C) Homogenates (H) of CHO cells treated with 25-hydroxycholesterol (2.5 μg/ml) or control solvent for 1 h were separated on discontinuous sucrose gradients, and individual fractions were assayed for PI4K activity and expressed relative to controls. Results are the mean and SEM of three experiments. (D) Fraction II was isolated from cells treated with 25-hydroxycholesterol for the indicated times and assayed for PI4K activity. Results are expressed relative to time-matched untreated controls (mean and SEM of 6 experiments).
Figure 9.
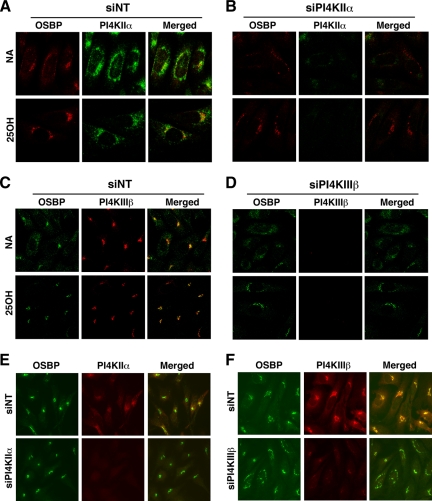
Knockdown of PI4KIIα or PI4KIIIβ does not prevent oxysterol-dependent Golgi localization of OSBP. CHO cells transfected with siNT (A and C), siPI4KIIα (B), or siPI4KIIIβ (D) were treated with ethanol solvent or 25-hydroxycholesterol (25OH; 2.5 μg/ml) for 1 h. Cells were then fixed and incubated with an OSBP polyclonal antibody and goat anti-rabbit Alexa Fluor488 (C and D) or Alexa Fluor594 (A and B) secondary antibodies, followed by PI4KIIα or PI4KIIIβ monoclonal antibodies and a goat anti-mouse Alexa Fluor594 C and D) or Alexa Fluor488 (A and B) secondary antibodies. Images are single confocal sections (0.4–0.2 μM) taken on an LSM510/AxioVert 100M inverted microscope equipped with a 100× oil immersion objective (NA 1.4). HeLa cells were transiently transfected with siPI4KIIα (E) or siPI4KIIIβ (F), treated with 25-hydroxycholesterol for 1 h, and immunostained for the corresponding PI4K and OSBP. Images were captured on an Axiovert 200M fluorescence microscope equipped with a 63× objective (NA 1.4).
We next tested whether PI4K activity in subcellular fractions from CHO cells was responsive to 25-hydroxycholesterol. Total PI4K activity in homogenates (H) of oxysterol-treated CHO cells was reduced by 20%, and the activity in fractions I, III, IV, and V was similar to that of controls (Figure 1C). However, PI4K activity in the Golgi/endosome-enriched fraction II from oxysterol-treated cells has increased by 40% compared with untreated controls. Increased enzyme activity in fraction II of 25-hydroxycholesterol-treated cells was not the result of increased PI4KIIα or PI4KIIIβ protein expression (Figure 1A). The time course for 25-hydroxycholesterol activation of PI4K activity in fraction II is shown in Figure 1D. Treatment of CHO cells for 60 min caused a maximal 75% increase in PI4K activity in fraction II that declined at 90 min.
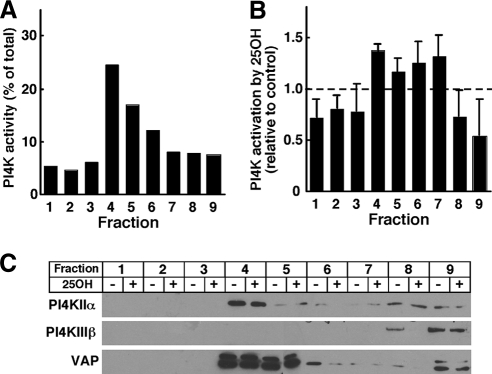
To confirm that membrane-associated PI4K activity was activated by 25-hydroxycholesterol, CHO cells were extracted with a β-OG/DOC containing buffer, pH 11.0, and fractionated on discontinuous sucrose gradients (Figure 2). This method separates soluble kinases from a buoyant β-OG/DOC–resistant membrane fraction enriched in PI4KIIα (Waugh et al., 2003). Similar to results with A431 cells (Waugh et al., 2003), PI4K activity in detergent extracts of CHO cells was enriched in fraction 4, which correspond to the 5–35% sucrose boundary (Figure 2A). Next, CHO cells were treated with or without 25-hydroxycholesterol (2.5 μg/ml) for 1 h, solubilized in β-OG/DOC buffer, separated on sucrose gradients, and assayed for PI4K activity (Figure 2B). Compared with untreated controls, oxysterols increased the PI4K activity in fraction 4 by 35% and caused variable activation in fractions 5–7 that had less kinase activity. Immunoblotting of sucrose gradient fractions from control and oxysterol-treated cells confirmed that fraction IV was enriched in PI4KIIα and that oxysterol treatment did not affect expression of the kinase (Figure 2C). β-OG/DOC-resistant membranes were enriched in the ER protein VAP but were devoid of PI4KIIIβ. Thus, two independent fractionation methods show that a membrane-associated PI4K, possibly the IIα isoform, was activated in response to 25-hydroxycholesterol.
Figure 2.
