Reactive oxygen species (ROS) generated by the NADPH oxidase system have been shown to be necessary for the invadopodia formation and function. We show here that the abolishment of Src-mediated phosphorylation of NoxA1 and Tks4 blocks their binding, decreases Nox1-dependent ROS generation, and inhibits the invadopodia formation and ECM degradation.
Abstract
The NADPH oxidase family, consisting of Nox1-5 and Duox1-2, catalyzes the regulated formation of reactive oxygen species (ROS). Highly expressed in the colon, Nox1 needs the organizer subunit NoxO1 and the activator subunit NoxA1 for its activity. The tyrosine kinase c-Src is necessary for the formation of invadopodia, phosphotyrosine-rich structures which degrade the extracellular matrix (ECM). Many Src substrates are invadopodia components, including the novel Nox1 organizer Tks4 and Tks5 proteins. Nox1-dependent ROS generation is necessary for the maintenance of functional invadopodia in human colon cancer cells. However, the signals and the molecular machinery involved in the redox-dependent regulation of invadopodia formation remain unclear. Here, we show that the interaction of NoxA1 and Tks proteins is dependent on Src activity. Interestingly, the abolishment of Src-mediated phosphorylation of Tyr110 on NoxA1 and of Tyr508 on Tks4 blocks their binding and decreases Nox1-dependent ROS generation. The contemporary presence of Tks4 and NoxA1 unphosphorylable mutants blocks SrcYF-induced invadopodia formation and ECM degradation, while the overexpression of Tks4 and NoxA1 phosphomimetic mutants rescues this phenotype. Taken together, these results elucidate the role of c-Src activity on the formation of invadopodia and may provide insight into the mechanisms of tumor formation in colon cancers.
INTRODUCTION
Members of the Nox family are transmembrane proteins that catalyze the NADPH-dependent one-electron reduction of oxygen to form superoxide (Lambeth, 2004). To date, seven members of this family have been described: Nox1-5 and Duox (dual oxidase) 1 and 2. Historically, Nox enzymes have been viewed as transmembrane proteins expressed in leukocytes that play a vital role in host defense against microorganisms (Bokoch and Knaus, 2003; Geiszt and Leto, 2004). Although this is clearly an important function, it is now evident that different Nox enzymes play diverse roles in nonleukocyte cells and tissues.
Nox enzymes differ in both tissue distributions and mechanisms by which their activity is regulated (Krause, 2004; Lambeth et al., 2007). Nox2 is expressed by phagocytic leukocytes, and its activity is triggered by inflammatory mediators which induce the assembly of four cytosolic regulatory proteins (p40phox, p47phox, p67phox, and Rac2-GTPase) with the Nox2 core enzyme to stimulate superoxide formation. Nox1 and Nox3 are highly expressed in the colon epithelium and in the inner ear, respectively, and their activity is also regulated by Rac1-GTPase and by related cytosolic adaptors, known as the activator subunit NoxA1 (homologous to p67phox) and the organizer subunits NoxO1 (homologous of p47phox) (Banfi et al., 2003; Geiszt et al., 2003; Takeya et al., 2003). Recently, we have shown that tyrosine kinase c-Src substrate Tks4 and Tks5 proteins are novel members of the organizer superfamily (Gianni et al., 2009), which directly bind the N-terminal Proline-Rich Region (PRR) of NoxA1 (Gianni et al.). Finally, Nox4 is widely distributed in the kidney, bone and vascular cells and its activity is independent of Rac-GTPase.
All Nox enzymes have been implicated in physiological and pathophysiological processes (Bedard and Krause, 2007; Lambeth et al., 2008). Particularly, Nox1-dependent ROS generation has been shown to play a pivotal role in cell signaling, cell growth, angiogenesis, motility and blood pressure regulation (Sancho and Fabregat, 2010; Arbiser et al., 2002; Gavazzi et al., 2006; Komatsu et al., 2008; Sadok et al., 2008). Interestingly, ROS generated via Nox1 have been reported to contribute to a growing number of diseases, including cancer, atherosclerosis, hypertension, neurological disorders, and inflammation (Cave et al., 2006; Cifuentes and Pagano, 2006; Rokutan et al., 2006; Block, 2008; Nauseef, 2008; Ushio-Fukai and Nakamura, 2008).
Despite much investigation, very little is known about the signaling events regulating the formation of ROS by Nox proteins under normal, much less pathological, conditions. Increasing evidence suggests that phosphorylation of various Nox proteins or their regulatory cofactors or both may play important roles in regulating the activity of these enzymes (Bokoch et al., 2009). For instance, it has been shown that during neutrophil activation, the phosphorylation by protein kinase C (PKC) on serine (Ser) and threonine (Thr) residues of p47phox is critical to Nox2 priming and activation (El-Benna et al., 2008). This phosphorylation event induces the translocation of p47phox/p67phox complex to the plasma membrane, where it primes the oxidase for activation. On the other hand, our group has shown that protein kinase A (PKA) inhibits Nox1 activity in colon epithelial cells by phosphorylating NoxA1 at two distinct sites, Ser172 and Ser461 (Kim et al., 2007). On this phosphorylation event, NoxA1 is sequestered in the cytosol, thus resulting in inhibition of Nox1-dependent ROS generation. Finally, we have also described a signaling axis linking the activation of the kinase c-Src to increased Nox1-dependent ROS generation through the tyrosine phosphorylation-mediated activation of Rac1 exchange factor Vav2 (Gianni et al., 2008).
Strong evidence has highlighted the importance of Nox1-dependent ROS generation in mechanisms of cancer invasion (Arnold et al., 2001; Mitsushita et al., 2004; Kamata, 2009). Consistent with this, our group has shown that in human colon cancer cells Nox1-derived ROS are necessary for the formation of extracellular matrix (ECM)-degrading actin-rich cellular structures known as invadopodia (Gianni et al., 2009). Invadopodia appear as actin protrusions of the ventral plasma membrane and contain proteases capable of degrading the ECM (Linder, 2007; Gimona et al., 2008). Their formation in human cancer cells correlates with their invasiveness both in vitro and in vivo (Weaver, 2006). The tyrosine kinase c-Src is highly expressed and active in human colon epithelial tumors (Bolen et al., 1987; Irby and Yeatman, 2000). Consistent with this, c-Src activation is required for the formation of functional invadopodia (Lowe et al., 1993). There is substantial evidence for the importance of c-Src in invadopodia formation. Many Src substrates are obligate invadopodia components, including cortactin, Tks4, and Tks5 (Abram et al., 2003; Baldassarre et al., 2003; Buschman et al., 2009). Interestingly, Tks proteins (tyrosine kinase substrate with five or four SH3 domains) have been shown to be required for the formation of invadopodia and to promote cancer cell invasion (Seals et al., 2005; Blouw et al., 2008; Buschman et al., 2009). However, despite much effort, the signals and the molecular machinery involved in the redox-dependent regulation of Src-induced invadopodia formation remain unclear.
In this study, we investigate the role of Src phosphorylation in the redox-dependent formation of functional invadopodia in human colon cancer cells. First, we show that the interaction of NoxA1 and Tks proteins is dependent on Src activity. Moreover, the abolishment of Src-mediated phosphorylation of Tyr110 on NoxA1 and of Tyr508 on Tks4 blocks their binding and decreases Nox1-dependent ROS generation. Interestingly, the presence in human colon cancer cells of Tks4 and NoxA1 unphosphorylable mutants blocks SrcYF-induced invadopodia formation and ECM degradation, while the overexpression of Tks4 and NoxA1 phosphomimetic mutants rescues this phenotype.
