c-Jun mediates ROS production and apoptosis.
Abstract
c-jun, which is overexpressed in a number of human cancers encodes a critical component of the AP-1 complex. c-jun has been shown to either induce or inhibit cellular apoptosis. Germ line deletion of both c-jun alleles is embryonically lethal. To determine the role of the endogenous c-jun gene in apoptosis, we performed mammary epithelial cell–targeted somatic deletion using floxed c-jun (c-junf/f) conditional knockout mice. Laser capture microdissection demonstrated endogenous c-jun inhibits expression of apoptosis inducing genes and reactive oxygen species (ROS)-reducing genes (MnSOD, catalase). ROS have been implicated in apoptosis and undergo enzymatic elimination via MnSOD and CuZnSOD with further detoxification via catalase. c-jun–mediated survival was in part dependent on ROS production. c-jun–mediated repression of MnSOD and catalase occurred via mitochondrial complex I and NOX I. Collectively, these studies define a pivotal role of endogenous c-jun in promoting cell survival via maintaining mitochondrial integrity and expression of the key regulators of ROS production.
INTRODUCTION
The c-jun oncoprotein is an essential component of an activator protein transcription complex. On hetero-dimerization with other Jun/Fos members it forms an active activator protein -1 complex (AP-1), which regulates the expression of various target genes harboring AP-1–binding DNA elements within their promoters. AP-1 controls the expression of genes that regulate several crucial cellular functions such as cell cycle progression, migration, proliferation, and apoptosis. c-jun is activated upon phosphorylation by c-jun N-terminal kinase (JNK), which responds to diverse biological stress signals (Kyriakis, 1999).
c-jun functions as both a positive and negative regulator of apoptosis (Shaulian and Karin, 2002). Nerve growth factor (NGF) withdrawal induced neuronal apoptosis is reduced by c-jun inhibition, and mice expressing the c-jun phosphorylation site mutant (c-jun A63/73) are resistant to kainate seizure-induced apoptosis (Ham et al., 1995; Behrens et al., 1999). In contrast, c-jun inhibits TNF-α–induced apoptosis via p53/p21 in transformed fibroblasts (Eferl et al., 2003), and p53 is induced in c-jun−/− liver tumors. p53 however is not activated in c-jun−/− hepatocytes (Eferl et al., 2003), suggesting acute c-jun deficiency induces apoptosis eliminating p53-overexpressing cells.
Reactive oxygen species (ROS) are thought to play an important role in cell death: apoptosis, programmed necrosis, cellular proliferation, premature senescence, genomic instability, and cellular invasiveness. On one hand, ROS induced by oncogenic Ras or ErbB2 has been shown to induce antiproliferative signals that block cellular proliferation and growth (Dolado et al., 2007). ROS can cooperate with oncogenic signals and in some circumstances ROS production is associated with activation of growth receptors including transforming growth factor-β and platelet-derived growth factor (PDGF; Sattler et al., 1999; Balaban et al., 2005). The carcinogenic effect of ROS has been linked to increased cellular proliferation rate seen in many cancer cell lines (Fernandez-Pol et al., 1982; Church et al., 1992). Mitochondria are the major source of cellular ROS, derived from leakage of electrons from carriers in the electron transport chain (Scherz-Shouval et al., 2007) produced during the turnover of complex I and complex II. Molecular oxygen is converted to superoxide (O2−), which in turn develops into ROS. Nonmitochondrial ROS are derived from other compartments including peroxisomes, plasma membrane, the endoplasmic reticulum, Golgi complex, lysosomes, and the nucleus. ROS may be generated by reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, which includes a catalytic subunit of the NADPH oxidase (NOX) family (Lambeth, 2004). ROS formation and efflux may be altered by the mitochondrial inner transmembrane potential (Δψm), pH gradient and the outer mitochondrial membrane potential, O2− production is enhanced by high Δψm ROS leave the mitochondria either via diffusion or via active carriers such as the inner membrane anion channel (IMAC) or the voltage-dependent anion channel (VDAC; Boveris and Chance, 1973).
Superoxide dismutases are the antioxidant enzymes that catalyze the dismutation of O2 to H2O2. There are two forms of intracellular superoxide dismutases (SODs) in mammalian cells: nuclear and cytoplasmic CuZnSOD (SOD-1) and mitochondrial matrix localized MnSOD (SOD-2).
In addition to passive or active diffusion from mitochondria, ROS undergo enzymatic elimination via MnSOD (Melov, 2000) or CuZnSOD (Okado-Matsumoto and Fridovich, 2001). Further detoxification is undertaken by glutathione peroxidase (GPX; Nohl and Jordan, 1980) which uses reduced glutathione (GSH) and/or catalase in which converts H2O2 to H2O (Radi et al., 1991).
The c-jun gene plays a central role in the diverse functions of the AP-1 complex. Although the number of studies is small, analysis of acute loss of target genes have revealed distinct results compared with germ line deletion studies (Sage et al., 2003). The current studies were conducted first, therefore, to determine the signaling pathways regulated by endogenous c-jun in vivo. To this end, c-junfl/fl mice were deployed, and excision was conducted using cre recombinase. Laser capture microdissection (LCM) was conducted of cells deleted of c-jun, and genome-wide expression analysis was conducted to determine signaling pathways regulated by c-jun in vivo. Second, JNK and the c-jun genes have been linked to both the inhibition and induction of cellular apoptosis (Xia et al., 1995; Kuan et al., 1999; Xia and Karin, 2004). The current studies were therefore conducted to examine the effect of acute c-jun excision on cellular survival.
MATERIALS AND METHODS
Transgenic Mice and Expression Plasmids
Transgenic animals carrying floxed c-jun alleles, c-junf/f, and mouse mammary tumor virus (MMTV)-Cre were previously described (Zenz et al., 2003). The mammary gland mRNA was obtained from virgin female mice at 2 mo of age. All experimental procedures conducted on these mice were approved by the ethics committee of Thomas Jefferson University. Quantitation of mammary epithelial cell number was conducted as described (Atabai et al., 2005). Apoptosis in situ was determined using the TdT in situ apoptosis kit (DAB TUNEL-based Apoptosis Detection Assay; R&D Systems, Minneapolis, MN). The murine embryonic fibroblasts (MEFs) were produced as previously described (Albanese et al., 1999) and cultured following the 3T3 protocol. The expression plasmids for adenovirus Cre (Ad-Cre) or control (Ad-Null) were previously described (Wang et al., 1995). The rat c-jun was subcloned in murine sarcoma cytomegalovirus (MSCV)-internal ribosome entry site (IRES)-green fluorescent protein (GFP) to form MSCV-c-jun-IRES-GFP.