PI4K activity in detergent-resistant membranes is stimulated by 25-hydroxycholesterol. (A) Untreated CHO cells were solubilized in β-OG/DOC buffer, pH 11.0, separated on discontinuous sucrose gradients, and individual fractions were assayed for PI4K activity. (B) β-OG/DOC extracts of CHO cells treated with 25-hydroxycholesteorl (25OH) or control solvent were separated on sucrose gradients and assayed for PI4K activity. Results are expressed relative to solvent-treated controls and are the mean and SEM of four experiments. (C) Equivalent volumes of fractions prepared as described in B were immunoblotted for PI4KIIα, PI4KIIIβ, and VAP.
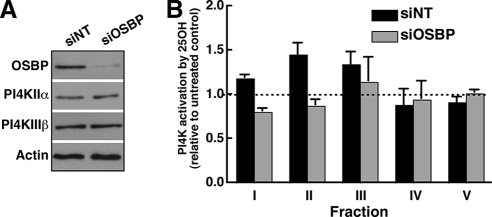
To test whether 25-hydroxycholesterol activation of PI4K activity required OSBP, CHO cells were transfected with siNT or siOSBP for 48 h, treated with or without 25-hydroxycholesterol, homogenized, fractionated on sucrose gradients, and assayed for PI4K activity (Figure 3). Compared with nontargeting controls, siOSBP transfection reduced expression by >90% but did not affect PI4KIIα or PI4KIIIβ (Figure 3A). A comparison of 25-hydroxycholesterol-dependent activation of PI4K activity in individual fractions revealed that fraction II from siNT-transfected cells displayed the expected 40% increase in PI4K activity in response to 25-hydroxycholesterol (Figure 3B). However, PI4K activity in this fraction from OSBP-depleted cells was resistant to activation by 25-hydroxycholesterol. Fraction I also displayed a slight increase in PI4K activity that was suppressed by knockdown of OSBP.
Figure 3.
PI4K activation by 25-hydroxycholesterol is dependent on OSBP. (A) CHO cells were transfected with siNT or siOSBP for 48 h and subsequently cultured in the absence or presence of 25-hydroxycholesterol (2.5 μg/ml) for 1 h. Expression of OSBP, PI4KIIα, or PI4KIIIβ in cell homogenates from siNT- and siOSBP-transfected CHO cells was determined by immunoblotting. (B) Homogenates of control and OSBP-depleted cells, treated with or without 25-hydroxycholesterol, were separated on sucrose gradients and fractions were assayed for PI4K activity. Activation of PI4K activity by oxysterol is expressed relative to solvent-treated controls (mean and SEM of 4 experiments).
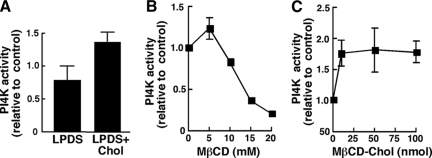
Palmitoylation of a CCPCC motif in the catalytic region of PI4KIIα mediates cholesterol-dependent activity in the TGN (Barylko et al., 2001; Waugh et al., 2003, 2006; Minogue et al., 2010), suggesting it is a candidate for regulation by OSBP and sterols. We tested whether the PI4K activity in fraction II was due to the IIα isoform by examining sensitivity to manipulation of cholesterol levels using methyl β-cyclodextrin (MβCD) or MβCD complexed with cholesterol as described previously (Waugh et al., 2006). To determine whether altering cholesterol affected Golgi PI4K activity, CHO cells were treated with MβCD for 30 min followed by incubation in lipid-depleted medium with or without cholesterol supplementation (Figure 4A). Fraction II was isolated from these cells, assayed for PI4K activity and compared with control cells that were not pretreated with MβCD but cultured under identical conditions. PI4K activity in fraction II from cholesterol-depleted cells cultured in lipoprotein-deficient serum (LPDS) was slightly reduced. However, culturing cells in media supplemented with MβCD-cholesterol increased PI4K activity by 50% compared with cells that received only LPDS. Similar to previous results using a Golgi/endosomal fraction from A431 cells (Waugh et al., 2006), extraction of cholesterol from fraction II with MβCD caused a dose-dependent inhibition of PI4K activity (Figure 4B). Supplementation of fraction II membranes with MβCD–cholesterol complex increased PI4K activity to a maximum of 1.5-fold at 100 nmol cholesterol/assay (Figure 4C). Interestingly, addition of 60 μM 25-hydroxycholesterol directly to fraction II membranes also stimulated PI4K activity by 40–50% (data not shown).
Figure 4.
In vitro regulation of Golgi/endosomal PI4K activity by cholesterol. (A) CHO cells were treated with or without 5 mM MβCD in DMEM for 30 min, washed twice in DMEM, and incubated in DMEM with 5% LPDS with or without MβCD-cholesterol (100 μM) complex (LPDS+Chol). After 2 h, cells were harvested, and fraction II was isolated and assayed for PI4K activity. PI4K activity is expressed relative to cells that were not treated with MβCD. Results are the mean and SEM of three experiments. (B and C) Golgi-enriched fraction II from CHO cells was treated with the indicated concentrations of MβCD (B) or MβCD-cholesterol complex (C) for 30 min at 37°C before assaying for PI4K activity. Results are expressed relative to buffer-treated controls and are the mean and SEM of three experiments.