MATERIALS AND METHODS
DNA Plasmids, Reagents, and Antibodies
Cell culture medium, fetal bovine serum, supplements, and Hank's Balanced Salt Solution (HBSS, Cat# 24020-117) were from Invitrogen (Carlsbad, CA). Nox1, SrcYF, SrcKM, NoxO1, and Rac1-Q61L expression plasmids were previously described (Gianni et al., 2008). Myc- and Flag-tagged NoxA1 and Tks4 and Tks5 expression plasmids were described previously (Gianni et al., 2009). GST-tagged NoxA1 and Tks4 were described previously (Gianni et al., 2009). The NoxA1 Y110, Tks4 Y25, Y373, and Y508 mutant constructs were generated inserting the following mutations: Y110A and Y110E for NoxA1, and Y25F, Y373F, Y508F, and Y508E for Tks4 by using QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) as described (Kao et al., 2008). Plasmids for transfection were purified using the Qiagen Qiafilter system. DLD1 and HT29 colonic adenocarcinoma cells (Cat# CCL-221 and HTB-38) were purchased from ATCC, Manasass, VA. DPI (D2926), horseradish peroxidase (HRP) (77330), PP2 Src inhibitor (529576), its nonfunctional analog PP3 (529574) and luminol (09253) were purchased from Sigma, St. Louis, MO. The following antibodies were purchased as indicated: anti-phosphotyrosine (p-Tyr) antibody 4G10 (05–321) from Millipore, Billerica, MA, monoclonal cortactin antibody 4F10 from Millipore. Anti-mouse Alexa-Fluor-568 and Alexa-Fluor-568 phalloidin were purchased from Invitrogen, Carlsbad, CA. 9E10 anti-myc antibody was prepared in-house; polyclonal anti-NoxA1 antibody was generated in house as part of the Centers for Disease Control Program PO1 CI000095; rabbit polyclonal Tks4 and Tks5 antibodies were obtained from Dr. Sara Courtneidge (Sanford Burnham Medical Research Institute) and were previously described in (Lock et al., 1998; Buschman et al., 2009). Protein G-Sepharose and Glutathione beads were from GE Health Care, Piscataway, NJ.
Cell Culture, Transfection, and Treatments
Human embryonic kidney HEK293 and human DLD1 colonic adenocarcinoma cells were maintained in DMEM (Invitrogen) containing 10% heat-inactivated fetal bovine serum (Invitrogen), 2 mM glutamine, and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in 5% CO2. For transfection, cells were plated in either six-well plates (for ROS measurements and microscopy analysis) or in 10 cm-diameter plates (for coimmunoprecipitation experiments) at appropriate density, grown overnight, and then transfected by using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Sixteen hours after transfection, cells were processed accordingly.
For inhibitor studies, cells were treated with 10 μM DMSO-dissolved Src inhibitor PP2 or its nonfunctional analog PP3 or DMSO alone for 16 h. Calf intestinal phosphatase (CIP) was purchased from New England BioLabs, Ipswich, MA and used according to the manufacturer's instructions.
Western Blot and Immunoprecipitation
For the preparation of total cell extracts, monolayer cultures were washed in cold PBS and lysed in appropriate amount of RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1%, Nonidet P-40, 0.25% sodium deoxycholate, and 1 mM EDTA) supplemented with 1 mM leupeptin, 1 mM aprotinin, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride (PMSF). The extracts were clarified by centrifugation at 16,000 × g at 4°C, and the protein concentration was estimated using the Bio-Rad assay according to the manufacturer's instructions. For immunoprecipitations, 1–2 μl of specific antibodies were incubated with 1 mg of protein lysates for 2 h at 4°C, followed by 30 min incubation with 20 μl of Protein G–Sepharose (GE Healthcare, Piscataway, NJ). The samples were incubated for an additional 30 min after adding 20 μl of protein G plus-Sepharose. Immunoprecipitates were washed three times in lysis buffer and proteins released by boiling in Laemmli SDS sample buffer, and samples were resolved by 10% SDS-PAGE. Gels were transferred onto nitrocellulose membranes using the electrophoretic transfer cell (Bio-Rad, Hercules, CA) at 100 V for 1 h. After blocking with nonfat dry milk (5%), proteins were probed overnight using antibodies at appropriate dilution. Anti-Myc and anti p-Tyr dilution was (1:1000), anti-GST was used at a dilution of (1:10,000). Rabbit polyclonal antibodies against NoxA1, Tks4, and Tks5 were used at a dilution of (1:5000). The excess antibody was removed by sequential washing of the membranes in Tween-PBS, and then a 1:5000 dilution of the appropriate horseradish peroxidase-conjugated secondary antibody (Pierce Chemical) was added to the filters followed by incubation for 1 h at room temperature. After sequential washing of the membranes in T-PBS to remove excess secondary antibody, the signals were detected by chemiluminescence using the ECL system (Pierce Chemical). Blots were stripped and reprobed as necessary.
Kinase Assay
In vitro kinase assay was performed as described previously (DerMardirossian et al., 2006) using as substrates NoxA1 wt and NoxA1 Y110A proteins immunoprecipitated from HEK293 transfected accordingly. Immunoprecipitated proteins were washed three times in lysis buffer and two additional times in kinase buffer (50 mM HEPES pH = 7.5, 10 mM MgCl2, 2 mM MnCl2, 0.2 mM DTT). Recombinant active Src (0.5 μg) from Millipore (Cat # 14-326) was incubated with the immunoprecipitated proteins resuspended in kinase buffer for 30 min at 30°C in presence of 100 μM ATP per reaction. Proteins were released by boiling in Laemmli SDS sample buffer, and samples were resolved by 10% SDS-PAGE and processed accordingly.
Expression of Recombinant Proteins and GST-Pull Downs
The recombinant NoxA1 and Tks4 were expressed and purified as GST-fusion proteins by isopropyl-thiogalactoside (IPTG) induction of Escherichia coli cultures harboring the corresponding pGEX-4T1 vector. Affinity purification of the GST-fusion proteins was performed on Glutathione-Sepharose resin using standard isolation protocols. For the pulldown experiments, equal amounts of fusion proteins (10 μg) were bound to glutathione-Sepharose beads (10 μl) and challenged with 10 μg of HEK293 cell lysate transfected as indicated in figure legends. Unbound proteins were removed by washing the beads three times with RIPA buffer, whereas retained proteins were resolved by SDS-PAGE and analyzed by Western blot.
Measurement of ROS
This assay was performed as previously described (Gianni et al., 2008). Briefly, 16 h after transfection, 5 × 105 HEK293 cells per assay were dispensed in white 96-well plate (Berthold) and mixed with 250 μM luminol and 1U horseradish peroxidase HRP in 200 μl total final volume in each well. Chemiluminescence was recorded using 96-well plate luminometer (Berthold) 5 min after the addition of HRP/luminol mixture for 30 min at room temperature without any stimulation.
Confocal and Epifluorescence Microscopy
Twenty-four hours after transfection, DLD1 cells plated on glass or FITC-labeled gelatin-coated coverslips were fixed in 4% paraformaldehyde (PFA) at room temperature for 10 min. Successively, cells were permeabilized in 0.5% Triton for 10 min and blocked in 2% BSA in PBS for 45 min at room temperature. Cells were then immunolabeled as indicated in the figure legends with appropriate primary and Alexa-Fluor 568–conjugated secondary antibodies. F-actin was detected by using Alexa-Fluor 568-conjugated phalloidin. Cells were mounted on slides with Mowiol mounting medium (Invitrogen) according to the manufacturer's instructions. Epifluorescence and confocal images of fixed cells were acquired using the same setup described in (Gianni et al., 2009).
ECM Degradation Assays
Fluorescently labeled gelatin-coated coverslips were prepared as previously described (Blouw et al., 2008). Twenty-four hours after transfection, cells were trypsinized and plated on FITC-labeled gelatin-coated coverslips. Forty-eight hours later, cells were fixed in 4% PFA, stained with Alexa-Fluor-568 phalloidin, and visualized by epifluorescence microscopy (×60).
Statistical Analysis
In this study overall, representative experiments from at least three independent experiments are shown. Results for each experiment are given as the mean of triplicates ± SD. Statistically significant differences between sample groups were determined using two-tailed t tests (Microsoft Excel, Redmond WA). p value <0.01 was considered significant, unless differently indicated (see legend to Figure 7B).
Figure 7.
