SUMMARY
Plexins are a family of single pass transmembrane proteins that serve as cell surface receptors for Semaphorins during the embryonic development of animals. Semaphorin-Plexin signaling is critical for many cellular aspects of organogenesis, including cell migration, proliferation and survival. Until recently, little was known about the function of PlexinD1, the sole member of the vertebrate-specific PlexinD (PlxnD1) subfamily. Here we review novel findings about PlxnD1’s roles in the development of the cardiovascular, nervous and immune systems and salivary gland branching morphogenesis and discuss new insights concerning the molecular mechanisms of PlxnD1 activity.
MEET THE PLEXINS
In 1995 the Xenopus cell surface axonal antigen B2 was cloned and shown to encode a novel single-pass type I transmembrane protein with calcium-dependent homophilic cell adhesion properties and three extra-cellular cysteine-rich clusters similar to those found in the oncogenic family of MET/HGF (Mesenchymal-Epithelial Transition factor/Hepatocyte Growth Factor) tyrosine kinase receptors (Ohta et al., 1995). The new molecule was renamed Plexin (Plxn) to highlight its role in organizing the plexiform layers of the neural retina (Ohta et al., 1992; Satoda et al., 1995).
Soon the first mammalian Plxns were discovered and found to bind to Semaphorins (Semas), a large family of related extracellular proteins that includes both secreted (class 2 and 3) and membrane-tethered (classes 1, 4–7 and V) forms (Comeau et al., 1998; Kameyama et al., 1996a, b; Maestrini et al., 1996). The first human Plxns were identified in 1996 (Maestrini et al., 1996). The cloning of human Plxns was completed in 1999 and the current system of Plxn classification which groups these proteins into one of four subfamilies (A, B, C and D) based on the sequence similarity of their ectodomains was simultaneously devised (Tamagnone et al., 1999). In 1998 studies revealed that Drosophila PlxnA, which is expressed in the developing nervous system, functions as a Sema-responsive axon guidance receptor, thus uncovering the role of Plxns as Sema receptors (Winberg et al., 1998).
Today we know that Sema-Plxn signaling is important during eumetazoan development not only for axonal guidance, but also for the patterning of many other tissues and organs (see (Roth et al., 2009; Yazdani and Terman, 2006)). In this review we focus on PlxnD1 (Tamagnone et al., 1999), the sole member of the vertebrate-specific D subfamily of Plxn receptors and a key player in vascular, neuronal and immune system development implicated the etiology of congenital defects and cancer.
THE DEVELOPMENTAL BIOLOGY OF PLXND1
It is in your blood (vessels)
plxnD1 is dynamically expressed in many embryonic tissues. In particular, plxnD1 transcripts are prominent in endothelial cells of the developing vasculature (Fig. 1A–C). For example, in E9.5–12.5 mouse embryos plxnD1 expression is found in most, if not all, of the vascular endothelium. By E14.5–18.5 plxnD1 continues to be expressed in both the endothelium and the heart’s endocardium. Similarly, early embryonic plxnD1 expression in the zebrafish is mostly endothelial-specific (Cheng et al., 2001; Gitler et al., 2004; Gu et al., 2005; Torres-Vázquez et al., 2004; van der Zwaag et al., 2002; Zhang et al., 2008).
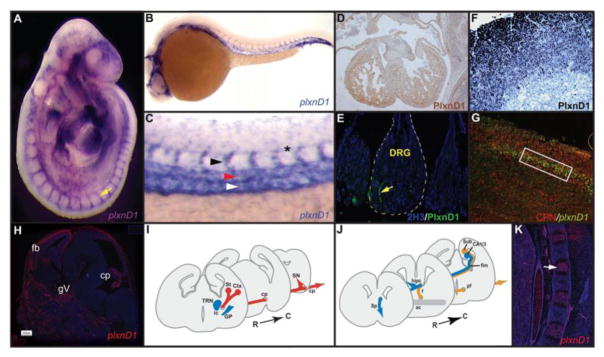
Fig. 1. Expression of plxnD1 mRNA and PlxnD1 protein in the cardiovascular, immune, nervous and skeletal systems.
A–E, Endothelial-specific expression of plxnD1 mRNA (A, purple; B–C, blue) and PlxnD1 protein (D–E). A, E9.5 mouse embryo (left lateral view). The head is up. Anterior dorsal side, left. plxnD1 is detected in the entire vasculature. Segmental vessel, yellow arrow. B, C, 28 hpf zebrafish embryo. Left lateral views. Anterior, left. Dorsal is up. B, plxnD1 is detected in the entire vasculature. C, Trunk detail. Segmental Arteries (SeAs; black arrowhead). Dorsal Aorta (DA; red arrowhead). Posterior Cardinal Vein (PCV; yellow arrowhead). Floorplate's hypochord, black asterisk. D, E11.5 murine heart cross-section. PlxnD1 (brown). E, Murine dorsal root ganglia (DRG; blue, delimited by yellow dotted line). Vessel with PlxnD1 (green) expression, yellow arrow. F, Murine immune system (paraffin section of the thymus). Cortical thymocytes expressing PlxnD1, dark blue. G–J, Expression of plxnD1/PlxnD1 in the murine nervous system. G, P8 cortex section. Only some cortical commissural neurons (callosal projection neurons; CPN, red) express plxnD1 (green) (rectangle). H, E14.5 murine section of the brain. Forebrain, fb. Trigeminal ganglion, gv. Choroid plexus, cp. plxnD1, red. I–J, Diagrams of coronal sections of E17.5 murine forebrains showing sites were Sema3E (blue) and PlxnD1 (red) are expressed and locales where PlxnD1 and Nrp1 are co-expressed (light orange). Rostral, left. Caudal, right. Dorsal is up. I, PlxnD1 is expressed along the corticofugal and striatonigral projections that originate from the ventrolateral cortex (Ctx) and striatum (St), respectively and which transverse both the internal capsule (ic) and the cerebral peduncle (cp). At the level of the midbrain the striatonigral projections leave the cp and terminate in the substantia nigra (SN), while corticofugal axons continue into the pons (not shown). Robust Sema3E expression is observed in the globus pallidus (GP) and thalamic reticular nucleus (TRN). J, PlxnD1 and Nrp1 are co-expressed in the subiculo-mammillary tract, which projects from the subiculum (Sub) through the fimbria (fim), fornix (f) and postcommissural fornix (pf). Sema3E expression is observed in the hippocampal pyramidal cell layers of the cornus ammonis 1 and 3 (CA1/3) adjacent to the Sub. The anterior commissure (ac), hippocampal commissure (hipc) and septum (Sp) are indicated. K, E18.5 murine vertebral bodies, lateral cross-section. Ossification center, oc. plxnD1, red. Credits for reproduced and/or modified images: A, D and E from (Gitler et al., 2004); B and C from (Torres-Vázquez et al., 2004); F from (Choi et al., 2008); G from (Molyneaux et al., 2009a); H and K from (Zhang et al., 2008); I-J from (Chauvet et al., 2007).
Sema3-PlxnD1 signaling guides angiogenic pathfinding
The reproducible anatomy of the vasculature ensures homeostasis and survival by enabling the adequate distribution of gases, metabolites, hormones and immunity factors through the body (Jain, 2003; Weinstein, 1999). Others and we have shown that the stereotypical organization of the blood vessels is guided by the same genetic mechanisms that guide axons (reviewed in (Carmeliet and Tessier-Lavigne, 2005). For example, vertebrate-specific Semas of the class 3 (Sema3s) inhibit the migration of neuronal growth cones expressing both Plxns and Neuropilins (Nrps/Npns) to restrict their navigation pathways (reviewed in (Casazza et al., 2007; Shim and Ming, 2007). Nrps are cell surface transmembrane proteins that are expressed in both the nerves and the endothelium that serve as Sema3 and VEGF (Vascular Endothelial Growth Factor) co-receptors for Plxns and VEGFR-2 (VEGF Receptor 2/Flk1/Kdr/), respectively (reviewed in (Geretti et al., 2008; Schwarz and Ruhrberg, 2010)).
Pioneer in vitro and in vivo experiments revealed that endothelial cells also respond to Sema3 cues. Given that Nrps act as VEGF co-receptors during cardiovascular development, the inhibitory effect of Sema3s on angiogenic sprouting and endothelial cell migration was attributed to Sema3/VEGF competition for Nrp binding (Bates et al., 2003; Miao et al., 1999; Shoji et al., 2003). However, an alternative molecular mechanism for explaining the effects of Sema3s on cardiovascular development was suggested by the endothelial expression of plxnD1 in both mouse and zebrafish embryos (Torres-Vázquez et al., 2004; van der Zwaag et al., 2002). By following upon this observation we, along with others, were able to demonstrate that paracrine Sema3 signaling via PlxnD1 guides the anatomical patterning of specific subsets of angiogenic vessels (Gitler et al., 2004; Gu et al., 2005).
For example, in our studies we used time-lapse confocal imaging to visualize the developing zebrafish vasculature in embryos carrying endothelial-specific fluorescent reporters. In wild type animals angiogenic Segmental Arteries (SeAs) sprout next to the somite boundaries from both the left and right sides of the Dorsal Aorta (DA) at approximately 21 hours post-fertilization (hpf). These nascent vessels extend dynamic filopodia-like processes as they grow dorsally acquiring a characteristic chevron shape that reflects their trajectory, which tracks along most of the somite boundary. By 30–32 hpf the SeAs have extended past the roof of the neural tube. Here they branch in an antero-posterior manner, interconnecting with their ipsilateral neighbors to form each of the two Dorsal Longitudinal Anastomotic Vessels (DLAVs) that run along the trunk dorsally to the neural tube (Fig. 2C) (Isogai et al., 2003; Torres-Vázquez et al., 2004).
Fig. 2. Sema/PlxnD1 signaling functions to pattern the cardiovascular, skeletal and nervous systems.
