Abstract
Toll-like receptor 4 (TLR4) is an innate immune receptor that is constitutively and inducibly activated in monocytes. Although TLR4 is expressed at very low levels on human B cells from healthy individuals, recent reports showed that TLR4 expression and function is elevated in B cells from inflammatory disease patients. New data showed that TLR4 expression on B cell is increased upon stimulation through surface Igμ and CD40 in combination with IL-4. In contrast, monocyte stimulation through CD40 and IL-4 receptors decreased TLR4 surface expression. Analysis of molecular signatures of TLR4 activation in stimulated B cells suggested that TLR4 is regulated by different mechanisms in B cells compared to monocytes. PU.1 and interferon regulatory factor association with the TLR4 promoter are sufficient for TLR4 transcription, but are not sufficient for surface TLR4 expression on B cells. In contrast, the PU.1/IRF combination is sufficient for surface TLR4 expression on monocytes. These data identify mechanisms that can activate B cell TLR4 expression in inflammatory disease patients, and demonstrate that B cells have additional layers of TLR4 regulation absent in monocytes.
Keywords: B cells, gene expression, human inflammatory disease, monocytes, Toll-like receptor 4
1. INTRODUCTION
TLR4 is a generally pro-inflammatory pattern recognition receptor of the innate immune system. Many studies implicate elevated levels of TLR4 ligands and TLR4 activity in a wide array of inflammatory diseases, including type 2 diabetes, periodontal disease, and Crohn’s Disease (CD) (Al-Attas et al., 2009; Caradonna et al., 2000; Creely et al., 2007; Dasu et al., 2010; Gardiner et al., 1995; Wellmann et al., 1986)(McDonnell et al., in press). For example, mice with a naturally occurring TLR4-inactivating mutation are more insulin sensitive (i.e. less “diabetic”) and have less vascular inflammation than TLR4-sufficient mice in high-fat diet feeding experiments (Kim et al, 2007; Poggi et al, 2007; Suganami et al, 2007; Tsukumo et al, 2007). TLR4-sufficient mice also have more oral bone destruction than TLR4-null mice in an in vivo model of periodontal disease (Hou et al., 2000). Finally, although TLR4-null mice develop a colitis that is indistinguishable from wild-type mice (Ohkawara et al., 2005), competing pro- and anti-inflammatory TLR4 functions have been more recently identified that associate TLR4 function with CD (Gonzalez-Navajas et al., 2010; Liu et al., 2010).
We and others have recently shown that inflammatory disease associates with changes in TLR expression and function on B cells, a non-traditional arm of the innate immune system (Ganley-Leal et al., 2006; Mansson et al., 2006; Noronha et al., 2009). Although B cells from healthy donors express little to no surface TLR4 (Bourke et al., 2003; Hornung et al., 2002; Muzio et al., 2000; Shin et al., 2009; Zarember and Godowski, 2002), our analyses of inflammatory disease patient samples demonstrated that TLR4 expression modestly increases on circulating B cells from periodontal disease and type 2 diabetes patients, and more dramatically increases on B cells from CD patients (Shin et al., 2009). More importantly, B cell TLR4 has unexpected functions that fail to recapitulate TLR4 roles defined by studies on myeloid cells. Perhaps the most biologically important function of B cell TLR4 in inflammatory disease patients is its ability to decrease production of IL-10 (Jagannathan et al., 2009), a critical anti-inflammatory cytokine that plays roles in inflammation onset and resolution. In B cells from periodontal disease patients, a TLR4 ligand-induced decrease in IL-10 is coupled with TLR4-induced increases in pro-inflammatory cytokine production to indicate that B cell TLR4 contributes to systemic inflammation through multiple mechanisms (Jagannathan et al., 2009). Finally, TLR4 function on B cells is disease-dependent, as evidenced by the demonstration that TLR4 engagement inhibits constitutive IL-8 production by B cells from CD patients (McDonnell et al, in press). Overall, these data indicate that understanding the expression and function of human B cell TLR4 is critical for identifying targetable sources of inflammation in multiple diseases.
Initial studies have identified stimuli that can up regulate human B cell TLR4 expression. For example, B cell stimulation with TLR2 or TLR9 ligand significantly increases surface TLR4 levels (Jagannathan et al., 2009). This finding suggests that elevated serum concentrations of TLR ligands in inflammatory disease patients can promote B cell TLR4 expression. Single TLR ligands generally activate only a subset of circulating B cells, specifically memory B cells (Bernasconi et al., 2003), which comprise <20% of the circulating B cells (Chong et al., 2005). Taken together, these studies suggest that TLR2 or TLR9 ligand activates TLR4 expression only on the minority memory B cell population. However, up to 99% of peripheral B cells in inflammatory disease patients express TLR4 (McDonnell et al., in press; Shin et al., 2009), and TLR4 is equivalently up regulated on the memory and non-memory B cell subsets from patients (Shin et al., 2009). These data suggest that additional unidentified mechanisms promote B cell TLR4 expression on the non-memory B cell population. New findings herein show that TLR4 activation is regulated by transcriptional and post-transcriptional mechanisms in B cells from inflammatory disease patients. In contrast, “healthy” monocytes appear to regulate TLR4 surface expression primarily through transcriptional mechanisms. These findings identify a new focus for studies aimed at artificially regulating pro-inflammatory TLR4 functions in inflammatory disease. Importantly, the results also indicate that vaccine adjuvants designed to regulate TLR4 function/expression must be tested on cells from the expanding population of inflammatory disease patients to avoid unexpected immunological consequences of widespread immunotherapy use.