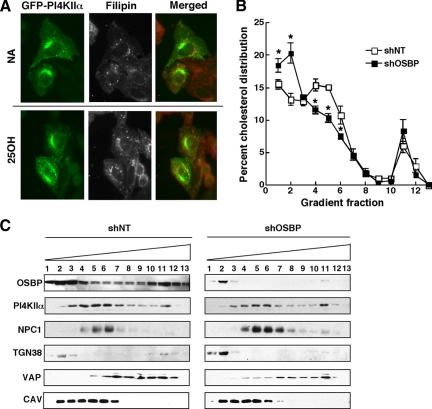
Cholesterol sensitivity of PI4KIIα suggested it could be associated with a cholesterol-enriched cellular compartment. Consistent with its previously described endosomal/TGN localization (Balla et al., 2002; Salazar et al., 2005; Minogue et al., 2006; Craige et al., 2008), GFP-PI4KIIα was present in perinuclear vesicles in control and 25-hydroxycholesterol–treated cells (Figure 5A). Filipin staining of cholesterol in the endosomes/lysosomes was also similar in control and 25-hydroxycholesterol–treated CHO cells but did not overlap with GFP-PI4KIIα. We also observed that filipin distribution was unaffected by OSBP depletion with siRNA (data not shown), suggesting it is not a good probe for assessing the relationship between cholesterol distribution and PI4KIIα. We also measured whether activation by 25-hydroxycholesterol and OSBP affected the cholesterol content of fraction II. However, cholesterol mass in fraction II isolated from control and OSBP-depleted cells treated with or without 25-hydroxycholesterol was similar (data not shown). Although the sucrose density gradient method is useful for quantifying changes in PI4K activity, it does not have the resolution to identify subtle changes in the cholesterol content of Golgi and endosomal membranes. For this purpose, cholesterol and PI4KIIα distribution in control and OSBP-depleted CHO cells were determined by fractionation of postnuclear supernatants on Opti-Prep gradients (Figure 5, B and C). CHO cells were stably depleted of OSBP by expression of a lentiviral short hairpin RNA (shRNA) that afforded >95% knockdown of OSBP protein compared with nontargeting control (shNT) (Figure 5C). PI4KIIα in shOSBP and ShNT cells was distributed primarily to fractions containing the endosomal marker NPC1 and partially overlapped with TGN38 (Figure 5C). Control shNT CHO cells have a cholesterol peak in fractions 4–6 that comigrated with NPC1and PI4KIIα (Figure 5B). In OSBP-depleted cells, there was a significant shift in cholesterol distribution from fractions 4–6 to fractions 1 and 2 that contained TGN38 and caveolin. Although cholesterol distribution was significantly altered by OSBP silencing, the distribution of PI4KIIα was unaffected. PI4KIIα localization to a TGN/endosomal compartment that is subject to cholesterol depletion when OSBP expression is reduced further supports the conclusion that this kinase is regulated by a sterol- and OSBP-dependent mechanism.
Figure 5.
OSBP depletion reduces the cholesterol content of Golgi/endosomal membranes containing PI4KIIα. (A) The localization of cholesterol was determined by filipin staining of CHO cells transiently expressing PI4KIIα-GFP and treated with or without 25-hydroxycholesterol (2.5 μg/ml) for 60 min. (B) Equivalent amounts (2–2.5 mg protein) of the postnuclear supernatants from CHO cells expressing shOSBP or a nontargeting control (shNT) were fractionated on an Opti-Prep gradient and assayed for unesterified cholesterol content as described in Materials and Methods. The cholesterol content of individual fraction is expressed as a percentage of the total cholesterol recovered from the gradient and is the mean and SEM of three experiments (*p < 0.05). (C) Distribution of the organelle markers NPC1 (late endosomes), TGN38 (Golgi), VAP (ER), and caveolin (plasma membrane/endosomes), as well as OSBP and PI4KIIα, were determined by immunoblotting of equivalent volumes of each fraction.
PI4KIIα Is Required for Activation of SM Synthesis by 25-Hydroxycholesterol
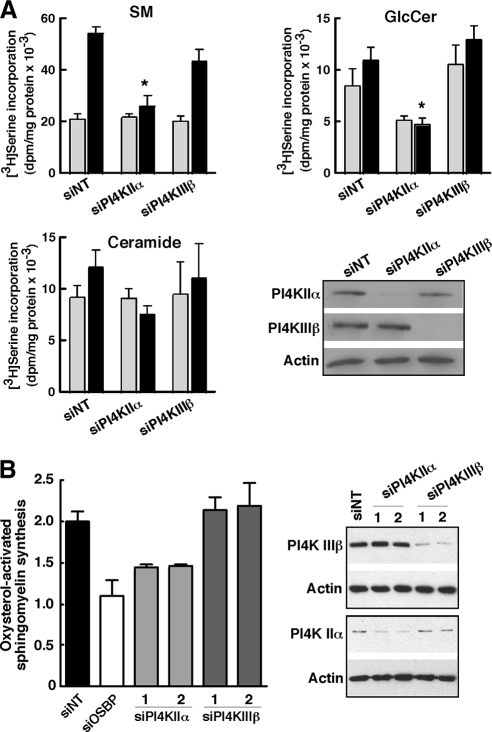
OSBP coordinates cholesterol transport and/or signaling with SM synthesis by activation of CERT activity between the ER and Golgi apparatus (Perry and Ridgway, 2006). PI4KIIα is a good candidate for mediating CERT recruitment to the Golgi apparatus, but other PI4Ks could be involved. Indeed, PI4KIIIβ is abundant in the Golgi apparatus and has a dominant, although apparently not exclusive role in CERT-dependent ceramide transport to the Golgi apparatus under unstimulated conditions (Toth et al., 2006; D'Angelo et al., 2007). To test whether PI4KIIα or PI4KIIIβ was involved in oxysterol regulation of SM synthesis, their expression was silenced in CHO cells with siRNAs followed by metabolic labeling of sphingolipids with [3H]serine in the presence and absence of 25-hydroxycholesterol (Figure 6A). Treatment of siNT-transfected cells with 25-hydroxycholesterol resulted in a 2.5-fold increase in [3H]serine incorporation into SM without affecting [3H]ceramide and [3H]GlcCer levels. CHO cells in which PI4KIIα expression was reduced by >80% had basal [3H]SM synthesis that was similar to siNT controls; however, activation of [3H]SM synthesis by oxysterol was blocked. Silencing of PI4KIIα also reduced synthesis of [3H]GlcCer in control and oxysterol-treated CHO cells, an effect that was not observed after OSBP and CERT depletion (Perry and Ridgway, 2006). In contrast, silencing of PI4KIIIβ did not affect basal or 25-hydroxycholesterol-activated SM synthesis. To confirm these results, oxysterol-activated SM synthesis was quantified in HeLa cells using two different siRNAs directed against PI4KIIα and PI4KIIIβ (Figure 6B). 25-Hydroxycholesterol activation of SM synthesis in HeLa cells is suppressed by OSBP silencing. Moreover, knockdown of PI4KIIα with two different siRNAs suppressed oxysterol-activated SM synthesis by ∼50%, but silencing of PI4KIIIIβ had no affect.