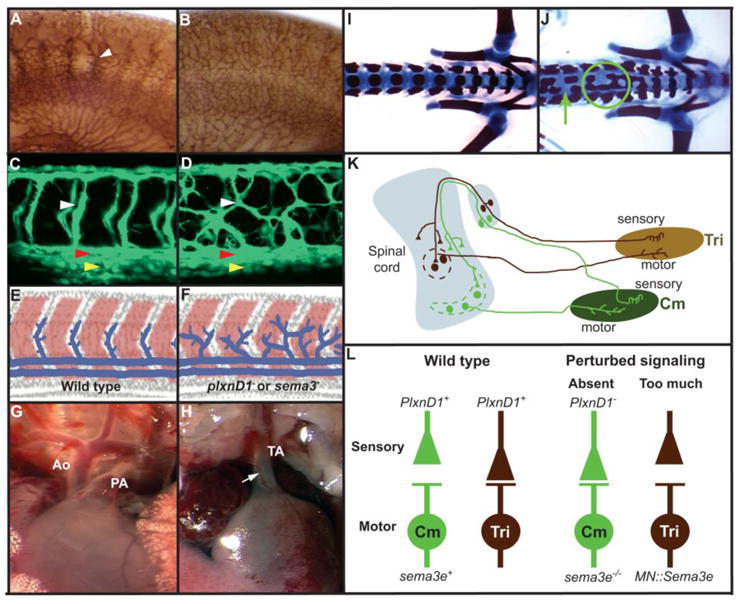
A–B, E11.5 murine trunks. Lateral views. Dorsal is up. Endothelium, dark brown. A, Phenotypically wild type sema3E+/− heterozygote. Segmental vessel, white arrow. Note loss of avascular areas. B, sema3E−/− homozygous mutant displaying disorganized Segmental vessels. Notably, plxnD1 −/− homozygous mutants display identical vascular patterning defects (not shown). C, D, 48 hpf zebrafish embryos. Lateral views. Anterior, left. Dorsal is up. Endothelium, green. Segmental Arteries (SeAs), white arrowheads. Dorsal Aorta (DA), red arrowheads. Posterior Cardinal Vein (PCV), yellow arrowheads. C, Wild type. D, Animal lacking plxnD1 activity. Note aberrant vascular pattern, resembling that of sema3E−/− homozygous mutant mice (B). E, Model for the evolutionarily conserved role of Sema-PlxnD1 signaling in shaping the Segmental vasculature. Paracrine Sema signals from the somites (pink) are sensed by PlxnD1 receptors expressed in the endothelium (blue) to guide the patterning of nascent Se vessels via a repulsive mechanism. F, Without Sema-PlxnD1 signaling Se vesels grow aberrantly. G–H, plxnD1 activity is required for proper formation of the murine heart’s outflow tract. Images of P0-stage hearts. G, Wild type heart showing two major vessels, the aorta (Ao) and the pulmonary artery (PA). H, plxnD1 mutant displaying persistent truncus arteriosus (TA; a single great vessel), a form of congenital heart disease. In addition, the coronary artery (white arrow) displays an abnormal origin. I–J, P0 murine lumbar skeletons (dorsal views) Anterior, left. Mineralized bone, red. Cartilage, blue. I, Wild type. J, plxnD1−/− mutant pup showing axial skeletal defects and malformations such as splitting (green arrow) and fusion (green circle) of the vertebral bodies. K, Connectivity patterns between sensory and motor neurons in the triceps (Tri, dark brown) and cutaneous maximus (Cm, light green) reflex arcs in the mouse. Muscles, ovals (Tri; light brown, Cm; dark green). Dotted lines surround synapses between sensory afferents and motor neurons in these reflex arcs. L, Diagram summarizing the role of Sema3E-PlxnD1 repulsive signaling in establishing the fine synaptic specificity between sensory afferents (top) and motor neurons (bottom) in the Cm (green) and Tri (brown) arcs. Left half, wild type wiring pattern. Normally, plxnD1 is expressed in both Cm and Tri sensory neurons and sema3E is expressed only in Cm motor neurons. Therefore only the Tri motor neurons receive direct proprioceptive inputs from Tri sensory neurons (homonymous connectivity). The right half of the figure shows the rewiring of homonymous connectivity resulting from perturbing Sema3E–PlxnD1 signaling. Absence of either plxnD1 activity or sema3E expression induces abnormal homonymous monosynaptic Cm connectivity (left). In contrast, ectopic sema3E expression in the Tri motorneurons (right) prevents the establishment of monosynaptic connections in the Tri reflex arc. Notably, perturbing Sema3E–PlxnD1 signaling does not induce heteronymous sensory–motor connections (Cm to Tri or vice versa). This indicates that additional factors regulate motor pool specificity. Credits for reproduced and/or modified images: A–B from (Gu et al., 2005); C–F from (Torres-Vázquez et al., 2004); G–H from (Gitler et al., 2004); I–J from (Zhang et al., 2008); K adapted from (Maro et al., 2009) L adapted from (Pecho-Vrieseling et al., 2009).
In contrast, plxnD1-deficient embryos, such as out of bounds (obd) homozygous mutants and wild type animals injected with anti-plxnD1 morpholinos show dramatic defects in SeA development, such as premature and ectopic sprouting, aberrant pathfinding accompanied by the formation of abnormally long filopodia and improper branching (Fig. 2D). In contrast, plxnD1 is not required for DLAV positioning, vascular lumenization or circulatory blood flow (Childs et al., 2002; Torres-Vázquez et al., 2004).
The class 3 Semas encoded by the zebrafish sema3aa/3a1 (Shoji et al., 1998) and sema3ab/3a2 (Shoji et al., 2003) genes are expressed in the developing somites, in overlapping patterns that are largely complementary to the paths followed by growing SeA sprouts (Fig. 2E). Zebrafish embryos with reduced Sema3a function display disorganized SeAs, similar to those found in plxnD1-deficient animals. Conversely, somite-specific over-expression of Sema3s inhibits SeA growth in a plxnD1-dependent manner (Torres-Vázquez et al., 2004). Together, our findings indicate that paracrine repulsive Sema3-PlxnD1 signaling from the somites to the developing vasculature shapes the reproducible anatomy of the SeAs by regulating fundamental aspects of their angiogenic development, such as their sprouting schedule, launching position, pathfinding and branching morphogenesis (Fig. 2F).
Importantly, studies about the role of PlxnD1 (Gitler et al., 2004; Gu et al., 2005; Zhang et al., 2008) and its canonical murine ligand, Sema3E (Gitler et al., 2004; Gu et al., 2005) fully support this model. For example, knockout mouse embryos lacking either PlxnD1 (plxnD1KO) or Sema3E activity show identical Se vessel phenotypes (Fig. 2A–B), which parallel those of obd mutants. Accordingly, Sema3E induces the collapse of PlxnD1-expressing COS-7 cells and vascular repulsion in chick (Gitler et al., 2004; Gu et al., 2005). Finally, two lines of evidence indicate that Sema3-PlxnD1 signaling is required in the endothelium for proper Se vessel development. First, endothelial-specific inactivation of murine plxnD1 (plxnD1ECKO) using a floxed allele induces all of the cardiovascular defects observed in plxnD1KO mice (Zhang et al., 2008). Second, we found that SeA defects of obd mutants are rescued when wild type, exogenous plxnD1 cDNA is provided in an endothelial-specific manner (Tomasz Zygmunt and Jesús Torres-Vázquez, unpublished results). However, the cell autonomy of plxnD1 in the endothelium has not been defined. Nonetheless, these observations indicate that Sema3-PlxnD1 signaling plays an evolutionarily conserved role in shaping the anatomy of the Se vessels.
Beyond Se vessel development
plxnD1KO mouse embryos also display hypervascularization of the heart’s epicardium (Gitler et al., 2004), ectopic vascular branching in the hindbrain (Vieira et al., 2007) and reduction of the fourth and sixth aortic arch arteries. Furthermore, the pups exhibit mispatterned intercostal vessels and persistent truncus arteriosus (PTA), a congenital form of heart disease caused by defective remodeling of the heart’s outflow tract (OT). Specifically, a lack of conotruncal septation fails to separate the pulmonary artery and the aorta, leading to perinatal cyanosis and lethality (Fig. 2G–H).
Notably, migration of cardiac neural crest cells (which also express plxnD1) is not impaired in plxnD1KO embryos, thus suggesting that the PTA’s etiology is endothelial-specific. Similar OT abnormalities are observed in animals lacking Sema3C or with impaired Nrp activity, but not in sema3e-deficient animals, suggesting that loss of Sema3C-PlxnD1/Nrp1 signaling is the cause for these abnormalities. Accordingly, Sema3C binds to Nrps in an enhanced manner in the presence PlxnD1 (Gitler et al., 2004) (see also (Banu et al., 2006; Gu et al., 2003; Toyofuku et al., 2008).
In contrast to mice, zebrafish lack OT septation. This allows the survival of obd mutants and enables the analysis of phenotypes related to loss of plxnD1 activity through life (Childs et al., 2002; Gitler et al., 2004). We have found that in adult obd mutants the stereotypical organization of the fin vasculature is disrupted (Jesús Torres-Vázquez, unpublished observations).
Surprisingly, even the morphogenesis of some vascular beds that do not exhibit a fixed anatomical pattern is also plxnD1-dependent. For example, obd mutant embryos display retinal (Alvarez et al., 2009; Alvarez et al., 2007) and subintestinal vessels that are aberrantly branched (Jesús Torres-Vázquez, unpublished observations) as well as abnormal remodeling of the caudal vein plexus (Torres-Vázquez et al., 2004). Importantly, similar defects in retinal vasculature organization are observed in mice with endothelial-specific deletion of plxnD1 activity (Zhang et al., 2008). Overall, these observations indicate that endothelial PlxnD1 activity plays an evolutionarily conserved role in shaping many different vascular beds, including vessels with stereotypical and non-stereotypical anatomies.
Besides Sema3C and Sema3E, the transmembrane Sema4A is also a PlxnD1 ligand in mice, although of low affinity. Sema4A is expressed in the heart’s ventricle and the Se vessels of E10.5 embryos. Although Sema4A appears to suppress angiogenesis in a PlxnD1-dependent manner, Sema4A does not appear to be involved in shaping the vasculature, since Sema4A-deficient mice display normally patterned vessels. Instead, Sema4A-deficient adult mice exhibit enhanced wound-induced neo-angiogenesis (Kumanogoh et al., 2005; Toyofuku et al., 2007).
Finally, endothelial PlxnD1 signaling appears to be required for proper development of the heart’s myocardium and bones. For example, plxnD1ECKO mouse embryos display atrial defects and ventricular septal abnormalities. These phenotypes are likely due to disrupted endocardial-myocardial communication. In addition, plxnD1KO and plxnD1ECKO mice display defects in the patterning of the axial skeleton (Fig. 2I–J), such as hemivertebrae and vertebral fusions, which likely stem from improper bone vascularization (Kanda et al., 2007; Toyofuku et al., 2007).