Reagents and Antibodies
The following reagents and antibodies were used: H2DCFDA (Invitrogen, Carlsbad, CA); anti-Cre (EMD Biosciences, San Diego, CA); anti-c-jun (H-79), anti-survivin, and anti-rho-guanine nucleotide dissociation inhibitor (GDI; Santa Cruz Biotechnology, Santa Cruz, CA); VDAC-1 (Abcam, Cambridge, MA); and DAPI (4′,6′-diamino-2-phenylindole; Sigma-Aldrich, St. Louis, MO). Amplex Red H2O2 detection kit was from Invitrogen. Reagents and their final concentration used in experiments were N-acetyl-l-cysteine (NAC; 5 mM), diphenyleneiodonium chloride (DPI; 10 μM), ammonium pyrrolidinedithiocarbamate (PDTC; 10 μM), rotenone (1 μg/ml), and H2O2 were purchased from Sigma-Aldrich. Measurements of H2O2 production were conducted using Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit from Invitrogen (Mishin et al., 2010). Catalase activity was determined using the Catalase Assay Kit from Caymen Chemicals (Ann Arbor, MI; Sinha, 1972), and the In Gel MnSOD activity was done as previously described (Beauchamp and Fridovich, 1971).
Cell Culture, Viral Cell Transduction, and Reporter Gene Assays
Cells were maintained under standard tissue culture conditions in DMEM supplemented with 10% FBS and 100 mg/ml each of penicillin-streptomycin at 37°C with 5% CO2. Adenovirus propagation was previously described (Wang et al., 2003). Infection was done at a multiplicity of infection (MOI) of 50, cells were cultured overnight, and media were changed before experimental analysis. Retroviral infections were conducted as previously described (Wang et al., 2003). Transfections were conducted using Genejuice transfection reagent (EMD Biosciences, San Diego, CA) as described (Bouras et al., 2005). Statistical analysis was conducted using the Mann-Whitney U test.
Immunofluorescence and Confocal and Electron Microscopy
c-junf/f cells were grown on chamber slides and then treated with either Ad-Null or Ad-Cre. Cells were then stained with antibodies against cre-recombinase and c-jun, and DAPI was used as nuclear stain. The microscopic images were captured using the 20× and 63× objectives of an Olympus LSM-5 Meta laser confocal scanning microscope (Olympus America, Center Valley, PA). Briefly, confluent c-junf/f cells treated with adenoviruses were harvested and washed with PBS. Cells were fixed in paraformaldehyde and permeabilized with Triton X-100, followed by a PBS wash, and were stained with primary antibodies and consequently with fluorophore-tagged secondary antibodies.
For electron microscopy (EM) virus-treated cells were plated on fibronectin-coated glass coverslips and grown to approximately 80% confluence. The cells were then rinsed with PBS and fixed in 2.5% glutaraldehyde/0.1 M for 2 hr at room temperature, postfixed in 1% osmium tetroxide for 1 hr, en bloc stained with uranyl acetate, and processed in situ for conventional electron microscopy araldite embedding procedure. Layers of plastic-embedded cells were removed from the coverslips, re-embedded in plastic, and ultrathin sections were cut parallel to the original plane of the coverslip. Sections were then poststained with uranyl acetate and lead citrate, and observed and photographed with a Hitachi H-7600 transmission electron microscope (Hitachi, Tokyo, Japan).
Assessment of ROS Production
H2DCFDA staining for ROS was performed on fibroblasts treated with Ad-Null and Ad-Cre according to the manufacturer's instructions. DCFDA, at 5 mM, was used for staining the cells for 30 min, followed by fluorescence-activated cell sorting (FACS) sorting of stained cells at excitation and emission wavelengths of 492–495 and 517–527 nm, respectively. H2DCFDA is a cell-permeant indicator for ROS that is nonfluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell.
Assays for Assessment of Cellular Apoptosis
FACS-based cell cycle assays were performed using propidium iodide staining of the virus-treated cells in citrate buffer. Assays of cellular apoptosis were performed using annexin V staining on living cells using the annexin V kit from BD Biosciences (San Jose, CA) followed by the FACS sorting of fluorescent apoptotic cells.
Real-Time PCR and ELISA
All gel-based and real-time quantitative RT-PCR (qRT-PCR) assays were performed as previously described (Katiyar et al., 2007). Briefly, RNA extracted using standard GITC method was RQ1 DNase I (Promega, Madison, WI) treated, phenol-chloroform extracted, and quantitated using a Nanodrop spectrophotometer (Nanodrop Tech, Wilmington, DE). An equal quantity of RNA from each sample was reverse-transcribed using Iscript Reverse transcriptase (Bio-Rad, Hercules, CA). Primers for all the genes and gene transcripts including housekeeping control genes and gene transcripts are listed in Table 1.
Table 1.