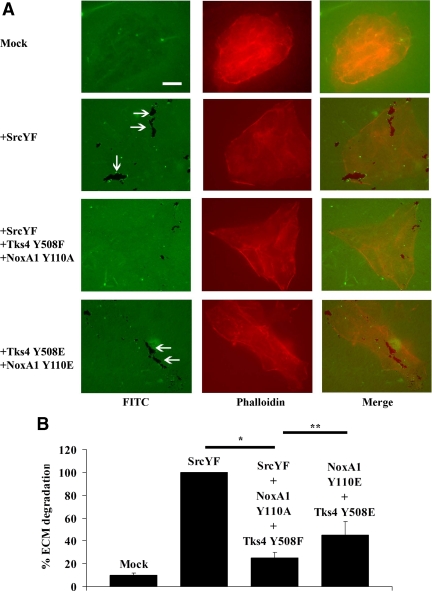
The presence of Tks4 and NoxA1 unphoshorylable mutants blocks SrcYF-induced ECM degradation in DLD1 cells, whereas the presence of their phosphomimetic mutants partially rescues this phenotype. (A) The analysis of the ability of DLD1 cells to degrade the ECM shows that the presence of Tks4 and NoxA1 unphoshorylable mutants blocks SrcYF-induced ECM degradation in DLD1 cells, whereas the presence of their phosphomimetic mutants partially rescues this phenotype. DLD1 cells were transfected as indicated, and 24 h later they were trypsinized and plated on FITC-labeled gelatin-coated coverslips. After 48 h, cells were fixed in 4% PFA, stained with Alexa-Fluor-568 phalloidin, and visualized by epifluorescence microscopy (×60). The white arrows indicate areas in which cells (in red) degrade the ECM (in green). The merge is shown in the right column. Scale bars, 10 μm. One representative image from three separate experiments is shown. (B) Quantification from three independent biological experiments shown in A is given: for each experiment, the total degradation area was obtained as sum of degradation areas calculated using Metamorph software from 25 random fields and reported as percentage (SrcYF was set as 100%). In the graph, error bars represent SEM. *p < 0.01; **p <0.05 (Mann–Whitney U test).
RESULTS
The Interaction between NoxA1 and Tks Proteins Is Dependent on Src Activity
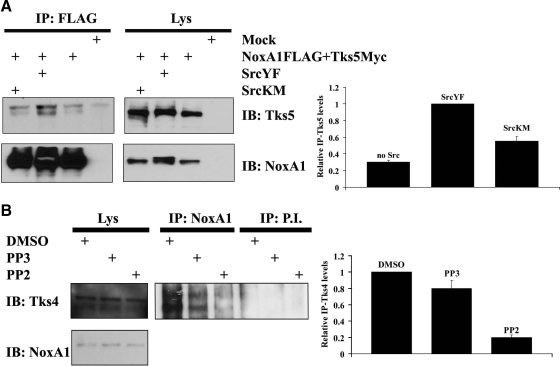
We had previously shown that Tks proteins are novel members of p47phox organizer superfamily as they can support Nox1-dependent ROS generation by binding the N-terminal PRR of the activator protein NoxA1. Our group has also demonstrated that the presence of activated Src in different cell lines induces Nox1-dependent ROS generation (Gianni et al., 2008). To further investigate the mechanisms by which c-Src induces Nox1-dependent ROS generation, we tested whether the interaction between Tks proteins and NoxA1 was dependent on Src activity. To this aim, coimmunoprecipitation experiments were performed in which HEK293 cells were cotransfected with Flag-tagged NoxA1 and Myc-tagged Tks5 expression vectors in presence of constitutive active or dominant negative Src (SrcYF or SrcKM, respectively). We observed an increased interaction between NoxA1 and Tks5 in presence of SrcYF, as shown in Figure 1A (left panel) and in the quantification of three independent experiments in Figure 1A (right panel). Although SrcKM overexpression showed some residual activity, its effect on inducing the interaction between these two proteins was substantially less than that of SrcYF. To confirm our findings, we repeated similar coimmunoprecipitation experiments using this time Flag-tagged Tks5 and Myc-tagged NoxA1 expression vectors to transfect HEK293 cells. Again, we observed that the presence of SrcYF increased the affinity between NoxA1 and Tks5, while the overexpression of SrcKM did not cause the same effect, as indicated in Supplemental Figure S1.
Figure 1.
The interaction between NoxA1 and Tks proteins is dependent on Src activity. (A) The interaction between NoxA1 and Tks5 is dependent on Src activity. In the left panel, HEK293 cells were transfected as indicated with Myc-tagged Tks5 and with Flag-tagged NoxA1 in presence of constitutive active or dominant negative Src (SrcYF or SrcKM respectively). After 24 h, cells were lysed and immunoprecipitation (IP) was carried out (see Material and Methods) using Flag antibody. Lys indicates lysate before IP was performed. The interaction between NoxA1 and Tks5 was tested by immunoblot (IB) using Tks5 antibody (upper section), while comparable expression levels of transfected Flag-tagged NoxA1 in cell lysates and immunoprecipitation efficiency was assessed by reblotting the membrane with NoxA1 specific antibody (lower section). One representative experiment from three separate experiments is shown. In the right panel, the results from three independent experiments performed as above were quantified. The levels of immunoprecipitated Tks5 (IP-Tks5) for each condition were normalized to the respective total Tks5 present in the Lys. The IP-Tks5 levels in presence of SrcYF were set to 1 (mean ± SEM from three independent experiments). (B) The interaction between NoxA1 and Tks4 is dependent on Src activity. In the left panel, DLD1 cells were treated as indicated with DMSO control, Src inhibitor PP2, or its nonfunctional analog PP3. After 16 h, cells were lysed and IP was carried out using NoxA1-specific antibody or pre-immune serum (P.I.). Specific interaction between endogenous Tks4 and NoxA1 was detected using Tks4-specific antibody (upper section), while the presence of NoxA1 in cell lysates was verified by reblotting the membrane with NoxA1-specific antibody (lower section). One representative experiment from three separate experiments is shown. In the right panel, the results from three independent experiments performed as above were quantified. The levels of immunoprecipitated Tks4 (IP-Tks4) for each condition were normalized to the respective total Tks4 present in the Lys. The IP-Tks4 levels in DMSO treatment condition were set to 1 (mean ± SEM from three independent experiments).
Human DLD1 colon cancer cells represent an ideal system to study the dependency on Src activity of the endogenous interaction between Tks4 protein and NoxA1. In fact, we previously established that these cells i) express a high level of active Src and ii) endogenously express only Nox1, NoxA1 and Tks4 (Gianni et al., 2009). In addition, we have demonstrated that in DLD1 cells Tks4 and NoxA1 interact directly, thus regulating Nox1-mediated formation of invadopodia and ECM degradation. To confirm that the endogenous interaction between Tks4 and NoxA1 is dependent on Src activity, we performed coimmunoprecipitation experiments in DLD1 cells treated with DMSO control, Src inhibitor PP2, or its nonfunctional analog PP3. As shown in Figure 1B (left panel), PP2 treatment abolished the interaction between Tks4 and NoxA1, while PP3 or DMSO treatment did not cause significant changes (see the quantification of three independent experiments in Figure 1B, right panel). Our data indicate that the extent of the interaction between endogenous or ectopically-expressed NoxA1 and Tks proteins is dependent on Src activity.
c-Src Directly Phosphorylates NoxA1 on Tyr110
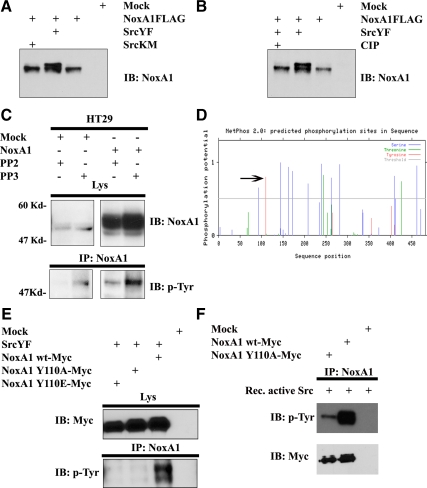
It was previously established by our group that the activation of the kinase c-Src leads to increased Nox1-dependent ROS generation through the tyrosine phosphorylation-mediated activation of Rac1 exchange factor Vav2 (Gianni et al., 2008). However, it cannot be excluded that c-Src might directly target other regulatory components of Nox1 pathway such as the activator protein NoxA1. To test this hypothesis, HEK293 cells were transfected with Flag-tagged NoxA1 in presence of SrcYF or SrcKM and protein extracts were analyzed by SDS-PAGE using a specific NoxA1 antibody. As shown in Figure 2A, we observed the presence of a doublet corresponding to NoxA1 only when this was coexpressed with SrcYF, suggesting that NoxA1 might be phosphorylated by Src under these conditions. To confirm this, cell extracts from HEK293 cells cotransfected with NoxA1 and SrcYF were treated with or without calf intestinal phosphatase (CIP). Figure 1B shows that the phosphorylation doublet was not detected when cell extracts were pre-treated with CIP, indicating that NoxA1 can be phosphorylated.
Figure 2.
Src phosphorylates NoxA1 on Tyr110. (A and B) NoxA1 is targeted by Src-mediated phosphorylation. One representative experiment from three separate experiments is shown. (A) HEK293 cells were transfected with Flag-tagged NoxA1 along with SrcYF or SrcKM as indicated. After 24 h, cells were lysed and cell extract were resolved by SDS-PAGE using NoxA1-specific antibody. (B) HEK293 cells were transfected as indicated and cell extracts were treated or not with calf intestinal phosphatases (CIP) as described in Material and Methods. Proteins were resolved by SDS-PAGE and the presence of a phosphorylation doublet corresponding to NoxA1 was detected using NoxA1-specific antibody. (C) Endogenous and ectopically-expressed NoxA1 is Tyr-phosphorylated by Src in HT29 cells. HT29 cells were transfected with empty vector or with NoxA1 expression vector and treated as indicated with PP2 or PP3. After 24 h, cells were lysed and IP was performed using NoxA1-specific antibody. The levels of endogenous or ectopically-expressed NoxA1, which was Tyr-phosphorylated, were analyzed using anti-p-Tyr antibody (lower panel), while the presence of NoxA1 in cell lysates was verified by reblotting the membrane with NoxA1-specific antibody (upper section). One representative experiment from three separate experiments is shown. (D) A schematic representation of the predicted phosphorylation sites on human NoxA1 protein (accession number NM_006647) using bioinformatics tools available online (www.cbs.dtu.dk). The phosphorylation potential represents the likeness of a residue to be phosphorylated. Its value is included between 0 (low phosphorylation potential) and 1 (high phosphorylation potential). Phosphorylation potential (y axis) is plotted for each amino acid residue of NoxA1 (x axis). The blue and the green lines indicate serine (Ser) and threonine (Thr) residues which could be targeted by Ser/Thr kinases. The red lines indicate tyrosine (Tyr) residue which can be phosphoryalted by Tyr-kinase. The black arrow indicates the Tyr110 of NoxA1 as the residue with the highest phosphorylation potential for Tyr-kinase (=0.87). (E) Src phosphorylates NoxA1 on Tyr110. HEK293 cells were transfected as indicated with SrcYF and with Myc-tagged wild type NoxA1, unphosphorylable NoxA1 Y110A and phosphomimetic NoxA1 Y110E. After 24 h, cells were lysed and IP was performed using NoxA1-specific antibody. The levels of Tyr-phosphorylated NoxA1 were analyzed using anti-p-Tyr antibody (lower panel), while comparable expression levels of transfected Myc-tagged NoxA1 mutants was verified by reblotting the membrane with Myc antibody (upper section). One representative experiment from three separate experiments is shown. (F) Src phosphorylates NoxA1 directly. NoxA1 wt and NoxA1 Y110A were transfected in HEK293 cells and immunoprecipitated using NoxA1 specific antibody. An in vitro kinase assay was performed using recombinant active Src as in Materials and Methods. The levels of Tyr-phosphorylated NoxA1 were analyzed using anti-p-Tyr antibody (upper panel), while immunoprecipitation efficiency was verified by reblotting the membrane with Myc antibody (lower section).
Human HT29 colon cancer cells were also reported to endogenously express NoxA1 and high level of active Src. To test whether NoxA1 is targeted by Src under more physiological conditions, HT29 cells were treated with Src inhibitor PP2 or PP3, endogenous or ectopically-expressed NoxA1 was immunoprecipitated and the levels of tyrosine (Tyr)-phosphorylated NoxA1 were analyzed using p-Tyr antibody. As shown in Figure 2C, PP2 treatment strongly diminished the levels of Tyr-phosphorylation of both endogenous and ectopically-expressed NoxA1 compared with PP3 treatment.
To identify the Tyr residue(s) on NoxA1 targeted by Src, we used bioinformatics tools available online (www.cbs.dtu.dk) and scanned human NoxA1 protein (accession number NM_006647) for predicted phosphorylation sites. As illustrated in Figure 2D, several threonine (Thr) and serine (Ser) phosphorylation sites are predicted with high score. Of note, among those are included the Ser172 and Ser461, which our group has shown to be the sites for PKA phosphorylation (Kim et al., 2007). However, only Tyr110 on NoxA1 was recognized as a high-score phosphorylation site for tyrosine kinases. To verify whether Tyr110 was the site for c-Src phosphorylation on NoxA1, we generated NoxA1 phosphomimetic and unphosphorylable mutants (NoxA1-Y110E and NoxA1-Y110A, respectively), and used them to transfect HEK293 cells along with SrcYF. As indicated in the Western blot analysis in Figure 2E, (as expected) SrcYF phosphorylates wild-type NoxA1, while the disruption of Y110 residue on NoxA1 blocked its SrcYF-induced Tyr-phosphorylation. These results confirm that c-Src phosphorylates NoxA1 on its Tyr110 residue.
c-Src has been reported to activate several cellular tyrosine kinases (Ingley, 2008), which in turn could phosphorylate NoxA1. To rule out this possibility, we performed an in vitro kinase assay in which recombinant active Src was incubated in presence of immunoprecipitated NoxA1 wt and NoxA1 Y110A mutant. As shown in Figure 2F, Src strongly phosphorylates NoxA1 wt whereas Tyr-phoshorylation of NoxA1 Y110A is significantly decreased. These data indicate that Src directly phosphorylates NoxA1 on Tyr110.
The Abolishment of NoxA1 Phosphorylation on Tyr110 Blocks Its Binding to Tks4 and Decreases Nox1-Dependent ROS Generation
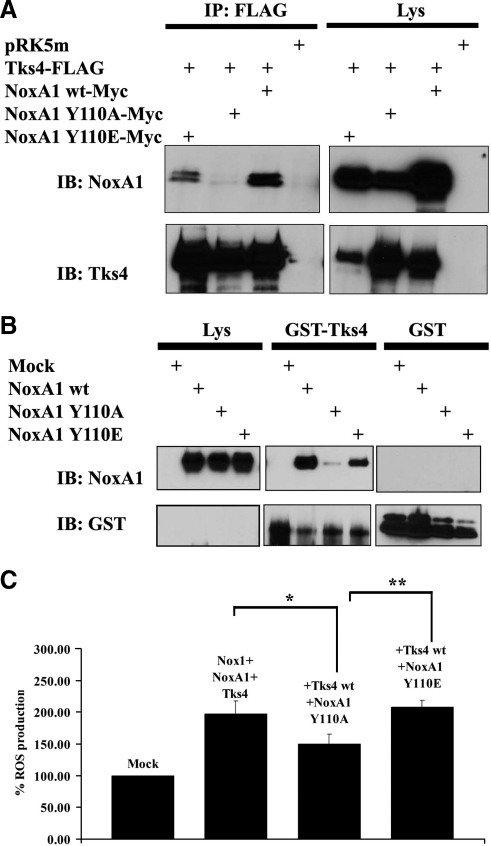
To verify whether Src-mediated phosphorylation of NoxA1 on its Tyr110 had an effect on its binding to Tks4, HEK293 cells were cotransfected with Flag-tagged Tks4 and either with NoxA1 wt, NoxA1 Y110E, and NoxA1 Y110A mutant. As clearly indicated in the coimmunoprecipitation experiments in Figure 3A, NoxA1 wt bound Tks4 as expected, whereas the presence of NoxA1 Y110A unphosphorylable mutant completely abolished its binding to Tks4. Conversely, NoxA1 Y110E phosphomimetic mutant restored this interaction. To validate this finding, we pursued an alternative biochemical approach, performing a pulldown using recombinant GST-tagged Tks4 protein to probe HEK293 cell extracts expressing either NoxA1 wt, NoxA1 Y110E and NoxA1 Y110A mutants. Figure 3B confirms that GST-Tks4 bound NoxA1 wt and NoxA1 Y110E but not NoxA1 Y110A.
Figure 3.