Getting nervous
plxnD1 transcription occurs also in the nervous system (Fig. 1G–H). In the E14.5–18.5 mouse embryo plxnD1 is expressed in the CNS at the forebrain, trigeminal and dorsal root ganglia and choroid plexus (Zhang et al., 2008). At E15.5–E17.5 plxnD1 is expressed in the cortex’s ventrolateral regions (piriform, perirhinal, and insular cortices), striatum and the pyramidal layer of the subiculum during the formation of the forebrain’s descending axon tracts. In particular, at E17.5 plxnD1 is expressed in the cortifugal and striatonigral projections that grow through the mesencephalic cerebral peduncle to innervate lower brain centers as well as the subiculo-mammillary tract, the main output structure of the hippocampus (Chauvet et al., 2007). In addition, postnatal day 1 (P1) mice display plxnD1 expression in the hippocampus (outer molecular layer of the dentate gyrus and the stratum lacunosum moleculare layers of the cornua ammonis 1 and 2). In P14 mice plxnD1 is expressed in a subset of callosal projection neurons (Cheng et al., 2001; Molyneaux et al., 2009b) and in adults it is expressed in the amygdala (D’Souza et al., 2008). Finally, plxnD1 is expressed in the neocortex of both mature mice and Japanese macaques in a laminar distribution that is complementary to that of its ligand, sema3e (Watakabe et al., 2006).
Wiring the brain
Sema-Plxn signaling is important for both axonal guidance and pruning (reviewed in (Waimey and Cheng, 2006)). Accordingly, both Sema3E and PlxnD1 have been implicated in the organization of neuronal circuits. For example, in the E17.5 forebrain PlxnD1 is expressed in the descending cortifugal and striatonigral tracts (Fig. 1I). Both tracts share a common caudal projecting pathway, crossing through the internal capsule and the cerebral peduncle to reach their lower brain centers targets (the midbrain’s substantia nigra and the brain stem’s pons, respectively). A second PlxnD1+ axonal pathway (the subiculo-mamillary tract) begins in the subiculum, transverses the fimbria, fornix and postcommisural fornix to reach the mammillary bodies in the caudal hypothalamus (Fig. 1J).
The axons of these two descending pathways extend near Sema3E-expressing cells but display sharply different behaviors. The corticofugal and striatonigral axons avoid Sema3E sources (the thalamic ventricular neurons and the globus pallidus) by growing between them (through the internal capsule). In contrast, the subiculo-mammillary axons are attracted to Sema3E and grow along the axons of Sema3E-expressing neurons of the pyramidal cell layers in the CA1 and CA3 hyppocampal fields. Notably, the behavior of these axonal tracts correlates with differences in Nrp1 expression. Nrp1 is absent in the neurons that interpret Sema3E as a repellent (the cortifugal and striatonigral tracts) but it is present in the neurons that sense Sema3E as a chemoattractant (the subiculo-mamillary tract). In experiments with cultured, dissociated neurons both the repulsive and attractive effects of Sema3E are PlxnD1-dependent, with Nrp1 enabling or “gating” Sema3E-induced axonal attraction. Remarkably, Sema3E-induced axonal attraction involves a PlxnD1/Nrp1/VEGFR2 ternary complex in which PlxnD1 functions as the ligand binding subunit and VEGFR2 as the signal transducing subunit (Fig. 3D) (Bellon et al., 2010; Chauvet et al., 2007).
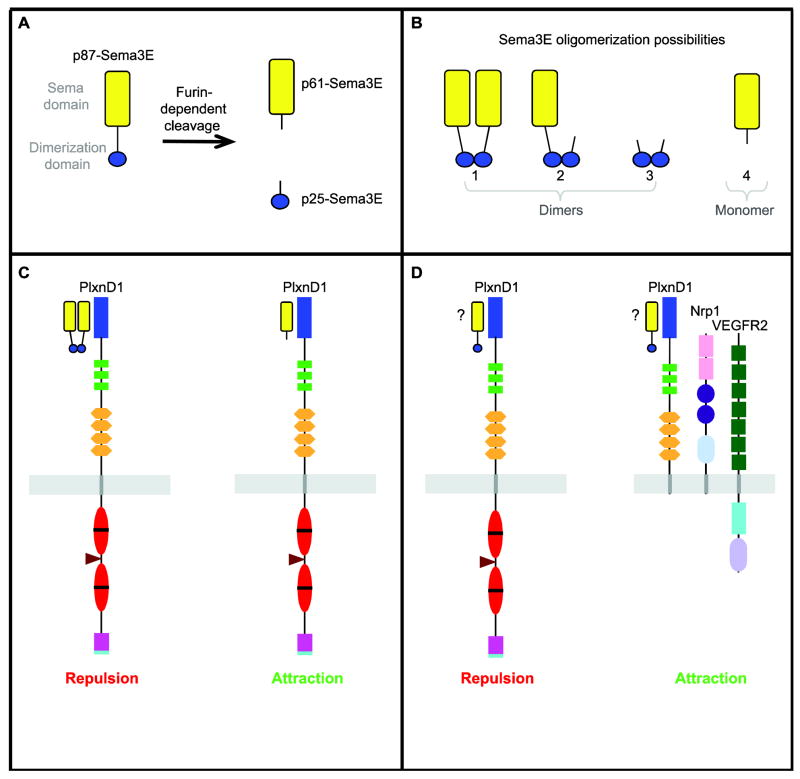
Fig. 3.
Sema3/PlxnD1 signaling is capable of promoting attraction and repulsion in both the vascular and nervous systems. A, In adenocarcinoma cells the full-length Sema3E (p87-Sema3E) is cleaved by a furin-like endoprotease to yield two products. The longer one contains the Sema domain (p61-Sema3E) while the shorter one contains the dimerization domain (p25-Sema3E). B, As a result, adenocarcinoma cells secrete three combinations of Sema3E dimers (1–3) and one monomer (4) (Christensen et al., 2005). C, In the vascular system (left) full-length Sema3E dimers induce repulsion via PlxnD1. However, the p61-Sema3E monomer containing the Sema domain can function as an attractant in some endothelial lines expressing PlxnD1 (right). However, the requirement for PlxnD1 to mediate this attractive response has not been tested (Christensen et al., 2005). D, In the nervous system, Sema3E acts as a repellant when signaling through PlxnD1 alone (left). However, in neurons expressing also Nrp1 and the VEGF receptor VEGFR2, binding of Sema3E elicits an attractive/growth-promoting axonal response (right). Remarkably, this attractive response requires only the extracellular domains of PlxnD1 and Nrp1 (as shown), with PlxnD1 functioning as the ligand-binding subunit of the complex. In contrast, VEGFR2’s intracellular tail is required in this context as the signal transducing subunit (Bellon et al., 2010). Note however, that the precise Sema3E oligomerization form that mediates these responses is unknown.
Although the possible link between Sema3E-induced axonal attraction and p61Sema3E expression has not been explored in this context, the authors of these studies found that the dual Sema3/VEGF co-receptor Nrp1 as well as the VEGF receptor VEGFR2 reduce in half the number of Sema3E-binding sites of PlxnD1-expressing cells in vitro. Thus, the presence of either co-receptor might reduce the access of Sema3E dimers to PlxnD1 (Bellon et al., 2010; Chauvet et al., 2007) and/or enable the binding of p61Sema3E monomers, despite the fact that p61Sema3E does not appear to bind to Nrp1 (Casazza et al., 2010). Interestingly, the endothelial cell line used to show that p61Sema3E functions as an attractant for endothelial cells is known to express both PlxnD1 and Nrp1; whether it also expresses VEGFR2 has not been experimentally verified (Christensen et al., 2005; Matthies et al., 2002).
Sensory-motor circuit connectivity
Sema3E and PlxnD1 are also key determinants for establishing the fine synaptic specificity that matches particular subsets of sensory afferents (proprioceptive sensory neurons or PSNs) with their cognate motor neurons (MNs) in two different spinal sensory-motor circuits of the mouse: the triceps (Tri) and the cutaneous maximus (Cm) reflex arcs (Fig. 2K–L). In the Tri reflex arc PSNs provide monosynaptic inputs to MNs. In contrast, Cm’s sensory afferents do not synapse with MNs. The majority of PSNs in both reflex arcs express PlxnD1. However, Sema3e expression is restricted to the Cm’s MNs. In a series of beautiful experiments, the loss of SEMA3E-PLXND1 signaling was found to induce differential effects on synaptic connectivity. When either Sema3e or PlxnD1 activity is eliminated, the PSNs and MNs of the Cm wire together, but Tri PSN-MN connectivity are unaffected. Conversely, forced SEMA3E expression in Cm MNs prevents PSN-MN connectivity in the Cm arc. Together, these observations indicate that sensory-motor connectivity is based on a system that employs SEMA3E-induced, PLXND1-mediated repulsion for blocking synapse formation (Pecho-Vrieseling et al., 2009) see also (Yoshida et al., 2006).
Glandular branching morphogenesis
Formation of the salivary and mammary glands, the lungs and the kidneys is orchestrated by interactions between epithelial cells organized in tubular buds and the mesenchyme that surrounds them, which leads to the growth and branching of the epithelial primordium (Andrew and Ewald, 2009).
Recently, it was found that epithelial bud cleft formation in the developing murine submandibular gland (SMG) requires Sema3A, Sema3C, PlxnD1, PlxnA1 and Nrp1. RNA in situ hybridization reveals that the transcripts of these five molecules are expressed within the epithelial bud during SMG branching morphogenesis. Moreover, loss-of-function experiments using cultured SMG explants indicate that the activity of these five proteins is required for epithelial bud cleft formation. SMG branching morphogenesis involves cleft formation, a process that is thought to occur via the local collapse of epithelial cells. Surprisingly, the canonical PlxnD1 ligand Sema3E (which is expressed in the epithelial bud) is not required for this process (Chung et al., 2007).
Notably, plxnD1 transcripts are also detected in the mouse developing mammary gland at the terminal end buds and the stroma and in the embryonic adrenal gland. Thus, it is likely that PlxnD1 is involved in the branching of these organs as well (Morris et al., 2006) (Zhang et al., 2008).
Thymocyte development
Thymocyte maturation requires their proper migration within the thymus. Repulsive Sema3E-PlxnD1 signaling controls the migration of CD4+CD8+ double positive (DP) thymocytes. Expression of both plxnD1 mRNA and PlxnD1 protein is observed in cortical thymocytes (Fig. 1F). In contrast, Sema3E mRNA is detected in a reciprocal pattern, at the medulla, where mature CD8+ single positive (SP) thymocytes (which lack detectable PlxnD1 expression) are located. CCL25-CCR9 chemokine signaling promotes thymocyte migration into the medula. Thymocyte migration experiments indicate that Sema3E-PlxnD1 signaling inhibits CCL25-CCR9 chemokine signaling, consistent with the fact that during the DP-SP transition thymocytes down regulate PlxnD1 expression (Choi et al., 2008). Reduced levels of PlxnD1 expression are also observed in leukemic thymocytes (Calvo, 2005; Guijosa, 2007).