2. METHODS
2.1. Patients
This study was approved by the Institutional Review Board of Boston University and was performed in accordance with the declaration of Helsinki. Human samples were obtained following informed consent. Healthy donors (N=10) and donors with a diagnosis of Crohn’s Disease (N=3) were recruited from the Boston Medical Center Community and the BMC Center for Gastroenterology, respectively. Fifty mls of blood was collected from these donors into heparinized tubes by venous puncture. Additional samples from healthy donors were obtained commercially as leukopacks (N=3) for monocyte purification only. The monocyte cell line Mono-Mac-6 was cultured as published (Liang et al, 2006) and used where indicated. Surgically excised tonsil (N=11) or spleen (N=l) were obtained from the National Disease Research Interchange. Tonsils were excised due to chronic inflammation; spleen was removed due to trauma. B cells or monocytes from all sources were purified from PBMCs or the mononuclear fraction of minced tissue by negatively selecting magnetic bead isolation kits (Miltenyi;(Noronha et al., 2009; Shin et al., 2009). The majority of contaminating cells in all B cell and monocyte preparations were T cells; monocyte contamination in the purified B cells and B cell contamination of purified monocytes was below the level of flow cytometric detection.
2.2. Biochemical analyses
Purified cells were stimulated for 24–72 hours with the following alone or in combination: anti-human CD40 (1 μg/ml); anti-human Igμ F(ab)′2 (2 μg/ml), or recombinant human cytokines (20 ng/ml IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IFN-γ). Quantification of protein/DNA interactions by chromatin immunoprecipitation (ChIP) were performed as described (Liang et al., 2006) using 500,000 purified cells per precipitation. Transcription factor-associated DNA was quantified using TLR4-specific ChIP primers 5′-GCTAAGGTTGCCGCTTTCAC-3′ and 5′-CTTCCTCGAGCCGCCC-3′ that recognize the TLR4 gene 5′ to the transcription start site. ChIP-competent antibodies from Santa Cruz Biotechnology (Santa Cruz, CA) were: anti-histidine tag or anti-GST antibody (sc-803; sc-459) as a non-specific species-matched control, anti-cJun (sc-45), anti-IRF8 (sc-13043), and anti-PU.1 (sc-352). mRNA was quantified by RT-PCR using the Superscript III One-step RT-PCR System and Platinum Taq DNA polymerase (Invitrogen). Alternatively, RNA was prepared with the RNeasy mini kit (Qiagen) and treated with RNAase-free DNAase prior to RT. Primer sequences for PCR were: (β-actin: 5′-TCATGAAGTGTGACGTTGACATCCGT-3′ and 5′-CCTAGAAGCATTTGCGGTGCACGATC-3′; TLR4: 5′-TGGATACGTTTCCTTATAAG-3′ and 5′-GAAATGGAGGCACCCCTTC-3′. mRNA was quantified by ImageJ and TLR4 mRNA was normalized to β-actin mRNA. TLR4 mRNA levels in variously stimulated B cells were plotted relative to levels in unstimulated B cells from the same individual. Differences in relative mRNA levels were determined by t test.
2.3. Flow cytometry
Antibodies to the following antigens were purchased from BD Pharmingen: CD3, CD14 and CD19-FITC. α-TLR4 was purchased from ebioscience. Cells were stained for 20 min at 4°C, then washed with 0.2% BSA/PBS and resuspended in PBS. Data were collected on a FACScan or FACSCalibur (BD Biosciences) using CellQuest software. Analysis of results used WinMDI (Joseph Trotter, The Scripps Institute, Palo Alto, CA) or FloJo (Tree Star) software.
3. RESULTS
3.1. Molecular signatures of an active TLR4 gene are similar in B cells from inflammatory disease patients compared to monocytes
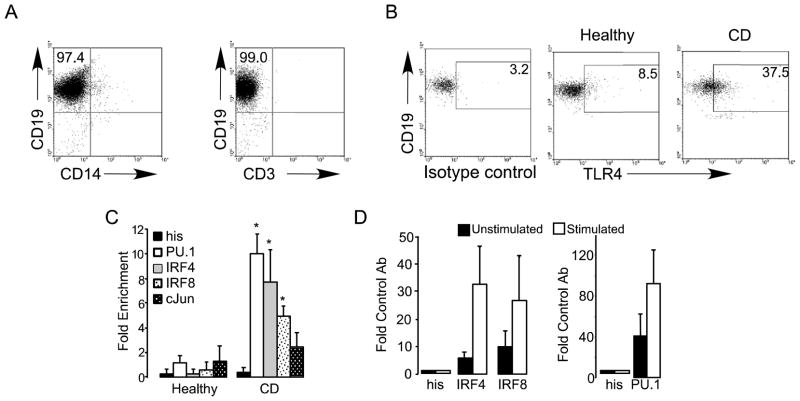
B cells from healthy donors express little to no surface TLR4 (Bourke et al., 2003; Hornung et al., 2002; Muzio et al., 2000; Shin et al., 2009; Zarember and Godowski, 2002). These findings explain, at least in part, the general demonstration that human B cells respond poorly to TLR4 ligand (Jagannathan et al., 2009; Jagannathan et al., 2010). However, we previously reported that the chromatin structure of the TLR4 locus is primed for activation in B cells (Shin et al., 2009), and that ligands for other TLR family members, including Pam3CSK4 (TLR2) and CpG (TLR9), induce expression of TLR4 on B cells (Jagannathan et al., 2009). These data begin to mechanistically explain the source of elevated surface TLR4 expression on B cells from inflammatory disease patients. To more rigorously identify mechanisms that explain an increased percentage of TLR4-positive B cells in inflammatory disease patients, we quantified known molecular signatures of TLR4 promoter up regulation in freshly purified B cells. For these studies, we focused on highly purified B cells (Fig. 1A) from CD patients. An elevated percentage of B cells from CD patients are surface TLR4-positive, on average, as compared to B cells from healthy donors (Fig. 1B and McDonnell et al., in press). The elevated percentage of TLR4-positive CD19-negative cells in CD patients (Fig. 1B, right panel) was consistently present but was not further investigated. Chromatin immunoprecipitation (ChIP) analyses showed robust, constitutive association of the known TLR4-activating transcription factors PU.1, IRF4 and IRF8 with the TLR4 promoter in B cells from CD patients but not healthy donors (Fig. 1C). The transcription factor cJun associated modestly with the TLR4 promoter in B cells from CD patients, consistent with the demonstration that PU.1 and IRFs, but not cJun, are critical for TLR4 gene expression (Pedchenko et al., 2005; Rehli et al., 2000). Control analyses on monocytes, a cell type widely demonstrated to express functional surface TLR4, confirmed that PU.1, IRF4 and IRF8 association with the TLR4 promoter identifies an active TLR4 locus that is further activated in response to LPS stimulation (Fig. 1D). These findings raise the possibility that similar molecular mechanisms may regulate surface TLR4 expression on B cells from inflammatory disease patients and normal monocytes.