Figure 6.
siRNA silencing of PI4KIIα prevents 25-hydroxycholesterol activation of SM synthesis. (A) CHO cells were transfected with siNT, siPI4KIIα, or siPI4KIIIβ for 48 or 72 h. Cells were then treated with solvent (gray bars) or 25-hydroxycholesterol (2.5 μg/ml; black bars) for 6 h. During the last 2 h of oxysterol treatment, cells were pulse labeled with [3H]serine. and isotope incorporation into SM, ceramide and GlcCer was quantified as described in Materials and Methods. Results are the mean and SEM of three to six separate experiments. The extent of PI4KIIα and PI4KIIIβ knockdown was determined by immunoblotting of whole cell lysates by using actin as a load control. *p < 0.05 compared with oxysterol-treated siNT. (B) HeLa cells were transfected with two different siRNAs against PI4KIIα or PI4KIIIβ, or siOSBP, for 48 h. 25-Hydroxycholesterol–stimulated SM synthesis was then measured as described in A. Results are expressed relative to solvent treated controls and are the mean and SEM of three separate experiments.
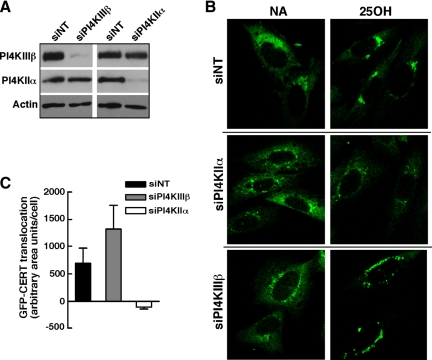
Increased SM synthesis in oxysterol-treated cells is accompanied by Golgi translocation of CERT and delivery of a fluorescent-labeled ceramide analogue from the ER (Perry and Ridgway, 2006). Thus, we tested whether CERT recruitment to the Golgi was also dependent on expression of PI4KIIα. GFP-CERT was stably expressed in CHO cells, and localization to the Golgi apparatus was visualized after depletion of PI4KIIα or PI4KIIIβ by RNA interference (RNAi) and treatment with 25-hydroxycholesterol (Figure 7). siRNAs were effective in knocking down PI4KIIα and IIIβ protein expression by >90% in CHO cells expressing GFP-CERT (Figure 7A). GFP-CERT was in a diffuse and perinuclear compartment in solvent-treated siNT cells but displayed strong Golgi localization in cells exposed to 25-hydroxycholesterol (Figure 7B). Depletion of PI4KIIα did not affect the distribution of CERT in untreated control cells; however, the addition of 25-hydroxycholesterol failed to promote translocation to the Golgi apparatus (Figure 7B). In PI4KIIIβ-depleted cells, the distribution of GFP-CERT was similar to untreated siNT controls and addition of 25-hydroxycholesterol increased the translocation of GFP-CERT to the Golgi apparatus. However, CERT-GFP was slightly dispersed in PI4KIIIβ knockdown cells compared with siNT controls, indicating fragmentation of the Golgi stacks. The quantification of GFP-CERT localization confirmed that siNT- and siPI4KIIIβ-transfected cells treated with 25-hydroxycholesterol had increased perinuclear Golgi staining compared with PI4KIIα knockdown cells (Figure 7C). These result show that, consistent with effects on SM synthesis, PI4KIIα mediates 25-hydroxycholesterol–dependent recruitment of CERT to the Golgi apparatus without affecting its distribution under basal conditions.
Figure 7.
Knockdown of PI4KIIα prevents oxysterol-mediated translocation of GFP-CERT to the Golgi apparatus. (A) Knockdown of PI4KIIα or PI4KIIIβ in CHO cells stably expressing GFP-CERT was confirmed by immunoblotting as described in Materials and Methods. (B) GFP-CERT was visualized by confocal microscopy in CHO cells in which PI4KIIα or PI4KIIIβ was knocked down by >90%. Images are single optical sections (0.4–0.2 mM) captured using an LSM510/AxioVert 100M inverted microscope equipped with a 100× oil immersion objective (NA 1.4). In some experiments, the absence of PI4KIIα and PI4KIIIβ expression also was confirmed by coimmunofluorescence as described in the legend to Figure 9. (C) Localization of GFP-CERT to perinuclear Golgi structures was quantified in 63× wide-field images of control and oxysterol-treated CHO cells transfected with siNT, siPI4KIIα, or siPI4KIIIβ as described in Materials and Methods. Results are the mean and SEM of three experiments.