Sema-PlxnD1 signaling: Going new places
plxnD1 is expressed in many other organs and tissues where its role has not yet been addressed. For example, in the murine podocytes (the visceral epithelial cells of the kidney that function as the glomerular filtration barrier), in both the adrenal and mammary glands, the lung mesenchyme, the ossification centers of vertebral bodies (Fig. 1K), osteoblastic cells and bone tissues of both newborn and adult mice; the smooth muscle of the small intestine and macrophages (as in humans). plxnD1 is also prominently expressed in the floorplate’s hypochord of the zebrafish embryo (Choi et al., 2008; Chung et al., 2007; Guan et al., 2006; Kanda et al., 2007; Roodink et al., 2008; Roodink et al., 2005; Torres-Vázquez et al., 2004; Zhang et al., 2008) (Morris et al., 2006).
PLXND1 AND DISEASE
Congenital defects: CHARGE syndrome
plxnD1 is necessary for key aspects of murine cardiovascular development (Gitler et al., 2004; Gu et al., 2005; Zhang et al., 2008). Some of these defects, notably OT abnormalities, are incompatible with postnatal survival and likely explain why mutations in plxnD1 have not been causally linked to human cardiovascular birth defects.
In contrast, Sema3E-null mice are viable and recapitulate most, but not all, of the cardiovascular defects observed in plxnD1-deficient animals (Chauvet et al., 2007; Gu et al., 2005). Accordingly, a missense mutation in SEMA3E was recently identified in a patient suffering from CHARGE syndrome (Lalani et al., 2004). This is a rare and genetically heterogenous condition named after the constellation of features first used to diagnose it (Coloboma of the eye, Heart defects, Atresia of the choanae, Retardation of growth and/or development, Genital and/or urinary abnormalities, and Ear abnormalities and deafness) (Pagon et al., 1981). Notably, CHARGE patients and both mouse sema3e and plxnD1 knockouts show parallel abnormalities in the cardiovascular and nervous systems. Moreover, CHARGE patients and mice lacking plxnD1 activity display defective skeletal development (Lalani et al., 2004; Song et al., 2008) (see also http://chargesyndrome.org/about-charge.asp). Together, these observations suggest that impaired Sema3E-PlxnD1 signaling is an etiological factor in CHARGE syndrome.
Cancer
Tumors hijack the programs that modulate angiogenesis to gain the blood supply that enables their survival, growth and metastasis (Carmeliet, 2005). Recent findings suggest that Sema3-PlxnD1 signaling antagonizes tumor angiogenesis and tumor development (Christensen et al., 2005; Roodink et al., 2005). For example, Sema3E is highly expressed in the melanocytes of early-stage, non-invasive in situ melanomas but only in 36% of melanoma metastases. In addition, melanoma cell lines with high Sema3E expression inhibit tumor angiogenesis in vitro and fail to metastasize in a murine tumor transplantation model (Kigel et al., 2008; Roodink et al., 2008). Although these experiments did not address if the Sema3E effects are PlxnD1-dependent, these results are consistent with the known role of Sema3E as a PlxnD1-dependent repulsive cue for endothelial cells during mammalian embryogenesis (Gitler et al., 2004; Gu et al., 2005).
However, while full-length dimeric forms of Sema3s act as growth-repelling cues, furin-dependent cleavage of Sema3s reduces their repelling activity. Consistent with this notion, blocking Furin activity abolishes the invasiveness and tumorigenicity of human cancer cells (Adams et al., 1997; Bassi et al., 2001; Klostermann et al., 1998; Koppel and Raper, 1998). Furin cleaves Sema3E into two fragments (Fig. 3A). The large one contains the N-terminal Sema domain, while the short C-terminal fragment includes the Cys residue involved in Sema dimerization. Thus, furin-induced Sema3E cleavage generates four Sema3E isoforms of different size and oligomerization state (Fig. 3B).
Surprisingly, the cleaved Sema3E monomer (p61Sema3E) acts as a pro-angiogenic attractant in vitro (Fig. 3C), promotes lung metastasis in mouse xenografts and binds to PlxnD1. In fact, the main Sema3E is form secreted by mouse mammary Aden carcinoma cells is p61Sema3E. Adenocarcinoma cells that lack endogenous sema3E expression and metastatic potential can form tumors when transected with vectors that drive the expression of either wild type sema3E cDNA or an artificial p61Sema3E is form (Christensen et al., 2005). Moreover, in healthy human adults PlxnD1 is expressed only in very few organs and at low levels. However, the tumor vasculature and the malignant cells of a wide range of tumors (including both primary and metastatic brain tumors and melanomas) display dramatically enhanced PlxnD1 expression. Thus, PlxnD1 is the only known protein whose expression increases in both tumor compartments: the neoplasm and its vasculature (Roodink et al., 2005; Roodink et al., 2009).
Therefore, a recent study analyzed whether the effects of p61Sema3E on tumor metastasis and tumor angiogenesis are PlxnD1-dependent. The authors found that in a collection of 60 different human colon carcinoma samples all of them expressed PlxnD1 (Casazza et al., 2010). Accordingly, in a different study plxnD1 was found to be upregulated in colorectal cancer (Galamb et al., 2008). Interestingly, the first study found that Sema3E expression is selectively enriched in primary tumors associated with metastatic disease (88%) and in 100% of liver metastases from colon carcinomas. This indicates that Sema3E and PlxnD1 levels are higher in metastases than in primary tumors. Similarly, both the level and number of melanoma cells expressing Sema3E increases with tumor grade (Casazza et al., 2010). Consistent with this notion another study reported a parallel trend for PlxnD1 abundance in melanoma (Roodink et al., 2008). Together, these observations indicate that increased levels of both Sema3E and PlxnD1 correlate with the potential for metastatic spreading.
Accordingly, in tumor xenograft assays using mouse hosts reducing the level of Sema3E or PlxnD1 in the human carcinoma cells impairs their metastasis without affecting tumor growth. Conversely, forced expression of either Sema3E or p61Sema3E in tumor cells increases the incidence of lung metastasis. Indeed, elevated levels of both Sema3E and PlxnD1 are associated with enhanced risk for metastatic progression in colon cancer patients. Interestingly, p61Sema3E-PlxnD1 autocrine signaling in tumor cells appears to promote tumor metastasis by increasing both tumor cell migration and extravasation via enhanced extracellular matrix (ECM) degradation/invasion (Casazza et al., 2010). Finally, it is worth noting that Sema4D, an alternative PlxnD1 ligand also promotes tumor development. Sema4D belongs to a class of cleavable transmembrane Semas that yield soluble forms, further underscoring the importance of proteolytic cleavage events as determinants for Sema activity (Basile et al., 2006; Serini et al., 2009; Sierra et al., 2008).
An unhealthy partnership: PlxnD1 and ErbB2
Remarkably, the pro-metastatic effects of p61Sema3E/PlxnD1 require the activity of the oncogenic receptor tyrosine kinase ErbB2 (Erythroblastic leukemia viral oncogene homolog 2). p61Sema3E does not bind to ErbB2, but rather induces the formation of a phosphotyrosinated PlxnD1-ErbB2 complex in human lung carcinoma cells. Interestingly, each receptor is required for the phosphorylation of the other: ErbB2 phosphorylation is PlxnD1-dependent and PlxnD1 phosphorylation requires ErbB2’s kinase activity. The p61Sema3E-PlxnD1 mediated metastasis of human lung carcinoma cells is ErbB2-dependent (Casazza et al., 2010). Consistent with these findings, ErbB2 induces the expression of the invasion-promoting matrix metalloprotease MT1-MMP (Miyamori et al., 2000). Moreover, knockdown of MT1-MMP in human fibrosarcoma cells in vitro leads to lower plxnD1 levels (Rozanov et al., 2008). These observations suggest that p61Sema3E/PlxnD1/ErbB2 signaling is modulated via positive feedback.
Despite these important advances, key controversies remain. Studies report opposite correlations between Sema3E abundance and melanoma and contrasting effects of p61Sema3E on the chemotactic response of endothelial cells (Christensen et al., 2005) (Kigel et al., 2008; Roodink et al., 2008) (Casazza et al., 2010). Undoubtedly future studies will clarify these discrepancies. Nonetheless, these observations suggest that targeting Sema-PlxnD1 signaling could provide the basis for novel anti-cancer therapies.
PLXND1: STRUCTURE AND MOLECULAR MECHANISMS OF ACTIVITY
The view from the outside: the ectodomain
PlxnD1 contains all the domains and motifs generally found in other Plxns (Figs. 4A, 5A-D). The variably sized (~860–1400 aa) ectodomain of Plxns contains two regions with homology to the Sema, MET and integrin protein families. The first is the amino-terminal ~500 aa Sema domain, which displays a seven-bladed Beta-propeller topology structurally resembling that of integrins. The second is comprised of three cysteine-rich motifs known as MET-Related Sequences (MRS repeats or PSI -Plexins, Semas and Integrins- domains) that are ~50 aa long each. Notably, PlxnD1’s third MRS is atypical because it has six, rather than eight, cysteines. The function of the MRS repeats is unknown. Finally, the last stretch of the PlxnD1 ectodomain contains four IPT domains (Immunoglobin-like fold shared by Plexins and Transcription factors domains; also found in MET family members). Each IPT domain contains glycine and proline rich repeats. The role of the IPT domains is unknown, but there is evidence that they are functionally important: in PlxnA3 a missense mutation in one of them renders the receptor inactive (Bork et al., 1999; Tamagnone et al., 1999; Tanaka et al., 2007; van der Zwaag et al., 2002; Winberg et al., 1998); (Gherardi et al., 2004; Love et al., 2003).
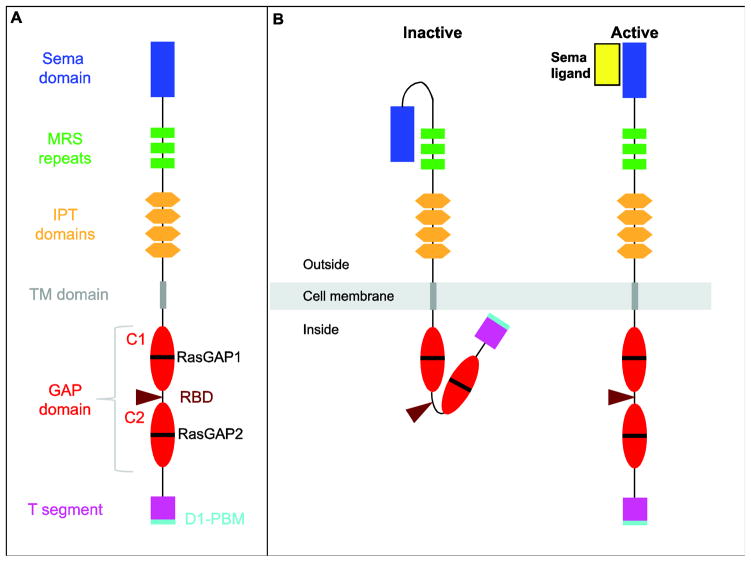
Fig. 4.