Figure 1.
TLR4-activating transcription factors associate with the TLR4 promoter in B cells from CD patients and in monocytes. (A) Reanalysis of negatively selected B cells for purity. Shown is representative of re-analysis done on all purified B cell preparations. CD19 is a definitive B cell marker, CD14 labels monocytes and CD3 labels T cells. In no case was B cell purity <95%. (B) Representative analysis of TLR4 surface expression on CD19-positive B cells from a healthy donor (middle panel) or CD patient (rightmost panel). Left shows isotype control stain for the CD sample. Plot is one of >50 similar results from CD patients showing, on average, an elevated percentage of TLR4-positive B cells in CD patients (McDonnell et al., in press). (C) Chromatin immunoprecipitation (ChIP) measuring association between transcription factors indicated in the key and the TLR4 promoter in purified B cells from healthy donors (left bars) or CD patients (right bars). Association is shown relative to background detected with an anti-histidine tag control antibody. Values less than 2 demonstrate a lack of association. Association values for PU.1, IRF4 and IRF8 were significantly greater (*, p≤0.05) in B cells from CD patients compared to B cells from healthy donors, as determined by unpaired t test. (D) ChIP shows association between the transcription factor indicated and the TLR4 promoter in unstimulated (black bars) or stimulated (E. coli LPS, 100ng/ml, 1 hr.; white bars) Mono-Mac-6 monocytes. “His” represents values from the negative control antibody whose value was set to=1. Shown are average and range of 2–3 independent analyses.
3.2. B cells up regulate surface TLR4 expression in response to combinations of stimuli
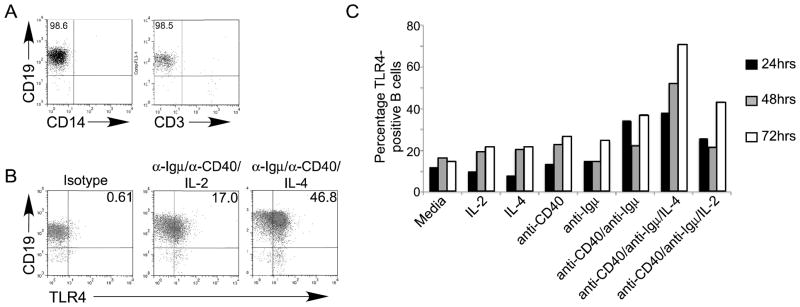
Inflammatory disease patients naturally maintain high levels of many B cell activators that could promote changes in surface TLR4 expression and function in B cells. For example, B cell-activating cytokines are elevated in the serum of inflammatory disease patients (Desfaits et al., 1998; Iacopino, 2001; Koss et al., 2000; Tsiavou et al., 2004). We predicted that cytokines, perhaps in combination with more traditional naïve B cell stimuli (i.e. stimulation through surface Igμ and CD40; Ruprecht and Lanzavecchia, 2006), may also induce TLR4 expression/function. To test this possibility, we treated purified TLR4-negative tonsil B cells (Fig. 2A) with a variety of stimuli alone or in combination, then assessed surface TLR4 expression 24–72 hours post-stimulation by flow cytometry (Figs. 2B&C). Tonsil B cells stimulated through the B cell receptor (anti-Igμ), CD40 (with anti-CD40), or IL-2, -6, -7, -10, -12 or interferon-γ (IFN-γ) alone or in combination had a similar percentage of TLR4-positive cells as media-stimulated cells (Fig. 2C, Tables 1&2). By contrast, IL-4 significantly enhanced TLR4 surface expression on B cells co-stimulated with anti-CD40 and anti-Igμ (Fig. 2C and Table 1), and recapitulated the high level of TLR4-positive B cells in many inflammatory disease patients. Increased surface TLR4 expression was first detected at the earliest time point tested (24hr., Fig. 2C, black bars), and continued to increase up to the final time point tested, 72 hrs (white bars). Results were similar in experiments that used healthy human spleen or peripheral blood as a source of B cells (data not shown). We conclude that B cell stimulation through the classical “signal 1+signal 2/Igμ+CD40”, in combination with IL-4, maximally increases the percentage of TLR4 surface-positive B cells, and identifies a new mechanism that can drive surface TLR4 up regulation on human B cells.
Figure 2.