PI4KIIα and PI4KIIIβ Are Not Required for Golgi Localization of OSBP
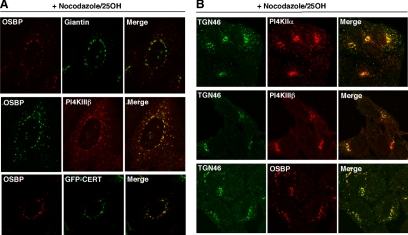
Results shown in Figures 6 and 7 indicated that OSBP is an upstream regulator of PI4KIIα. However, PI4KIIIβ and PI4KIIα have been implicated in recruitment of the GFP-PH domain of OSBP to the Golgi apparatus (Wang et al., 2003; Balla et al., 2005), implying that PtdIns(4)P production also could initiate or enhance OSBP interaction with the Golgi apparatus. Whereas previous studies used the isolated OSBP PH domain as a PtdIns(4)P reporter, siRNA silencing was used here to establish the role of PI4KIIα and PI4KIIIβ in 25-hydroxycholesterol–dependent recruitment of endogenous OSBP to the Golgi apparatus. First, the localization of OSBP, PI4KIIIβ, PI4KIIα, and CERT was identified after activation with 25-hydroxycholesterol and fragmentation of the Golgi apparatus into mini-stacks with nocodazole (Godi et al., 2004; Figure 8). In CHO cells (Figure 8A), OSBP did not colocalize with giantin in the cis/medial-Golgi but strongly colocalized with PI4KIIIβ in the cis/medial- (Weixel et al., 2005) or trans-Golgi/TGN (Hausser et al., 2005). OSBP also partially localized with endogenous PI4KIIα in the endosomes/TGN (Figure 9; Salazar et al., 2005; Minogue et al., 2006; Craige et al., 2008), and strongly costaining with GFP-CERT, which is also reported in the TGN (Fugmann et al., 2007). Because antibodies to TGN resident proteins reacted poorly with CHO cells, we confirmed the localization of OSBP and the PI4-kinases with TGN46 in HeLa cells (Figure 8B). Consistent with the implied TGN localization in CHO cells, OSBP and PI4KIIIβ strongly colocalized with TGN46. Perinuclear PI4KIIα also costained with TGN46, but there was still a portion that did not and, based on cell fractionation shown in Figure 5C, probably corresponds to the endosomes.
Figure 8.
Colocalization of OSBP, CERT, PI4KIIα, and PI4KIIIβ at the TGN. (A) CHO cells were treated with 25-hydroxycholesterol (2.5 μg/ml) for 30 min followed by addition of nocodazole (2 μg/ml) for an additional 30 min. Endogenous OSBP was detected with a polyclonal antibody followed by goat anti-rabbit Alexa Fluor488- or Alexa Fluor594-conjugated secondary antibodies. Cells were costained for giantin or PI4KIIIβ by using corresponding Alexa Fluor488- or Alexa Fluor594-conjugated secondary antibodies. CHO cells transiently expressing GFP-CERT were immunostained for OSBP as described above using a goat anti-rabbit Alexa Fluor594-conjugated secondary antibody. (B) HeLa cells were immunostained with a sheep anti-goat TGN46 antibody and a goat anti-sheep Alexa488-conjugated secondary antibody. This was followed by polyclonal or monoclonal antibodies against OSBP, PI4KIIα, or PI4KIIβ and appropriate Alexa Fluor594-conjugated secondary antibodies. Images are single confocal sections (0.2–0.4 μm) obtained as described in Materials and Methods and the legend to Figure 7.
Next, RNAi was used to determine whether PI4KIIα or PI4KIIIβ was required for translocation of OSBP to the Golgi apparatus in response to 25-hydroxycholesterol (Figure 9). In siNT-transfected CHO cells, OSBP was diffusely localized to the cytoplasm and perinuclear structures but shifted to the Golgi apparatus after addition of 25-hydroxycholesterol for 30 min (Figure 9, A and C). Endogenous PI4KIIα in siNT-transfected cells was distributed in punctate structures and in the perinuclear region, a pattern that was not affected by oxysterol (Figure 9A). OSBP in CHO cells with reduced PI4KIIα expression was localized to the perinuclear Golgi apparatus in the presence of 25-hydroxycholesterol (Figure 9B), indicating that OSBP does not depend on PtdIns(4)P generated by this PI4K for its translocation.
The localization of OSBP was examined in CHO cells depleted of PI4KIIIβ, which has been implicated in Golgi localization of proteins with PtdIns(4)P-specific PH domains (Balla et al., 2005; Toth et al., 2006; Figure 9, C and D). 25-Hydroxycholesterol did not affect the constitutive Golgi localization of PI4KIIIβ (Figure 9C). OSBP translocated to the Golgi apparatus in PI4KIIIβ-depleted cells but tended to be more dispersed than controls, suggesting fragmentation of the Golgi stacks or partial suppression of OSBP translocation (Figure 9D). Similar to results with CHO cells, we observed that suppression of PI4KIIα did not affect the prominent Golgi localization of OSBP in HeLa cells treated with 25-hydroxycholesterol (Figure 9E). OSBP also was localized to the Golgi apparatus in HeLa cells with reduced PI4KIIIβ expression, but again the distribution was slightly fragmented compared with nontargeting controls (Figure 9F). Although we cannot discount other sources of PtdIns(4)P, the lack of involvement of the major Golgi-associated PI4Ks in OSBP recruitment to the Golgi apparatus confirms it is an upstream regulator of PI4KIIα activity.
DISCUSSION
This study demonstrates that OSBP stimulates a sterol-regulated PI4KIIα activity in the TGN/endosome compartment resulting in recruitment of CERT and increased SM synthesis. Mechanistically, this could involve maintenance or stabilization of a cholesterol gradient in the late-Golgi endosomal pathway that is required for PI4KIIα activity by the high-affinity sterol binding and transfer activities of OSBP. By coupling this function with ceramide transfer through CERT recruitment, SM and cholesterol content would be maintained within an optimal range in the early secretory pathway.
The yeast OSBP homologue Osh4p (Prinz, 2007), as well as OSBP and ORP9L (Ngo and Ridgway, 2009), catalyzed the in vitro PtdIns(4)P- and PtdIns(4,5)P2-dependent transfer of cholesterol between liposomes. In vivo, this activity could alter cholesterol or oxysterol distribution in the ER and Golgi membranes with resultant changes in the activity of membrane-associated proteins such as PI4KIIα, which associates with Golgi and endosomal membranes by a cholesterol-dependent mechanism (Waugh et al., 2006; Minogue et al., 2010). In support of this model, PI4K activity in Golgi/endosome-enriched fractions and OG/DOC-resistant membranes from CHO cells was stimulated by 25-hydroxycholesterol and OSBP, and sensitive to in vitro manipulation of cholesterol using MβCD. PI4KIIα was required for Golgi recruitment of CERT and increased SM synthesis in 25-hydroxycholesterol–treated CHO and HeLa cells, activities that are also dependent on OSBP (Perry and Ridgway, 2006). PI4KIIα is not associated with lipid rafts (Waugh et al., 1998; Barylko et al., 2001) but is confined to a TGN/endosomal environment that is relatively cholesterol poor but exquisitely sensitive to addition and extraction of cholesterol (Minogue et al., 2010). Based on density gradient fractionation, OSBP-depleted of CHO cells caused a significant redistribution of cholesterol from endosome fractions, which contained the bulk of PI4KIIα, into lighter fractions enriched in TGN and caveolin but devoid of PI4KIIα. These data suggest that OSBP is required to maintain an membrane cholesterol environment for PI4PIIα that is optimal for synthesis of PtdIns(4)P involved in CERT recruitment and SM synthesis. This conclusion is slightly at odds with previous work showing that PtdIns(4)P made by PI4KIIIβ was primarily involved in constitutive ceramide transport and SM synthesis (Toth et al., 2006; D'Angelo et al., 2007). However, these studies differed in the use of a pharmacological inhibitor and [3H]sphingosine as a biosynthetic precursor, which could be incorporated into ceramide independently of the de novo biosynthetic pathway in the ER. Moreover, our experimental design was for measurement of sterol-activated SM synthesis rather then acute changes in constitutive ceramide transport and SM synthesis.
Although it is established that the PH domain is essential for Golgi localization of OSBP (Lagace et al., 1997), our study shows that neither PI4KIIα nor PI4KIIIβ affected the distribution of OSBP in the presence or absence of oxysterols. Knockdown of PI4KIIIβ caused a slight dispersion of OSBP Golgi staining (Figure 9), but this was due to the requirement of this PI4K for Golgi integrity rather than inhibition of OSBP translocation (Godi et al., 1999). The apparent insensitivity to PI4K expression was unexpected considering that these kinases were required for Golgi localization of the OSBP-PH domain fused to GFP (Wang et al., 2003; Balla et al., 2005) and PI4KIIIβ colocalized with OSBP in the TGN (Figure 8). There are several possible explanations for this result: PtdIns(4)P generated by another PI4K could be substituting for reduced synthesis via PI4KIIα or PI4KIIIβ, other lipid or protein ligand(s) for the PH domain mediate Golgi localization under conditions of limiting PtdIns(4)P, or other OSBP domains facilitate localization by PtdIns(4)P-independent mechanisms. The relative lack of sensitivity of OSBP to Golgi PI4Ks is, however, consistent with an initiating role in recruitment of other PH domain proteins such as CERT. 25-Hydroxycholesterol also promotes Nir2 recruitment to the Golgi apparatus and increased its interaction with VAP (Peretti et al., 2008), suggesting that this PtdIns-transfer protein is required to replenish substrate pools for PtdIns(4)P synthesis following OSBP activation of PI4KIIα.
The identification of PI4KIIα as a target for oxysterol and OSBP regulation provides a plausible mechanism for integration of cholesterol and SM metabolism at the Golgi apparatus. In this scenario, cholesterol or oxysterol transfer by OSBP would provide an optimal membrane environment for PI4KIIα in the endosomes or TGN leading to increased PtdIns(4)P synthesis, recruitment of CERT to the Golgi apparatus and increased ceramide delivery for SM synthesis, thus counterbalancing increased sterol content of the Golgi, post-Golgi membranes, or both. The equilibrium would reestablish once excess sterol was adsorbed into SM-enriched membranes. The localization of OSBP, CERT, PI4KIIα, and PI4KIIIβ at the TGN, facilitated by VAP and Nir2 (Perry and Ridgway, 2006; Peretti et al., 2008), in oxysterol-stimulated cells suggests that coregulated lipid transfer by this complex involves targeted short-range diffusion or contact sites between the ER and TGN (Olkkonen and Levine, 2004). 25-Hydroxycholesterol or cholesterol could be equally effective in this model because like cholesterol, 25-hydroxycholesterol forms liquid-ordered raft domains in vitro (Massey and Pownall, 2006). Also, oxysterols synthesized as a consequence of increased cellular cholesterol or taken up by cells could act as surrogate ligands for OSBP, effectively stimulating SM synthesis more rapidly than cholesterol due to their increased solubility and affinity for OSBP.
ACKNOWLEDGMENTS
We thank Robert Zwicker for excellent technical assistance and Dr. Claudia Weidemann for mAb 1C4. This work was supported by Canadian Institutes of Health Research operating grant MOP 15284 (to N.D.R.) and Biotechnology and Biological Sciences Research Council research grant BB/G021163/1 (to S. M.). S. B. and C.-A.R. are the recipients of a fellowship and studentship, respectively, from the Izaak Walton Killiam Children's Health Centre. C. L. is the recipient of a Beattie Summer studentship.
Abbreviations used:
- β-OG
β-octylglucoside
- CERT
ceramide transfer protein
- DOC
deoxycholate
- EEA1
early endosomal antigen 1
- ER
endoplasmic reticulum
- FFAT
two phenylalanines in an acid tract
- GlcCer
glucosylceramide
- LPDS
lipoprotein deficient serum
- MβCD
methyl β-cyclodextrin
- OSBP
oxysterol binding protein
- PH
pleckstrin homology
- PBS
phosphate-buffered saline
- PI4K
phosphatidylinositol 4-kinase
- SM
sphingomyelin
- siRNA
small interfering RNA
- TGN
trans-Golgi network
- VAP
vesicle-associated membrane protein-associated protein.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-05-0424) on September 29, 2010.
REFERENCES
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Balla A., Tuymetova G., Barshishat M., Geiszt M., Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 2002;277:20041–20050. doi: 10.1074/jbc.M111807200. [DOI] [PubMed] [Google Scholar]
- Balla A., Tuymetova G., Tsiomenko A., Varnai P., Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol. Biol. Cell. 2005;16:1282–1295. doi: 10.1091/mbc.E04-07-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barylko B., Gerber S. H., Binns D. D., Grichine N., Khvotchev M., Sudhof T. C., Albanesi J. P. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J. Biol. Chem. 2001;276:7705–7708. doi: 10.1074/jbc.C000861200. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bowden K., Ridgway N. D. OSBP negatively regulates ABCA1 protein stability. J. Biol. Chem. 2008;283:18210–18217. doi: 10.1074/jbc.M800918200. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Craige B., Salazar G., Faundez V. Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol. Biol. Cell. 2008;19:1415–1426. doi: 10.1091/mbc.E07-12-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo G., et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- Fugmann T., Hausser A., Schoffler P., Schmid S., Pfizenmaier K., Olayioye M. A. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J. Cell Biol. 2007;178:15–22. doi: 10.1083/jcb.200612017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A., Di Campli A., Konstantakopoulos A., Di Tullio G., Alessi D. R., Kular G. S., Daniele T., Marra P., Lucocq J. M., De Matteis M. A. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- Godi A., Pertile P., Meyers R., Marra P., Di Tullio G., Iurisci C., Luini A., Corda D., De Matteis M. A. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Halter D., Neumann S., van Dijk S. M., Wolthoorn J., de Maziere A. M., Vieira O. V., Mattjus P., Klumperman J., van Meer G., Sprong H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J. Cell Biol. 2007;179:101–115. doi: 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hausser A., Storz P., Martens S., Link G., Toker A., Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat. Cell Biol. 2005;7:880–886. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino S., Lusa S., Somerharju P., Ehnholm C., Olkkonen V. M., Ikonen E. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl. Acad. Sci. USA. 2000;97:8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- Lagace T. A., Byers D. M., Cook H. W., Ridgway N. D. Altered regulation of cholesterol and cholesteryl ester synthesis in Chinese-hamster ovary cells overexpressing the oxysterol-binding protein is dependent on the pleckstrin homology domain. Biochem. J. 1997;326:205–213. doi: 10.1042/bj3260205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace T. A., Byers D. M., Cook H. W., Ridgway N. D. Chinese hamster ovary cells overexpressing the oxysterol binding protein (OSBP) display enhanced synthesis of sphingomyelin in response to 25-hydroxycholesterol. J. Lipid Res. 1999;40:109–116. [PubMed] [Google Scholar]
- Lange Y. Disposition of intracellular cholesterol in human fibroblasts. J. Lipid Res. 1991;32:329–339. [PubMed] [Google Scholar]
- Leppimaki P., Kronqvist R., Slotte J. P. The rate of sphingomyelin synthesis de novo is influenced by the level of cholesterol in cultured human skin fibroblasts. Biochem. J. 1998;335:285–291. doi: 10.1042/bj3350285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T. P., Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- Massey J. B., Pownall H. J. Structures of biologically active oxysterols determine their differential effects on phospholipid membranes. Biochemistry. 2006;45:10747–10758. doi: 10.1021/bi060540u. [DOI] [PubMed] [Google Scholar]
- Minogue S., Chu K. M., Westover E. J., Covey D. F., Hsuan J. J., Waugh M. G. Relationship between phosphatidylinositol 4-phosphate synthesis, membrane organisation and lateral diffusion of PI4KIIalpha at the trans-Golgi Network. J. Lipid Res. 2010;51:2314–2324. doi: 10.1194/jlr.M005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue S., Waugh M. G., De Matteis M. A., Stephens D. J., Berditchevski F., Hsuan J. J. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J. Cell Sci. 2006;119:571–581. doi: 10.1242/jcs.02752. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Catt K. J., Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl. Acad. Sci. USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo M., Ridgway N. D. Oxysterol binding protein (OSBP)-related protein 9 (ORP9) Is a cholesterol transfer protein that regulates Golgi structure and function. Mol. Biol. Cell. 2009;20:1388–1399. doi: 10.1091/mbc.E08-09-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen V. M., Levine T. P. Oxysterol binding proteins: in more than one place at one time? Biochem. Cell Biol. 2004;82:87–98. doi: 10.1139/o03-088. [DOI] [PubMed] [Google Scholar]
- Peretti D., Dahan N., Shimoni E., Hirschberg K., Lev S. Coordinated lipid Transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol. Biol. Cell. 2008;19:3871–3884. doi: 10.1091/mbc.E08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. J., Ridgway N. D. Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol. Biol. Cell. 2006;17:2604–2616. doi: 10.1091/mbc.E06-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porn M. I., Slotte J. P. Reversible effects of sphingomyelin degradation on cholesterol distribution and metabolism in fibroblasts and transformed neuroblastoma cells. Biochem. J. 1990;271:121–126. doi: 10.1042/bj2710121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz W. A. Non-vesicular sterol transport in cells. Prog. Lipid Res. 2007;46:297–314. doi: 10.1016/j.plipres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S., Im Y. J., Hurley J. H., Prinz W. A. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J. Cell Biol. 2006;173:107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway N. D. Interactions between metabolism and intracellular distribution of cholesterol and sphingomyelin. Biochim. Biophys. Acta. 2000;1484:129–141. doi: 10.1016/s1388-1981(00)00006-8. [DOI] [PubMed] [Google Scholar]
- Ridgway N. D., Dawson P. A., Ho Y. K., Brown M. S., Goldstein J. L. Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding. J. Cell Biol. 1992;116:307–319. doi: 10.1083/jcb.116.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway N. D., Lagace T. A., Cook H. W., Byers D. M. Differential effects of sphingomyelin hydrolysis and cholesterol transport on oxysterol-binding protein phosphorylation and Golgi localization. J. Biol. Chem. 1998;273:31621–31628. doi: 10.1074/jbc.273.47.31621. [DOI] [PubMed] [Google Scholar]
- Salazar G., Craige B., Wainer B. H., Guo J., De Camilli P., Faundez V. Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles. Mol. Biol. Cell. 2005;16:3692–3704. doi: 10.1091/mbc.E05-01-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheek S., Brown M. S., Goldstein J. L. Sphingomyelin depletion in cultured cells blocks proteolysis of sterol regulatory element binding proteins at site 1. Proc. Natl. Acad. Sci. USA. 1997;94:11179–11183. doi: 10.1073/pnas.94.21.11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Aasland R., Toh B. H., D'Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem. 1996;271:24048–24054. doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- Toth B., Balla A., Ma H., Knight Z. A., Shokat K. M., Balla T. Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J. Biol. Chem. 2006;281:36369–36377. doi: 10.1074/jbc.M604935200. [DOI] [PubMed] [Google Scholar]
- Urano Y., Watanabe H., Murphy S. R., Shibuya Y., Geng Y., Peden A. A., Chang C. C., Chang T. Y. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proc. Natl. Acad. Sci. USA. 2008;105:16513–16518. doi: 10.1073/pnas.0807450105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani L., Simoni R. D. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J. Biol. Chem. 1990;265:1919–1923. [PubMed] [Google Scholar]
- Wang Y. J., Wang J., Sun H. Q., Martinez M., Sun Y. X., Macia E., Kirchhausen T., Albanesi J. P., Roth M. G., Yin H. L. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- Waugh M. G., Lawson D., Tan S. K., Hsuan J. J. Phosphatidylinositol 4-phosphate synthesis in immunoisolated caveolae-like vesicles and low buoyant density non-caveolar membranes. J. Biol. Chem. 1998;273:17115–17121. doi: 10.1074/jbc.273.27.17115. [DOI] [PubMed] [Google Scholar]
- Waugh M. G., Minogue S., Blumenkrantz D., Anderson J. S., Hsuan J. J. Identification and characterization of differentially active pools of type IIalpha phosphatidylinositol 4-kinase activity in unstimulated A431 cells. Biochem. J. 2003;376:497–503. doi: 10.1042/BJ20031212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh M. G., Minogue S., Chotai D., Berditchevski F., Hsuan J. J. Lipid and peptide control of phosphatidylinositol 4-kinase IIalpha activity on Golgi-endosomal Rafts. J. Biol. Chem. 2006;281:3757–3763. doi: 10.1074/jbc.M506527200. [DOI] [PubMed] [Google Scholar]
- Weixel K. M., Blumental-Perry A., Watkins S. C., Aridor M., Weisz O. A. Distinct Golgi populations of phosphatidylinositol 4-phosphate regulated by phosphatidylinositol 4-kinases. J. Biol. Chem. 2005;280:10501–10508. doi: 10.1074/jbc.M414304200. [DOI] [PubMed] [Google Scholar]
- Wyles J. P., McMaster C. R., Ridgway N. D. Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J. Biol. Chem. 2002;277:29908–29918. doi: 10.1074/jbc.M201191200. [DOI] [PubMed] [Google Scholar]
- Wyles J. P., Ridgway N. D. VAMP-associated protein-A regulates partitioning of oxysterol-binding protein-related protein-9 between the endoplasmic reticulum and Golgi apparatus. Exp. Cell Res. 2004;297:533–547. doi: 10.1016/j.yexcr.2004.03.052. [DOI] [PubMed] [Google Scholar]