The abolishment of NoxA1 phosphorylation on Tyr110 blocks its binding to Tks4 and decreases Nox1-dependent ROS generation. (A and B) The integrity of Tyr110 of NoxA1 is necessary for its binding to Tks4. One representative experiment from three separate experiments is shown. (A) Coimmunoprecipitation analysis was performed in HEK293 cells transfected as indicated with Flag-tagged Tks4 and Myc-tagged wild type NoxA1, unphosphorylable NoxA1 Y110A, and phosphomimetic NoxA1 Y110E. After 24 h, cells were lysed and immunoprecipitation (IP) was carried out using Flag antibody. The interaction between NoxA1 and Tks4 was tested by immunoblot (IB) using NoxA1 antibody (upper section), while comparable expression levels of transfected Flag-tagged Tks4 in cell lysates and immunoprecipitation efficiency was assessed by reblotting the membrane with Tks4 specific antibody (lower section). (B) HEK293 cells were transfected as indicated with empty vector or with wild-type NoxA1, unphosphorylable NoxA1 Y110A, and phosphomimetic NoxA1 Y110E. After 24 h, cells were lysed and cell lysates were used as the source of different NoxA1 proteins and incubated as indicated with equal amounts of GST alone or GST-fusion Tks4 protein, which were prebound to glutathione-Sepharose beads. GST pulldown was performed as described in Material and methods. The interaction between GST-fusion Tks4 and different NoxA1 proteins and comparable levels of NoxA1 proteins in cell lysates was tested using the NoxA1 antibody (upper panel). The reblot using GST-specific antibody in the lower panel indicates that GST-fusion protein were present at similar levels in the GST pulldown analysis. (C) The abolishment of NoxA1 phosphorylation on Tyr110 decreases Tks4-mediated Nox1-dependent ROS generation. ROS generation was monitored in HEK293 cells transfected as indicated using luminol-based chemiluminescence (CL) assay. One representative experiment from three separate experiments is shown, and data are given as mean of triplicates ± SD. *p < 0.008; **p < 0.01.
To test the functional relevance of NoxA1 Tyr-phosphorylation by Src on Nox1-dependent ROS generation, HEK293 cells were cotransfected with Nox1, Tks4, and either NoxA1 wt, NoxA1 Y110E, or NoxA1 Y110A constructs and ROS formation was monitored using luminol-based chemiluminescence (CL) assay. As shown in Figure 3C, we observed a consistent 30% decrease of ROS formation when NoxA1 Y110A mutant was expressed, but the presence of NoxA1 Y110E construct did not change the levels of ROS production compared with NoxA1 wt used as a positive control. These experiments strengthen the findings illustrated in Figure 3, A and B and support the hypothesis that Src-mediated phosphorylation of NoxA1 on Tyr110 affects its binding to Tks4 and Nox1-dependent ROS generation.
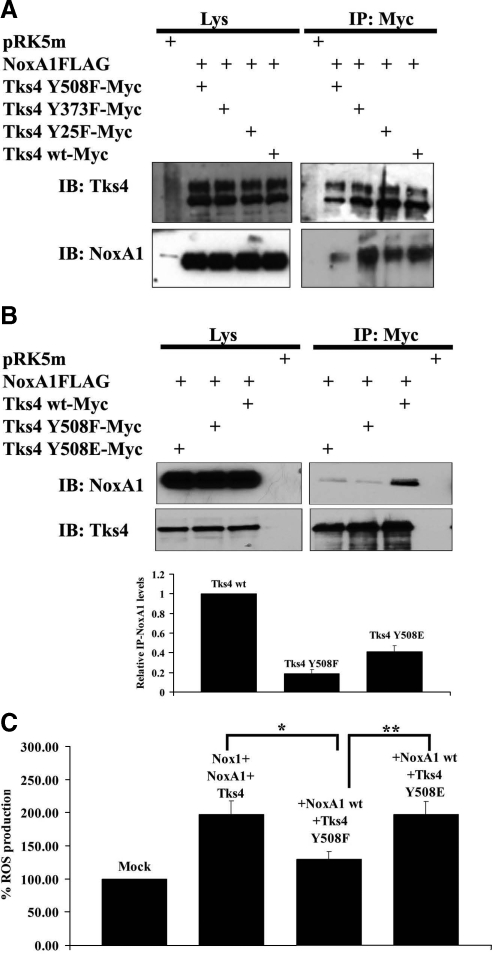
The Integrity of Tyr508 on Tks4 Is Necessary for Its Binding to NoxA1 and for Nox1-Dependent ROS Generation
It has been reported that Tks4 is Tyr-phosphorylated by Src in Src-3T3 mouse fibroblasts and other cell systems (Buschman et al., 2009). The authors demonstrated that Src-mediated phosphorylation of Tks4 was reduced when Tyr 25, 373, and 508 were individually mutated to phenylalanine (Phe), and essentially absent when the mutations were combined. To test whether Src-mediated phosphorylation of Tks4 affects its binding to NoxA1, we generated the same Tks4 unphosphorylable single mutants (Tks4-Y25F, Tks4-Y373F, and Tks4-Y508F) and tested them in coimmunoprecipitation experiments along with Flag-tagged NoxA1. As shown in Figure 4A, only Tks4 Y508F bound NoxA1 with less efficiency than the other Tks4 unphoshorylable mutants as well as Tks4 wild-type used as a positive control. To confirm our finding, we generated the Tks4 Y508E phosphomimetic mutant construct and used it to test its ability to bind NoxA1 in the coimmunoprecipitation analysis illustrated in Figure 4B (upper panel) and in the GST-pulldown experiments shown in Supplemental Figure S2. In both cases, we observed that the presence of Tks4 Y508F unphosphorylable mutant blocked the interaction with NoxA1 as expected, whereas the Tks4 Y508E phosphomimetic mutant partially restored this binding. Again, wild-type Tks4 expression vector was used as a positive control. Figure 4B (lower panel) shows the quantification of three independent coimmunoprecipitation experiments.
Figure 4.
The integrity of Tyr508 on Tks4 is necessary for its binding to NoxA1 and for Nox1-dependent ROS generation. (A) Only the disruption of Tyr508 of Tks4 blocks its binding to NoxA1. Coimmunoprecipitation analysis was performed in HEK293 cells transfected as indicated with Myc-tagged Tks4 Tyr-phosphorylation mutants and Flag-tagged NoxA1. After 24 h, cells were lysed and immunoprecipitation (IP) was carried out using Myc antibody. The interaction between NoxA1 and Tks4 mutants and comparable expression of NoxA1 in cell lysates was tested by immunoblot (IB) using NoxA1 antibody (lower section). Similar expression levels of transfected Myc-tagged Tks4 mutants in cell lysates and immunoprecipitation efficiency was assessed by reblotting the membrane with Tks4 specific antibody (upper section). One representative experiment from three separate experiments is shown. (B) The abolishment of phosphorylation of Tyr508 of Tks4 blocks the interaction between NoxA1 and Tks4. In the upper panels, HEK293 cells were transfected as indicated with Flag-tagged NoxA1 and Myc-tagged wild type Tks4, unphosphorylable Tks4 Y508F and phosphomimetic Tks4 Y508E. After 24 h, cells were lysed and immunoprecipitation (IP) was carried out using Myc antibody. The interaction between NoxA1 and Tks4 mutants and comparable expression of NoxA1 in cell lysates was tested by immunoblot (IB) using NoxA1 antibody (upper section). Similar expression levels of transfected Flag-tagged Tks4 in cell lysates and immunoprecipitation efficiency was assessed by reblotting the membrane with Tks4 specific antibody (lower section). One representative experiment from three separate experiments is shown. In the lower panel, the results from three independent experiments performed as above were quantified. The levels of immunoprecipitated NoxA1 (IP-NoxA1) for each condition were normalized to the respective total NoxA1 present in the Lys. The IP-NoxA1 levels in presence of wild type Tks4 were set to 1 (mean ± SEM from three independent experiments). (C) The abolishment of Tks4 phosphorylation on Tyr508 decreases Nox1-dependent ROS generation. ROS generation was monitored in HEK293 cells transfected as indicated using CL assay. One representative experiment from three separate experiments is shown, and data are given as mean of triplicates ± SD. *p < 0.005; **p < 0.01.
Finally, we examined whether Src-mediated phosphorylation of Tks4 on Tyr508 had an effect on Nox1-dependent ROS generation. To this aim, HEK293 cells were transfected with Nox1, NoxA1, and either with Tks4 wt, Tks4 Y508E, and Tks4 Y508F constructs, and ROS formation was monitored using a luminol-based CL assay. As shown in Figure 4C, we noticed a 45% decrease of ROS formation when Tks4 Y508F mutant was expressed, while the presence of Tks4 Y508E construct restored the levels of ROS production observed with Tks4 wt used as a positive control. Interestingly, the coexpression of Tks4 Y508F along with Tks4 Y508E mutant did not decrease the superoxide generation compared with the condition in which only the phosphomimetic mutant was expressed.
The Presence of NoxA1 and Tks4 Phosphomimetic Mutants Reinforces Their Interaction and ROS Generation
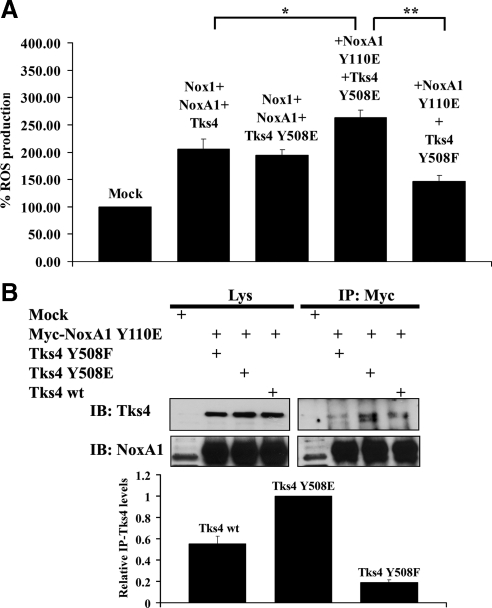
Our data indicate that the Src-mediated phosphorylation of NoxA1 on Y110 and of Tks4 on Tyr508 is important for ROS generation and their reciprocal binding. Next, we wanted to test whether the presence of NoxA1 and Tks4 phosphomimetic mutant had an effect on Nox1-dependent ROS generation. To this aim, superoxide production was monitored in HEK293 cells cotransfected with Nox1, with NoxA1 Y110E or NoxA1 wt and with Tks4 wt, Tks4 Y508F, or Tks4 Y508E. As shown in Figure 5A, the presence of Tks4 and NoxA1 phosphomimetic mutants caused a modest but consistent 25% increase in ROS generation compared with the conditions in which both wild-type proteins or Tks4 Y508E and NoxA1 wt were expressed. Of note, the overexpression of Tks4 Y508F decreased ROS generation compared with the condition in which both phosphomimetic constructs are expressed.
Figure 5.
(A) The contemporary presence of NoxA1 and Tks4 phosphomimetic mutants reinforces Nox1 dependent ROS generation. ROS generation was monitored in HEK293 cells transfected as indicated using CL assay. One representative experiment from three separate experiments is shown, and data are given as mean of triplicates ± SD. *p < 0.01; **p < 0.004. (B) The contemporary presence of NoxA1 and Tks4 phosphomimetic mutants reinforces their interaction. In the upper panels, HEK293 cells were transfected as indicated with Myc-tagged NoxA1 and wild-type Tks4, unphosphorylable Tks4 Y508F, and phosphomimetic Tks4 Y508E. After 24 h, cells were lysed and immunoprecipitation (IP) was performed using Myc antibody. The interaction between NoxA1 and Tks4 mutants and comparable expression of Tks4 proteins in cell lysates was tested by immunoblot (IB) using Tks4 antibody (upper section). Similar expression levels of transfected Myc-tagged NoxA1 in cell lysates and immunoprecipitation efficiency was assessed by reblotting the membrane with NoxA1 specific antibody (lower section). One representative experiment from three separate experiments is shown. In the lower panel, the results from three independent experiments performed as above were quantified. The levels of immunoprecipitated Tks4 (IP-Tks4) for each condition were normalized to the respective total Tks4 present in the Lys. The IP-Tks4 Y508E levels were set to 1 (mean ± SEM from three independent experiments).
To confirm the relevance of our findings on the reciprocal interaction between NoxA1 and Tks4, HEK293 cells were transfected with Myc-tagged NoxA1 Y110E phosphomimetic mutant along with either Tks4 wt, Tks4 Y508E, or Tks4 Y508F constructs and coimmunoprecipitation experiments were carried out as shown in Figure 5B (upper panel). We observed that NoxA1 Y110E interacted more strongly with Tks4 Y508E than with Tks4 wt, whereas the presence of Tks4 Y508F diminished the extent of this interaction. The lower panel shows the quantification of three independent experiments.
Next, we asked whether the presence of Tks4 and NoxA1 unphosphorylable mutants could block their SrcYF-induced interaction and Nox1-dependent ROS generation. To this aim, HEK293 cells were cotransfected with Nox1, SrcYF, Tks4, and either NoxA1 wt or NoxA1 Y110A and coimmunoprecipitation analysis (shown in Supplemental Figure S3, upper panel) or ROS generation measurements (shown in Supplemental Figure S3, lower panel) were performed. We noticed that, in presence of SrcYF, Tks4 and NoxA1 strongly interacted thus inducing ROS generation as expected. Interestingly, only the contemporary presence of NoxA1 and Tks4 unphosphorylable mutants completely blocked SrcYF-induced interaction between NoxA1 and Tks4 and Nox1-dependent ROS generation. These results are in agreement with those illustrated in Figure 5, A and B and support the hypothesis that Src induces the interaction between Tks4 and NoxA1 by phosphorylating the Tyr110 of NoxA1 and the Tyr508 of Tks4, thus increasing Nox1-dependent ROS generation.
Src Induces the Formation of Functional Invadopodia in Human DLD1 Colon Cancer Cells by Phosphorylating NoxA1 on Tyr110 and Tks4 on Tyr508
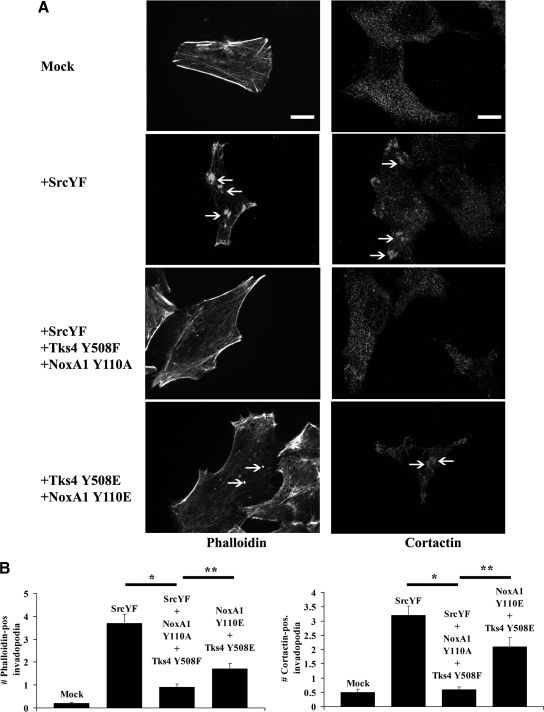
It has been reported that active Src is necessary for the formation of functional invadopodia in many cancer cells (Clark et al., 2007). Our group has shown that Src-induced ROS formation by Nox1 is important for invadopodia formation and ECM degradation in DLD1 cells. We hypothesized that Src could trigger ROS-dependent formation of functional invadopodia in DLD1 cells by phosphorylating NoxA1 on Tyr110 and Tks4 on Tyr508. To evaluate this, DLD1 cells were cotransfected with empty vector, SrcYF or cotransfected with SrcYF, Tks4 Y508F and NoxA1 Y110A, or only with Tks4 Y508E and NoxA1 Y110A mutants. After 48 h, the cells were fixed and stained for invadopodia markers, including F-actin (Figure 6A, left panels) and cortactin (Figure 6A, right panels). The number of phalloidin- and cortactin-positive invadopodia was analyzed by confocal microscopy. As indicated by the white arrows in Figure 6A and by the quantification of three independent experiments in Figure 6B, we noticed that SrcYF overexpression induced the formation of phalloidin- and cortactin-positive invadopodia as expected. Interestingly, this effect was significantly blocked in presence of Tks4 and NoxA1 unphosphorylable mutant. Importantly, the overexpression of Tks4 and NoxA1 phosphomimetic mutants was able to partially restore the formation of invadopodia, even in absence of SrcYF. Of note, in these experiments cells were transfected with a ratio indicated plasmid to GFP empty vector of 5:1 and only GFP-transfected cells were analyzed as shown in Supplemental Figure S4.
Figure 6.
The presence of Tks4 and NoxA1 unphoshorylable mutants blocks SrcYF-induced invadopodia formation in DLD1 cells, whereas the presence of their phosphomimetic mutants partially rescues this phenotype. (A) The analysis of the number of phalloidin- and cortactin-positive invadopodia indicates that the presence of Tks4 and NoxA1 unphoshorylable mutants blocks SrcYF-induced invadopodia formation in DLD1 cells, whereas the presence of their phosphomimetic mutants partially rescues this phenotype. DLD1 cells were plated on glass coverslips and after 24 h cells were transfected as indicated. Forty-eight hours after transfection, cells were fixed in PFA 4% and stained with Alexa-Fluor-568 phalloidin (left column) or cortactin antibody, followed by Alexa-Fluor 568-conjugated secondary antibody (right column) and visualized by confocal microscopy (×100). White arrows indicate phalloidin-positive (right column) or cortactin-positive (left column) invadopodia. Scale bars, 5 μm. One representative picture from three separate experiments is shown. (B) Quantification from three independent biological experiments shown in A is given: the number of phalloidin positive-invadopodia (left panel) or cortactin positive-invadopodia (right panel) was counted and averaged from 50 cells/condition for each experiment. Error bars represent SEM. *p < 0.001; **p < 0.01.
The formation of functional invadopodia relates to the ability of cells to degrade the ECM (Baldassarre et al., 2003; Weaver, 2006). To further confirm that Src induces the formation of functional invadopodia in DLD1 cells by phosphorylating NoxA1 on Tyr110 and Tks4 on Tyr508, we tested the effect of these Tks4 and NoxA1 unphosphorylable mutants on SrcYF-induced ECM degradation in DLD1 cells. DLD1 cells were transfected as indicated in Figure 7A, plated on FITC-labeled gelatin-coated coverslips, and stained with phalloidin. The analysis of the ability of cells (in red) to degrade the matrix (in green) revealed that, as expected, SrcYF overexpression induced the ECM degradation in DLD1 cells. Interestingly, we also observed that the presence of Tks4 and NoxA1 unphosphorylable mutants blocked this effect. Again, the overexpression of Tks4 and NoxA1 phosphomimetic mutants was able to partially restore the ability of these cells to degrade the ECM. The quantification of three independent experiments is shown in Figure 7B. These results support the idea that Src induces the formation of functional invadopodia in human DLD1 colon cancer cells by phosphorylating NoxA1 on Tyr110 and Tks4 on Tyr508.
DISCUSSION
NADPH oxidases represent a family of enzymes that catalyze the regulated formation of ROS. Nox isoforms are expressed in virtually every tissue and ROS generated by Nox enzymes have been implicated in such biological processes as cell growth, apoptosis and cancer, angiogenesis and blood pressure regulation, innate immunity and inflammation, cell signaling, motility, and transcription (Bedard and Krause, 2007). The homologue Nox1 is highly expressed in the colon epithelium and Nox1-dependent ROS generation in the colon has been implicated in cell growth, migration and invasion. Consistent with these observations, many studies have reported increased Nox1 expression in colon cancers and Nox1-derived ROS have been implicated in mechanisms inducing cell division and angiogenesis (Lambeth, 2007). Nox1 requires for its activity the activator subunit NoxA1 and the organizer subunit NoxO1, both highly expressed in the colon epithelium. Recently, we have shown that the widely expressed Tks proteins can also serve as organizers to support Nox1 activity. Rac1-GTPase is also required for full Nox1 activation (Cheng et al., 2006). At this point, very little is known about how the activities of the Nox proteins are regulated under normal, much less pathological, conditions.
Increasing evidence suggests that phosphorylation of various Nox proteins or their regulatory cofactors or both may play important roles in regulating the activity of these enzymes. In general, the Nox family proteins and their respective regulatory proteins all contain sites for potential regulatory phosphorylation(s) by various kinases, as predicted by a number of sequence-analysis algorithms. Consistent, with this, it has been shown that a variety of kinases and phosphorylation-mediated mechanisms regulate the activity of several Nox enzymes (Finkel, 2001; Jagnandan et al., 2007; Bokoch et al., 2009). These mechanisms include i) direct phosphorylation of Nox or Nox regulatory components; ii) the phosphorylation-induced binding of regulatory 14-3-3 proteins; and iii) stimulation of Rac GTPase activation. In this study, we describe additional effects by which c-Src induces Nox1-dependent ROS generation in colon cancer cells. To investigate this, we chose human DLD1 colon cancer cells as model system as they express a high level of active Src and endogenously express only Nox1 (among members of the NADPH oxidase family), NoxA1, and Tks4 (among members of the p47phox organizer superfamily). Here, we demonstrate that Src activity augments the affinity of the interaction between the ectopically-expressed or endogenous activator protein NoxA1 and the organizer Tks4. The residue Tyr508 located between the third and the fourth SH3 domain of Tks4 has already been shown to be target of Src phosphorylation in different cell systems (Buschman et al., 2009). However, the biological relevance of such phosphorylation event on Tks4 function has never been fully understood. On the contrary, no Src-mediated phosphorylation sites have been described for NoxA1 yet. In this study, we have identified Tyr110 of NoxA1 as a key amino acid targeted by Src. The phosphorylation of both Tyr110 of NoxA1 and Tyr508 of Tks4 by Src increases the interaction between NoxA1 and Tks4. Nevertheless, Nox1-dependent ROS generation is only slightly increased by the presence of NoxA1 Y110E and Tks4 Y508E indicating that there might be additional Src target protein(s) which are needed for full Nox1 activation. Consistent with this, our group has previously shown that the activation of the kinase c-Src leads to increased Nox1-dependent ROS generation through the tyrosine phosphorylation-mediated activation of Rac1 exchange factor Vav2 (Gianni et al., 2008).
Increased c-Src activity is also a characteristic of both premalignant and progressively advanced colon neoplasia, and it has been correlated with the conversion of benign polyps into malignant metastatic tumors (Summy and Gallick, 2003). c-Src was previously shown to be involved in signaling events stimulated by ROS production (Abe et al., 1997). c-Src itself undergoes activation on cellular ROS formation through multiple mechanisms (Alvarez et al., 2006). Src activity can be regulated through the control of its phosphorylation status by protein tyrosine phosphatases (PTPases) (Nakashima et al., 2002). The PTPases, which contain sulfhydryl groups on cysteine residues in the catalytic domain that undergo oxidative modification to impair catalytic activity, are well-known targets of ROS (Stone and Dixon, 1994).
We have previously shown that in human DLD1 colon cancer cells and other cancer cell lines there is a correlation between the levels of c-Src activity and their ability to generate ROS (Gianni et al., 2008), yet the exact relationships between Src activity, ROS formation, carcinogenesis, and metastasis remain poorly defined. Metastatic cancer cells have the ability to both migrate to and degrade the ECM, and invasiveness has been correlated with the presence of invadopodia (Linder, 2007; Gimona et al., 2008), as well as ROS production (Okada et al., 2006). Src activity and production of ROS via NADPH oxidase have been shown to be required for the formation of functional invadopodia in several cancer cells, including human DLD1 colon cancer cells. However, the signals and molecular mechanisms by which Src induces the redox-dependent generation of invadopodia are still a matter of investigation. In this study, we elucidate the relationship between Src phosphorylation of Tks4 and NoxA1 and the Nox1-dependent formation of invadopodia and ECM degradation in DLD1 cells. Interestingly, we show that the overexpression of Tks4 and NoxA1 unphoshorylable mutants reduces the ability of DLD1 cells to form ECM-degrading invadopodia in presence of active Src. On the contrary, the presence of Tks4 and NoxA1 phosphomimetic mutants is able to partially restore this phenotype even in absence of active Src.
This article describes the signaling events required for Src-induced ROS-dependent formation of functional invadopodia in human colon cancer cells and implies that Src-mediated regulation of the Nox1 activity is an important mean of controlling Nox1-dependent biological functions. Our results suggest the possibility to modulate Nox1 function indirectly by pharmacologic intervention using specific and effective Src inhibitors well tolerated in vivo.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Bruce Fowler and Benjamin Bohl for technical help, Michael Howell for helpful discussion, Janett Schwarz for sharing reagents, and other members of Bokoch/DerMardirossian laboratory for helpful suggestions in revising this manuscript. We are grateful to Dr. Sara Courtneidge (Sanford-Burnham Medical Research Institute) for Tks4 and Tks5 antibodies. We dedicate this paper to the memory of our mentor and colleague Gary M. Bokoch. This work was supported by National Institutes of Health Grant HL48008 (to G.M.B. and C.D.M.).
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-08-0685) on October 13, 2010.
REFERENCES
- Abe J., Takahashi M., Ishida M., Lee J. D., Berk B. C. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J. Biol. Chem. 1997;272:20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- Abram C. L., Seals D. F., Pass I., Salinsky D., Maurer L., Roth T. M., Courtneidge S. A. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 2003;278:16844–16851. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- Alvarez R. H., Kantarjian H. M., Cortes J. E. The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer. 2006;107:1918–1929. doi: 10.1002/cncr.22215. [DOI] [PubMed] [Google Scholar]
- Arbiser J. L., Petros J., Klafter R., Govindajaran B., McLaughlin E. R., Brown L. F., Cohen C., Moses M., Kilroy S., Arnold R. S., Lambeth J. D. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Natl. Acad. Sci. USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. S., Shi J., Murad E., Whalen A. M., Sun C. Q., Polavarapu R., Parthasarathy S., Petros J. A., Lambeth J. D. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre M., Pompeo A., Beznoussenko G., Castaldi C., Cortellino S., McNiven M. A., Luini A., Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol. Biol. Cell. 2003;14:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi B., Clark R. A., Steger K., Krause K. H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Block M. L. NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci. 2008;9:S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouw B., Seals D. F., Pass I., Diaz B., Courtneidge S. A. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur. J. Cell Biol. 2008;87:555–567. doi: 10.1016/j.ejcb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Diebold B., Kim J. S., Gianni D. Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases. Antioxid. Redox. Signal. 2009;11:2429–2441. doi: 10.1089/ars.2009.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G.M., Knaus U.G. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., DeSeau V., Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc. Natl. Acad. Sci. USA. 1987;84:2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman M. D., Bromann P. A., Cejudo-Martin P., Wen F., Pass I., Courtneidge S. A. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol. Biol. Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave A. C., Brewer A. C., Narayanapanicker A., Ray R., Grieve D. J., Walker S., Shah A. M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox. Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- Cheng G., Diebold B. A., Hughes Y., Lambeth J. D. Nox1-dependent reactive oxygen generation is regulated by Rac1. J. Biol. Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- Cifuentes M. E., Pagano P. J. Targeting reactive oxygen species in hypertension. Curr. Opin. Nephrol. Hypertension. 2006;15:179–186. doi: 10.1097/01.mnh.0000214776.19233.68. [DOI] [PubMed] [Google Scholar]
- Clark E. S., Whigham A. S., Yarbrough W. G., Weaver A. M. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C., Rocklin G., Seo J. Y., Bokoch G. M. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol. Biol. Cell. 2006;17:4760–4768. doi: 10.1091/mbc.E06-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Benna J., Dang P. M., Gougerot-Pocidalo M. A. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52:3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- Gavazzi G., Banfi B., Deffert C., Fiette L., Schappi M., Herrmann F., Krause K. H. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Geiszt M., Lekstrom K., Witta J., Leto T. L. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- Geiszt M., Leto T. L. The Nox family of NAD(P)H oxidases: host defense and beyond. J. Biol. Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- Gianni D., Bohl B., Courtneidge S. A., Bokoch G. M. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol. Biol. Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni D., Dermardirossian C., Bokoch G. M. Direct interaction between Tks proteins and the N-terminal proline-rich region (PRR) of NoxA1 mediates Nox1-dependent ROS generation. Eur. J. Cell Biol. 2010 doi: 10.1016/j.ejcb.2010.05.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni D., Diaz B., Taulet N., Fowler B., Courtneidge S. A., Bokoch G. M. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci. Signal. 2009;2:ra54. doi: 10.1126/scisignal.2000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M., Buccione R., Courtneidge S. A., Linder S. Assembly and biological role of podosomes and invadopodia. Current opinion in cell biology. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim. Biophys. Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Irby R. B., Yeatman T. J. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- Jagnandan D., Church J. E., Banfi B., Stuehr D. J., Marrero M. B., Fulton D. J. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J. Biol. Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009;100:1382–1388. doi: 10.1111/j.1349-7006.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao Y. Y., Gianni D., Bohl B., Taylor R. M., Bokoch G. M. Identification of a conserved Rac-binding site on NADPH oxidases supports a direct GTPase regulatory mechanism. J. Biol. Chem. 2008;283:12736–12746. doi: 10.1074/jbc.M801010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Diebold B. A., Babior B. M., Knaus U. G., Bokoch G. M. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14–3-3 binding. J. Biol. Chem. 2007;282:34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- Komatsu D., Kato M., Nakayama J., Miyagawa S., Kamata T. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene. 2008;27:4724–4732. doi: 10.1038/onc.2008.102. [DOI] [PubMed] [Google Scholar]
- Krause K. H. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Japan. J. Infect. Dis. 2004;57:S28–S29. [PubMed] [Google Scholar]
- Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Rad. Biol. Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J. D., Kawahara T., Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Rad. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J. D., Krause K. H., Clark R. A. NOX enzymes as novel targets for drug development. Semin. Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Lock P., Abram C. L., Gibson T., Courtneidge S. A. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C., Yoneda T., Boyce B. F., Chen H., Mundy G. R., Soriano P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc. Natl. Acad. Sci. USA. 1993;90:4485–4489. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushita J., Lambeth J. D., Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- Nakashima I., Kato M., Akhand A. A., Suzuki H., Takeda K., Hossain K., Kawamoto Y. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid. Redox. Signal. 2002;4:517–531. doi: 10.1089/15230860260196326. [DOI] [PubMed] [Google Scholar]
- Nauseef W. M. Nox enzymes in immune cells. Semin. Immunopathol. 2008;30:195–208. doi: 10.1007/s00281-008-0117-4. [DOI] [PubMed] [Google Scholar]
- Okada F., Kobayashi M., Tanaka H., Kobayashi T., Tazawa H., Iuchi Y., Onuma K., Hosokawa M., Dinauer M. C., Hunt N. H. The role of nicotinamide adenine dinucleotide phosphate oxidase-derived reactive oxygen species in the acquisition of metastatic ability of tumor cells. Am. J. Pathol. 2006;169:294–302. doi: 10.2353/ajpath.2006.060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokutan K., Kawahara T., Kuwano Y., Tominaga K., Sekiyama A., Teshima-Kondo S. NADPH oxidases in the gastrointestinal tract: a potential role of Nox1 in innate immune response and carcinogenesis. Antioxid. Redox. Signal. 2006;8:1573–1582. doi: 10.1089/ars.2006.8.1573. [DOI] [PubMed] [Google Scholar]
- Sadok A., Bourgarel-Rey V., Gattacceca F., Penel C., Lehmann M., Kovacic H. Nox1-dependent superoxide production controls colon adenocarcinoma cell migration. Biochim Biophys. Acta. 2008;1783:23–33. doi: 10.1016/j.bbamcr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Sancho P., Fabregat I. The NADPH oxidase NOX1 controls autocrine growth of liver tumor cells through up-regulation of the epidermal growth factor receptor pathway. J. Biol. Chem. 285:24815–24824. doi: 10.1074/jbc.M110.114280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D. F., Azucena E. F., Jr, Pass I., Tesfay L., Gordon R., Woodrow M., Resau J. H., Courtneidge S. A. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Stone R. L., Dixon J. E. Protein-tyrosine phosphatases. J. Biol. Chem. 1994;269:31323–31326. [PubMed] [Google Scholar]
- Summy J. M., Gallick G. E. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- Takeya R., Ueno N., Kami K., Taura M., Kohjima M., Izaki T., Nunoi H., Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M., Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A. M. Invadopodia: specialized cell structures for cancer invasion. Clin. Exper. Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.