List of oligonucleotide primers used in PCR, RT-PCR, real-time qRT-PCR analysis, and chromatin immunoprecipitation assays
| Gene | Orientation | Sequence 5′→3′ |
|---|---|---|
| c-jun genotyping | Forward | CTC ATA CCA GTT CGC ACA GGC GGC |
| Reverse | CCG CTA GCA CTC ACG TTG GTA GGC | |
| Reverse | CAG GGC GTT GTG TCA CTG AGC T | |
| RPL-19 (DNA PCR) | Forward | AAT GCT CGG ATG CCT GAG AA |
| Reverse | CTC CAT GAG GAT GCG CTT GT | |
| Cre recombinase | Forward | TGC TCT GTC CGT TTG CCG |
| Reverse | ATC GTG TCC AGA CCA GGC | |
| RPL-19 (for RT-PCR) | Forward | CTG AAG GTC AAA GGG AAT GTG |
| Reverse | GGA CAG AGT CTT GAT GAT CTC | |
| c-jun (for RT-PCR) | Forward | AGA GCG GTG CCT ACG GCT ACA GTA A |
| Reverse | CGA CGT GAG AAG GTC CGA GTT CTT G | |
| MnSOD | Forward | GCA CAT TAA CGC GCA GAT CA |
| Reverse | AGC CTC CAG CAA CTC TCC TT | |
| CuZnSOD | Forward | AAG GCC GTG TGC GTG CTG AA |
| Reverse | CAG GTC TCC AAC ATG CCT CT | |
| GPX | Forward | CCT CAA GTA CGT CCG ACC TG |
| Reverse | CAA TGT CGT TGC GGC ACA CC | |
| Catalase | Forward | GCA GAT ACC TGT GAA CTG TC |
| Reverse | GTA GAA TGT CCG CAC CTG AG | |
| Rac 1 | Forward | CTG CCT GCT CAT CAG TTA CACG |
| Reverse | GGA CAG AGA ACC GCT CGG ATA | |
| Rac 2 | Forward | CAG GTC AGG AGG ACT ATG ACC G |
| Reverse | GAT TGC CTC ATC GAA GAC GGT | |
| p40phox | Forward | CAA AGT CTA CAT GGG CGC AAA |
| Reverse | TGT CTT CAT AGA AGT AGC ATC GTA GCC | |
| p67phox | Forward | CTA TCT GGG CAA GGC TAC GGT T |
| Reverse | CAC AAA GCC AAA CAA TAC GCG | |
| gp91phox | Forward | AGT CGG GAT TTC TGA CCG GTA T |
| Reverse | TCC AGT CTC CAA CAA TAC GGA TAT G | |
| 18S r-RNA | Forward | AGG AAT TCC CAG TAA GTG CG |
| Reverse | GCC TCA CTA AAC CAT CCA A | |
| AP-1 elements | Forward | AGA AGT GAG TGG ATG TGA TGC CCA |
| Reverse | AGT ACA TCG TTG ACT GCA CGA CCT | |
| Negative (4) | Forward | TTC ATT TGC TGT CTG TCA CCG GG |
| Reverse | TGC AGA TAG TCC CAG CAT TGG GTA | |
| Oligos used for creating AP-1 deletion in MnSOD promoter constructs | Forward | CAG GGC ATA AAT TAA GAA GGC CCC TG |
| Reverse | CAG GGG CCT TCT TAA TTT ATG CCC TG |
Laser Capture Microdissection
Mammary glands from the following bitransgenic mice genotypes: wild-type (cre+/c-junwt/wt; resulting genotype: c-jun+/+), heterozygotes (cre+/c-junf/w; resulting genotype: c-jun+/−), or knockouts (cre+/c-junf/f; resulting genotype: c-jun−/−) were frozen in optimal temperature compound on dry ice. The frozen tissue blocks were trimmed and cryosectioned at −20°C in a cryostat machine onto cold, RNase-free sterile glass slides. Each slide was mounted in the LCM machine, and ∼200–1000 mammary epithelial cells were picked by firing lasers on to the resin-bearing caps. RNA was isolated using microRNAeasy RNA extraction kits (Qiagen, Valencia, CA) followed by DNase treatment and concentrated in a small volume using DNA-free kit (Zymo Research, Orange, CA). RNA quantity and quality was measured in a Nanodrop 1000 spectrophotometer. This RNA was then subjected to hybridization to Affymetrix RNA chips (Santa Clara, CA) for assessing the relative expression of genes affected by c-jun excision.
Gene Expression Arrays and Pathways Analysis
RNA samples extracted from laser capture microdissected mammary epithelial cells from wild-type c-jun+/+ and knockout c-jun−/− bitransgenic mice mammary glands were processed for Affymetrix 430 2.0 arrays to determine gene expression. RNA quality was determined by gel electrophoresis. Probe synthesis and hybridization were performed as previously described (Li et al., 2008). Analysis of the arrays was performed using the R statistics package (www.r-project.org) and the limma library of the Bioconductor software package (www.bioconductor.org). Arrays were normalized using robust multiarray analysis, and a p of 0.05 was applied as statistical criterion for differentially expressed genes (Zhou et al., 2010). These genes were then grouped using hierarchical clustering with “complete” agglomeration. Expression profiles are displayed using Treeview (http://taxonomy.zoology.gla.ac.uk/ROD/treeview.html). Classification and clustering for pathway level analysis used ASSESS (Analysis of Sample Set Enrichment Scores) (Edelman et al, 2006).
RNA amplification was performed from 5 ng of total RNA followed by cDNA production and its purification using a DNA cleaning kit from Zymo Research. The purified cDNA was then quantified on a Nanodrop ND-1000 Spectrophotometer (Thermo Scientific, Waltham, MA) and 5 μg of amplified cDNA was fragmented and chemically labeled with biotin to generate biotinylated cDNA using FL-Ovation cDNA biotin labeling module V2 (NuGen Technologies, San Carlos, CA).
Low-Density Apoptosis Arrays
The cDNA prepared from purified RNA extracted c-junf/f fibroblasts treated with either adenovirus null (wild type [WT]) or adenovirus expressing cre-recombinase (knockout [KO]) was amplified on prefabricated, low-density, 384-well apoptosis arrays and run according to the manufacturer's instructions (SA Bioscience, Gaithersburg, MD).