B cell surface TLR4 increases maximally in response to a combination of stimuli: anti-Igμ, anti-CD40 and IL-4. Purified B cells (A) were stimulated as indicated and surface TLR4 expression was measured as shown in panel B. (C) Percentage of TLR4-positive B cells in cultures incubated for the indicated time (black, gray, and white bars represent 24, 48 and 72 hrs, respectively) and stimulated as shown below X axis. One representative of nine analyses is shown. Note Table 1 includes values of a second representative analysis for comparison. Similar results were obtained with B cells from healthy human spleen (N=1) or peripheral blood (N=2; not shown).
Table 1.
| Stimulation | %TLR4+ B cells |
|---|---|
| Media | 11.6 |
| α-CD40 | 15.5 |
| α-Igμ | 18.4 |
| α-Igμ/α-CD40 | 39 |
| IL-2 | 12.8 |
| IL-10 | 10.9 |
| IL-4 | 16.4 |
| IL-6 | 12.8 |
| α-CD40/IL-4 | 47 |
| α-CD40/IL-2 | 12.8 |
| α-Igμ/IL-2 | 40.7 |
| α-CD40/IL-6 | 14.6 |
| α-Igμ/IL-6 | 15 |
| α-CD40/α-Igμ/IL-2 | 18.6 |
| α-CD40/α-Igμ/IL-6 | 39.3 |
| α-CD40/α-Igμ/IL-4 | 55.8 |
| α-Igμ/IL-4 | 37.8 |
| IL-7 | 17.4 |
| IL-2/IL-7 | 11.1 |
| IL-2/IL-6 | 12.4 |
| IL-2/IL-4 | 17.3 |
IL-2, IL-6, IL-10 and combinations of cytokines fail to up regulate B cell surface TLR4. Shown is one of two similar results. IL-7 and IL-10 failed to change the percentage of TLR4-positive B cells in α-Igμ plus α-CD40-stimulated B cells (not shown).
Table 2.
| Stimulation | %TLR4+ B cells |
|---|---|
| Media | 11.5 |
| α-Igμ | 31.45 |
| α-CD40 | 34.9 |
| IL-12 | 16.5 |
| IFN-γ | 15.91 |
| α-Igμ/α-CD40 | 67 |
| α-Igμ/IL-12 | 20.3 |
| α-Igμ/IFN-γ | 27.9 |
| α-CD40/IL-12 | 47.1 |
| α-CD40/IFN-γ | 44.3 |
| α-Igμ/CD40/IL-12 | 68.5 |
| α-Igμ/IFN-γ | 39.98 |
IL-12 and IFN-γ fail to up regulate B cell surface TLR4. Shown is one of two similar results. Note response to a combination of α-Igμ and α-CD40 is relatively robust for this particular sample.
3.3. PU.1 and IRF association is insufficient for TLR4 surface expression in B cells
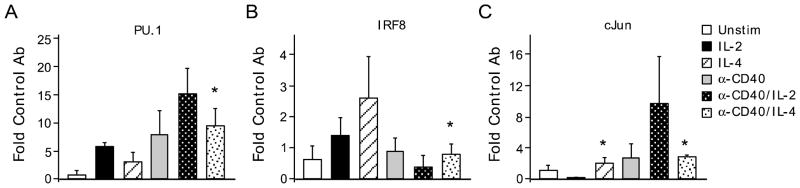
To test whether ex vivo stimulation recapitulated the molecular events responsible for TLR4 up regulation on B cells from inflammatory disease patients (and monocytes, Fig. 1), we quantified association of TLR4 transcriptional activators with the TLR4 promoter in tonsil B cells at 72 hrs. post-stimulation. B cells stimulated with IL-2, alone or in combination with anti-CD40 and anti-Igμ, failed to induce significant association of PU.1 and IRF8 with the TLR4 promoter (Figs. 3A&B). Instead, addition of IL-2 to anti-Igμ/anti-CD40 treated B cells trended towards decreased PU.1/TLR4 association (Fig. 3A; compare gray bar and black stippled bar). Although constitutive cJun association (Fig. 3C, white bar) is consistent with our demonstration that the TLR4 promoter is poised for activation on B cells, but not T cells or non-hematopoietic cells (Jagannathan et al., 2010; Shin et al., 2009), changes in cJun/TLR4 association in differentially activated cells were overall statistically insignificant (p>0.08). However, the trend towards decreased cJun/promoter association in anti-Igμ/anti-CD40/IL-2 vs. anti-Igμ/anti-CD40/IL-4 stimulated B cells is intriguing (Fig. 3C; compare stippled bars). Overall, these data provide a mechanistic explanation for the failure of IL-2, alone or as a cocktail, to increase B cell surface TLR4 expression (Figs. 2B&C).
Figure 3.
IL-4 alone induces transcription factor association with the TLR4 promoter in the absence of TLR4 surface expression. ChIP performed as outlined in Fig. 1 with tonsil B cells stimulated for 72 hrs. as indicated in the key. Transcription factor association assayed was (A) PU. 1; (B) IRF8; (C) cJun. Shown is average and range of (A,B) 2–3 or (C) 2 independent determinations, “*”highlights values that differ significantly (p≤0.05) in paired t tests; “#” trends towards significance (p≤0.1). Symbols above graphs show differences in stimulated vs. unstimulated cells. “*” below graphs show differences in anti-Igμ/anti-CD40/IL-4 stimulated vs. anti-Igμ/anti-CD40/IL-2 stimulated cells. “##” below panel A highlights a difference between anti-Igμ/anti-CD40 vs. anti-Igμ/anti-CD40/IL-2 stimulation that trends towards significant (p=0.062); in panel B, ## below panel highlights a difference between anti-Igμ/anti-CD40 and anti-Igμ/anti-CD40/IL-4 stimulated B cells that trends towards significance (p=0.052). Differences in values for cJun association (panel C) were not significant (p>0.14).