Structure of the Sema receptor PlxnD1. A, PlxnD1 is a type I transmembrane protein. The extracellular N-terminal portion contains a Sema domain (blue), likely involved in mediating ligand binding. Following the Sema domain there are three MRS (MET-Related Sequence) repeats (green), four IPT (Immunoglobin-like fold shared by Plexins and Transcription factors) domains (orange) and the transmembrane domain (brown). The cytosolic tail of PlxnD1 is also known as the Sex and Plexins (SP) domain. It contains a split GAP (GTPase Activating Protein) domain with two highly conserved C regions (C1 and C2; red). Each C region contains a Ras GAP motif (RasGAP1 and RasGAP2; black), each of which includes conserved arginine residues required to inhibit the activity of R-Ras proteins. A Rho GTPase-binding domain (RBD, beige) is located between the C1 and C2 regions. Finally, the GAP domain is followed by a short C-terminal region that lacks any resemblance to known protein domains and which is highly conserved between members of the same Plxn subfamily. Here we designate this region as the terminal (T) segment (pink). The T segment of PlxnD1 ends in a short PDZ-binding motif (D1-PBM; aqua) that physically associates with GIPC1. B, Activation model of PlxnD1. In the absence of its Sema ligands, PlxnD1 is in a conformationally inactive folded state, in which the Sema domain contacts the rest of the extracellular portion and the GAP domain is non-functional. Upon Sema binding PlexinD1 undergoes conformational changes that activate its GAP domain and likely enable additional protein-protein interactions.
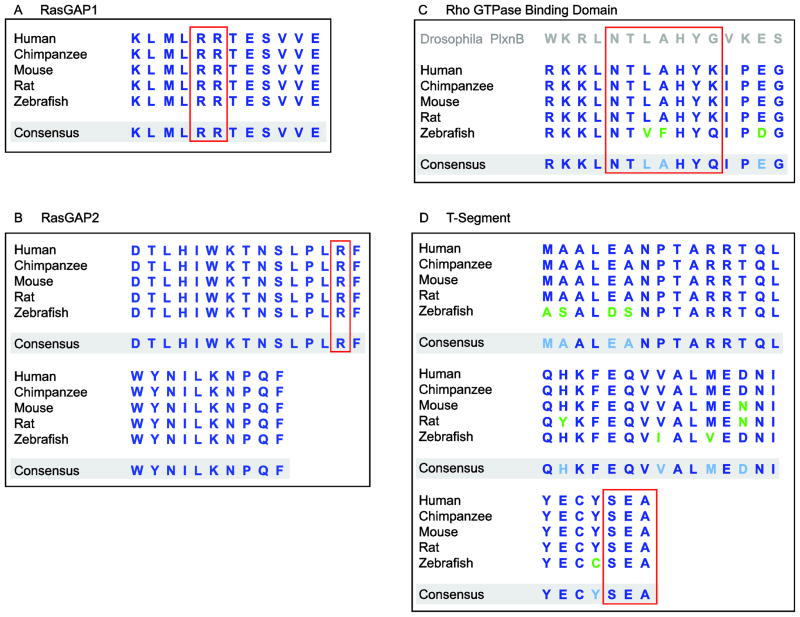
Fig. 5.
Sequence conservation of PlxnD1’s intracellular domains and motifs across vertebrates. A, B, The two RasGAP motifs are one-hundred-percent conserved amongst the species shown, including the arginine residues (outlined in red) essential to functionally antagonize R-Ras family members via GAP activity and/or sequestration. C, The Rho-GTPase binding domains are highly conserved and show a high degree of similarity to the seven amino acid sequence of Drosophila PlxnB (outlined in red) that is required for binding to active, GTP-bound Rac (Hu et al., 2001). D, The PlxnD1-specific T-segment is highly conserved. It includes an invariable PDZ-binding motif SEA-COOH (outlined in red) that mediates the physical interaction between PlxnD1 and the PDZ-containing protein GIPC1. The accession numbers of the PlxnD1 proteins used for sequence analysis are: human/Homo sapiens (AAI50281), chimpanzee/Pan troglodytes (XP_001144444), mouse/Mus musculus (NP_080652), rat/Rattus norvegicus (NP_001101351) and zebrafish/Danio rerio (AAT64905).
Functions of PlxnD1’s Sema domain
Overall, the Sema domain is the extracellular Plxn region that is best understood. The only demonstrated role for PlxnD1’s Sema domain is the physical interaction with the ectodomain of Npns (Gitler et al., 2004). However, Nrps are not required for Sema3E-PlxnD1 binding and the PlxnD1-Nrp interaction is Sema3E-independent. Nonetheless, the PlxnD1-Nrp1 interaction switches axonal Sema3E-PlxnD1 signaling from repulsion to attraction during brain development (Chauvet et al., 2007; Gu et al., 2005).
Studies of other Plxns suggest additional roles for PlxnD1’s Sema domain. These include ligand binding (see Box 1 to learn more about PlxnD1’s ligands and co-receptors), maintaining the receptor in an inactive state and mediating Plxn-Plxn associations. For example, ligand binding by PlxnB1 requires its Sema domain and other C-terminal ectodomain regions (Tamagnone et al., 1999). In addition, a missense mutation in the Sema domain of PlxnA3 inactivates it (Tanaka et al., 2007). Accordingly, the extra-cellular region of mammalian PlxnD1 is required for Sema3E binding (Gu et al., 2005; Watakabe et al., 2006). Structure/function studies of PlxnA1 indicate that the association of the Sema domain with the adjacent C-terminal half of the ectodomain keeps the receptor inactive. This auto-inhibition is relieved upon ligand binding, likely via an activating conformational change that extends into the intracellular domain of the receptor as in Fig. 4A–B. Thus, PlxnA1 forms lacking either the Sema domain or the entire ectodomain show constitutive activity (Takahashi and Strittmatter, 2001). Similarly, PlxnD1 forms without the ectodomain display ligand-independent constitutive activity in heterologous assays (Uesugi et al., 2008). Finally, homophilic interactions between the Sema domains of B Plxns appear to mediate homophilic interactions in cis and cell-cell adhesion in trans (Hartwig et al., 2005; Ohta et al., 1995).
Box 1. PlxnD1 ligands and co-receptors.
Sema3E is the canonical mammalian PlxnD1 ligand. Binding studies with cultured cells and mouse tissue sections indicate that Sema3E binds to PlxnD1 but not to other Plxns. Typically, type 3 Semas bind and signal via Neuropilin (Npn/Nrp)-Plxn co-receptor complexes (reviewed in (Geretti et al., 2008; Schwarz and Ruhrberg, 2010)). Co-immunoprecipitation experiments show that Npn-1 and PlxnD1 associate with each with or without Sema3E and that this interaction requires the Sema domain of PlxnD1. PlxnD1 also associates with Npn-2. However, Sema3E-PlxnD1 binding is Npn-independent. For example, an Alkaline Phosphatase-Sema3E fusion protein (AP-Sema3E) binds to COS1 cells expressing PlxnD1, but not to COS1 cells transfected with Npn-1 or Npn-2 expression vectors. Accordingly, AP-Sema3E binds to COS7 cells and induces their collapse in a PlxnD1-dependent manner. Furthermore, AP-Sema3E still binds to the endothelium of mouse embryos in which Npn-1 is only available as Npn-1Sema-, an engineered Npn-1 form unable to bind to Sema3s but which retains VEGF165 binding. Moreover, npn-1Sema-; npn-2 double mutant mice lack Se vessel patterning defects, the hallmark phenotype of plxnD1 mutants, thus indicating that Sema3E-induced, PlxnD1-mediated endothelial cell repulsion is Nrp1-independent (Fig. 6A–B). However, although Npn-1 is not required for Sema3E-PlxnD1 binding, in COS7 cells its presence halves Sema3E's Bmax without affecting Sema3E’s binding affinity or PlxnD1’s surface levels (Chauvet et al., 2007; Gitler et al., 2004; Gu et al., 2005; Stöhr et al., 2002; Watakabe et al., 2006). Interestingly, p61Sema3E also binds to PlxnD1 but not to Npn-1 (Casazza et al., 2010).
Neto1 (Neuropilin tolloid-like 1; also known as Btcl1) is a transmembrane protein expressed in the brain and retina with two extracellular CUB (complement C1r/C1s, Uegf, Bmp1) domains related to those found in Npns and Tolloid (Michishita et al., 2003; Ng et al., 2009) that like Npns, also associates with PlxnD1. Specifically, Neto1 and PlxnD1 form a Sema3F-specific co-receptor: Neto1 binds to Sema3F only in the presence of PlxnD1 and associates with PlxnD1 in a Sema3F-dependent manner. Experiments with COS7 cells co-expressing both Neto1 and PlxnD1 and with explants of Neto1−/− subicular neurons suggest that Sema3F-Neto1/PlxnD1 signaling is repulsive. Notably, the ectodomain of Neto1 is sufficient to rescue the Sema3F-responsivennes of Neto1−/− subicular neurons. Overall, Neto1 seems to modulate the ligand binding specificity of PlxnD1 (Gingrich et al., 2009).
Besides Nrps and Nrp-related proteins, Plxns also associate with other receptors and transmembrane proteins. These include Met/Ron (Artigiani et al., 2004; Conrotto et al., 2004; Conrotto et al., 2005; Giordano et al., 2002; Swiercz et al., 2008), OTK/Off-track kinase/, VEGFR2 (Bellon et al., 2010; Catalano et al., 2009; Toyofuku et al., 2004; Winberg et al., 2001), L1CAM/L1 Cell Adhesion Molecule (Wang et al., 2008), TREM/TREM-like receptors (Triggering Receptor Expressed on Myeloid cells) (Ford and McVicar, 2009; Takegahara et al., 2006), ErbB2/erythroblastic leukaemia viral oncogene homologue 2 (Casazza et al., 2010; Swiercz et al., 2002; Swiercz et al., 2004) and Abelson 2 (Shimizu et al., 2008). Some of these Plxn partners also associate with Nrps and/or are active receptor tyrosine kinases (Met/Ron, VEGR2, ErbB2; OTK lacks tyrosine kinase activity; reviewed in (Franco and Tamagnone, 2008). Among these, the only transmembrane proteins known to associate with PlxnD1 are VEGFR2 and ErbB2. Remarkably, VEGFR2 reduces in half Sema3E's Bmax without affecting Sema3E’s binding affinity or PlxnD1’s surface levels (Bellon et al., 2010; Casazza et al., 2010).
Mice laking the canonical PlxnD1 ligand Sema3E are viable but loss of plxnD1 induces perinatal lethality, suggesting the existence of additional PlxnD1 ligands (Chauvet et al., 2007; Gu et al., 2005). Accordingly, Sema3C, Sema3F and Sema4A also function PlxnD1 ligands. Sema3C binds to both Npn-1 and Npn-2 directly, but its binding is enhanced by PlxnD1, while Sema4A-PlxnD1 binding is Npn-independent (Gitler et al., 2004; Toyofuku et al., 2007). Finally, Sema3F specifically binds to PlxnD1/Neto1 co-receptor complexes (Gingrich et al., 2009).
The ectodomain of PlxnD1 is N-glycosilated
N-glycosylation is a common post-translational modification that occurs in the endoplasmic reticulum membrane in which polysaccharide chains are attached to target proteins. N-glycosylation plays diverse functions, such as providing specificity to molecular recognition events, promoting protein stability and enhancing protein folding (reviewed in (Yan and Lennarz, 2005)). The PlxnD1 ectodomain includes many predicted Asparagine (N)-linked glycosylation sites. These fit the consensus tripeptide sequon Asn-X-Ser/Asn-X/Thr (where X is any residue except Pro) (Yan and Lennarz, 2005). The role that N-glycosylation serves for PlxnD1 is unknown. However, the glycosylation of Asn500, a predicted N-glycosylation site in hPlxnD1, has been experimentally confirmed (Liu et al., 2005).
The inside story: The intracellular tail
The ~630 aa cytosolic tail of PlxnD1 resembles that of other Plxns (Figs. 4A, 5A–D). Plxns contain an intracellular tail with a “Sex and Plexins” SP domain that harbors two highly conserved C1 and C2 regions (Tamagnone et al., 1999). Collectively, the two C regions are known as the RasGAP domain because each includes a short motif with sequence similarity to a group of Guanosine triphosphatase (GTPase)-Activating Proteins (GAPs) (Figs. 4A, 5A–B) with specificity for small monomeric GTPases of the R-Ras subfamily (reviewed in (Pasterkamp, 2005)). These two RasGAP motifs (RasGAP1 and RasGAP2) are the only known homology between Plxns and a catalytic domain. Each includes conserved R residues essential for the catalytic activity of RasGAPs. Specifically, the RasGAP1 motif at the N-terminal C1 region includes the invariant R residue found at the finger-loop of p120 RasGAP that inserts into the Ras active center. The RasGAP2 motif in the C2 region includes the R residue that stabilizes the finger-loop (Rohm et al., 2000). Notably, Plxns are the only transmembrane receptors known to directly associate with small GTPases. A Rho GTPase-Binding Domain (RBD) is sandwiched between the C regions (Figs. 4A, 5C) (Barberis et al., 2005; Driessens et al., 2001; Hu et al., 2001; Oinuma et al., 2004a; Oinuma et al., 2004b; Rohm et al., 2000; Turner et al., 2004; Vikis et al., 2000; Zanata et al., 2002). The RBD of Plxns displays a ubiquitin-like fold conformation. In PlxnB1 the RBD appears to be dimeric. However, in PlxnD1 it appears to be monomeric (He et al., 2009; Tong and Buck, 2005; Tong et al., 2007), (http://www.rcsb.org/pdb/explore.do?structureId=3H6N). Finally, the C2 region of Plxns is followed by a C-terminal stretch of ~40–60 aa that includes the COOH terminus and lacks any resemblance to known protein domains. Here we designate this C-terminal region, which has not been previously described, as the T-segment (Fig. 5D).
Plxns as RasGAPs
Members of the R-Ras subfamily are GTPases, molecular switches that oscillate between active (GTP-bound) and inactive (GDP-bound) states. GAPs inactivate GTPases by increasing their intrinsic rate of GTP hydrolysis. In the active state R-Ras GTPases enhance integrin-mediated cell adhesion to the extracellular matrix (ECM; reviewed in (Kinbara et al., 2003)).
Accordingly, biochemical assays using cultured neuronal or COS7 cells indicate that Plxns inactivate R-Ras related proteins by acting as GAPs and that this effect requires the highly-conserved R residues found at their RasGAP-like motifs. The GAP activity of Plxns abrogates integrin-mediated cell-ECM adhesion, which in cultured neurons reduces neurite outgrowth (Ito et al., 2006; Oinuma et al., 2004a; Oinuma et al., 2006; Rohm et al., 2000; Uesugi et al., 2008). Ras activity also stimulates phosphoinositide 3-kinase (PI3K) signaling, a modulator of cell survival, growth and migration (reviewed in (Cantley, 2002)). Accordingly, down regulation of PI3K activity by neuronal Sema-Plxn signaling (Atwal et al., 2003; Chadborn et al., 2006; Gallo, 2008; Orlova et al., 2007) requires the RasGAP activity of Plxns, as demonstrated for Sema4D-PlxnB1 (Ito et al., 2006; Oinuma et al., 2006).
With the exception of PlxnC1, Plxns do not function as constitutively active GAPs (Uesugi et al., 2008). Instead, expression of the Plxn’s GAP activity requires two events. One of them is Sema binding to the Plxn ectodomain, which “primes” the receptor likely by inducing an intracellular conformational change. The second event is the binding of activated monomeric GTPases of the Rho family (Rac, Cdc42 and Rnd subfamilies; Rho-like GTPases or RLGs) to the RBD. RBD-RLG binding disrupts the inhibitory association between the C1 and C2 regions, thus enabling the GAP activity of Plxns.
In some Plxns the RBD also plays a second role, that of preventing the bound RLG from interacting with its downstream effectors. For example, in fly PlexB the physical interaction of the RBD with GTP-bound Rac/Cdc42 (RacGTP) sequesters RacGTP away from its effector p21-activated kinase (PAK), thus blocking PAK activation. This antagonizes the stabilization of actin filaments and promotes neuronal growth cone collapse (Barberis et al., 2005; Driessens et al., 2001; Hu et al., 2001; Oinuma et al., 2004a; Oinuma et al., 2004b; Rohm et al., 2000; Turner et al., 2004; Vikis et al., 2000; Zanata et al., 2002).
PlxnD1 has GAP activity
Several observations indicate that similar to other Plxns, PlxnD1 acts as a RasGAP to antagonize both integrin-mediated cell-ECM adhesion and PI3K signaling. For instance, Sema3E treatment of cultured endothelial cells decreases the phosphorylation of Focal Adhesion Kinase (FAK), a key molecule that regulates the turnover of integrin-containing focal adhesions. In addition, Sema3E-treated COS-7 cells grown on integrin ligands such as collagen or fibronectin, collapse if transfected with constructs for the expression of wild type PlxnD1. In contrast, COS-7 cells grown on poly-L-lisine or expressing PlxnD1 forms with mutated RasGAP motifs fail to collapse upon Sema3E treatment (Sakurai et al., 2010). In addition, unpublished evidence suggests that repulsive Sema3E-PlxnD1 signaling in neurons downregulates PI3K activity in a RasGAP-dependent manner (Eickholt, 2008).
Activation of the RasGAP activity of PlxnB1 and PlxnA1 requires RLGs of the Rnd subfamily, which are unique in that they exist in a constitutively-active GTP-bound state due to their lack of intrinsic GTPase activity (Foster et al., 1996; Ito et al., 2006; Nobes et al., 1998; Oinuma et al., 2004a; Oinuma et al., 2003; Oinuma et al., 2006; Tong et al., 2007; Toyofuku et al., 2005). Therefore, the ability of Rnd1, 2 and 3 to bind to the intracellular tail of murine PlxnD1 (mPlxnD1) was recently tested. Extracts from transfected COS-7 cells indicate that these three Rnds can bind to PlxnD1. However, Rnd2 (Rho7/RhoN/ARHN) exhibits preferential PlxnD1 binding. The Rnd2-binding region of PlxnD1 maps to the N-terminal half of the cytosolic tail, which contains both the C1 region and the RBD. Hence, these observations suggest that the PlxnD1-Rnd2 physical interaction is mediated by PlxnD1’s RBD.
Moreover, different lines of evidence support the idea that Rnd2 is required for the activation of the RasGAP activity of PlxnD1 in vivo. First, Sema3E blocks axon elongation in cultured cortical neurons in an Rnd2-dependent manner. Second, endogenous PlxnD1-Rnd2 complexes can be isolated from these cells via co-immunoprecipitation (Uesugi et al., 2008). Additional observations provide insights into the molecular mechanisms by which Rnd proteins might modulate PlxnD1 function. First, formation of a PlxnD1-Rnd2 complex occurs even without Sema3E stimulation in transfected COS-7 cells. Second, formation of a PlxnD1-Rnd2 complex does not result in the expression of PlxnD1's GAP activity. Third, the GAP activity of a membrane-targeted form of PlxnD1 lacking its extracellular domain is Sema3-independent but Rnd2-dependent (Uesugi et al., 2008).
There are conflicting reports about the effects of p61Sema3E on endothelial cell migration (Christensen et al., 2005) (Kigel et al., 2008; Roodink et al., 2008) (Casazza et al., 2010). Nonetheless, it is interesting to note that p61Sema3E provided by human carcinoma cells inhibits tumor vascularization by the murine hosts. Accordingly, p61Sema3E collapses and inhibits the migration of HUVECs (Human Vein Endothelial Cells) in a manner that requires both PlxnD1 and Rnd2 (Casazza et al., 2010; Gitler et al., 2004). Thus, these experiments suggest that Sema3E and p61Sema3E-induced cell repulsion are Rnd2-dependent.
Sema3E, the canonical ligand for mammalian PlxnD1, induces both repulsive and attractive responses in axons (Fig. 3D) (Bellon et al., 2010; Chauvet et al., 2007). Interestingly, Rnd2 also mediates opposite effects in neuronal morphology. Rnd2’s differential effects depend on its choice of partner. Rnd2 can bind to Pragmin (pragma for Rnd2), a neuronal molecule that can associate with RhoA simultaneously. Pragmin promotes Rho activity to stimulate cell contraction via a poorly defined non-GEF mechanism (Govek et al., 2005; Tanaka et al., 2006). However, Rnd2 is also capable of binding to Rapostlin (apostle of Rnd2), a microtubule-binding protein. Rapostlin induces neurite branching in PC12 cells in the presence of constitutively active Rnd2 (Fujita et al., 2002). These observations suggest that Rnd2 might be involved in both repulsive and attractive responses downstream of Sema3E. However, it is unlikely that Sema3E-induced axonal attraction/growth requires a PlxnD1-Rnd2 interaction, since these effects are independent of the intracellular tail of PlxnD1 (Bellon et al., 2010). Nonetheless, the factors that modulate Rnd2's choice of partner, whether Pragmin or Rapostlin associate individually and/or simultaneously with PlxnD1-bound Rnd2 in vivo and the precise role of these interactions remain unexplored.
The substrate specificity of PlxnD1's GAP activity was recently explored using COS-7 cells transfected with two different R-Ras subfamily members, TC21 and M-Ras. Under these conditions PlxnD1 exhibits Rnd2-dependent GAP activity only towards M-Ras (Uesugi et al., 2008). This suggests that M-Ras is involved in Sema3E-PlxnD1 signaling in the brain (Bellon et al., 2010; Chauvet et al., 2007), since M-Ras is predominantly expressed in the CNS (Kimmelman et al., 1997). However, given PlxnD1’s prominent role in cardiovascular development (Gitler et al., 2004; Gu et al., 2005; Torres-Vázquez et al., 2004; Zhang et al., 2008) another likely substrate for PlxnD1’s GAP activity is R-Ras, which is enriched in endothelial cells (Komatsu and Ruoslahti, 2005). However, the involvement of R-Ras in Sema-PlxnD1 signaling has not been directly tested.
Finally, it is possible that PlxnD1 antagonizes the activity of R-Ras family members non-catalytically, by sequestering them in a manner that still requires the RasGAP1/2 motifs (Sakurai et al., 2010).
A general model for Sema-PlxnD1 signaling
Based on the above observations we propose the following model (Fig. 6A–B). Upon Sema3E stimulation PlxnD1 in pre-existing PlxnD1-Rnd2/RLG complexes undergoes an Rnd2/RLG-dependent intracellular conformation change that translates the concentration and distribution of extracellular Sema3 cues into an intracellular gradient of distinct PlxnD1 activities.
Fig. 6.
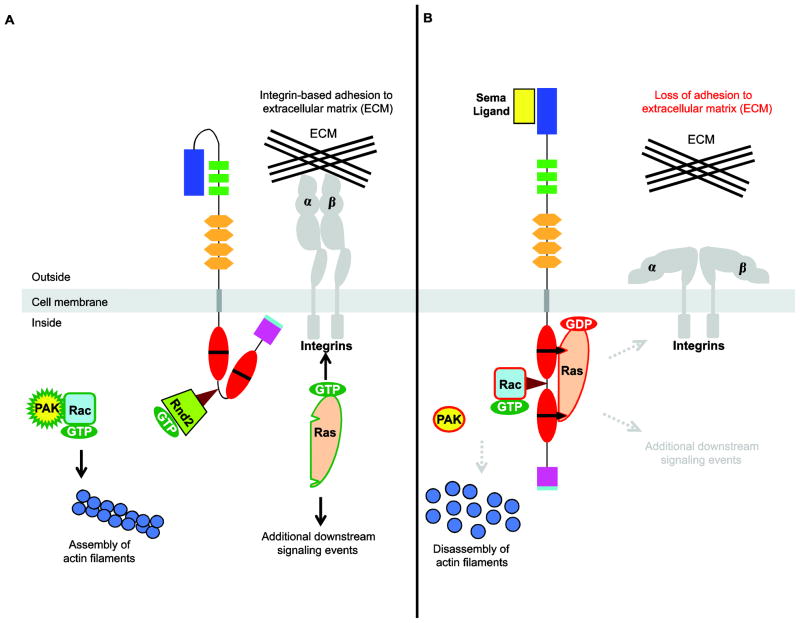
A general model of Sema3/PlxnD1-mediated repulsion. A, In the absence of ligand-mediated activation PlxnD1 is in a conformation that enables its association with GTP-bound Rnd2 but prevents its interaction with active, GTP-bound Rac and R-Ras. Thus, GTP-bound Rac is able to bind to PAK (p21-activated kinase) to stimulate the assembly of actin filaments to support cell migration while active GTP-bound Ras promotes integrin-mediated adhesion to the extracellular matrix (ECM) and mediates additional downstream signaling events. B, Upon binding of its Sema3 ligand, PlxnD1 undergoes a conformational change and binds the active forms of both Rac and R-Ras GTPases. By sequestering Rac, PlxnD1 leads to the inactivation of PAK and the collapse of the actin-based cytoskeleton leading to retraction and/or turning responses. PlxnD1 inactivates R-Ras GTPases by either enhancing GTP hydrolysis (as shown) or by sequestering them resulting in the loss of integrin-based adhesion to the ECM (Ito et al., 2006; Oinuma et al., 2004a; Oinuma et al., 2006; Rohm et al., 2000; Sakurai et al., 2010; Uesugi et al., 2008) and likely reducing as well other R-Ras mediated signaling events.
In cells interpreting Sema cues as repulsive, such as endothelial cells and neurons (Bellon et al., 2010; Chauvet et al., 2007; Gitler et al., 2004; Gu et al., 2005; Torres-Vázquez et al., 2004; Zhang et al., 2008), these activities would be the RasGAP1/2-dependent inhibition of members of the R-Ras subfamily to antagonize integrin-mediated adhesion, PI3K signaling and the RhoA/Pragmin dependent collapse of the cytoskeleton.
In contrast, when Sema3E functions as an attractive cue, as it does for some mammalian axon tracts, the intracellular domain of PlxnD1 is not required, thus indicating that this effect is mediated by a different molecular mechanism. Indeed, it was recently shown that Sema3E-induced axonal attraction/growth occurs via a PlxnD1/Nrp1/VEGFR2 trimeric complex that increases PI3K signaling in a VEGFR2-dependent manner (Bellon et al., 2010; Chauvet et al., 2007; Eickholt, 2008; Sakurai et al., 2010). Whether the same mechanism is responsible for the pro-angiogenic effects of specific Sema3E isoforms secreted by some tumors (see (Christensen et al., 2005) or whether these effects are promoted by the simultaneous interaction of Rnd2/RLGs with both PlxnD1 and with Rapostlin -or a functionally similar molecule- remains to be elucidated.
Sema-Plxn signaling: Output specificity
The inactivation of R-Ras related proteins and the regulation of cytoskeletal dynamics are common Plxn outputs. Yet each Plxn plays seemingly distinct biological roles. This is likely due to the unique expression pattern of each Plxn, their differential ligand specificity and/or affinity and the specific pathway repertoire active in each tissue. However, another key contributing factor is that individual Plxns also engage specific suites of modulators and/or effectors via subfamily-specific intracellular binding motifs. For instance, B Plxns contain additional motifs between their C1 and C2 regions besides the short RBD shared by all Plxns: Drosophila PlxnB binds and promotes RhoA activity via a 40 aa motif that is absent from other Plxn subfamilies (Hu et al., 2001). Similarly, both human PlxnB1 and B2 contain a Cdc42/Rac interactive binding-like motif (CRIB-LM) that binds active Rac in a ligand-dependent manner (Vikis et al., 2000). In contrast, PlxnA1, A2 and D1 lack a CRIB-LM and do not bind to active Rac in vitro (Driessens et al., 2001) (see also (Turner et al., 2004). Moreover, except for the RBD, the sequence between the halves of the SP domain is poorly conserved across Plxn subfamilies. Hence, novel subfamily-specific molecular determinants of Plxn activity are likely to reside here.
The T-segment is a candidate region for providing signaling specificity to each Plxn. We have found through sequence comparisons that T-segments are highly conserved within Plxn subfamilies but very dissimilar across subfamilies. Thus T-segment sequence conservation parallels that of the ectodomains, the criteria for Plxn subfamily classification (see (Tamagnone et al., 1999). For example, the only significant hits obtained by BLASTing (McGinnis and Madden, 2004) the ~37 aa T-segment of hPlxnD1 are the T-segments of PlxnD1 proteins from mammals, birds, amphibians and fish, which are 94–100% identical and 100% similar. In contrast, the T-segment of hPlxnD1 lacks any similarity with that of PlxnC1 and is only 25–53% identical and 45–69% similar to the T-segments of A and B Plxns.
Accordingly, in B Plxns the T-segment terminates in a short protein-protein interaction motif that mediates binding to the PDZ (PSD-95, Dlg, ZO-1) domain of Leukemia-Associated Rho Guanine exchange factor (LARG or PDZ-RhoGEF/Leukemia). This PDZ-binding motif (B-PBM) seems to be essential for LARG binding, since Plxns from the other three subfamilies lack this motif and fail to associate with LARG. The B/PDZ-BM dependent recruitment of LARG is essential for the ability of B Plxns to activate RhoA (Aurandt et al., 2002; Driessens et al., 2002; Hirotani et al., 2002; Hu et al., 2001; Oinuma et al., 2003; Perrot et al., 2002; Swiercz et al., 2002).
PlxnD1 contains a unique binding motif in its T-segment
By comparing the sequence of Plxn T-segments we uncovered a highly conserved, PlxnD1-specific C-terminal sequence similar to a motif (S/T-X-A/V/L/I) known for binding to proteins containing a PDZ type I domain (De Vries et al., 1998; Hu et al., 2003) reviewed in (Jele et al., 2003). We thus refer to this sequence as the D1-PBM (PlxnD1 PDZ-binding motif; Figs. 4A, 5D). The consensus D1-PBM sequence, derived from the PlxnD1 proteins of fish, amphibians, birds and mammals, is SEA-COOH. Importantly, we found that the D1-PBM is essential for PlxnD1's ability to physically associate with the PDZ domain of GIPC1 (GAIP Interacting Protein, C terminus; also known as Synectin among many other names) (Carl M. Gay, Brant M. Weinstein and Jesús Torres-Vázquez; unpublished observations), see also (Linhares and Gutkind, 2005). GIPC1 is an intracellular protein that, like PlxnD1, plays key roles during angiogenesis (Cai and Reed, 1999; Chittenden et al., 2006; Lanahan et al., 2010; Ren et al., 2010; Wang et al., 2006). Thus it is likely that GIPC1 functions as a PlxnD1-specific modulator/effector (see Box 2).
Box 2. Other potential intracellular effectors/modulators of PlxnD1.
GIPC1 is an scaffolding protein that associates with many single-pass type I transmembrane proteins via its central PDZ-domain. For example, GIPC1 binds to the cytosolic tail of Nrp1 via its C-terminal PDZ-binding motif SEA-COOH. This event promotes the association between Nrp1 and VEGFR2 and enhances VEGF-induced angiogenesis. For example, gipc1 morphants display stunted SeAs (Cai and Reed, 1999; Chittenden et al., 2006; Lanahan et al., 2010; Ren et al., 2010; Wang et al., 2006).
Remarkably, we isolated the entire gipc1 cDNA twice as a prey in a yeast-two-hybrid screen for proteins that bind to the PlxnD1 cytosolic tail. We have found that the PlxnD1-GIPC1 interaction is mediated by GIPC1’s PDZ domain and PlxnD1’s PDZ-binding motif, which is located in the T-segment and is identical to the Nrp1 motif (Brant Weinstein, Carl M. Gay and Jesús Torres-Vázquez, unpublished results), (see also (Linhares and Gutkind, 2005). These observations suggest that GIPC1 might play additional roles in the vasculature, such as specifically modulating Sema3-PlxnD1 signaling or enabling the integration of Sema3-PlxnD1 and VEGF-Nrp1/VEGFR2 signals. The potential importance of GIPC1 in the context of Sema-PlxnD1 signaling is further underscored by bioinformatic predictions that suggest the existence of alternatively spliced plxnD1 isoforms in human and chimpanzee. Among the various sequence changes that distinguish these predicted PlxnD1 variants is the substitution of the D1-PBM for a 43 aa stretch without identifiable PDZ-binding motifs. Overall, these observations suggest that PlxnD1’s T-segment engages unique effectors/modulators, including GIPC1, to fulfill its biological roles.
The MICALs (from Molecule Interacting with CasL) (Suzuki et al., 2002) are a family of multidomain cytosolic proteins with flavoenzymatic redox activity expressed in the vertebrate and Drosophila nervous systems. Fly MICAL is required for axonal repulsion by Sema-1a/PlexA and the C-terminal coiled coil domain (cc) of fly MICAL binds to a fragment of fly PlexA containing both the C2 region and the T-segment (Terman et al., 2002). MICAL also binds to cytoskeletal components, small GTPases and to CRMPs, another group of A Plxn associated proteins (Kolk and Pasterkamp, 2007). In flies Sema-1a/1b-PlexA signaling binds and destabilizes F-actin via MICAL’s redox activity to modulate the morphology of bristle and axon growth cones (Hung et al., 2010). However, whether MICAL’s oxidoreductase activity involves direct REDOX modification of its substrates or occurs via the generation of reactive oxygen species remains unclear (reviewed in (Kolk and Pasterkamp, 2007).
The MICAL-Like proteins are structurally similar to MICALs but lack the monooxygenase domain required for MICAL-mediated F-actin destabilization (Hung et al., 2010; Terman et al., 2002). MICAL-Like proteins have a cc domain, but it is not known if they bind to Plxns. Interestingly, MICAL-Like proteins associate with actin binding proteins (Nakatsuji et al., 2008), function as modulators of the actin cytoskeleton during neurite outgrowth (Sakane et al., 2010), promote cell migration (Kanda et al., 2008) and are found in tissues where Plxns, including PlxnD1, are expressed, such as the nervous (Terman et al., 2002) and vascular systems (Weber et al., 2005). MICAL-L proteins have been implicated in the endocytic recycling of some transmembrane proteins (Sharma et al., 2009; Terai et al., 2006) and thus they might exert a similar effect on Plxn receptors.
The Collapsin Response Mediator Proteins (CRMPs) is a family of tetrameric phosphoproteins expressed in the nervous system that function as positive effectors of repulsive Sema-PlxnA signaling. Despite sequence and structural similarities with dihydropyrimidinases and metal-dependent amidohydrolases CRMPs lack enzymatic activity. Biochemical evidence indicates that CRMPs interact physically with MICALs and suggests that they link Plxn signaling with membrane endocytosis during growth cone steering (reviewed in (Schmidt and Strittmatter, 2007; Shih et al., 2003).
Nervy belongs to the Myeloid Translocation Gene (MTG) family of A kinase Anchoring Proteins (AKAPs). It is expressed in the Drosophila CNS and associates with the intracellular region of fly PlexA containing the C2 region and the T-segment. Nervy is postulated to act as a negative cytosolic regulator of Sema1a/PlxnA-induced axonal repulsion by linking PlxnA with the cAMP (cyclic adenosine monophosphate)-dependent protein kinase A (PKA) (Terman and Kolodkin, 2004). Indeed, vertebrate PlxnA1 and PlxnA3 are phosphorylated in vitro by PKA and the vertebrate Nervy homologue MTG16b specifically interacts physically with Plxns A1 and A3 but not PlxnB1 (Fiedler et al., 2010). The mechanism by which Nervy antagonizes PlxnA signaling remains controversial because Nervy and its vertebrate homologs (the myeloid translocation genes MTG8/ETO, MTG16 and MTGR1) also localize to the nucleus and act as transcriptional co-repressors, thus raising the possibility that Nervy’s effects on Sema-Plxn signaling have a transcriptional basis (Terman and Kolodkin, 2005; Wildonger and Mann, 2005).
Arf6 (ADP-ribosylation factor 6) is a small GTPase that regulates both clathrin-dependent and clathrin-independent endocytic pathways as well as actin and membrane remodeling (reviewed in (D'Souza-Schorey and Chavrier, 2006)). Accordingly, Arf6 has been shown to regulate the trafficking of 1 integrins, which play important roles in angiogenic development. Moreover, downregulation of Arf6 impairs cell adhesion and migration (Dunphy et al., 2006; Mettouchi and Meneguzzi, 2006; Powelka et al., 2004). Interestingly, Sema3E-PlxnD1 signaling reduces endothelial cell adhesion to the ECM by promoting the disassembly of focal adhesions, which are integrin-based adhesive structures. These effects seem to be in part related to the activation of Arf6, since Sema3E-PlxnD1 signaling in COS-7 cells elevates active Arf6 levels in a manner that requires the RasGAP motifs of PlxnD1. Conversely, in COS-7 cells a dominant negative form of Arf6 blocks Sema3E/PlxnD1-mediated cell collapse. However, the mechanistic link between R-Ras inhibition and Arf6 activation remains unclear and there is no evidence supporting a direct physical interaction between Arf6 and PlxnD1 (Sakurai et al., 2010).
Additional candidate effectors and modulators of PlxnD1
Besides Rnd2, M-Ras (Uesugi et al., 2008) and GIPC1 (Carl M. Gay, Brant M. Weinstein and Jesús Torres-Vázquez; unpublished observations), (Linhares and Gutkind, 2005), the other intracellular proteins that associate with the cytosolic tail of PlxnD1 are ACF7 (Actin Cross-linking Family Protein 7), FLNA (Filamin A) and CKAP1 (Cytoskeleton Associated Protein 1). These three candidate PlxnD1 effectors/modulators are the first cytoskeletal proteins known to interact directly with any Plxn (see Box 3).
Box 3. Cytoskeletal proteins that interact physically with PlxnD1.
ACF7 is a large spectraplakin cytoskeletal crosslinking protein. It contains an N-terminal head domain with two actin-binding (ABD) Calponin-homology (CHD) domains and a plakin-like globular domain. Its central region includes a rod domain with many dystrophin-like spectrin repeats and an active ATPase region. Finally, the C-terminus harbors two putative calcium-binding EF-hand motifs and a microtubule-binding region with homology to Gas2 (growth arrest-speci c protein 2/GAR) (Bernier et al., 1996; Byers et al., 1995; Karakesisoglou et al., 2000; Leung et al., 1999; Sawamura et al., 1990; Sun et al., 1999). ACF7 was identified as a PlxnD1-binding protein in a large yeast-two-hybrid screen for interactions between the cytosolic tails of large transmembrane human brain proteins and other human proteins. Specifically, a bait containing aa 1352–1985 of hPlxnD1 trapped an N-terminal 317 aa ACF7 fragment (Nakayama et al., 2002) (see also http://www.kazusa.or.jp/huge/ppi/), suggesting PlxnD1's cytosolic tail binds to ACF7's ABDs.
In the murine skin epidermis ACF7 coordinates the growth of microtubules along F-actin by directing them to focal adhesions and enhancing their turnover during cell migration (Leung et al., 1999; Wu et al., 2008). ACF7 also promotes Wnt signaling (Chen et al., 2006). ACF7 is expressed in many murine embryonic tissues, including the nervous system (Bernier et al., 2000) and is also likely expressed in the endothelium, since its transcription is up regulated in human coronary artery endothelial cells by laminar shear stress (Chu and Peters, 2008). Thus, it seems likely that ACF7 and plxnD1 are co-expressed in some tissues, consistent with the potential involvement of ACF7 in PlxnD1-mediated cytoskeleton modulation.
FLNA is a large cytoplasmic, non-muscle actin-binding protein that forms filamentous v-shaped dimers which cross-link cortical actin filaments into a dynamic orthogonal network. Like ACF7, its N-terminus contains two CHDs and an ABD, which is followed by 24 Filamin repeat modules of ~100 aa which together form two rod domains joined by a pair of flexible hinge regions. In addition to F-actin, FLNA binds more than 30 other partners, including PlxnD1 (via modules 10 and/or 11) as well as other molecules implicated in Plxn signaling, like integrins and Rho family GTPases. FLNA’s repeats function as protein-protein interaction modules that belong to four sequence-based subgroups with presumably distinct ligand-binding specificity. It has been suggested that this property enables FLNA to function as a scaffold for clustering interacting receptors and their effectors at the cell surface and link their activity to the regulation of the cytoskeleton. Thus, it is tempting to speculate that FLNA serves to bring together PlxnD1 with its effectors/modulators (Feng and Walsh, 2004; Horowitz, 2007; Ithychanda et al., 2009; Popowicz et al., 2006; Robertson, 2005; Stossel et al., 2001). (See also (Lu et al., 2007)).
FLNA mutations are associated with human genetic diseases characterized by abnormalities in the development of the nervous, skeletal and cardiovascular systems (reviewed in (Popowicz et al., 2006; Robertson, 2005). In the murine embryonic cardiovascular system FLNA transcripts are enriched in the endothelium and the heart's endocardial cushion, outflow tract and cardiac valves. FLNA null mouse embryos dye at midgestation and display massive hemorrhage, misguided Se vessels, PTA and incomplete cardiac septation (Feng et al., 2006). Notably, the cardiovascular defects of FLNA null mouse embryos parallel those found in mouse plxnD1 knockouts (Gitler et al., 2004; Gu et al., 2005; Zhang et al., 2008), consistent with the putative involvement of FLNA in PlxnD1 signaling.
CKAP1 is a widely expressed 250 aa protein whose relatives are implicated in tubulin dynamics, such as folding, heterodimer formation, storage, dissociation and degradation. It is abundant in neuroblasts and enriched at the growth cone transition zone. CKAP1 contains an N-terminal ubiquitin (UBL)-like domain, a central coiled-coil region and a C-terminal cytoskeleton-associated protein glycine-rich (CAP-Gly) domain. CAP-Gly domains are common in proteins that bind to the C-terminus of alfa-tubulin (although CKAP1’s ability to bind alfa-tubulin is controversial) and also mediate other protein-protein interactions and regulate intracellular signaling, vesicle transport, cell migration and polarity (Grynberg et al., 2003; Kortazar et al., 2007; Lopez-Fanarraga et al., 2007; Steinmetz and Akhmanova, 2008; