The nuclear pore complex (NPC) is characterized by a long-lived membrane-lined channel connecting the inner and outer nuclear membranes. This stabilized membrane channel, within which the nuclear pore is built, has little evolutionary precedent. In this report we demonstrate and map the inner/outer nuclear membrane fusion in NPC assembly.
Abstract
Nuclear pore complexes (NPCs) are large proteinaceous channels embedded in double nuclear membranes, which carry out nucleocytoplasmic exchange. The mechanism of nuclear pore assembly involves a unique challenge, as it requires creation of a long-lived membrane-lined channel connecting the inner and outer nuclear membranes. This stabilized membrane channel has little evolutionary precedent. Here we mapped inner/outer nuclear membrane fusion in NPC assembly biochemically by using novel assembly intermediates and membrane fusion inhibitors. Incubation of a Xenopus in vitro nuclear assembly system at 14°C revealed an early pore intermediate where nucleoporin subunits POM121 and the Nup107-160 complex were organized in a punctate pattern on the inner nuclear membrane. With time, this intermediate progressed to diffusion channel formation and finally to complete nuclear pore assembly. Correct channel formation was blocked by the hemifusion inhibitor lysophosphatidylcholine (LPC), but not if a complementary-shaped lipid, oleic acid (OA), was simultaneously added, as determined with a novel fluorescent dextran-quenching assay. Importantly, recruitment of the bulk of FG nucleoporins, characteristic of mature nuclear pores, was not observed before diffusion channel formation and was prevented by LPC or OA, but not by LPC+OA. These results map the crucial inner/outer nuclear membrane fusion event of NPC assembly downstream of POM121/Nup107-160 complex interaction and upstream or at the time of FG nucleoporin recruitment.
INTRODUCTION
Membrane fusion is among the most fundamental and tightly controlled processes in life. Membranes merge during intracellular trafficking, organelle biogenesis, tissue formation, fertilization, and viral infection (Mohler et al., 2002; Jahn et al., 2003; Earp et al., 2005). The formation of the eukaryotic nuclear envelope (NE) also requires membrane fusion events. The nuclear envelope is composed of an outer nuclear membrane and an inner nuclear membrane, both populated with a variety of transmembrane proteins connecting nuclear membrane to cytoskeleton, nuclear lamina and chromatin (Gruenbaum et al., 2005; Dechat et al., 2008; Taimen et al., 2009). The nuclear membranes are separated by a lumen and joined by nuclear pore complexes. Much of our knowledge of the mechanism of vertebrate nuclear envelope assembly has been gained from a cell-free system derived from Xenopus egg extracts (Lohka and Masui, 1983; Newport, 1987; Newport and Dunphy, 1992; Higa et al., 2006). This has allowed the in vitro dissection of NE formation into distinct steps: recruitment of membrane vesicles or tubules to the chromatin surface, fusion of these vesicles/tubules to form double nuclear membranes, nuclear pore complex (NPC) assembly, and expansion of the NE to its full size (D'Angelo and Hetzer, 2006; Harel et al., 2003a; Hetzer et al., 2000; Macaulay and Forbes, 1996; Anderson and Hetzer, 2007). The formation in vitro of a double nuclear membrane around chromatin requires extensive vesicle–vesicle fusion, promoted by the small GTPase Ran, p97, and αSNAP, and regulated by importin β and transportin (Hetzer et al., 2000; Zhang and Clarke, 2000; Kalab et al., 2002; Harel et al., 2003a; Walther et al., 2003b; Baur et al., 2007; Kalab and Heald, 2008; Lau et al., 2009; Rafikova et al., 2009).
Nuclear pore complexes form at two times in the vertebrate cell cycle: (1) NPCs double in number in G1-S phase, then (2) disassemble in prophase and reform at the end of mitosis. The most current model of postmitotic NPC assembly argues that only the initial steps of NPC assembly occur on chromatin, followed by double nuclear membrane assembly and concurrent nuclear pore assembly in these nuclear membranes (D'Angelo and Hetzer, 2008; Kutay and Hetzer, 2008). Indeed, cell-free experiments using the NPC assembly inhibitor BAPTA or an NPC insertion assay show that NPCs can form in vitro in completely closed nuclear membranes, presumably through opposing bilayer fusion (Macaulay and Forbes, 1996; D'Angelo et al., 2006). This in vitro mechanism mirrors the inner/outer nuclear membrane fusion process that occurs in vivo in vertebrate G1-S-phase nuclei, as well as in yeast nuclei at all stages of the cell cycle. In all of these, new nuclear pores form in pre-existing nuclear membranes.
It has been shown that the fusion of biological membranes is driven by integral membrane proteins. Examples of this include enveloped virus entry into cells, SNARE-dependent intracellular fusion, and cell–cell fusion as found in myogenesis and sperm/egg fusion (Blobel et al., 1992; Mayer, 2002; Mohler et al., 2002; Jahn et al., 2003; Schwander et al., 2003; Earp et al., 2005; Jackson and Chapman, 2006; Kielian and Rey, 2006; Podbilewicz et al., 2006). A recent study showed that the vesicle–vesicle fusion event required for forming the double nuclear membranes in Xenopus egg extracts is dependent on SNARE proteins (Baur et al., 2007).
In vivo, a second fusion event between the inner and outer nuclear membranes must be required to form the large proteinaceous nuclear pore complex (Macaulay and Forbes, 1996; Goldberg et al., 1997; Harel et al., 2003b; Guttinger et al., 2009). Importantly, the mechanism for and timing of inner/outer nuclear membrane fusion, however, has not been revealed. It is likely that one or more integral membrane proteins play a key role in this fusion event. To date, POM121, gp210, and NDC1 are the only known integral membrane pore proteins in metazoans (Gerace et al., 1982; Hallberg et al., 1993; Madrid et al., 2006; Mansfeld et al., 2006; Stavru et al., 2006a). RNAi depletion of these transmembrane nucleoporins has shown that POM121 and NDC1 are essential for nuclear pore complex assembly (Antonin et al., 2005; Mansfeld et al., 2006; Stavru et al., 2006a; Stavru et al., 2006b; Funakoshi et al., 2007); however, their potential role in inner/outer nuclear membrane fusion has not been investigated. One possibility is that these proteins may work either alone or together to promote the fusion of the inner and outer nuclear membranes. Alternatively, an as yet unidentified transmembrane protein may mediate inner/outer nuclear membrane fusion in nuclear pore assembly.
The most accepted molecular model for general membrane fusion events is the “stalk-pore” mechanism. This mechanism was first identified on artificial protein-free bilayers, then later in viral fusion, intracellular fusion and, most recently, developmental cell fusion (Kozlov and Markin, 1983; Chernomordik et al., 1987; Chernomordik et al., 1993; Lu et al., 2005; Reese et al., 2005; Xu et al., 2005; Podbilewicz et al., 2006; Sapir et al., 2007). In the stalk-pore mechanism, membrane fusion begins with the bending of two membranes toward one another and proceeds through a hemifusion intermediate, which consists of a stalk-like connection that involves only the contacting membrane leaflets of the two fusing bilayers (Supplemental Figure 1). Hemifusion proceeds to complete fusion, which involves the progression to a fusion pore that connects all four leaflets (Supplemental Figure 1). Subsequent expansion of the fusion pore leads to a fully fused entity. To date, fusion proteins have been found to provide the driving force that induces hemifusion in biological membranes.
However, the fusion reaction also shows a striking sensitivity to membrane lipid composition (Chernomordik et al., 1993). For biological membranes, hemifusion intermediates are strongly sensitive to the shapes of the lipid molecules in the membrane leaflets. Inverted cone-shaped lipids, such as LPC, inhibit bending of leaflets toward one another and thus inhibit hemifusion. This is true for both viral and intracellular fusion events (Chernomordik and Kozlov, 2003; Melia et al., 2006). Cone-shaped lipids, such as oleic acid (OA), promote this type of bending, thereby stabilizing hemifusion but blocking full fusion. When LPC and OA are added together, however, their complementary shapes neutralize one another and fusion occurs unimpeded. In consequence, lipids such as LPC have been used as a tool to identify when a membrane fusion event occurs, as LPC has been shown to act as a universal inhibitor of hemifusion. In contrast, post-hemifusion stages, those that involve opening and expansion of the fusion pore, are found to be more protein dependent (Chernomordik and Kozlov, 2003; Lu et al., 2005; Reese et al., 2005; Xu et al., 2005).
In analysis of nuclear pore assembly, the membrane fusion step has been difficult to biochemically address, and its temporal order with regard to specific vertebrate nucleoporin recruitment has not been demonstrated. Using experimental approaches used previously to model membrane fusion reactions throughout the cell (Chernomordik et al., 1998; Markosyan et al., 2003; Henderson and Hope, 2006), here we address questions with respect to the fusion event between the inner and outer nuclear membranes during nuclear pore formation. Specifically, we describe a novel cold- and lipid-sensitive intermediate that occurs in a completely closed nuclear envelope. This intermediate is formed downstream from the acquisition of early nucleoporins on the chromatin surface, but before observation of NPC structures containing channels functional for diffusion and before the recruitment of FG nucleoporins.
MATERIALS AND METHODS
Antibodies
The antibodies used in this study included affinity-purified anti-hNup133 (Vasu et al., 2001), anti-Xenopus POM121 (Harel et al., 2003b), which was raised to Xenopus POM121 aa144-435, the anti-FG nucleoporin antibody mAb414 (Covance, Berkeley, CA), and anti-dsDNA antibody ab27156 (Abcam, Cambridge, MA). Note that mAb414 recognizes most FG nucleoporins in Xenopus, but not Xenopus POM121, either on immunoblots or by immunofluorescence, presumably due to an inexact match of the FG epitopes in xPOM121.
Nuclear Reconstitution and Immunofluorescence
Xenopus egg extracts, membranes, and demembranated chromatin were prepared as described previously (Harel et al., 2003a). Nuclei were reconstituted either at room temperature or at 14°C in a water bath, using constant temperature monitoring with an ethanol thermometer and temperature maintenance by ice addition for the 14°C reaction. Reactions were prepared on ice by mixing 20 μl Xenopus egg extract, 1 μl ATP-regeneration system (ATP 0.2 M + phosphocreatine 1 M + creatine phosphokinase 5 mg/ml; in proportions 1:1:2, respectively), 1 μl of membranes, and 2 μl of ELB (10 mM HEPES, pH 7.8, 2.5 mM MgCl2, 50 mM KCl, 250 mM sucrose). Ingredients were mixed using prechilled pipette tips. Chromatin (1 μl) was added from a stock of 50,000 U chromatin/μl (2500 U of chromatin per reaction) using prechilled large-orifice pipette tips and gently mixed using the same tips. Reactions were initiated by placing the reaction tubes at room temperature or into a 14°C water bath (or at times in PCR machine), both calibrated with a Traceable Digital Thermometer (Fisher Scientific #15-078-38), Fair Lawn, N.J.
For direct immunofluorescence, affinity purified anti-Nup133, anti-POM121, mAb414, or anti-dsDNA antibodies were coupled to Alexa fluor dyes per manufacturer protocol (Molecular Probes, Eugene, OR). Nuclear assembly reactions were stopped on ice at different times after the start of assembly, and 10-μl aliquots were transferred to new Eppendorf tubes prechilled on ice using ice-cold large-orifice pipette tips. The samples were either fixed with 2.5% ice-cold paraformaldehyde for 30 min on ice, following by incubation with 0.5 μg directly labeled antibodies (or 0.2 μg of anti-dsDNA antibody) for 30 min on ice, or incubated with antibody and then fixed. We observed no difference between the two protocols in the patterns observed, but observed brighter staining with the latter method for the anti-DNA antibody, perhaps because of increased accessibility before fixation of the chromatin. To monitor nuclear assembly by confocal microscopy samples were prepared as follows: slides, cover glasses, and pipette tips were prechilled on ice. An aliquot of the reaction was mixed with half a volume of ice-cold Vectashield with DAPI and 10 μg/ml the lipophilic dye 3,3-dihexyloxacarbocyanine iodide (DHCC) (Eastman Kodak, Rochester, NY), covered with ice cold cover glasses, and kept on ice until monitoring with a confocal microscope.
Nuclei were visualized with an Axioskop 2 microscope (×63 objective; Carl Zeiss, Thornwood, NY). Images were also acquired using an Axiovert 200M confocal microscope (Carl Zeiss, Thornwood, NY) at a magnification of ×63 using an oil objective (Carl Zeiss) with a 1.3 numerical aperture at 23°C and with Immersol 518F (Carl Zeiss) as the imaging medium. Images were recorded using a Coolsnap HQ (Photometerics, Tucson, AZ) camera and Metavue software (Molecular Devices Corporation, Downingtown, PA).
Application of Exogenous Lipids
Stock solutions of lysophosphatidylcholine (LPC; 1-lauroyl-2-hydroxy-sn-glycero-3-phosphocholine 12:0; Cat. #855475p; Avanti Polar Lipids, Alabaster, AL) and oleic acid (OA; Sigma Chemical Co., St. Louis, MO) were freshly prepared as 10 mM solutions in PBS or ELBS and 25 mM ethanolic solution, respectively. At the indicated time, an assembly reaction was stopped on ice and LPC or LPC plus OA were added directly to the samples at a final concentration of 600 μM each by rapid injection of the stock solution into the tube under continuous flicking. The samples were returned to the working temperature (14 degrees or room temperature, as noted), and after further incubation they were processed for direct immunofluorescence as previously described. It should be noted that in artificial membranes or cells the presence of LPC in only one of two fusing entities was sufficient to inhibit membrane fusion (Chernomordik et al., 1997; Chernomordik et al., 1998).
To test the effect of the membrane fluidizer benzyl alcohol (BA; Fisher Scientific Catalogue #100-51-6) on nuclear reconstitution and NPC formation, BA was added at t = 0 min or, alternately, to nuclei preassembled at 14°C for 30 min. Concentrations of BA were 0.05%, 0.1%, or 0.2%, as described specifically for each experiment. To test the effect of phosphatidylethanolamine (PE) (egg yolk-derived, Sigma Catalogue #P7943) on nuclear reconstitution, PE powder was mixed with Egg Lysis Buffer + Sucrose (ELBS) buffer to 15 mM at high temperature (50°C) to place PE above its transition temperature. This was sonicated, which produces a mixture of PE liposomes and micelles. We added different concentrations of this PE liposome suspension to Xenopus nuclear reconstitution reactions containing LPC at t = 0′ and incubated at room temperature for 60′ or, alternatively, we added PE plus or minus LPC to 30 min cold intermediates and incubated for a further 90 min.
Diffusion Assay
The presence of diffusion channels through the nuclear envelope was assayed by the ability of Alexa488-10 kDa or Alexa488-3 kDa dextrans to diffuse into the nucleus. Specifically, at the indicated time a nuclear assembly reaction was stopped on ice, and 1 μg Alexa488-10 kDa or -3 kDa dextrans (Molecular Probes, Eugene, OR) was added to a 10-μl reaction sample and incubated for 15 min on ice. The samples were fixed with 2.5% paraformaldehyde for 20 min, and then the Alexa fluorescence external to the nucleus was quenched by the addition of 5 μg anti-Alexa Fluor 488 antibody for an additional 15 min. The samples were either directly monitored by confocal microscopy, or were diluted to 200 μl in ELB buffer with 1 mM DTT, and 100 μg/ml cycloheximide. These latter were subsequently centrifuged through a cushion of 30% (wt/vol) sucrose in ELB onto poly-L-Lysine-coated coverslips and then monitored by confocal microscopy. This assay was usually performed simultaneously with an Alexa568-anti-DNA antibody assay. Only intact nuclei (i.e., nuclei that excluded the anti-DNA antibody) were assessed for their ability to exclude the labeled dextrans. Nuclear intermediates that excluded 3-kDa and 10-kDa dextrans were concluded to be lacking in diffusion channels. Under conditions where nuclei contained mature nuclear pores (for example, nuclei formed at room temperature for 30 min with no inhibitors, or nuclei formed at 14°C for 60 min; see text), the Alexa 488-labeled 3 and 10-kDa dextrans showed diffusion into the nuclear interior. In these cases, intranuclear dextran fluorescence was observed and was unquenchable by added 150 kDa anti-Alexa488 antibody, which is too large to pass through even a mature nuclear pore.
Expression and Application of Exogenous WTαSNAP and Δ80NαSNAP Proteins
His-tagged WTαSNAP and Δ80NαSNAP were expressed and purified as described previously (Baur et al., 2007). Proteins eluted from NTA-Ni-agarose beads were dialyzed against ELB supplemented with 10% glycerol, and concentrated using Amicon Ultra-4 Centrifugal Filter Devices (Millipore, Billerica, MA). Concentrated proteins were frozen in aliquots in liquid nitrogen and stored in –80°C.
Nuclei were reconstituted at room temperature or at 14°C as described above. The WTαSNAP or Δ80NαSNAP proteins were added at the beginning of the reaction or to nuclei reconstituted at 14°C for 30 min to a final concentration of 47 μM and incubated for the time and at the temperature stated for each experiment.
RESULTS
A Temperature-Sensitive Intermediate in Nuclear Pore Assembly That Is Deficient for FG Nups
In vitro nuclear assembly using extracts of Xenopus laevis eggs is normally performed at ∼22–23°C (room temperature), which is a physiological temperature for Xenopus laevis (Newport and Forbes, 1987; Fort et al., 2004). However, the fast rate of nuclear envelope formation and nuclear pore assembly both in the in vitro Xenopus system and in cells at physiological temperatures (Dultz et al., 2008; Katsani et al., 2008) creates significant difficulties for those wishing to isolate and explore early pore assembly intermediates. In other systems, lowered temperatures are known to affect the lateral mobility of fusion proteins and impair general membrane fusion (Saraste et al., 1986; Henderson and Hope, 2006; Baur et al., 2007). Indeed, lower temperatures have been used to stabilize and identify important steps in membrane fusion events such as virus-cell fusion, endocytosis, and cell–cell fusion (Stegmann et al., 1990; Schoch et al., 1992; Markosyan et al., 2003; Baur et al., 2007; Gattegno et al., 2007). Any step in nuclear assembly involving membranes could theoretically be influenced by temperature. Baur and colleagues (Baur et al., 2007) found nuclear assembly in Xenopus extracts occurred normally at 16°C, albeit slowly.
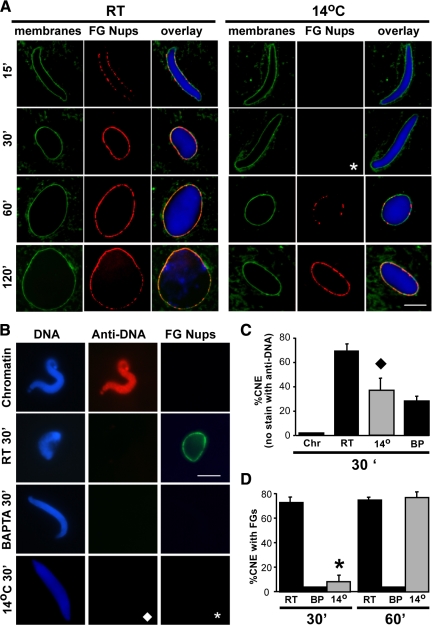
With the goal of identifying new intermediates in nuclear pore assembly, we tested lowered temperatures for an ability to reveal temperature sensitive steps in nuclear membrane or pore assembly. At room temperature in the Xenopus nuclear reconstitution system, nuclear membranes form around chromatin by 15 min after initiation of the nuclear assembly reaction and expand over time, as visualized with the membrane dye DHCC (Figure 1A, RT, green). At room temperature, nuclear pore complexes (NPCs) also appeared by 15 min, as visualized with the anti-FG nucleoporin antibody mAb414 (Figure 1A, RT, red). Import-competent nuclei became readily apparent by 30 min (data not shown) and fully-grown nuclei were observed by 60–120 min (Figure 1A, RT). When assembly was performed at 4°C, vesicle binding to chromatin was observed but vesicle–vesicle fusion was inhibited, resulting in no nuclear membranes (data not shown).
Figure 1.
Cold temperature produces a novel intermediate in NPC assembly at 30 min that lacks FG Nups. (A) The progression of nuclear envelope assembly is slowed down at 14°C. Nuclear assembly reactions containing chromatin, cytosol, and membranes were set up at different temperatures. Reactions were stopped at the indicated time points and analyzed by confocal microscopy after direct immunofluorescence with the Alexa568-labeled anti-FG Nup antibody mAb414 (red). Nuclear membranes were visualized with the fluorescent membrane dye DHCC (green), and chromatin was stained with DAPI (blue). Scale bar, 10 μm. (B) A DNA antibody exclusion assay was used to determine the formation of closed nuclear envelopes (CNE). For this, nuclear assembly reactions containing chromatin, cytosol and membranes were set up and incubated for 30 min at room temperature (RT), for 30 min at 14°C, and for 30 min at RT in the presence of BAPTA before fixation. Alexa568-labeled anti-DNA antibody (red) and Alexa488-labeled anti-FG Nup antibody (green) were added and the nuclei examined. Chromatin plus cytosol (no membranes) served as a positive control for anti-DNA antibody binding (top panel). Scale bar, 10 μm. (C) The formation of CNE was quantified for at least 100 randomly chosen structures in three independent experiments. (D) The percentage of CNE containing FG Nups, detected as a green rim with Alexa488-labeled anti-FG Nup antibody at 30 min and 60 min in three different experiments was also plotted. Errors bars indicate the SD. Nuclei reconstituted in equivalent conditions of 30 min 14°C are marked with a diamond for anti-DNA antibody (♦) and an asterisk (*) for anti-FG Nup antibody in panels A–D.
We found that incubation at the intermediate temperature of 14°C was permissive for nuclear membrane assembly and NPC formation over the long-term (Figure 1A, 14°C, 60 min and 120 min). This lower temperature, however, affected the kinetics of nuclear pore formation. Strikingly, although intact nuclear membranes formed, the FG nucleoporin rim staining characteristic of mature nuclear pores was not detected at 30 min of assembly at 14°C (asterisk, Figure 1A). Surface views and quantitation of NPCs/μm2 (Supplemental Figure 2) confirmed the lack of FG-staining nuclear pores in the 30 min 14°C cold intermediate. Nuclei containing FG nucleoporins became abundant only after 60 min at 14°C. This indicated that nuclear assembly proceeds at 14°C, but at a slower rate compared with room temperature.
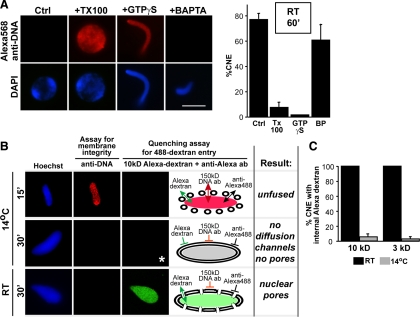
To analyze more carefully the molecular events of nuclear assembly at 14°C, we focused first on double nuclear membrane formation. Vesicle–vesicle fusion to form double nuclear membranes clearly occurred at 14°C, as characterized by the smooth nuclear rim visible at early time points (Figure 1A, 14°C, green). To determine whether the nuclear membranes completely enclosed the chromatin at 14°C, we used an Alexa568-labeled anti-DNA antibody assay, characterized in Figure 3A. Nuclear assembly reactions were initiated on chromatin templates with the addition of Xenopus egg membrane vesicles and cytosol. At the end of the reaction, Alexa568-labeled anti-DNA antibody was added, with the presumption that it could only access the chromatin if the NE was not fully closed. Both positive and negative controls for this reaction were performed first. When nuclei were assembled for 60 min at room temperature, the fluorescently labeled anti-DNA antibody did not have access to the chromatin in the majority of nuclei, as indicated by the absence of a red Alexa568 signal in the nucleus (Figure 3A, Ctrl). In contrast, if Triton X-100 was added at the end of the reaction to permeabilize the nuclei, or if GTPγS was added at the beginning of the reaction to prevent vesicle–vesicle fusion (Boman et al., 1992), chromatin substrates showed uniform staining with the anti-DNA antibody (Figure 3A, +TX100 or +GTPγS,). Lastly, addition of the calcium chelator BAPTA, known to allow formation of a closed nuclear membrane but to inhibit nuclear pore formation (Macaulay and Forbes, 1996), gave closed nuclear structures that excluded entry of the anti-DNA antibody (Figure 3A, +BAPTA). Quantitation of multiple experiments is also shown graphically in Figure 3A.
Figure 3.
The 30-min cold intermediate containing POM121 and the Nup107-160 complex lacks diffusion channels. (A) An anti-DNA antibody exclusion assay was used to assess the formation of completely enclosed nuclear envelopes. Nuclei were assembled at room temperature for 60 min with chromatin, membranes, and either control cytosol, GTPγS-treated cytosol, or BAPTA-treated cytosol. Control nuclei were either visualized alone (Ctrl) or permeabilized with Triton X-100 (TX100) to allow entry of anti-DNA antibody. Positive labeling of the chromatin with Alexa568-anti-DNA antibody in the GTPγS nuclei indicated that a fully closed NE did not form under this condition; absence of anti-DNA antibody staining for Control and BAPTA nuclei indicated that a fully closed NE formed in these conditions. Scale bar, 10 μm. The percentage of nuclei showing a lack of DNA antibody staining in their nuclear interior (% CNE) was quantified for ∼100 randomly chosen nuclear intermediates each in three independent experiments, as shown in the histogram. Error bars indicate SD. (B) The anti-Alexa488 quenching assay described in Supplemental Figure 4 was used to determine the presence of 10-kDa competent diffusion channels in closed nuclear envelopes reconstituted for 15 min and 30 min at 14°C. The 15 min, 14°C intermediates were not fully membrane enclosed, as they showed anti-DNA staining (red) and quenching by anti-Alexa488 antibody (black) (top panels). By 30 min at 14°C, many intact nuclei had formed, as judged by a lack of DNA staining, but these showed no entry of Alexa488-10 kDa dextran indicating no diffusion channels (middle panels). In contrast, by 30 min at room temperature, the nuclei were intact (no anti-DNA staining) but had developed nuclear pores capable of allowing Alexa488-dextran entry, enabling its protection from the quenching antibody (lower panels). (C) The percentage of 14°C 30 min enclosed nuclear intermediates (i.e., with no anti-DNA staining) that showed diffusion of Alexa488-10 kDa or Alexa488-3 kDa dextrans was measured in three different experiments each and plotted. We found that the great majority of enclosed 14°C 30 min nuclear intermediates did not show diffusion channels. Errors bars indicate the SD.
Having established that the anti-DNA antibody correctly monitored formation of completely fused nuclear membranes, we used this assay to characterize nuclei assembled for 30 min at 14°C. Nearly half of the nuclei showed no labeling by the anti-DNA antibody (diamonds, Figure 1, B and C), indicating that these nuclei were indeed membrane-enclosed by 30 min. Furthermore, the enclosed 30 min 14°C intermediates, when tested, did not contain FG nucleoporins (asterisks, Figure 1, B and D). By 60 min, however, the nuclei had acquired FG nucleoporins (Figure 1D, 60 min, light gray; see also Figure 1A). A membrane-enclosed FG Nup-minus intermediate is not observed during room temperature incubation, as parallel experiments indicate that abundant NPCs form at RT long before nuclear membrane enclosure (7 min vs. 12.5 min; Supplemental Figure 3). We concluded that the lower incubation temperature of 14°C causes a deceleration in the rate of nuclear pore assembly, but not in the rate of nuclear membrane assembly. This thus provides a novel nuclear intermediate that can be exploited to probe the mechanism of nuclear pore assembly.
Recruitment of POM121 and Nup133 into Punctate Structures Characteristic of a Partial Pore Intermediate on the Inner Nuclear Membrane
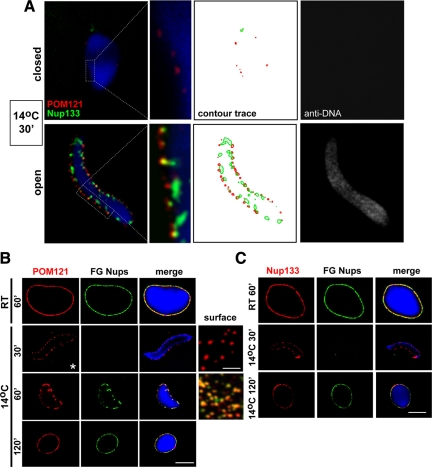
The cold intermediate formed at 30 min at 14°C lacked the FG Nups that are the hallmark of mature nuclear pores. We next asked whether other nucleoporins were present in this cold intermediate. In addition, we used an experimental strategy designed to distinguish whether nucleoporins, when present, were on the inner or outer nuclear surface. Directly labeled antibodies, each labeled with a different fluorophore were added to 30 min 14°C cold intermediates. Anti-Nup133 (Alexa488, green), anti-POM121 (Alexa568, red), and anti-dsDNA (Alexa647, far-red, digitally modified to gray in all figures) were used. On closed 30 min 14°C nuclear intermediates (CNE), i.e., nuclei that did not stain with Alexa647-labeled anti-DNA antibody (Figure 2A, top right panel), we observed no Nup133 staining (Figure 2A, top left panel, green) and a negligible number of POM121-punctate spots on the same intact nuclei (Figure 2A, top left panel, red). However, if we looked at the subset of 30 min 14°C cold intermediates that were accessible to anti-DNA antibody (Figure 2A, lower right panel), we observed strong Nup133 and POM121 staining (Figure 2A, lower left panel). These latter nuclei presumably contain gaps that allow the access of all three labeled antibodies. Strikingly, in such open nuclei the Nup133 stain appeared interior to the POM121 stain. POM121 and Nup133 staining overlapped significantly, but not completely at this time point (see inset and contour, Figure 2A, lower left panel).
Figure 2.
The 30-min cold intermediate contains punctate POM121 and Nup133 on the inner face of the nuclear envelope. (A) A nuclear assembly reaction was incubated for 30 min at 14°C and analyzed by confocal microscopy after direct immunofluorescence on ice with Alexa568-anti-POM121 (red) and Alexa488-anti-Nup133 (green) antibodies (left panels). The staining of the same nuclear intermediates with Alexa647-anti-DNA antibody (gray) is shown in the right panels; this latter staining was used to assess whether an individual nucleus contained a fully closed (top panels) or still open (bottom panels) nuclear envelope. Merged images of POM121/Nup133/DAPI stain are shown at normal (left panels) and high (adjacent panels) magnification. We note that POM121 and Nup133 could only be stained in intermediates where the nuclear envelope was open (i.e., accessible to anti-DNA antibody). A contour trace of the POM121 and Nup133 staining is shown in the 3rd set of panels, allowing comparison of their localization. Scale bar, 10 μm. (B) Nuclei with accessible interiors were analyzed by direct immunofluorescence with Alexa568-anti-POM121 (red) and Alexa488-anti-FG Nup (green) antibodies. The merged images (yellow) are shown at the right. Chromatin was stained with DAPI (blue). Scale bar, 10 μm. Individual NPCs were separately visualized at high magnification on the surface of 14°C nuclear intermediates assembled for 30 min and 60 min (surface). Scale bar, 1 μm. (C) The assembly reactions were analyzed in parallel with Alexa568-anti-Nup133 (red) and Alexa488-anti-FG Nup (green) antibodies. Scale bar, 10 μm.
POM121 is an integral membrane nuclear pore protein (Hallberg et al., 1993) recruited to the chromatin with the membrane vesicles. Nup133 is a signature member of the Nup107-160 complex, known to be key to the initiation of NPC assembly, which is recruited to chromatin by the pore-targeting chromatin-binding protein ELYS (Mishra et al., 2010; Li et al., 1995; Goldstein et al., 1996; Belgareh et al., 2001; Griffis et al., 2003; Walther et al., 2003a; Blower et al., 2005; Fernandez and Piano, 2006; Galy et al., 2006; Orjalo et al., 2006; Rasala et al., 2006; Franz et al., 2007; Ratner et al., 2007; Chakraborty et al., 2008; Davuluri et al., 2008; de Jong-Curtain et al., 2009; Lau et al., 2009; Rotem et al., 2009) Observation of Nup133 is taken as indicative of the presence of the entire Nup107-160 complex on the chromatin (Vasu et al., 2001; Boehmer and Schwartz, 2007; Rotem et al., 2009). A time course of the recruitment of POM121 compared to that of FG nucleoporins (Figure 2B), and Nup133 compared to FG nucleoporins (Figure 2C) was performed on non-intact nuclei. We found that POM121 was present in the 30-min cold intermediate (Figure 2B), as was Nup133 (Figure 2C). Overall, we conclude that (i) both POM121 and Nup133 are present in the 30 min cold intermediate, while FG nucleoporins are largely not detectable, (ii) POM121 and Nup133 are almost exclusively in or near the inner nuclear membrane, and (iii) that POM121 and Nup133 are often colocalized. Because this POM121/Nup133 complex is not accessible to antibody unless the nuclei are permeabilized or the membranes have not fully closed, we think that this is a “prepore” complex that forms specifically between POM121 in the inner nuclear membrane and the Nup107-160 complex (Nup133) on the surface of the chromatin.
A Diffusion Channel Is Lacking in the POM121+/Nup133+ Partial Pore Nuclear Intermediate
Traditional transmission EM (TEM) has been used in the field to reveal/verify three different types of uniform structures in in vitro nuclear assembly: (i) structures blocked before vesicle–vesicle fusion (by GTPγS or αSNAP addition), (ii) structures where the nuclear membranes completely enclose the nucleus, but nuclear pores do not form (by BAPTA or importin β45–462 addition), and (iii) complete nuclei with mature NPCs present (as in standard nuclear reconstitution reactions or rescue reactions). FEISEM is also adept at distinguishing the three uniform stages above, but not that useful for revealing a potential tiny fusion pore. A serious problem is that EM becomes uninformative in any situation in vitro that produces a mixed population of closed and open nuclear membrane intermediates, such as the case for the 30-min cold intermediate in the present study, and for some of the partially enclosed ER tubule-intermediates of (Anderson and Hetzer, 2007). In these situations there is a mixture of (i) partially fused intermediates, which contain vesicles and tubules intermixed with small double-membrane sheets side by side on the chromatin surface, and (ii) a population of completely membrane-enclosed chromatin structures (Supplemental Figure 1C). If one were to look at the cross-sections of the chromatin surface with transmission EM to identify inner/outer membrane fusion, one could not distinguish a narrow channel formed in early inner/outer membrane fusion (if such a channel could be stabilized) from two closely adjacent vesicles or membrane sheets bound to the chromatin. Thus, it became necessary to develop biochemical/fluorescence assays to detect inner/outer membrane fusion.
An important event that must occur for nuclear pore assembly in intact nuclear membranes, either in vivo or in vitro, is the opening of a channel by fusion between the inner and outer nuclear membranes. This channel presumably would begin in its simplest form as an initial fusion pore, which becomes an expanded fusion pore, and eventually enlarges by nucleoporin assembly into the ∼900 Å wide passage (Hinshaw et al., 1992) that connects the nucleus to the cytoplasm. Nuclear pore complexes, which mediate active nucleocytoplasmic traffic of large nuclear proteins and RNAs, also contain a small effective diffusion channel that allows the nonspecific passive diffusion of smaller macromolecules (Feldherr and Akin, 1997; Keminer and Peters, 1999; Naim et al., 2007). Dextrans or proteins of 20 kDa (∼33A in diameter) (Peters, 1984; Keminer and Peters, 1999; Lenart et al., 2003) can freely diffuse in and out of the nucleus through mature nuclear pores using this diffusion channel.
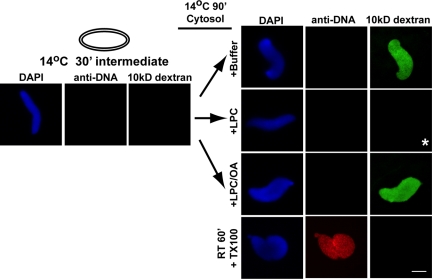
To characterize whether the POM121+/Nup133+ partial pore cold nuclear intermediate contains open channels capable of diffusion, we developed a modified diffusion assay designed to detect small channels. The necessity for this more sensitive assay was that in traditional dextran exclusion assays one looks for a dark hole in a bright background. While this works reasonably well for very large nuclei, it is not clearly distinguishable for smaller diameter spheres and cylinders. Development of an assay where a bright nuclear signal could be observed over a black external signal was deemed preferable. The new assay, characterized first at room temperature on control nuclei, is as follows: Nuclear assembly reactions were initiated at room temperature on chromatin templates and incubated for 30 min, then stopped on ice. Completely membrane-enclosed nuclei were formed by this point. Alexa488-labeled 10 kDa dextran was added for 15 min, and then the nuclei were fixed. Next, an antibody to Alexa488 was added to quench the fluorescence of any accessible Alexa488-labeled dextran. The rationale for this Alexa-488 dextran quenching assay is that when the dextran gains access to the nuclear interior, either through small diffusion channels or through fully formed nuclear pores, it will be inaccessible to the large anti-Alexa488 antibody. In contrast, Alexa488-labeled dextran outside of nuclei will be quenched by the anti-Alexa488 antibody. Indeed, in 30-min room temperature control nuclei, where nuclear pores are known to be present, Alexa488-10 kDa dextran was visualized inside the nuclei, but was quenched outside the nuclei (Supplemental Figure 4, RT, 30 min, upper panels; see also Figure 3B, RT, lower center panel). In contrast, in pore-free nuclei formed in the presence of BAPTA, which has been shown to prevent inner and outer nuclear membrane fusion and pore assembly (Macaulay and Forbes, 1996), no Alexa488-10 kDa dextran was observed in the nuclear interior (Supplemental Figure 4; BAPTA, lower panels).
Using this novel quenching assay to detect the presence of channels, we assessed the 14°C cold intermediates. At 15 min, the anti-DNA antibody gained access to the chromatin, indicating large gaps between the forming membrane sheets (Figure 3B, upper panel, red channel). Alexa488-labeled dextran, when added, was quenched both outside and inside this intermediate, as the anti-Alexa488 antibody had full access (Figure 3B, upper panel, green channel). However, when 30-min cold intermediates were examined, all membrane-enclosed nuclei, characterized by the exclusion of the Alexa568-labeled DNA antibody, also excluded the Alexa488-labeled 10-kDa dextran. A representative nucleus is shown in Figure 3B (14°C, 30 min, asterisk). The data are quantitated in Figure 3C (10 kDa). These data indicate that the 30-min cold intermediate lacks diffusion channels capable of 10 kDa dextran entry. To determine whether a smaller diffusion channel might be present, we repeated the above experiment using Alexa488-labeled 3-kDa dextran. This smaller dextran was also excluded from the 30-min cold intermediate (Figure 3C, 3-kDa dextran). Taken together, these findings demonstrate that the POM121+/Nup133+ partial pore cold intermediate lacks channels accessible to even 3-kDa dextrans [∼2 nm diameter (Peters, 1983; Lang et al., 1986)].
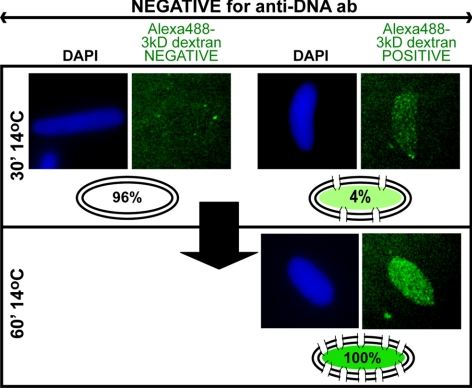
When such reactions were allowed to proceed to 60 min (Figure 4), we observed that 100% of the cold intermediates excluding anti-DNA antibody were now permeable to Alexa488-3 kDa and 10 kDa dextrans (Figure 4, 3 kDa; and data not shown, 10 kDa). Thus, between 30 min and 60 min at cold temperature, diffusion channels formed in the double nuclear membranes. This development of channels coincided with the recruitment of FG nucleoporins (Figure 1A). Stated differently, a POM121/Nup133 positive, channel-negative, FG Nup-negative intermediate was observed in the early stages of nuclear pore assembly (14°C, 30 min). This then matured into a channel-positive, FG Nup-positive mature nuclear pore by 60 min.
Figure 4.
Channel formation is a late event in NPC assembly. To ask whether the nuclear intermediates that did not show diffusion channels by 30 min at 14°C were competent to develop such channels, the diffusion assay in Figure 3B was performed for both 30 min and 60 min at 14°C. Only nuclei showing closed nuclear envelopes (CNE) according to the anti-DNA antibody staining assay were analyzed for 3-kDa dextran uptake and were quantitated. At 30 min at 14°C, only 4% of intact nuclear intermediates showed internal Alexa488-3 kDa dextran. By 60 min at 14°C, however, 100% of the intact nuclear intermediates showed internal protected Alexa488-3 kDa dextran. Scale bars, 10 μm.
The Membrane Fusion Inhibitor LPC Blocks Correct Diffusion Channel Formation
We believed from the results above that the 30-min cold intermediate had not undergone fusion between the inner and outer nuclear membranes. If so, then this intermediate should be sensitive to membrane fusion inhibitors, such as LPC. Inverted cone-shaped lipids, like LPC, inhibit the bending of the contacting leaflets of fusing biological membranes (Chernomordik and Kozlov, 2003), a bending which is required to transition to the well-known hemifusion intermediate.
As a control for the efficacy of LPC in the in vitro Xenopus system, we first tested for an effect on nuclear membrane assembly. LPC was added to a nuclear assembly reaction consisting of chromatin, cytosol, and membrane vesicles at t = 0 min at room temperature and assembly was allowed to proceed for 60 min. The addition of LPC did not prevent membrane vesicles from binding to the chromatin, but blocked their fusion into double nuclear membranes. A discontinuous membrane stain by the membrane dye DHCC was observed in the presence of LPC (Supplemental Figure 5, A and B; LPC). A smooth nuclear membrane stain was observed in control nuclei at 60 min (Supplemental Figure 5, A and B; Ctrl, RT, 60 min). The LPC block to vesicle–vesicle fusion was indistinguishable from a block caused by the chemical membrane fusion inhibitor GTPγS (Supplemental Figure 5A, GTPγS) (Boman et al., 1992). This LPC block to vesicle–vesicle fusion could be counteracted by adding a cone-shaped lipid such as oleic acid (OA) to offset the presence of the inverted cone-shaped lipid LPC in the membrane (Chernomordik and Kozlov, 2005). Specifically, when oleic acid was added with LPC at t = 0 min, formation of a continuous nuclear membrane was observed (Supplemental Figure 5, A and B, LPC/OA). We conclude that LPC and OA, when added simultaneously, neutralize one another and allow the vesicle–vesicle fusion required for nuclear membrane formation to occur, as expected from their combined effect on other known biological hemifusion reactions.
The critical question for the present study, however, was whether we could identify an LPC-sensitive step in nuclear pore formation itself. To ask this, we started with the completely membrane-enclosed 30-min cold intermediate described above, which contains POM121 and Nup133 associated with the inner nuclear membrane but lacks both FG nucleoporins and dextran-accessible diffusion channels.
Cold intermediates were formed for 30 min at 14°C, then buffer, LPC, or LPC+OA (≤0.06 volume) were added (Figure 5). The reactions were allowed to proceed for an additional 90 min at 14°C. At this time, the nuclear intermediates were assayed for the presence of 10-kDa–accessible channels using the Alexa488-10 kDa dextran quenching assay, concomitant with Alexa568-anti DNA antibody to assay nuclear integrity. In the buffer control, the intermediate proceeded to a channel-positive state after 90 min further incubation, as fluorescent dextran could be observed in the nuclei (Figure 5, top right panel, 10-kDa dextran). The presence of LPC, however, inhibited the formation of 10 kDa dextran-competent channels (Figure 5, +LPC, asterisk). The inclusion of OA with LPC counteracted the LPC inhibition, i.e., 10-kDa dextran-competent channels were formed and the nuclei appeared green (Figure 5, +LPC/OA). These results demonstrate that LPC prevents diffusion channel formation. The specific counteraction of the LPC block to diffusion channel formation by OA reinforces this conclusion.
Figure 5.
LPC blocks diffusion channel formation. Nuclei were assembled for 30 min at 14°C, and the preparation was verified for the absence of diffusion channels, as in Figure 3B, with Alexa488-10 kDa dextran (left panels). The reaction was then supplemented with buffer, LPC, or LPC plus OA, followed by incubation for 90 min at 14°C to assess the affect of LPC on channel formation. The nuclei were assayed for channel formation with Alexa488-10 kDa dextran. A block to channel formation was observed with LPC (asterisk). When LPC and OA were added together, however, diffusion of Alexa488-10 kDa dextran into the nuclei was now observed. Control nuclei assembled at room temperature for 60 min that were the treated with Triton X-100 before the addition of antibodies served as a positive control for the anti-DNA antibody assay and for the dextran quenching assay (bottom panels). Scale bar, 10 μm.
As described in the Introduction, in myriad cellular fusion studies the inverted-cone and cone shapes of LPC and OA, respectively, have been concluded to be the source of their effects on hemifusion and fusion and also the source of their counteraction when included together. We believe this to be the case with respect to the nuclear pore assembly experiments conducted here. A possible alternate theory, however, might be that cold and/or LPC act as a membrane “rigidifier” and OA as a “fluidizer.” If so, known membrane fluidizers such as benzyl alcohol might be predicted to reverse the cold or LPC phenotype. However, we did not find this to be the case; benzyl alcohol could not reverse the NPC assembly defects induced by cold or LPC (Supplemental Figures 6 and 7).
We next added a natural cone-shaped lipid, phosphatidylethanolamine (PE) that contains one saturated and one unsaturated fatty acid tail (Supplemental Figure 1B). When this form of PE (0.9 mM) was added we found that it could suppress LPC inhibition equally as well as OA did if assayed at room temperature (Supplemental Figure 8, A and B). When PE was added with LPC to a 30-min cold intermediate, PE promoted NPC assembly, albeit to a lesser extent than LPC + OA: 14% of the intermediates now had FG Nup-containing pores (Supplemental Figure 8D). Together, these results strongly argue that the shape of LPC and OA lipids are indeed the source of their action on nuclear pore assembly.
Wild-Type SNAP Does Not Inhibit Nuclear Pore Formation in POM121+/Nup133+ Cold Intermediates
When thinking about the process of nuclear pore assembly, one possible caveat that needed to be ruled out was that the late steps in pore assembly might require an additional recruitment of membrane vesicles and that LPC blocks pore assembly by inhibiting such late vesicle recruitment. To address this, we sought to specifically block membrane vesicle addition and then assess the effect on NPC assembly. It has been shown that the SNARE protein α-SNAP is involved in the vesicle–vesicle fusion that occurs during nuclear membrane assembly in Xenopus extracts. Indeed, excess wild-type α-SNAP inhibits vesicle–vesicle fusion, presumably by acting in a dominant negative manner by interfering with the function of the endogenous SNAREs [(Baur et al., 2007); Supplemental Figure 9]. A truncated fragment, Δ80 α-SNAP, which lacks 80 amino acids from its N terminus does not have this inhibitory effect on vesicle fusion [(Baur et al., 2007); Supplemental Figure 9].
To ask whether excess wild-type α-SNAP blocks nuclear pore assembly, we first made use of the pore-free membrane-enclosed nuclear intermediates that form in the presence of the BAPTA (Macaulay and Forbes, 1996). Such BAPTA nuclei can normally be rescued by dilution 1:10 into fresh cytosol (see Figure 6A, Ctrl panels). Strikingly, when we included excess wild-type α-SNAP together with the fresh cytosol, no inhibition of NPC assembly was observed, as measured by the clear presence of FG nucleoporins at the nuclear rim (Figure 6A, green).
Figure 6.
The cold intermediate contains all the membrane proteins required for NPC assembly. To determine whether the 14°C 30-min cold intermediate is able to go on to form nuclear pores without the addition of extra membranes, WTαSNAP was added as a vesicle–vesicle fusion inhibitor. Δ80NαSNAP served as a negative control. (A) We first looked at BAPTA-arrested pore-free nuclei which had been formed at room temperature for 30 min (left panels). Because neither SNAP protein is membrane permeable, we expected that WTαSNAP should inhibit addition of new vesicles to the forming nuclei, but could not inhibit inner-outer nuclear membrane fusion, thus permitting nuclear pore formation. Consistent with this, 30-min BAPTA-arrested pore-free nuclei which had been formed at room temperature, when diluted 1:10 into cytosol supplemented with buffer, WTαSNAP, or Δ80NαSNAP and incubated for a further 30 min, all became FG Nup–positive, as detected by Alexa488-anti-FG Nup antibody (green). (B) We performed the same type of experiment with nuclei reconstituted at 14°C for 30 min, at which time the reaction contained ∼50% closed, FG-negative nuclear intermediates. (Because of SNAP inhibition of vesicle–vesicle fusion, the open intermediates did not progress further.) The 14°C 30 min nuclei were diluted 1:10 into cytosol containing buffer, WTαSNAP (47 μM), or Δ80NαSNAP (47 μM) and incubated for a further 30 min at 14°C. Reactions were stopped on ice, fixed, and incubated on ice with Alexa488-anti-DNA (green) and Alexa568-anti-FG Nup (red) antibodies. All three reactions produced abundant anti-DNA–negative, FG-Nup–positive nuceli (red). Nuclear intermediates which had been assembled for 15 min at 14°C served as a negative control for the anti-FG Nup antibody (0%) and a positive control for the anti-DNA antibody (100%). A representative nucleus is shown in the bottom panels. The FG Nup–positive nuclei in B were quantified for each of three replicate experiments and the average percentage containing FG Nups is plotted in C. Errors bars indicate the SD.
We next asked whether α-SNAP or Δ80 α-SNAP would affect nuclear pore assembly when added to a 30-min 14°C reaction and incubated for another 30 min at 14°C. We focused on the closed POM121+/133+ nuclear intermediates. We found that neither excess α-SNAP nor Δ80 α-SNAP blocked nuclear pore assembly, i.e., both permitted robust FG nucleoporin recruitment at the nuclear rims of the closed nuclei (Figure 6, B and C). This result indicates that no further membrane vesicle recruitment is needed after assembly of closed 30-min cold intermediates for nuclear pores to form. Moreover, this experiment indicates that all the pore integral membrane proteins needed for active NPC assembly are already present in the 30 min cold intermediate.
The Fusion Inhibitors LPC and OA Individually Prevent FG Nup Assembly in the Cold Intermediate
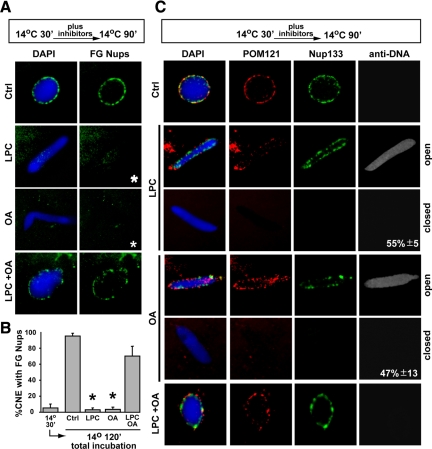
Having shown that LPC inhibited diffusion channel formation in the 30-min cold intermediate, even after 120-min incubation at 14°C (Figure 5), we asked whether this correlated with an inability to assemble FG nucleoporins at the nuclear rim. Indeed, we found that no FG signal was observed upon addition of either LPC or OA, even after 120 min of total incubation, indicating that detectable FG nucleoporins were not recruited (Figure 7, A and B; +LPC, asterisks). However, FG recruitment occurred if both OA and LPC were added together (Figure 7, A and B; +LPC/OA). We conclude that LPC or OA individually block the recruitment of detectable FG nucleoporins.
Figure 7.
The fusion inhibitors LPC and OA individually prevent FG Nup assembly in the 30 min cold intermediate. To ask whether membrane fusion inhibitors prevent formation of mature nuclear pores in the 30-min cold intermediate, intermediates were first formed at 14°C for 30 min and verified with Alexa647-anti-DNA and Alexa488-anti-FG Nup antibodies. Approximately 95% of the nuclei showed no FG Nup staining (B, left column). (A) At this point LPC, OA (final concentration 600 μM each), or a mix of both were added and the reactions were incubated at 14°C for an additional 90 min. The reactions were stopped on ice, fixed with 2.5% paraformaldehyde for 30 min on ice, followed by incubation for 30 min on ice with Alexa488-anti-FG Nup antibody (green). LPC or OA blocked FG recruitment (asterisks). (B) The data in A are quantitated, and error bars indicate SD. (C) In parallel, an aliquot of the nuclei in the fixed reactions from A were stained with a mixture of Alexa568-anti-POM121 (red), Alexa488-anti-Nup133 (green), and Alexa674-anti-DNA antibodies (gray). In A, LPC- or OA-treated nuclei at 120-min total incubation showed little FG Nup staining (A and B, asterisks), indicating no progression to an FG Nup–positive state. However, when LPC and OA were added together (A, B, and C), nuclear assembly occurred normally in that the majority of the 120 min closed nuclei showed POM121, Nup133, and FG Nup staining (LPC+OA).
We next asked whether the inhibitory lipids had an effect on the recruitment and localization of POM121 and the Nup107-160 complex, specifically looking at whether these nucleoporins appeared on the outer nuclear membrane as assembly proceeded in the presence of these fusion inhibitors. Assembly reactions were incubated for 30 min at 14°C, then supplemented with buffer, LPC, OA, or LPC+OA for a further 90 min at 14°C. These were then costained with Alexa568-anti-POM121 (red), Alexa488-anti-Nup133 (green), and Alexa647-anti-DNA (gray) antibodies. The staining revealed that POM121 and Nup133 were observed at the nuclear rim in control nuclei, even when the antibodies only had access to the outer nuclear membrane (i.e., negative for anti-DNA staining) (Figure 7C, top panels). A similar finding was obtained with LPC+OA (Figure 7C, bottom panels). In contrast, when LPC or OA were added alone to a 30-min 14°C reaction and incubated for 90 min at 14°C, POM121 and Nup133 were only detectable when the antibodies had access to the nuclear interior (open; positive for anti-DNA staining). The Nups were not detectable on closed nuclei (negative for anti-DNA staining) (middle panels, Figure 7C). This pattern of staining mirrored that shown by the original 14°C 30-min cold intermediate, i.e., POM121 and Nup133 on the inner nuclear membrane but not detectable on the outer nuclear membrane (see Figure 2A). We conclude that LPC or OA alone do not block the recruitment and localization of POM121 and Nup133 to the inner nuclear membrane; however, LPC or OA addition does block the recruitment of FG nucleoporins.
DISCUSSION
Mapping the Inner/Outer Membrane Fusion Step of Nuclear Pore Assembly
In this study, we used novel assays and the fusion inhibitors LPC and OA (i) to distinguish when fusion between the inner and outer nuclear membranes occurs in nuclear pore assembly in vertebrates and (ii) to begin to determine the molecular components that precede and follow the fusion step. We first identified a 14°C cold intermediate that contains POM121 and the Nup107-160 complex present on the inner but not the outer nuclear membrane (Figure 2). WT αSNAP experiments showed that this cold intermediate contains a complete set of the integral membrane components needed to eventually assemble mature nuclear pores. A dextran diffusion assay, together with the fusion inhibitors LPC and OA, allowed us to show that diffusion channel formation has not yet occurred in the 30 min cold intermediate. Subsequent fusion leading to channel formation appears to coincide with or precede recruitment of the FG Nups.
The early stages of in vitro nuclear reconstitution in Xenopus egg extracts resemble postmitotic nuclear envelope assembly in dividing cells. Our findings here reinforce previous studies which argue that NPC assembly occurs by fusion between the two nuclear membranes (Rasala et al., 2008; Hetzer and Wente, 2009; Rotem et al., 2009). Importantly, the sensitivity of channel formation to LPC that we see and its reversibility by OA or PE are consistent with a model where a hemifusion intermediate occurs between the outer and inner nuclear membranes during NPC assembly. These data argue against an alternate model which proposes that the bulk of the nuclear pore is assembled on the surface of chromatin and acquires membranes by being surrounded by expanding ER tubules and cisternae (reviewed in Anderson and Hetzer, 2007; Antonin et al., 2008).
The field is beginning to have an increasingly detailed view of the very early steps in postmitotic nuclear pore assembly. Specifically, the protein ELYS/MEL-28 acts by binding to chromatin via an AT-hook and at least one additional chromatin-binding domain. ELYS either recruits the Nup107-160 complex to chromatin or is recruited in a joint complex with it to initiate pore assembly (Rasala et al., 2006; Franz et al., 2007; Gillespie et al., 2007; Rasala et al., 2008; Lau et al., 2009; Rotem et al., 2009). In the absence of these proteins, the NPC integral membrane proteins POM121 and Ndc1 are not recruited to the nuclear membrane (Rasala et al., 2008). A recent study by (Doucet et al., 2010) used RNAi to partially knock down ELYS or POM121 in HeLa cells. They concluded that these two proteins are differentially rate-limiting in interphase and mitotic pore assembly. Their results are consistent with our biochemical demonstration that POM121 can bind and pull down the Nup107/160 complex from Xenopus egg extracts (Rasala et al., 2008) as well as with the findings here where POM121 and the Nup107/160 complex colocalize on the inner nuclear membrane in a very early step in post-mitotic nuclear pore assembly.
Inner/outer nuclear membrane fusion has previously proven to be a difficult step to detect or analyze. Assembly of nuclear pores in existing nuclear membranes, such as yeast nuclei or S-phase vertebrate nuclei in vivo, must by definition involve interaction between the lumenal leaflets of the inner and outer nuclear membranes. There hemifusion would precede full fusion and thus would be predicted to be sensitive to arrest by the membrane fusion-inhibiting lipids LPC and OA. In our in vitro nuclear assembly system, we could only add LPC or OA to the outer leaflet of the outer nuclear membrane of a cold intermediate, which is actually the leaflet facing away from the potential fusion event. However, multiple reports have shown that rapid interleaflet exchange of phospholipids occurs in ER-derived membranes via resident lipid flippases (Bishop and Bell, 1985; Herrmann et al., 1990; Buton et al., 1996; Marx et al., 2000; Papadopulos et al., 2007). Thus, when LPC or OA was added to our cold intermediate, it should quickly equilibrate between the leaflets of the outer nuclear membrane. The presence of LPC in only one of two fusing bilayers has been shown to be sufficient to block fusion (Chernomordik et al., 1997; Chernomordik et al., 1998). Indeed, we observed that LPC prevented 3-kDa channel formation and FG nucleoporin recruitment in the cold intermediate. OA treatment also inhibited FG nucleoporin recruitment in this intermediate, but when added together, the two lipids counteracted one another and diffusion channel formation and FG recruitment proceeded, fulfilling the prediction above. Thus, our data strongly argue that LPC and OA are acting to prevent inner/outer nuclear membrane fusion.
Is there a way in which cold, LPC, or OA could be blocking NPC assembly other than by blocking fusion? One speculative model might be that, normally, inner/outer nuclear membrane fusion occurs but results in formation of a tightly “closed” membranous tube that is so narrow as to be impermeable to 3-kDa dextrans. Cold, LPC, or OA could then all possibly act by inhibiting the postfusion dilation of such a tube. We think this unlikely, especially in light of their established role as fusion inhibitors in other studies. However, if true, this alternative model would still be consistent with our conclusion that correct channel formation is blocked by the inhibitor LPC and reversed by addition of its counteracting lipid OA.
Potential Fusogens and Candidates to Limit Expansion of the Fusion Pore in NPC Assembly
For inner/outer membrane fusion, a fusion machinery must of necessity bring the two membranes into close proximity. In structural terms, the 30–50 nm space (20–40 nm in yeast) must be bridged (Cohen et al., 2002; Crisp and Burke, 2008). Of the three human integral membrane nucleoporins, POM121, Ndc1, and gp210, the first two have a very small lumenal presence (33–39 aa and 21–30 aa) (Hallberg et al., 1993; Lau et al., 2006). Gp210 has a large lumenal domain (1782 aa) (Gerace et al., 1982), but has been found to be dispensable in some studies, both by RNAi depletion and in certain cells that naturally lack gp210 (Wozniak et al., 1989; Greber et al., 1990; Hallberg et al., 1993; Cotter et al., 1998; Drummond and Wilson, 2002; Liu et al., 2003; Eriksson et al., 2004; Olsson et al., 2004; Antonin et al., 2005; Lau et al., 2006; Mansfeld et al., 2006; Stavru et al., 2006a). Thus, neither Pom121, Ndc1, nor gp210 appears on its own to fill the requirements for bridging the lumenal gap needed for inner/outer membrane fusion.
The inner nuclear membrane SUN proteins and outer nuclear membrane KASH proteins together form long proteinaceous tethers across the intermembrane space and theoretically could be the bridge (Fridkin et al., 2004; Holaska and Wilson, 2006; Tzur et al., 2006; Stewart et al., 2007; Crisp and Burke, 2008; Burke and Roux, 2009). No involvement in nuclear membrane fusion events, however, has been observed for these proteins, but Sun1 at least has been found to be associated with the nuclear pore in mammalian cells (Liu et al., 2007).
The reticulon and Yop1/DP1 protein families, involved in ER membrane-bending, play a role in nuclear pore assembly in yeast (Voeltz et al., 2006; Hu et al., 2008; Shibata et al., 2008; Dawson et al., 2009). In addition, in the Xenopus nuclear assembly system, antibodies to Reticulon-4a inhibit new pore assembly (Dawson et al., 2009). Other proteins of the yeast ER and/or nuclear membrane have also been observed to affect nuclear pores at some level, including Apq12, Brr6, Snl1, and Acc1 (Hodge et al.,; Schneiter et al., 1996; de Bruyn Kops and Guthrie, 2001; Sondermann et al., 2002; Scarcelli et al., 2007). Interestingly, Apq12 null yeast are cold sensitive for growth. They and brr6-1 mutant cells appear to have electron dense NPC-like structures that are associated only with the inner nuclear membrane and not with the outer membrane (Hodge et al., 2010; Scarcelli et al., 2007). However, it is not yet known whether any of the above proteins have a connection to inner/outer membrane fusion. In summary, the fusion protein(s) for nuclear pore assembly await discovery.
The next question, beyond the issue of what causes fusion, is how the fusion pore becomes limited in its expansion to ∼900 Å, the width of the actual membrane passage that connects the nuclear interior to the cytoplasm. The Nup107-160 complex has a Y-shape (∼230 Å × ∼420 Å) and has been hypothesized to form a multimeric lattice that could line and stabilize this passageway (Siniossoglou et al., 2000; Lutzmann et al., 2002; Beck et al., 2004; Devos et al., 2004; Debler et al., 2008; Brohawn and Schwartz, 2009). In strong support of such a lattice model, crystallographic analysis of the Nup96/sec13 and Nup85/seh1 proteins, components of the Nup107-160 complex, are closely related in 3D structures to the sec31/sec13 complex that forms the lattice-like coat of COP II transport vesicles (Brohawn and Schwartz, 2009; DeGrasse et al., 2009). We would suggest that a ring of Nup107-160 complexes that is multimerized on the surface of the inner nuclear membrane and attached to the membrane by POM121 could act as a preset ring that limits fusion pore expansion. This Nup107-160 complex could also require other proteins for stabilization of the membrane channel, as in yeast the depletion of a subset of membrane nucleoporins (Ndc1, Pom152, and Pom34), together with Nup59 or Nup53 depletion, results in nuclear pores that are noticeably dilated (Onischenko et al., 2009).
Large membrane-lined channels connecting two entities are rare in evolution. Among the few known examples are the plasmodesmata channels that connect certain plant cells to one another, and the ring channels that connect Drosophila nurse cells to the oocyte that they supply (Huebner and Gutzeit, 1986; Cilia and Jackson, 2004; Mische et al., 2007). These two examples, however, are thought to result from incomplete cytokinesis, rather than by fusion pore expansion and stabilization as in the case of the NPC. The stabilized inner/outer membrane fusion product that is the nuclear pore complex may thus be a unique occurence in evolution.
A Model for the Early Fusion Step in Nuclear Pore Assembly
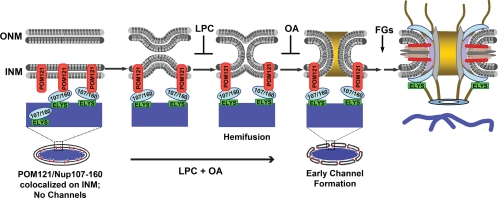
In conclusion, this study maps diffusion channel formation downstream from POM121 and Nup107-160 complex recruitment, and upstream or coincident with FG nucleoporin recruitment. These results thus order the important fusion step in NPC assembly with respect to other key steps (Figure 8): (i) AT-rich chromatin recruitment of ELYS and the Nup107-160 complex, (ii) colocalization of the Nup107-160 complex with POM121/Ndc1 at the inner nuclear membrane, followed by (iii) inner/outer membrane fusion to form a diffusion channel, and FG nucleoporin recruitment. The frontier of nuclear pore assembly now requires identification of the fusogen and determination of the order of addition of the myriad subunits that go on to build the pore complex within this evolutionarily unique membranous channel.
Figure 8.
A schematic model for inner/outer nuclear membrane fusion in the early steps of vertebrate NPC assembly. In previous studies, ELYS was shown to mediate the binding of the Nup107-160 complex to sites of AT-rich chromatin (blue) in early nuclear assembly. Simultaneous with this ER-derived membranes are recruited to the rim of developing nuclei. The data presented here argues that next: (1) the Nup107-160 complex (detected by anti-Nup133 antibody) interacts with POM121 at the inner nuclear membrane in an early nuclear intermediate (30 min, 14°C). Bending of the membranes toward one another would theoretically occur at or near this time. (2) This is followed by fusion of the inner and outer nuclear membranes to form a diffusion channel. (3) This fusion is simultaneous with or precedes recruitment of the bulk of FG nucleoporins (gold) and assembly of the mature nuclear pore, which involves expansion of the initial channel and its stabilization. In pioneering membrane-membrane fusion studies, progression to hemifusion was seen to be typically inhibited by cone-shaped lipids, such as LPC; here LPC was found to indeed block channel formation and FG Nup assembly in nuclear intermediates. Progression beyond hemifusion to full fusion has been observed to be inhibited by inverted cone-shaped lipids, such as OA; here we found that OA also blocks mature nuclear pore assembly. When added together LPC and OA are known to geometrically neutralize one another, and we observed that LPC+OA allow channel formation, i.e., inner/outer membrane fusion. (It is important to note that we do not believe that a completely sealed nuclear envelope is required before nuclear pores can form, but possession of such a sealed intermediate allowed us to distinguish and analyze the channel formation step of nuclear pore assembly.)
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Hemmo Meyer (Swiss Federal Institute of Technology, Zurich, Switzerland) for the kind gift of α-SNAP and Δ80 α-SNAP, Dr. Michael Elbaum (Weizmann Institute, Israel) and members of the Forbes lab for helpful discussions, and Michelle Gaylord for careful reading of the manuscript. This work was supported by an National Institutes of Health RO1 grant (GM-33279) (to D.F.) and in part by a grant from the United States – Israel Binational Science Foundation (to M.E. and D.F.).
Abbreviations used:
- BAPTA
1,2-Bis-(o-Aminophenoxy)-ethane-N, N, N′, N′-tetra acetic acid
- FG nucleoporins
phenylalanine-glycine repeat nucleoporins
- LPC
1-lauroyl-2-hydroxy-sn-glycero-3-phosphocholine 12:0
- OA
oleic acid.
Note added in proof.
After the acceptance of this manuscript, a report by Dultz and Ellenberg (2010) was published, which reported that the order of addition of POM121 and the Nup 107–160 complex is different for interphase NPC assembly than for post-mitotic NPC assembly. Their post-mitotic order of Nup 107–160 complex, then POM121, agrees with the post-mitotic results we report here.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-04-0309) on October 6, 2010.
REFERENCES
- Anderson D. J., Hetzer M. W. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell Biol. 2007;9:1160–1166. doi: 10.1038/ncb1636. [DOI] [PubMed] [Google Scholar]
- Antonin W., Ellenberg J., Dultz E. Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett. 2008;582:2004–2016. doi: 10.1016/j.febslet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- Antonin W., Franz C., Haselmann U., Antony C., Mattaj I. W. The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol. Cell. 2005;17:83–92. doi: 10.1016/j.molcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Baur T., Ramadan K., Schlundt A., Kartenbeck J., Meyer H. H. NSF- and SNARE-mediated membrane fusion is required for nuclear envelope formation and completion of nuclear pore complex assembly in Xenopus laevis egg extracts. J. Cell Sci. 2007;120:2895–2903. doi: 10.1242/jcs.010181. [DOI] [PubMed] [Google Scholar]
- Beck M., Forster F., Ecke M., Plitzko J. M., Melchior F., Gerisch G., Baumeister W., Medalia O. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science. 2004;306:1387–1390. doi: 10.1126/science.1104808. [DOI] [PubMed] [Google Scholar]
- Belgareh N., Rabut G., Bai S. W., van Overbeek M., Beaudouin J., Daigle N., Zatsepina O. V., Pasteau F., Labas V., Fromont-Racine M., Ellenberg J., Doye V. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J. Cell Biol. 2001;154:1147–1160. doi: 10.1083/jcb.200101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of the endoplasmic reticulum phospholipid bilayer: the phosphatidylcholine transporter. Cell. 1985;42:51–60. doi: 10.1016/s0092-8674(85)80100-8. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Wolfsberg T. G., Turck C. W., Myles D. G., Primakoff P., White J. M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992;356:248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Blower M. D., Nachury M., Heald R., Weis K. A Rae1-containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell. 2005;121:223–234. doi: 10.1016/j.cell.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Boehmer T., Schwartz T. U. Purification, crystallization and preliminary X-ray analysis of a Nup107-Nup133 heterodimeric nucleoporin complex. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2007;63:816–818. doi: 10.1107/S1744309107040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman A. L., Delannoy M. R., Wilson K. L. GTP hydrolysis is required for vesicle fusion during nuclear envelope assembly in vitro. J. Cell Biol. 1992;116:281–294. doi: 10.1083/jcb.116.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn S. G., Schwartz T. U. Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice. Nat. Struct. Mol. Biol. 2009;16:1173–1177. doi: 10.1038/nsmb.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Roux K. J. Nuclei take a position: managing nuclear location. Dev. Cell. 2009;17:587–597. doi: 10.1016/j.devcel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Buton X., Morrot G., Fellmann P., Seigneuret M. Ultrafast glycerophospholipid-selective transbilayer motion mediated by a protein in the endoplasmic reticulum membrane. J. Biol. Chem. 1996;271:6651–6657. doi: 10.1074/jbc.271.12.6651. [DOI] [PubMed] [Google Scholar]
- Chakraborty P., Wang Y., Wei J. H., van Deursen J., Yu H., Malureanu L., Dasso M., Forbes D. J., Levy D. E., Seemann J., Fontoura B. M. Nucleoporin levels regulate cell cycle progression and phase-specific gene expression. Dev. Cell. 2008;15:657–667. doi: 10.1016/j.devcel.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Frolov V. A., Leikina E., Bronk P., Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Kozlov M. M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Kozlov M. M. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123:375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Leikina E., Frolov V., Bronk P., Zimmerberg J. An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J. Cell Biol. 1997;136:81–93. doi: 10.1083/jcb.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Melikyan G. B., Chizmadzhev Y. A. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim. Biophys. Acta. 1987;906:309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Vogel S. S., Sokoloff A., Onaran H. O., Leikina E. A., Zimmerberg J. Lysolipids reversibly inhibit Ca(2+)-, GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 1993;318:71–76. doi: 10.1016/0014-5793(93)81330-3. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Zimmerberg J., Kozlov M. M. Membranes of the world unite! J. Cell Biol. 2006;175:201–207. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia M. L., Jackson D. Plasmodesmata form and function. Curr. Opin. Cell Biol. 2004;16:500–506. doi: 10.1016/j.ceb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cohen M., Tzur Y. B., Neufeld E., Feinstein N., Delannoy M. R., Wilson K. L., Gruenbaum Y. Transmission electron microscope studies of the nuclear envelope in Caenorhabditis elegans embryos. J. Struct. Biol. 2002;140:232–240. doi: 10.1016/s1047-8477(02)00516-6. [DOI] [PubMed] [Google Scholar]
- Cotter L. A., Goldberg M. W., Allen T. D. Nuclear pore complex disassembly and nuclear envelope breakdown during mitosis may occur by both nuclear envelope vesicularisation and dispersion throughout the endoplasmic reticulum. Scanning. 1998;20:250–251. [PubMed] [Google Scholar]
- Crisp M., Burke B. The nuclear envelope as an integrator of nuclear and cytoplasmic architecture. FEBS Lett. 2008;582:2023–2032. doi: 10.1016/j.febslet.2008.05.001. [DOI] [PubMed] [Google Scholar]
- D'Angelo M. A., Anderson D. J., Richard E., Hetzer M. W. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 2006;312:440–443. doi: 10.1126/science.1124196. [DOI] [PubMed] [Google Scholar]
- D'Angelo M. A., Hetzer M. W. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davuluri G., Gong W., Yusuff S., Lorent K., Muthumani M., Dolan A.C., Pack M. Mutation of the zebrafish nucleoporin elys sensitizes tissue progenitors to replication stress. PLoS Genet. 2008;4:e1000240. doi: 10.1371/journal.pgen.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. R., Lazarus M. D., Hetzer M. W., Wente S. R. ER membrane-bending proteins are necessary for de novo nuclear pore formation. J. Cell Biol. 2009;184:659–675. doi: 10.1083/jcb.200806174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn Kops A., Guthrie C. An essential nuclear envelope integral membrane protein, Brr6p, required for nuclear transport. EMBO J. 2001;20:4183–4193. doi: 10.1093/emboj/20.15.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong-Curtain T. A., Parslow A. C., Trotter A. J., Hall N. E., Verkade H., Tabone T., Christie E. L., Crowhurst M. O., Layton J. E., Shepherd I. T., Nixon S. J., Parton R. G., Zon L. I., Stainier D. Y., Lieschke G. J., Heath J. K. Abnormal nuclear pore formation triggers apoptosis in the intestinal epithelium of elys-deficient zebrafish. Gastroenterology. 2009;136:902–911. doi: 10.1053/j.gastro.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debler E. W., Ma Y., Seo H. S., Hsia K. C., Noriega T. R., Blobel G., Hoelz A. A fence-like coat for the nuclear pore membrane. Mol. Cell. 2008;32:815–826. doi: 10.1016/j.molcel.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Dechat T., Pfleghaar K., Sengupta K., Shimi T., Shumaker D. K., Solimando L., Goldman R. D. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrasse J. A., DuBois K. N., Devos D., Siegel T. N., Sali A., Field M. C., Rout M. P., Chait B. T. Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor. Mol. Cell Proteomics. 2009;8:2119–2130. doi: 10.1074/mcp.M900038-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D., Dokudovskaya S., Alber F., Williams R., Chait B. T., Sali A., Rout M. P. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2004;2:e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet C. M., Talamas J. A., Hetzer M. W. Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell. 141:1030–1041. doi: 10.1016/j.cell.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond S. P., Wilson K. L. Interference with the cytoplasmic tail of gp210 disrupts “close apposition” of nuclear membranes and blocks nuclear pore dilation. J. Cell Biol. 2002;158:53–62. doi: 10.1083/jcb.200108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dultz E., Zanin E., Wurzenberger C., Braun M., Rabut G., Sironi L., Ellenberg J. Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J. Cell Biol. 2008;180:857–865. doi: 10.1083/jcb.200707026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dultz E., Ellenberg J. Live imaging of single nuclear pores reveals unique assembly kinetics and mechanism in interphase. J. Cell Biol. 2010;191:15–22. doi: 10.1083/jcb.201007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp L. J., Delos S. E., Park H. E., White J. M. The many mechanisms of viral membrane fusion proteins. Curr. Top Microbiol. Immunol. 2005;285:25–66. doi: 10.1007/3-540-26764-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson C., Rustum C., Hallberg E. Dynamic properties of nuclear pore complex proteins in gp210 deficient cells. FEBS Lett. 2004;572:261–265. doi: 10.1016/j.febslet.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Akin D. The location of the transport gate in the nuclear pore complex. J. Cell Sci. 1997;110:3065–3070. doi: 10.1242/jcs.110.24.3065. [DOI] [PubMed] [Google Scholar]
- Fernandez A. G., Piano F. MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr. Biol. 2006;16:1757–1763. doi: 10.1016/j.cub.2006.07.071. [DOI] [PubMed] [Google Scholar]
- Fort D. J., Rogers R. L., Thomas J. H., Buzzard B. O., Noll A. M., Spaulding C. D. Comparative sensitivity of Xenopus tropicalis and Xenopus laevis as test species for the FETAX model. J Appl Toxicol. 2004;24:443–457. doi: 10.1002/jat.997. [DOI] [PubMed] [Google Scholar]
- Franz C., Walczak R., Yavuz S., Santarella R., Gentzel M., Askjaer P., Galy V., Hetzer M., Mattaj I. W., Antonin W. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 2007;8:165–172. doi: 10.1038/sj.embor.7400889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkin A., Mills E., Margalit A., Neufeld E., Lee K. K., Feinstein N., Cohen M., Wilson K. L., Gruenbaum Y. Matefin, a Caenorhabditis elegans germ line-specific SUN-domain nuclear membrane protein, is essential for early embryonic and germ cell development. Proc. Natl. Acad. Sci. USA. 2004;101:6987–6992. doi: 10.1073/pnas.0307880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T., Maeshima K., Yahata K., Sugano S., Imamoto F., Imamoto N. Two distinct human POM121 genes: requirement for the formation of nuclear pore complexes. FEBS Lett. 2007;581:4910–4916. doi: 10.1016/j.febslet.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Galy V., Askjaer P., Franz C., Lopez-Iglesias C., Mattaj I. W. MEL-28, a novel nuclear-envelope and kinetochore protein essential for zygotic nuclear-envelope assembly in C. elegans. Curr. Biol. 2006;16:1748–1756. doi: 10.1016/j.cub.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Gattegno T., Mittal A., Valansi C., Nguyen K. C., Hall D. H., Chernomordik L. V., Podbilewicz B. Genetic control of fusion pore expansion in the epidermis of Caenorhabditis elegans. Mol. Biol. Cell. 2007;18:1153–1166. doi: 10.1091/mbc.E06-09-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Ottaviano Y., Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J. Cell Biol. 1982;95:826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. J., Khoudoli G. A., Stewart G., Swedlow J. R., Blow J. J. ELYS/MEL-28 chromatin association coordinates nuclear pore complex assembly and replication licensing. Curr. Biol. 2007;17:1657–1662. doi: 10.1016/j.cub.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. W., Wiese C., Allen T. D., Wilson K. L. Dimples, pores, star-rings, and thin rings on growing nuclear envelopes: evidence for structural intermediates in nuclear pore complex assembly. J. Cell Sci. 1997;110(Pt 4):409–420. doi: 10.1242/jcs.110.4.409. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Snay C. A., Heath C. V., Cole C. N. Pleiotropic nuclear defects associated with a conditional allele of the novel nucleoporin Rat9p/Nup85p. Mol. Biol. Cell. 1996;7:917–934. doi: 10.1091/mbc.7.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U. F., Senior A., Gerace L. A major glycoprotein of the nuclear pore complex is a membrane-spanning polypeptide with a large lumenal domain and a small cytoplasmic tail. EMBO J. 1990;9:1495–1502. doi: 10.1002/j.1460-2075.1990.tb08267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis E. R., Xu S., Powers M. A. Nup98 localizes to both nuclear and cytoplasmic sides of the nuclear pore and binds to two distinct nucleoporin subcomplexes. Mol. Biol. Cell. 2003;14:600–610. doi: 10.1091/mbc.E02-09-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Margalit A., Goldman R. D., Shumaker D. K., Wilson K. L. The nuclear lamina comes of age. Nat. Rev. Mol. Cell Biol. 2005;6:21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- Guttinger S., Laurell E., Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat. Rev. Mol. Cell Biol. 2009;10:178–191. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- Hallberg E., Wozniak R. W., Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J. Cell Biol. 1993;122:513–521. doi: 10.1083/jcb.122.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A., Chan R. C., Lachish-Zalait A., Zimmerman E., Elbaum M., Forbes D. J. Importin beta negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol. Biol. Cell. 2003a;14:4387–4396. doi: 10.1091/mbc.E03-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A., Orjalo A. V., Vincent T., Lachish-Zalait A., Vasu S., Shah S., Zimmerman E., Elbaum M., Forbes D. J. Removal of a single pore subcomplex results in vertebrate nuclei devoid of nuclear pores. Mol. Cell. 2003b;11:853–864. doi: 10.1016/s1097-2765(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Henderson H. I., Hope T. J. The temperature arrested intermediate of virus-cell fusion is a functional step in HIV infection. Virol. J. 2006;3:36. doi: 10.1186/1743-422X-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann A., Zachowski A., Devaux P. F. Protein-mediated phospholipid translocation in the endoplasmic reticulum with a low lipid specificity. Biochemistry. 1990;29:2023–2027. doi: 10.1021/bi00460a010. [DOI] [PubMed] [Google Scholar]
- Hetzer M., Bilbao-Cortes D., Walther T. C., Gruss O. J., Mattaj I. W. GTP hydrolysis by Ran is required for nuclear envelope assembly. Mol. Cell. 2000;5:1013–1024. doi: 10.1016/s1097-2765(00)80266-x. [DOI] [PubMed] [Google Scholar]
- Hetzer M. W., Wente S. R. Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes. Dev. Cell. 2009;17:606–616. doi: 10.1016/j.devcel.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa M. M., Ullman K. S., Prunuske A. J. Studying nuclear disassembly in vitro using Xenopus egg extract. Methods. 2006;39:284–290. doi: 10.1016/j.ymeth.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hinshaw J. E., Carragher B. O., Milligan R. A. Architecture and design of the nuclear pore complex. Cell. 1992;69:1133–1141. doi: 10.1016/0092-8674(92)90635-p. [DOI] [PubMed] [Google Scholar]
- Hodge C. A., Choudhary V., Wolyniak M. J., Scarcelli J. J., Schneiter R., Col C. N. Integral membrane proteins Brr6 and Apq12 link assembly of the nuclear pore complex to lipid homeostasis in the endoplasmic reticulum. J. Cell Sci. 2010;123:141–151. doi: 10.1242/jcs.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska J. M., Wilson K. L. Multiple roles for emerin: implications for Emery-Dreifuss muscular dystrophy. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2006;288:676–680. doi: 10.1002/ar.a.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Shibata Y., Voss C., Shemesh T., Li Z., Coughlin M., Kozlov M. M., Rapoport T. A., Prinz W. A. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science. 2008;319:1247–1250. doi: 10.1126/science.1153634. [DOI] [PubMed] [Google Scholar]
- Huebner E., Gutzeit H. Nurse cell-oocyte interaction: a new F-actin mesh associated with the microtubule-rich core of an insect ovariole. Tissue Cell. 1986;18:753–764. doi: 10.1016/0040-8166(86)90075-3. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Chapman E. R. Fusion pores and fusion machines in Ca2+-triggered exocytosis. Annu. Rev. Biophys. Biomol. Struct. 2006;35:135–160. doi: 10.1146/annurev.biophys.35.040405.101958. [DOI] [PubMed] [Google Scholar]
- Jahn R., Lang T., Sudhof T. C. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Kalab P., Heald R. The RanGTP gradient - a GPS for the mitotic spindle. J. Cell Sci. 2008;121:1577–1586. doi: 10.1242/jcs.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab P., Weis K., Heald R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science. 2002;295:2452–2456. doi: 10.1126/science.1068798. [DOI] [PubMed] [Google Scholar]
- Katsani K. R., Karess R. E., Dostatni N., Doye V. In vivo dynamics of Drosophila nuclear envelope components. Mol. Biol. Cell. 2008;19:3652–3666. doi: 10.1091/mbc.E07-11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keminer O., Peters R. Permeability of single nuclear pores. Biophys. J. 1999;77:217–228. doi: 10.1016/S0006-3495(99)76883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Rey F. A. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M. M., Markin V. S. [Possible mechanism of membrane fusion] Biofizika. 1983;28:242–247. [PubMed] [Google Scholar]
- Kutay U., Hetzer M. W. Reorganization of the nuclear envelope during open mitosis. Curr. Opin. Cell Biol. 2008;20:669–677. doi: 10.1016/j.ceb.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang I., Scholz M., Peters R. Molecular mobility and nucleocytoplasmic flux in hepatoma cells. J. Cell Biol. 1986;102:1183–1190. doi: 10.1083/jcb.102.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. K., Delmar V. A., Chan R. C., Phung Q., Bernis C., Fichtman B., Rasala B. A., Forbes D. J. Transportin regulates major mitotic assembly events: from spindle to nuclear pore assembly. Mol. Biol. Cell. 2009;20:4043–4058. doi: 10.1091/mbc.E09-02-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. K., Delmar V. A., Forbes D. J. Topology of yeast Ndc1p: predictions for the human NDC1/NET3 homologue. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2006;288:681–694. doi: 10.1002/ar.a.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart P., Rabut G., Daigle N., Hand A. R., Terasaki M., Ellenberg J. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J. Cell Biol. 2003;160:1055–1068. doi: 10.1083/jcb.200211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li O., Heath C. V., Amberg D. C., Dockendorff T. C., Copeland C. S., Snyder M., Cole C. N. Mutation or deletion of the Saccharomyces cerevisiae RAT3/NUP133 gene causes temperature-dependent nuclear accumulation of poly(A)+ RNA and constitutive clustering of nuclear pore complexes. Mol. Biol. Cell. 1995;6:401–417. doi: 10.1091/mbc.6.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Prunuske A. J., Fager A. M., Ullman K. S. The COPI complex functions in nuclear envelope breakdown and is recruited by the nucleoporin Nup153. Dev. Cell. 2003;5:487–498. doi: 10.1016/s1534-5807(03)00262-4. [DOI] [PubMed] [Google Scholar]
- Liu Q., Pante N., Misteli T., Elsagga M., Crisp M., Hodzic D., Burke B., Roux K. J. Functional association of Sun1 with nuclear pore complexes. J. Cell Biol. 2007;178:785–798. doi: 10.1083/jcb.200704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983;220:719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhang F., McNew J. A., Shin Y. K. Membrane fusion induced by neuronal SNAREs transits through hemifusion. J. Biol. Chem. 2005;280:30538–30541. doi: 10.1074/jbc.M506862200. [DOI] [PubMed] [Google Scholar]
- Lutzmann M., Kunze R., Buerer A., Aebi U., Hurt E. Modular self-assembly of a Y-shaped multiprotein complex from seven nucleoporins. EMBO J. 2002;21:387–397. doi: 10.1093/emboj/21.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay C., Forbes D. J. Assembly of the nuclear pore: biochemically distinct steps revealed with NEM, GTP gamma S, and BAPTA. J. Cell Biol. 1996;132:5–20. doi: 10.1083/jcb.132.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid A. S., Mancuso J., Cande W. Z., Weis K. The role of the integral membrane nucleoporins Ndc1p and Pom152p in nuclear pore complex assembly and function. J. Cell Biol. 2006;173:361–371. doi: 10.1083/jcb.200506199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfeld J., Guttinger S., Hawryluk-Gara L. A., Pante N., Mall M., Galy V., Haselmann U., Muhlhausser P., Wozniak R. W., Mattaj I. W., Kutay U., Antonin W. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol. Cell. 2006;22:93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Markosyan R. M., Cohen F. S., Melikyan G. B. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell. 2003;14:926–938. doi: 10.1091/mbc.E02-09-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx U., Lassmann G., Holzhutter H. G., Wustner D., Muller P., Hohlig A., Kubelt J., Herrmann A. Rapid flip-flop of phospholipids in endoplasmic reticulum membranes studied by a stopped-flow approach. Biophys. J. 2000;78:2628–2640. doi: 10.1016/S0006-3495(00)76807-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. Membrane fusion in eukaryotic cells. Annu. Rev. Cell Dev. Biol. 2002;18:289–314. doi: 10.1146/annurev.cellbio.18.032202.114809. [DOI] [PubMed] [Google Scholar]
- Melia T. J., You D., Tareste D. C., Rothman J. E. Lipidic antagonists to SNARE-mediated fusion. J. Biol. Chem. 2006;281:29597–29605. doi: 10.1074/jbc.M601778200. [DOI] [PubMed] [Google Scholar]
- Mische S., Li M., Serr M., Hays T. S. Direct observation of regulated ribonucleoprotein transport across the nurse cell/oocyte boundary. Mol. Biol. Cell. 2007;18:2254–2263. doi: 10.1091/mbc.E06-10-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R. K., Chakraborty P., Arnaoutov A., Fontoura B. M., Dasso M. The Nup107-160 complex and gamma-TuRC regulate microtubule polymerization at kinetochores. Nat. Cell Biol. 2010;12:164–169. doi: 10.1038/ncb2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler W. A., Shemer G., del Campo J. J., Valansi C., Opoku-Serebuoh E., Scranton V., Assaf N., White J. G., Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev. Cell. 2002;2:355–362. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Naim B., Brumfeld V., Kapon R., Kiss V., Nevo R., Reich Z. Passive and facilitated transport in nuclear pore complexes is largely uncoupled. J. Biol. Chem. 2007;282:3881–3888. doi: 10.1074/jbc.M608329200. [DOI] [PubMed] [Google Scholar]
- Newport J. Nuclear reconstitution in vitro: stages of assembly around protein-free DNA. Cell. 1987;48:205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- Newport J., Dunphy W. Characterization of the membrane binding and fusion events during nuclear envelope assembly using purified components. J. Cell Biol. 1992;116:295–306. doi: 10.1083/jcb.116.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. W., Forbes D. J. The nucleus: structure, function, and dynamics. Annu. Rev. Biochem. 1987;56:535–565. doi: 10.1146/annurev.bi.56.070187.002535. [DOI] [PubMed] [Google Scholar]
- Olsson M., Scheele S., Ekblom P. Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp. Cell Res. 2004;292:359–370. doi: 10.1016/j.yexcr.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Onischenko E., Stanton L. H., Madrid A. S., Kieselbach T., Weis K. Role of the Ndc1 interaction network in yeast nuclear pore complex assembly and maintenance. J. Cell Biol. 2009;185:475–491. doi: 10.1083/jcb.200810030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjalo A. V., Arnaoutov A., Shen Z., Boyarchuk Y., Zeitlin S. G., Fontoura B., Briggs S., Dasso M., Forbes D. J. The Nup107-160 nucleoporin complex is required for correct bipolar spindle assembly. Mol. Biol. Cell. 2006;17:3806–3818. doi: 10.1091/mbc.E05-11-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopulos A., Vehring S., Lopez-Montero I., Kutschenko L., Stockl M., Devaux P. F., Kozlov M., Pomorski T., Herrmann A. Flippase activity detected with unlabeled lipids by shape changes of giant unilamellar vesicles. J. Biol. Chem. 2007;282:15559–15568. doi: 10.1074/jbc.M604740200. [DOI] [PubMed] [Google Scholar]
- Peters R. Nuclear envelope permeability measured by fluorescence microphotolysis of single liver cell nuclei. J. Biol. Chem. 1983;258:11427–11429. [PubMed] [Google Scholar]
- Peters R. Nucleo-cytoplasmic flux and intracellular mobility in single hepatocytes measured by fluorescence microphotolysis. EMBO J. 1984;3:1831–1836. doi: 10.1002/j.1460-2075.1984.tb02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbilewicz B., Leikina E., Sapir A., Valansi C., Suissa M., Shemer G., Chernomordik L. V. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev. Cell. 2006;11:471–481. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Rafikova E. R., Melikov K., Ramos C., Dye L., Chernomordik L. V. Transmembrane protein-free membranes fuse into xenopus nuclear envelope and promote assembly of functional pores. J. Biol. Chem. 2009;284:29847–29859. doi: 10.1074/jbc.M109.044453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasala B. A., Orjalo A. V., Shen Z., Briggs S., Forbes D. J. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc. Natl. Acad. Sci. USA. 2006;103:17801–17806. doi: 10.1073/pnas.0608484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasala B. A., Ramos C., Harel A., Forbes D. J. Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol. Biol. Cell. 2008;19:3982–3996. doi: 10.1091/mbc.E08-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner G. A., Hodel A. E., Powers M. A. Molecular determinants of binding between Gly-Leu-Phe-Gly nucleoporins and the nuclear pore complex. J. Biol. Chem. 2007;282:33968–33976. doi: 10.1074/jbc.M707911200. [DOI] [PubMed] [Google Scholar]
- Reese C., Heise F., Mayer A. Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature. 2005;436:410–414. doi: 10.1038/nature03722. [DOI] [PubMed] [Google Scholar]
- Rotem A., Gruber R., Shorer H., Shaulov L., Klein E., Harel A. Importin {:beta}: regulates the seeding of chromatin with initiation sites for nuclear pore assembly. Mol. Biol. Cell. 2009;20:4031–4042. doi: 10.1091/mbc.E09-02-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A., Choi J., Leikina E., Avinoam O., Valansi C., Chernomordik L. V., Newman A. P., Podbilewicz B. AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in. C. elegans. Dev. Cell. 2007;12:683–698. doi: 10.1016/j.devcel.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Temperature-sensitive steps in the transport of secretory proteins through the Golgi complex in exocrine pancreatic cells. Proc. Natl. Acad. Sci. USA. 1986;83:6425–6429. doi: 10.1073/pnas.83.17.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarcelli J. J., Hodge C. A., Cole C. N. The yeast integral membrane protein Apq12 potentially links membrane dynamics to assembly of nuclear pore complexes. J. Cell Biol. 2007;178:799–812. doi: 10.1083/jcb.200702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiter R., Hitomi M., Ivessa A. S., Fasch E. V., Kohlwein S. D., Tartakoff A. M. A yeast acetyl coenzyme A carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol. Cell. Biol. 1996;16:7161–7172. doi: 10.1128/mcb.16.12.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C., Blumenthal R., Clague M. J. A long-lived state for influenza virus-erythrocyte complexes committed to fusion at neutral pH. FEBS Lett. 1992;311:221–225. doi: 10.1016/0014-5793(92)81107-w. [DOI] [PubMed] [Google Scholar]
- Schwander M., Leu M., Stumm M., Dorchies O. M., Ruegg U. T., Schittny J., Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev. Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Shibata Y., Voss C., Rist J. M., Hu J., Rapoport T. A., Prinz W. A., Voeltz G. K. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J. Biol. Chem. 2008;283:18892–18904. doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou S., Lutzmann M., Santos-Rosa H., Leonard K., Mueller S., Aebi U., Hurt E. Structure and assembly of the Nup84p complex. J. Cell Biol. 2000;149:41–54. doi: 10.1083/jcb.149.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H., Ho A. K., Listenberger L. L., Siegers K., Moarefi I., Wente S. R., Hartl F. U., Young J. C. Prediction of novel Bag-1 homologs based on structure/function analysis identifies Snl1p as an Hsp70 co-chaperone in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:33220–33227. doi: 10.1074/jbc.M204624200. [DOI] [PubMed] [Google Scholar]
- Stavru F., Hulsmann B. B., Spang A., Hartmann E., Cordes V. C., Gorlich D. NDC 1, a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes. J. Cell Biol. 2006a;173:509–519. doi: 10.1083/jcb.200601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavru F., Nautrup-Pedersen G., Cordes V. C., Gorlich D. Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells. J. Cell Biol. 2006b;173:477–483. doi: 10.1083/jcb.200601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., White J. M., Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990;9:4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L., Roux K. J., Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–1412. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- Taimen P., Pfleghaar K., Shimi T., Moller D., Ben-Harush K., Erdos M. R., Adam S. A., Herrmann H., Medalia O., Collins F. S., Goldman A. E., Goldman R. D. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc. Natl. Acad. Sci. USA. 2009;106:20788–20793. doi: 10.1073/pnas.0911895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur Y. B., Wilson K. L., Gruenbaum Y. SUN-domain proteins: ‘Velcro’ that links the nucleoskeleton to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2006;7:782–788. doi: 10.1038/nrm2003. [DOI] [PubMed] [Google Scholar]
- Vasu S., Shah S., Orjalo A., Park M., Fischer W. H., Forbes D. J. Novel vertebrate nucleoporins Nup133 and Nup160 play a role in mRNA export. J. Cell Biol. 2001;155:339–354. doi: 10.1083/jcb.200108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz G. K., Prinz W. A., Shibata Y., Rist J. M., Rapoport T. A. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Walther T. C., Alves A., Pickersgill H., Loiodice I., Hetzer M., Galy V., Hulsmann B. B., Kocher T., Wilm M., Allen T., Mattaj I. W., Doye V. The conserved Nup107-160 complex is critical for nuclear pore complex assembly. Cell. 2003a;113:195–206. doi: 10.1016/s0092-8674(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Walther T. C., Askjaer P., Gentzel M., Habermann A., Griffiths G., Wilm M., Mattaj I. W., Hetzer M. RanGTP mediates nuclear pore complex assembly. Nature. 2003b;424:689–694. doi: 10.1038/nature01898. [DOI] [PubMed] [Google Scholar]
- Wozniak R. W., Bartnik E., Blobel G. Primary structure analysis of an integral membrane glycoprotein of the nuclear pore. J. Cell Biol. 1989;108:2083–2092. doi: 10.1083/jcb.108.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhang F., Su Z., McNew J. A., Shin Y. K. Hemifusion in SNARE-mediated membrane fusion. Nat. Struct Mol. Biol. 2005;12:417–422. doi: 10.1038/nsmb921. [DOI] [PubMed] [Google Scholar]
- Zhang C., Clarke P. R. Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science. 2000;288:1429–1432. doi: 10.1126/science.288.5470.1429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.