As expected, B cells that express surface TLR4 due to stimulation with a combination of anti-Igμ/anti-CD40/IL-4 were positive for association between the TLR4 promoter and both PU.1 and IRF8 (Fig. 3A&B, rightmost bars). Importantly, anti-Igμ/anti-CD40 stimulated cells significantly elevated PU.1, but not IRF8 association, with the TLR4 promoter association (Fig. 3A&B, gray bars), suggesting that the classical B cell stimulation through “signal 1 + signal 2” partially activated the TLR4 promoter. In contrast, IL-4 alone, which failed to induce significant TLR4 surface expression on tonsil B cells (Fig. 2C), unexpectedly induced association of both PU.1 and IRF8 with the TLR4 promoter (Fig. 3A&B, striped bars). These data indicate that the defined combination of transcription factors thought to activate the TLR4 promoter on monocytes (Rehli et al., 2000) is insufficient to induce surface TLR4 expression on B cells, despite the demonstration that these TLR4 activation signatures associated with surface TLR4 up regulation in B cells from inflammatory disease patients (Figs. 1B,C and 3A,B). These data suggest that the TLR4 promoter in IL-4-stimulated B cells is packaged into a “poised” structure, defined as a promoter that is bound by sequence-specific DNA binding proteins, regardless of the biologically important outcome of TLR4 surface expression, but that the poised structure alone cannot up regulate surface TLR4 expression in the absence of additional Igμ/CD40-mediated signals. Furthermore, the data support the possibility that initial activation of the TLR4 promoter with anti-Igμ/anti-CD40 and PU. 1 association combines with IL-4-induced IRF-8 association to achieve robust TLR4 promoter activation. We conclude that EL-4-dependent changes in transcription factor association with the TLR4 gene must combine with additional undefined signals activated by CD40 and/or Igμ engagement to increase surface TLR4 expression on B cells.
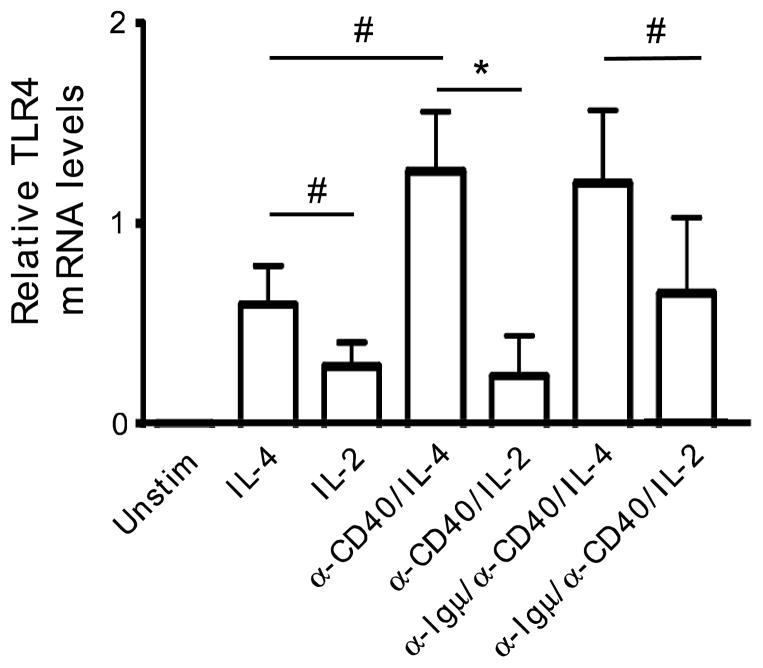
One explanation for the lack of B cell surface TLR4 expression despite transcription factor/TLR4 promoter association would be that IL-4 fails to induce B cell TLR4 transcription. To test this possibility, we stimulated B cells with IL-4, α-Igμ and α-CD40 alone or in combination, then measured TLR4 mRNA levels by RT-PCR. Stimuli that induced transcription factor/TLR4 promoter association, including IL-4 alone, also increased TLR4 mRNA levels (Fig. 4). IL-2, either in the presence or absence of additional stimulation through Igμ and CD40, induced lower levels of TLR4 mRNA compared to IL-4 alone or in combination, as indicated by statistical significance or trends towards significance (Fig. 4, *=p≤005; #=p≤0.08). These data are consistent with the demonstration that IL-2 alone or in combination fails to support association of standard TLR4 activating transcription factors with the TLR4 promoter (Fig. 3), but introduce the possibility that IL-2 may modestly activate TLR4 transcription through mechanisms outside the focus of our analyses. We conclude both TLR4 mRNA and TLR4 promoter/transcription factor association are induced by IL-4 alone, despite the inability of IL-4 to increase surface TLR4 expression on B cells. These data furthermore support the conclusion that post-transcriptional mechanisms controlled by surface Igμ and/or CD40 engagement play important roles in determining levels of surface TLR4 expression on B cells.
Figure 4.
IL-4, in combination with standard B cell stimulation (α-Igμ+ α-CD40), elicits maximum TLR4 mRNA levels that parallel transcript factor association (ChIP) results. RT-PCR amplified β-actin for 24 cycles or TLR4 25 cycles, and signal was quantified by ImageJ. TLR4 mRNA levels in stimulated B cells relative to unstimulated B cells, whose levels were set to =0 (far left). Bar shows average and SEM of independently analyzed samples from 6–7 donors. *=p≤0.05; #=p≤0.08 in the indicated comparisons as determined by paired t tests as appropriate.
3.4. Monocytes down regulate TLR4 expression in response to stimuli that activate TLR4 expression on B cells
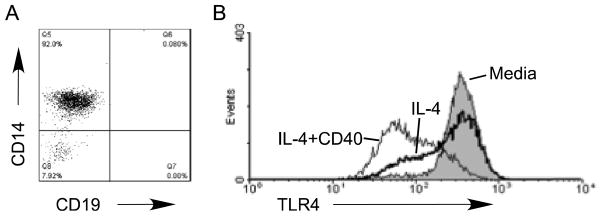
The failure of PU.1/IRF8 association (Fig. 3) and IL-4-induced TLR4 mRNA (Fig. 4) to culminate in B cell surface TLR4 expression may indicate that B cells and monocytes regulate TLR4 expression through fundamentally different mechanisms. To test this possibility, we stimulated purified blood monocytes (Fig. 5A) with IL-4 alone or in combination with α-CD40, then measured the effect of stimulation on surface TLR4 expression. Anti-Igμ was not used in these analyses due to lack of surface Igμ receptors on monocytes. Stimulation through IL-4 or CD40 receptors alone or in combination similarly decreased surface TLR4 expression on monocytes (Fig. 5B and data not shown). These data, in combination with data in Fig. 2, support the conclusion that TLR4 regulation differs significantly between B cells and monocytes. To more rigorously test this conclusion, we stimulated monocytes with anti-CD40, IL-4 and/or IL-2 alone or in combination, then assessed molecular signatures of TLR4 promoter activation by ChIP. Low levels of PU.1 and cJun association with the TLR4 promoter in IL-4 or α-CD40/IL-4 stimulated monocytes, as well as low IRF8 association with the TLR4 promoter in α-CD40/IL-4 stimulated monocytes contrasted with the elevated promoter/transcription factor association in B cells treated similarly (Fig. 6A–C compared to Fig. 3A–C). Differences between associations in B cells and monocytes were statistically significant (Fig. 6 “*”). IRF8 association with the TLR4 promoter in IL-4 stimulated monocytes was variable hence of limited interpretative value (Fig. 6B). Furthermore, monocytes responded to IL-2, alone or in combination with α-CD40, with increased association between the TLR4 promoter and both PU.1 and cJun (Fig. 6A–C). IRF8/TLR4 promoter association in response to IL-2 tended to increase, but this increase did not achieve significance (Fig. 6B, black bar). Taken together, these data confirm TLR4 surface expression data to demonstrate that fundamentally different mechanisms regulate IL-4 induced TLR4 surface expression in B cells and monocytes.
Figure 5.
IL-4 down regulates surface TLR4 on monocytes. (A) Re-analysis of isolated monocytes for purity by flow cytometry. (B) Purified monocytes were stimulated as shown then surface TLR4 fluorescence was measured after gating on the CD14-positive population. Data shown represents 5 determinations.
Figure 6.
IL-4 fails to induce transcription factor association with the TLR4 promoter in monocytes. Purified monocytes were stimulated for 72 hrs. as indicated in the key, then analyzed by ChIP for (A) PU.1, (B) IRF8, or (C) cJun association with the TLR4 promoter. Values that differ significantly in comparisons between monocytes and B cells are highlighted by “*”, which shows p≤0.05 as determined by unpaired t tests. Bars show the average and S.E.M. from 2–3 independent experiments.
4. DISCUSSION
Our data indicate that human B cells responding to antigen plus T cell help, as mimicked by anti-Igμ+α-CD40, have increased potential to up regulate surface TLR4, but the final levels of TLR4 expression and perhaps function is highly dependent on the cytokine milieu. The positive correlation between bioactive IL-4 (as measured by the surrogate eosinophilia) and the percentage of circulating TLR4-positive B cells in type 2 diabetes patients (p≤0.05, data not shown) suggests an unappreciated link between altered cytokine levels and expression/function of TLR4, that together may promote or regulate systemic inflammation in such individuals (McDonnell et al., in press; Jagannathan et al., 2009; Jagannathan et al., 2010). The data therefore significantly extend a previous demonstration that IL-4 up regulates B cell TLR4 (Mita et al., 2002) by linking elevated B cell TLR4 expression, thus mechanisms of B cell TLR4 activation, with inflammatory disease. Whether the cytokine milieu in patients also plays roles in establishing the unexpected pro-inflammatory functions of B cell TLR4 is under investigation.
Thus far, we have demonstrated that B cell TLR4 decreases TLR2-mediated IL-10 secretion (Jagannathan et al., 2009). B cell TLR4 engagement also decreases B cell IL-8 secretion in samples from CD patients, but B cell TLR4 responds to E. coli LPS by increasing IL-8 secretion by B cells from patients with ulcerative colitis (McDonnell et al., in press). These data indicate that B cell TLR4 up-regulation imparts unexpected disease-associated functions that likely affect the overall inflammatory milieu in affected individuals. Regardless, the data highlight the pitfalls of characterizing diseases such as CD, type 2 diabetes, and periodontal disease as IL-2-dominated (Th1) or IL-4-dominated (Th2) disease. Circulating cells from these patients have signatures of both IL-4 activity (elevated B cell TLR4 activation: Fig. 1, Shin et al., 2009; McDonnell et al., in press) and either increased IL-2 activity (elevated TLR4 activation in cultured monocytes, Dasu et al., 2010), or balanced IL-2/IL-4 activity (no change in blood monocyte TLR4 expression, (Jagannathan et al., 2010) defined herein.
The presence of an elevated percentage of TLR4-positive cells in the naïve B cell population of inflammatory disease patients (McDonnell et al., in press; Jagannathan et al., 2009) cannot be explained by the strong stimulatory conditions used in this study: memory, but not naïve B cells would have been exposed to such stimuli in vivo. However, published work has shown that peripheral B cell survival is absolutely dependent on low-level “tonic” signaling through the B cell receptor (Bannish et al., 2001; Lam et al., 1997) and that even naïve B cells have low-level activation of the Igμ signaling pathway (Fuentes-Panana et al., 2006). It is possible that Igμ-mediated tonic signaling, along with the elevated levels of soluble and/or cell-associated CD40, TLR ligands and cytokines present in inflammatory disease patients (Al-Attas et al., 2009; Cani et al., 2007; Creely et al., 2007; Jinchuan et al., 2004; Liu et al., 1999; Varo et al., 2003), are sufficient to induce B cell TLR4 activation in vivo, even in the absence of the activation history that characterizes the memory compartment.
Multiple mechanisms could explain differential TLR4 regulation in B cells and monocytes. Previous demonstrations that the TLR4 promoter is packaged into an equivalently active chromatin structure in monocytes and B cells from healthy or inflammatory disease patients (Jagannathan et al., 2009; Jagannathan et al., 2010) discount the likelihood that chromatin accessibility plays a critical role in differential TLR4 regulation highlighted by our IL-2/IL-4 experiments. We instead speculated that different concentrations of key TLR4 activating factors (PU.1 and IRFs; (Pedchenko et al., 2005; Rehli et al., 2000) in B cells and monocytes explained differential regulation of TLR4 surface levels in the two cell types. However, the ChIP data do not support this possibility: PU. 1 and IRFs associate with the TLR4 promoter approximately equivalently in B cells and monocytes despite higher protein levels of PU.1 and lower protein levels of IRFs in monocytes compared to B cells (DeKoter and Singh, 2000; Marecki et al., 1999; Nikolajczyk et al., 1997). Instead, the agreement between the ChIP and mRNA data, which both lack concordance with the surface staining data, indicates that post-transcriptional regulation plays a critical role in determining TLR4 surface expression on B cells. Post-transcriptional TLR4 regulation has not been attributed to monocytes by either our data or by published studies. However, differences between B cell and monocyte TLR4 regulation raised herein suggest more rigorous approaches are required to formally discount the possibility of additional layers of TLR4 regulation in monocytes. Regardless, different mechanisms of TLR4 regulation in B cells and monocytes are consistent with the finding that TLR4 is up regulated on B cells, but not monocytes or neutrophils, in fresh ex vivo samples from type 2 diabetes patients vs. non-diabetic donors (Jagannathan et al., 2010). We predict that further studies aimed at understanding post-transcriptional mechanisms of TLR4 regulation in immune system cells may shed light on the role these cells and receptors play in inflammatory disease, and identify new targets for next-generation anti-inflammatory treatments.
Acknowledgments
This study was supported by R01 AI54611 and a Research Grant from the American Diabetes Association (BN); Broad Medical Research Program of The Broad Foundation (LMG) and a BD Grant Award 2007 (LMG); T32HL007035 (MJB). We thank Dr. Hatice Hasturk for blood from non-diabetic donors. Yue Zhang provided expert technical assistance. Dr. Farraye has affiliations with Abbott, Centocor, Prometheus, Shire and UCB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Attas OS, Al-Daghri NM, Al-Rubeaan KA, da Silva NF, Sabico SL, Kumar S, McTernan PG, Harte AL. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc Diabetol. 2009;8:20. doi: 10.1186/1475-2840-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med. 2001;194:1583–96. doi: 10.1084/jem.194.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–63. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Caradonna L, Amati L, Leila P, Jirillo E, Caccavo D. Phagocytosis, killing, lymphocyte-mediated antibacterial activity, serum autoantibodies, and plasma endotoxins in inflammatory bowel disease. Am J Gastroenterol. 2000;95:1495–502. doi: 10.1111/j.1572-0241.2000.02085.x. [DOI] [PubMed] [Google Scholar]
- Chong Y, Ikcematsu H, Yamaji K, Nishimura M, Nabeshima S, Kashiwagi S, Hayashi J. CD27(+) (memory) B cell decrease and apoptosis-resistant CD27(−) (naive) B cell increase in aged humans: implications for age-related peripheral B cell developmental disturbances. Int Immunol. 2005;17:383–90. doi: 10.1093/intimm/dxh218. [DOI] [PubMed] [Google Scholar]
- Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–7. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- Dasu MR, Devaraj S, Park S, Jialal I. Increased Toll-like Receptor activation and TLR ligands in Recently Diagnosed Type 2 diabetes Subjects. Diabetes Care. 2010 doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKoter RP, Singh H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science. 2000;288:1439–41. doi: 10.1126/science.288.5470.1439. [DOI] [PubMed] [Google Scholar]
- Desfaits AC, Serri O, Renier G. Normalization of plasma lipid peroxides, monocyte adhesion, and tumor necrosis factor-alpha production in NIDDM patients after gliclazide treatment. Diabetes Care. 1998;21:487–93. doi: 10.2337/diacare.21.4.487. [DOI] [PubMed] [Google Scholar]
- Fuentes-Panana EM, Bannish G, Karnell FG, Treml JF, Monroe JG. Analysis of the individual contributions of Igalpha (CD79a)- and Igbeta (CD79b)-mediated tonic signaling for bone marrow B cell development and peripheral B cell maturation. J Immunol. 2006;177:7913–22. doi: 10.4049/jimmunol.177.11.7913. [DOI] [PubMed] [Google Scholar]
- Ganley-Leal LM, Liu X, Wetzler LM. Toll-like receptor 2-mediated human B cell differentiation. Clin Immunol. 2006;120:272–84. doi: 10.1016/j.clim.2006.04.571. [DOI] [PubMed] [Google Scholar]
- Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, Maxwell RJ, Rowlands BJ. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Navajas JM, Fine S, Law J, Datta SK, Nguyen KP, Yu M, Corr M, Katakura K, Eckman L, Lee J, Raz E. TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J Clin Invest. 2010 doi: 10.1172/JCI40055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- Hou L, Sasaki H, Stashenko P. Toll-like receptor 4-deficient mice have reduced bone destruction following mixed anaerobic infection. Infect Immun. 2000;68:4681–7. doi: 10.1128/iai.68.8.4681-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontal. 2001;6:125–37. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- Jagannathan M, Hasrurk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonnell ME, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. TLR Cross-Talk Specifically Regulates Cytokine Production by B Cells from Chronic Inflammatory Disease Patients. J Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan M, McDonnell M, Liang Y, Hasrurk H, Hetzel J, Rubin D, Kantarci A, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–71. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinchuan Y, Zonggui W, Jinming C, Li L, Xiantao K. Upregulation of CD40--CD40 ligand system in patients with diabetes mellitus. Clin Chim Acta. 2004;339:85–90. doi: 10.1016/j.cccn.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–96. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- Koss K, Satsangi J, Welsh KI, Jewell DP. Is interleukin-6 important in inflammatory bowel disease? Genes Immun. 2000;1:207–12. doi: 10.1038/sj.gene.6363658. [DOI] [PubMed] [Google Scholar]
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–83. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhang Y, McDevit M, Marecki S, Nikolajczyk B. The IL-1 beta gene is transcribed from a poised promoter architecture in monocytes. J Biol Chem. 2006;281:9227–9237. doi: 10.1074/jbc.M510700200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang Z, Wang L, Li J, Dong L, Yue W, Chen J, Sun X, Zhong L, Sun D. TLR4 monoclonal antibody blockade suppresses dextran-sulfate-sodium-induced colitis in mice. J Gastroenterol Hepatol. 2010;25:209–14. doi: 10.1111/j.1440-1746.2009.06046.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Colpaert S, D’Haens GR, Kasran A, de Boer M, Rutgeerts P, Geboes K, Ceuppens JL. Hyperexpression of CD40 ligand (CD 154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J Immunol. 1999;163:4049–57. [PubMed] [Google Scholar]
- Mansson A, Adner M, Hockerfelt U, Cardell LO. A distinct Toll-like receptor repertoire in human tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation. Immunology. 2006;118:539–48. doi: 10.1111/j.1365-2567.2006.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marecki S, Atchison ML, Fenton MJ. Differential Expression and Distinct Functions of IFN Regulatory Factor 4 and IFN Consensus Sequence Binding Protein in Macrophages. J Immunol. 1999;163:2713–2722. [PubMed] [Google Scholar]
- Mita Y, Dobashi K, Endou K, Kawata T, Shimizu Y, Nakazawa T, Mori M. Toll-like receptor 4 surface expression on human monocytes and B cells is modulated by IL-2 and IL-4. Immunol Lett. 2002;81:71–5. doi: 10.1016/s0165-2478(01)00328-5. [DOI] [PubMed] [Google Scholar]
- Muzio M, Bosisio D, Polentarutti N, D’Amico G, Stoppacciaro A, Mancinelli R, van’t Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- Nikolajczyk BS, Cortes M, Feinman R, Sen R. Combinatorial determinants of tissue-specific transcription in B cells and macrophages. Mol & Cell Biol. 1997;17:3527–3535. doi: 10.1128/mcb.17.7.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009 doi: 10.1189/jlb.0309203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara T, Takeda H, Nishihira J, Miyashita K, Nihiwaki M, Ishiguro Y, Takeda K, Akira S, Iwanaga T, Sugiyama T, Asaka M. Macrophage migration inhibitory factor contributes to the development of acute dextran sulphate sodium-induced colitis in Toll-like receptor 4 knockout mice. Clin Exp Immunol. 2005;141:412–21. doi: 10.1111/j.1365-2249.2005.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedchenko TV, Park GY, Joo M, Blackwell TS, Christman JW. Inducible binding of PU.1 and interacting proteins to the Toll-like receptor 4 promoter during endotoxemia. Am J Physiol Lung Cell Mol Physiol. 2005;289:L429–37. doi: 10.1152/ajplung.00046.2005. [DOI] [PubMed] [Google Scholar]
- Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, Tanti JF, Burcelin R, Alessi MC. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007 doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B. PU. 1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000;275:9773–81. doi: 10.1074/jbc.275.13.9773. [DOI] [PubMed] [Google Scholar]
- Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–6. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- Shin H, Zhang Y, Jagannathan M, Hasturk H, Kantarci A, Liu H, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. B cells from periodontal disease patients express surface Toll-like receptor 4. J Leukoc Biol. 2009;85:648–55. doi: 10.1189/jlb.0708428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–9. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res. 2004;24:381–7. doi: 10.1089/1079990041535665. [DOI] [PubMed] [Google Scholar]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–98. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- Varo N, Vicent D, Libby P, Nuzzo R, Calle-Pascual AL, Bernal MR, Fernandez-Cruz A, Veves A, Jarolim P, Varo JJ, Goldfine A, Horton E, Schonbeck U. Elevated plasma levels of the atherogenic mediator soluble CD40 ligand in diabetic patients: a novel target of thiazolidinediones. Circulation. 2003;107:2664–9. doi: 10.1161/01.CIR.0000074043.46437.44. [DOI] [PubMed] [Google Scholar]
- Wellmann W, Fink PC, Benner F, Schmidt FW. Endotoxaemia in active Crohn’s disease. Treatment with whole gut irrigation and 5-aminosalicylic acid. Gut. 1986;27:814–20. doi: 10.1136/gut.27.7.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]