RESULTS
Endogenous c-jun Inhibits Signaling Pathways Promoting Apoptosis In Vivo
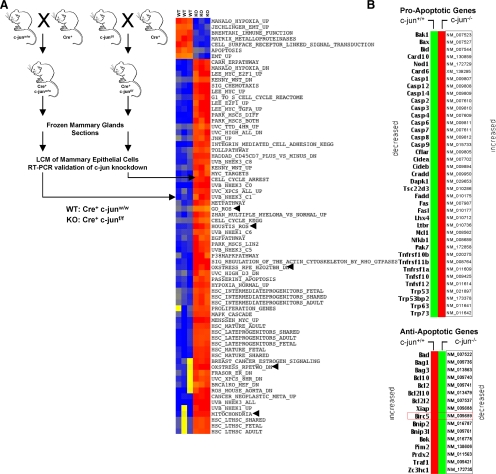
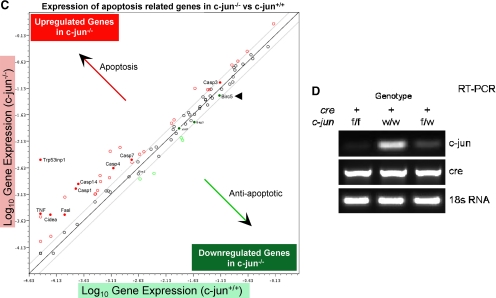
c-jun has been shown to either inhibit or induce cellular apoptosis in different cell types (Seaman, 1976; Udou et al., 2004; Vleugel et al., 2006; Hettinger et al., 2007; Podar et al., 2007). We conducted studies to determine the role of endogenous c-jun in mammary epithelial cell survival. Because c-jun somatic deletion is embryonically lethal, we intercrossed floxed c-jun (c-junf/f) transgenic mice with MMTV-Cre mice and conducted LCM of mammary epithelial cells from mouse mammary glands (Materials and Methods; Figure 1A). mRNA derived from mammary epithelial cells of six separate transgenic mice was subjected to microarray analysis and subsequently to pathways analysis using ASSESS (Figure 1A). Gene expression pathways and the induction of apoptosis associated with ROS production were induced upon c-jun deletion (Figure 1A). To examine whether c-jun deletion–regulated expression of specific pro- or antiapoptotic genes, we conducted RT-PCR–based expression analysis of 87 genes (using low-density apoptosis arrays) previously shown to be involved in apoptosis. Thirty-nine proapoptotic genes were induced, and 16 anti-apoptotic genes were repressed upon deletion of c-jun (Figure 1B). The relative change in gene expression of the antiapoptotic and proapoptotic genes are shown on a log scale (Figure 1C). Deletion of c-jun induced expression of apoptotic genes and reduced expression of antiapoptotic genes (Figure 1C). The inhibitor of apoptosis protein (IAP) BIRC5 (survivin) was repressed substantially upon deletion of endogenous c-jun (Figure 1C). RT-PCR–based analysis of c-jun gene expression within laser capture–microdissected mammary epithelial cells derived from Cre recombinase expressing bitransgenic mammary glands also showed reduction in c-jun expression (Figures 1D).
Figure 1.
Endogenous c-jun represses gene expression pathways governing cellular apoptosis and ROS production in vivo. (A) Schematic representation of intercrosses between MMTV-Cre and c-junf/f transgenic mice performed to produce c-jun+/+ and c-jun−/− mice. The mammary glands from these mice were subjected to laser capture microdissection (LCM) to enrich and purify mammary epithelial cells for performing microarray-based gene expression analysis. ASSESS pathway analysis was performed on the differentially expressed genes. The blue gradient coloration in the heat map shows down-regulation of the indicated pathways, whereas red depicts up-regulation of those pathways. (B) Heat map of differentially expressed pro- and antiapoptotic gene transcripts up- or down-regulated upon treatment of floxed c-jun cells with Ad-Null or Ad-Cre. (C) Scatter plot for the gene expression profiling performed using low-density apoptosis qRT-PCR arrays indicating expression of pro- and antiapoptosis genes, with the direction of gene expression show by red (up-regulated) and green (down-regulated) genes.
To determine whether the excision of c-jun within the mammary epithelial cells had affected mammary gland development and mammary ductal morphology, we performed mammary gland whole-mount analyses. Mammary glands squashes prepared by staining the entire #4 mammary glands from cre+/c-junf/f (resulting genotype: c-jun−/− or KO) and cre+c-junf/w (resulting genotype: c-jun+/−) to littermate controls cre+/c-junwt/wt (resulting genotype: c-jun+/+ or WT) were photographed and carefully compared (Supplemental Figure 1A). The morphological structure and architecture was unchanged between genotypes. Mammary gland ductal branching complexity number was also largely unaltered (Supplemental Figure 1A). Studies of the cellular components of the mammary gland of the mice were next conducted. The relative number of cells per field and the area covered by cells lining mammary glands were carefully assessed and characterized by pixels per field and demonstrated ∼50% reduction in the number of epithelial cells observed per field and the same for their area. (Supplemental Figure 1B).
Endogenous c-jun Inhibits ROS Production via Mitochondrial Complex I and NOX1
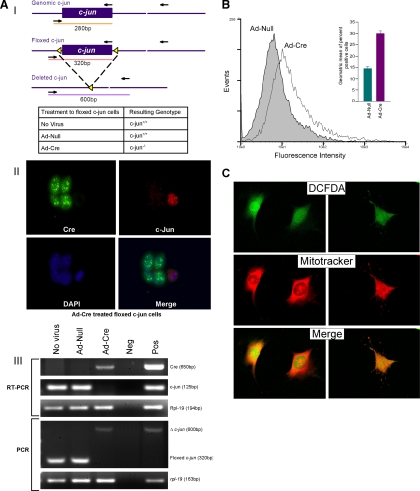
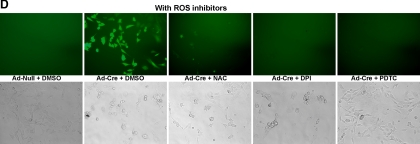
To determine the role of endogenous c-jun in ROS production, c-junfl/fl MEFs (Figure 2A) were transduced with an adenoviral expression vectors encoding cre (Ad-Cre) or a control vector (Ad-Null). The excision of c-jun was documented by RT-PCR analyses for c-jun mRNA normalized to the internal control Rpl-19 and deletion of the floxed site by PCR (Figure 2AIII). ROS production was visualized by 2′7′-dichlorofluorescein diacetate (DCFDA) and quantitated by FACS sorting (Figure 2, B and C), which demonstrated an approximate doubling of DCFDA positivity (Figure 2B). Confocal microscopy combining staining of mitochondria with mitotracker dye evidenced a colocalization of DCFDA with mitochondria (yellow; Figure 1C). Additional nonmitochondrial DCFDA was also seen. Reintroduction of c-jun by retroviral transduction into c-jun−/− cells reversed the increase in DCFDA positivity by FACS (data not shown). To examine further the source of endogenous ROS repressed by c-jun, ROS inhibitors were used. NAC and PDTC had been thought to act as antioxidants. PDTC inhibits IκB-ubiquitin ligase activity (Hayakawa et al., 2003). Rotenone, is an inhibitor of the mitochondrial electron transport chain, and DPI is an inhibitor of the NOX enzymes that are the major mediators of nonmitochondrial ROS (Kamata and Hirata, 1999). Addition of DPI, PDTC, or rotenone reduced DCFDA fluorescence (Figure 2D) suggesting both NOX- and mitochondrial-dependent sources of ROS in c-jun−/− cells.
Figure 2.
Somatic excision of c-jun induces mitochondrial and NOX1-dependent ROS production. (AI) Schematic diagram of the genomic wild-type (c-jun), floxed (c-junf/f), and deleted c-jun (c-junPΔ) locus with loxP sites (◀) and PCR primer binding sites (→). Adenovirus treatments to floxed c-jun cells, and resulting cellular genotypes. (II) Confocal microscopic images of Ad-Cre–treated c-junf/f fibroblasts showing expression of Cre-recombinase and c-jun. DAPI-stained nuclei. Note that the cells expressing Cre have lost c-jun expression. (III) RT-PCR showing expression of cre-recombinase, c-jun, and rpl-19 (housekeeping gene control) mRNA transcripts in RNA from no virus NS Ad-Null– and Ad-Cre–treated c-junf/f fibroblasts. PCR genotyping of DNA from no virus and Ad-Null– and Ad-Cre–treated c-junf/f cells showing floxed and excised c-jun alleles. (B) FACS-based quantification of DCFDA-stained ROS production in Ad-Null– and Ad-Cre–treated floxed c-jun cells (data are mean ± SEM). (C) High-resolution confocal microscopic images of the DCFDA staining of intracellular ROS in Ad-Cre–treated floxed c-jun cells. Mitotracker mitochondrial stain was used for visualization of mitochondria (data are mean ± SEM). (D) DCFDA staining of Ad-Null– or Ad-Cre–treated fibroblasts after addition of various ROS inhibitors.
Endogenous c-jun Maintains Mitochondrial Membrane Potential
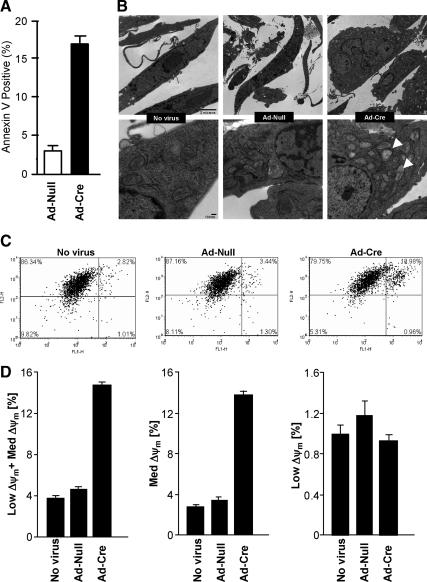
Apoptosis assessed by annexin V staining was increased fourfold in c-jun−/− cells (Figure 3A). EM of the mitochondria demonstrated mitochondrial blebbing (Figure 3B) characteristic of apoptotic cell death. The size of mitochondria assessed by mitotracker staining was unchanged in c-jun−/−cells (Figure 3C). The mitochondrial membrane potential (Dym) regulates ROS formation and efflux. O2− production increased the number of cells with medium and low Dym. Quantitation of JC-1 staining was conducted to measure Dym and demonstrated a 3–5-fold increase in staining in c-jun−/− cells (Figure 3D).
Figure 3.
c-jun determines mitochondrial membrane potential and apoptosis. (A) Annexin V staining for assessment of apoptotic cells after c-jun excision (data are mean ± SEM for n > 5). (B) Electron micrographs of the floxed c-jun cells with (No virus or Ad-Null) or without c-jun expression (Ad-Cre). (C) Mitochondrial membrane potential measurements using JC-1 mitochondrial probe after c-jun excision (data are mean ± SEM). (D) Quantitation of JC-1 staining and measurement of Dym, indicating a 3–5-fold increase in staining in c-jun−/− cells.
To determine the role of c-Jun and c-Jun–regulated ROS in cellular growth and apoptosis, cellular proliferation assays were conducted. c-jun−/− MEFs have a severe proliferative defect that can be passaged only a few times before entering premature senescence. To avoid any independent effects of serial passage and senescence, the studies were conducted acutely on early-passage MEFs. c-junf/f cells were transduced with an adenovirus expressing cre via an IRES to the GFP protein (Supplemental Figure 3A). Immunohistochemical staining confirmed cells transduced with Cre lacked c-Jun protein. GFP-positive cells were selected and analyzed. c-jun−/− MEFs demonstrated a reduced proliferation rate assessed by cellular counting (Supplemental Figure 3A) or by MTT assays (Supplemental Figure 3B). c-jun−/− MEFs displayed enhanced sensitivity to H2O2-induced apoptosis (Supplemental Figure 3C).
c-jun–dependent ROS Production Governs MnSOD, Catalase, and GPX Expression
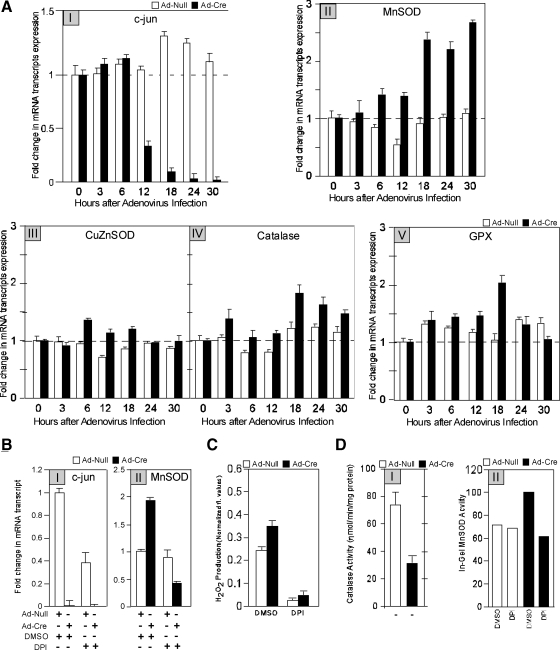
We examined further the mechanisms governing endogenous c-jun–mediated inhibition of ROS production. Increased ROS production in the c-jun−/− cells may be a consequence of either reduced ROS elimination or increased ROS production. O2− is converted to H2O2 by matrix manganese superoxide dismutase (MnSOD; Temkin and Karin, 2007), whereas catalase and GPX either separately or synergistically convert H2O2 to H2O. To determine the mechanism by which c-jun inhibited ROS production, we examined the role of c-jun in regulating MnSOD and catalase abundance. We examined the abundance of MnSOD by quantitative real-time RT-PCR upon acute excision of c-jun using Ad-Cre. As endogenous c-jun mRNA levels decreased (Figure 4AI), MnSOD mRNA transcript abundance increased (Figure 4AII). O2− released to the mitochondrial intramembrane space is dismutated by CuZnSOD (Okado-Matsumoto and Fridovich, 2001). ROS are further detoxified by GPX (Nohl and Jordan, 1980), which uses GSH in catalysis. The relative abundance of MnSOD, CuZnSOD, catalase, and GPX were assessed upon acute deletion of c-jun to determine the role of endogenous c-jun in regulating the key components involved in eliminating ROS (Figure 4A, III–V). Quantitative RT-PCR transcript analysis was conducted, and mean data of multiplicate studies demonstrated that c-jun excision induced MnSOD 2.5-fold. More modest changes occurred with catalase and GPX (1.5- to 2-fold). CuZnSOD mRNA expression was unchanged (Figure 4A, III–V). The finding that MnSOD mRNA increased and CuZnSOD was unchanged upon excision of c-jun in fibroblasts was confirmed by microarray analysis of the c-jun−/− mammary gland in which MnSOD was increased (1.6-fold, mean of n = 3), and CuZnSOD mRNA abundance was unchanged.
Figure 4.
c-jun excision induces MnSOD expression via a ROS-dependent mechanism. (A) qRT-PCR–based assessment of expression of c-jun (I), MnSOD (II), CuZnSOD (III), catalase (IV), and GPX (V) transcripts at various time points after treatment of floxed c-jun cells with Ad-Null or Ad-cre to excise the c-jun gene. The real-time qRT-PCR expression data for various targets were normalized to amplification of 18S rRNA housekeeping gene control in every sample. (B) Expression of c-jun and MnSOD transcripts after treatment of Ad-Null– or Ad-Cre–treated floxed c-jun cells with the ROS inhibitor DPI. (C) Quantitation of H2O2 levels after c-jun excision and DPI treatment. (D) Catalase enzyme activities and (E) MnSOD enzyme activity in Ad-Null– or Ad-Cre–treated floxed c-jun cells in the presence of various ROS inhibitors (data are mean ± SEM).
We had previously shown that ROS induces MnSOD expression (Tanaka et al., 2002). We therefore examined whether inhibition of ROS production in c-jun−/− cells was responsible for the increase in MnSOD. The addition of DPI (Figure 4B), rotenone, or PDTC (not shown) reversed the hyperactivation of MnSOD. Thus, increased ROS from NOX1 and mitochondrial sites produced in c-jun−/− cells enhances MnSOD expression. Consistent with an important role for MnSOD in converting O2 to H2O2, H2O2 production (Figure 4C) was reduced by DPI. Consistent with the induction of expression in c-jun−/− cells, MnSOD enzyme activity was also increased in c-jun−/− cells (Figure 4D). The induction of MnSOD activity in c-jun−/− cells was reduced by DPI (Figure 4D). To determine whether the increase in H2O2 abundance in c-jun−/−cells was due to reduced catalase activity, catalase activity assays were conducted. Catalase activity was reduced by 60% upon deletion of c-jun (Figure 4D). The reduction in catalase activity was partially reversed by DPI, suggesting NOX-dependent ROS are a source of reduced catalase activity in c-jun−/− cells.
Endogenous c-jun Induces Survivin (BIRC5) in Part via ROS
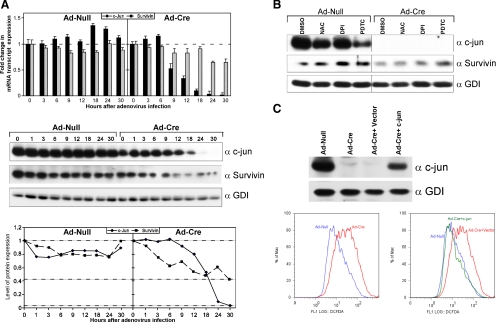
Microarray and RT-PCR analysis of mRNA from LCM of the mammary epithelial cell targeted MMTV-Cre/c-junfl/fl bitransgenics demonstrated a reduction in the abundance of several antiapoptotic genes (Figure 1, B and C). The abundance of BIRC5 (survivin), in particular, was down-regulated. To determine whether BIRC5 was also a direct target of endogenous c-jun, acute excision of c-jun was conducted and BIRC5 abundance was determined (Figure 5A). c-jun excision was associated with a decrease in BIRC5 (survivin) mRNA (by RT-PCR) and protein levels. Survivin abundance in c-jun−/− cells was reduced in part by the addition of ROS inhibitors (Figure 5B). DPI and PDTC treatment increased survivin abundance in c-jun−/− cells 3–5-fold (Figure 5B, lanes 5 vs. 7 and 8). Reintroduction of c-jun restored c-jun and survivin levels and reversed aberrant ROS production (Figure 5C).
Figure 5.
Endogenous c-jun maintains survivin abundance. (A) mRNA and protein abundance for c-jun and survivin determined after acute excision of c-jun using Ad-Cre treatment of c-junfl/fl cells quantitated by RT-PCR (data are mean ± SEM for n > 5). (B) Western blot analysis of surviving abundance in wild-type versus c-jun−/− cells after treatment with ROS inhibitors for 24 h as indicated. (C) Western blot and DCFDA analysis of c-jun−/− cells transduced with retrovirus encoding c-jun. c-jun restores endogenous c-jun levels and reverses the increased production of ROS.
Collectively these studies demonstrate acute c-jun excision increased proapoptosis and inhibited antiapoptotic gene expression in vivo. The mammary epithelium in vivo c-jun excision or c-junfl/fl cultured cells induced expression of genes that catalase ROS, mitochondrial membrane potential and both mitochondrial and NOX-based ROS, which induced MnSOD, and catalase expression. The inhibition of ROS production by endogenous c-jun reduced cellular apoptosis and promoted contact-independent growth. Because c-jun functions as a pivotal downstream regulator of diverse biological processes governing cellular proliferation and growth, these studies provide a direct link between c-jun and mitochondrial function.
DISCUSSION
The current studies demonstrate an essential role for endogenous c-jun in maintaining mammary epithelial cellular survival in vivo. Double transgenic mice (c-junfl/fl-MMTV-Cre) and conditional knockout MEFs were generated to identify the key signaling pathways regulated by endogenous c-jun in vivo. Genome-wide expression studies conducted of mRNA derived from laser capture–microdissected mammary epithelium from these double transgenic mice identified the key pathways regulated by endogenous c-jun. In the mammary epithelium and fibroblasts the key pathways regulated by endogenous c-jun govern cellular apoptosis and ROS production. RT-PCR analysis of 87 genes confirmed that 39 apoptotic genes were induced and 16 antiapoptotic genes were repressed upon deletion of c-jun.
Herein, acute c-jun excision increased apoptosis. The induction of cellular senescence, which occurs upon several cellular divisions, is associated with the induction of p53/p21CIP1 (MacLaren et al., 2004). To distinguish the effects of c-jun excision from the effects of cellular senescence the molecular events, accompanying acute somatic excision of c-jun were characterized. The apoptosis induced upon acute excision of c-jun herein was associated with increased annexin V staining, characteristic of phosphatidyl-serine exposure on the cellular membrane seen in apoptosis and features of caspase 3 cleavage and activation (Supplemental Data) indicative of the intrinsic mitochondrial pathways of apoptosis. Mitochondrial morphology reflected the characteristic feature of mitochondrial cristae swelling and inner mitochondrial membrane swelling.
The current studies define a central role for endogenous c-jun gene in maintaining ROS homeostasis. The decreased membrane potential of c-jun−/− cells would be anticipated to increase ROS production and enhance efflux (Tirosh et al., 2003). Acute c-jun excision enhanced ROS production and expression of the enzymes that catalase and detoxify ROS (MnSOD and GPX). The induction of gene expression occurred within 16–18 h. The induction of the cellular ROS-catalyzing enzymes was accompanied by a decrease in mitochondrial membrane potential. ROS are known to induce expression of MnSOD (Tanaka et al., 2002). The enhanced production of MnSOD in c-jun−/− cells was abolished by addition of DPI, indicating that ROS production from mitochondrial complex I and NOX are inhibited by endogenous c-jun. Thus, endogenous c-jun inhibits MnSOD production via NOX1 and mitochondrial complex 1.
Apoptosis is regulated via extrinsic pathways that are triggered by ligand binding, whereas the intrinsic apoptotic pathways involves mitochondria, which release cytochrome c that binds and activates Apaf1 (apoptotic protease activation factor 1). Apaf1 induces assembly of the apoptosome, leading to activation of caspase 9 members of the IAP family including survivin and others (ML-IAP, XIAP, CIAP1, CIAP2, NIAP, and Apollon). These factors block apoptosis downstream of cytochrome c release by interfering with caspase 9 activity and processing and inhibiting the terminal effectors caspase-3 and -7. Increased ROS production has been linked to cellular apoptosis through sustained JNK activation and an additional pathway (Kamata et al., 2005; Chang et al., 2006) that is inhibited by a prosurvival NFκB (nuclear factor κB) pathway (Temkin and Karin, 2007). The current studies extend these prior observations by demonstrating that the endogenous c-jun functions to inhibit ROS production (Supplemental Figure 3). The IAP BIRC5 (survivin) was reduced upon deletion of c-jun. Survivin is a 16.5-kDa protein, with a single baculovirus IAP repeat (BIR). Mitochondrial survivin orchestrates a novel pathway of apoptosis inhibition, which contributes to tumorigenesis (Dohi et al., 2004). On deletion of c-jun, ROS levels rise, and survivin and other apoptotic gene levels fall, inducing apoptosis (Supplemental Figure 3). c-jun induced survivin abundance in a ROS-dependent manner. ROS generated independently of c-jun and other factors also regulate survivin expression. The survivin gene is regulated by a variety of factors including chronic hypoxia, growth factors, HIF1α (hypoxia-inducible factor), and paracrine factors. The independent role of these factors in ROS signaling to the survivin gene remains to be determined.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health (NIH) Grants R01CA70896, R01CA75503, and R01CA86072 (R.G.P.). The Kimmel Cancer Center is supported by the NIH Cancer Center Core Grant P30CA56036 (R.G.P.). This project is funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust and a grant from Pennsylvania Department of Health (R.G.P.), which specifically disclaims responsibility for analyses, interpretations, or conclusions.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-08-0705) on October 6, 2010.
REFERENCES
- Albanese C., et al. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 1999;274:34186–34195. doi: 10.1074/jbc.274.48.34186. [DOI] [PubMed] [Google Scholar]
- Atabai K., et al. Mfge8 is critical for mammary gland remodeling during involution. Mol. Biol. Cell. 2005;16:5528–5537. doi: 10.1091/mbc.E05-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Behrens A., Sibilia M., Wagner E. F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- Bouras T., Fu M., Sauve A. A., Wang F., Quong A. A., Perkins N. D., Hay R. T., Gu W., Pestell R. G. SIRT1 deacetylation and repression of P300 involves lysine residues 1020/1024 within the cell-cycle regulatory domain 1. J. Biol. Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Kamata H., Solinas G., Luo J. L., Maeda S., Venuprasad K., Liu Y. C., Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Church S. L., Grant J. W., Meese E. U., Trent J. M. Sublocalization of the gene encoding manganese superoxide dismutase (MnSOD/SOD2) to 6q25 by fluorescence in situ hybridization and somatic cell hybrid mapping. Genomics. 1992;14:823–825. doi: 10.1016/s0888-7543(05)80202-2. [DOI] [PubMed] [Google Scholar]
- Dohi T., Beltrami E., Wall N. R., Plescia J., Altieri D. C. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J. Clin. Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolado I., Swat A., Ajenjo N., De Vita G., Cuadrado A., Nebreda A. R. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Edelman E., Porrello A., Guinney J., Balakumaran B., Bild A., Febbo P. G., Mukherjee S. Analysis of sample set enrichment scores: assaying the enrichment of sets of genes for individual samples in genome-wide expression profiles. Bioinformatics. 2006;22:e108–e116. doi: 10.1093/bioinformatics/btl231. [DOI] [PubMed] [Google Scholar]
- Eferl R., Ricci R., Kenner L., Zenz R., David J. P., Rath M., Wagner E. F. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pol J. A., Hamilton P. D., Klos D. J. Correlation between the loss of the transformed phenotype and an increase in superoxide dismutase activity in a revertant subclone of sarcoma virus-infected mammalian cells. Cancer Res. 1982;42:609–617. [PubMed] [Google Scholar]
- Ham J., Babij C., Whitfield J., Pfarr C. M., Lallemand D., Yaniv M., Rubin L. L. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Miyashita H., Sakamoto I., Kitagawa M., Tanaka H., Yasuda H., Karin M., Kikugawa K. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger K., Vikhanskaya F., Poh M. K., Lee M. K., de Belle I., Zhang J. T., Reddy S. A., Sabapathy K. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007;14:218–229. doi: 10.1038/sj.cdd.4401946. [DOI] [PubMed] [Google Scholar]
- Kamata H., Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Katiyar S., Jiao X., Wagner E., Lisanti M. P., Pestell R. G. Somatic excision demonstrates c-Jun induces cellular migration and invasion through induction of stem cell factor. Mol. Cell Biol. 2007;27:1356–1369. doi: 10.1128/MCB.01061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C. Y., Yang D. D., Samanta Roy D. R., Davis R. J., Rakic P., Flavell R. A. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M. Signaling by the germinal center kinase family of protein kinases. J. Biol. Chem. 1999;274:5259–5262. doi: 10.1074/jbc.274.9.5259. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang C., Jiao X., Katiyar S., Casimiro M. C., Prendergast G. C., Powell M. J., Pestell R. G. Alternate cyclin D1 mRNA splicing modulates p27KIP1 binding and cell migration. J. Biol. Chem. 2008;283:7007–7015. doi: 10.1074/jbc.M706992200. [DOI] [PubMed] [Google Scholar]
- MacLaren A., Black E. J., Clark W., Gillespie D. A. c-Jun-deficient cells undergo premature senescence as a result of spontaneous DNA damage accumulation. Mol. Cell. Biol. 2004;24:9006–9018. doi: 10.1128/MCB.24.20.9006-9018.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S. Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann. NY Acad. Sci. 2000;908:219–225. doi: 10.1111/j.1749-6632.2000.tb06649.x. [DOI] [PubMed] [Google Scholar]
- Mishin V., Gray J. P., Heck D. E., Laskin D. L., Laskin J. D. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic. Biol. Med. 2010;48:1485–1491. doi: 10.1016/j.freeradbiomed.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister P., et al. Cyclin D1 governs adhesion and motility of macrophages. Mol. Biol. Cell. 2003;14:2005–2015. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohl H., Jordan W. The metabolic fate of mitochondrial hydrogen peroxide. Eur. J. Biochem. 1980;111:203–210. doi: 10.1111/j.1432-1033.1980.tb06094.x. [DOI] [PubMed] [Google Scholar]
- Okado-Matsumoto A., Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- Podar K., et al. Up-regulation of c-Jun inhibits proliferation and induces apoptosis via caspase-triggered c-Abl cleavage in human multiple myeloma. Cancer Res. 2007;67:1680–1688. doi: 10.1158/0008-5472.CAN-06-1863. [DOI] [PubMed] [Google Scholar]
- Radi R., Turrens J. F., Chang L. Y., Bush K. M., Crapo J. D., Freeman B. A. Detection of catalase in rat heart mitochondria. J. Biol. Chem. 1991;266:22028–22034. [PubMed] [Google Scholar]
- Sage J., Miller A. L., Perez-Mancera P. A., Wysocki J. M., Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- Sattler M., Winkler T., Verma S., Byrne C. H., Shrikhande G., Salgia R., Griffin J. D. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93:2928–2935. [PubMed] [Google Scholar]
- Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman R. L. Comments on “propagation of plane waves through two parallel dielectric sheets.” IEEE Trans. Biomed. Eng. 1976;23:269–270. doi: 10.1109/tbme.1976.324643. [DOI] [PubMed] [Google Scholar]
- Shaulian E., Karin M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Sinha A. K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Matsumura I., Ezoe S., Satoh Y., Sakamaki T., Albanese C., Machii T., Pestell R. G., Kanakura Y. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol. Cell. 2002;9:1017–1029. doi: 10.1016/s1097-2765(02)00522-1. [DOI] [PubMed] [Google Scholar]
- Temkin V., Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol. Rev. 2007;220:8–21. doi: 10.1111/j.1600-065X.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- Udou T., Hachisuga T., Tsujioka H., Kawarabayashi T. The role of c-jun protein in proliferation and apoptosis of the endometrium throughout the menstrual cycle. Gynecol. Obstet. Invest. 2004;57:121–126. doi: 10.1159/000075701. [DOI] [PubMed] [Google Scholar]
- Vleugel M. M., Greijer A. E., Bos R., van der Wall E., van Diest P. J. c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer. Hum. Pathol. 2006;37:668–674. doi: 10.1016/j.humpath.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Wang C., et al. Cyclin D1 repression of peroxisome proliferator-activated receptor gamma (PPARgamma) expression and transactivation. Mol. Cell. Biol. 2003;23:6159–6173. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Anton M., Graham F. L., Bacchetti S. High frequency recombination between loxP sites in human chromosomes mediated by an adenovirus vector expressing Cre recombinase. Somat. Cell Mol. Genet. 1995;21:429–441. doi: 10.1007/BF02310209. [DOI] [PubMed] [Google Scholar]
- Xia Y., Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Xia Z., Dickens M., Raingeaud J., Davis R. J., Greenberg M. E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Zenz R., Scheuch H., Martin P., Frank C., Eferl R., Kenner L., Sibilia M., Wagner E. F. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev. Cell. 2003;4:879–889. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Zhou X., Gallazzini M., Burg M. B., Ferraris J. D. Contribution of SHP-1 protein tyrosine phosphatase to osmotic regulation of the transcription factor TonEBP/OREBP. Proc. Natl. Acad. Sci. USA. 2010;107:7072–7077. doi: 10.1073/pnas.1002795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.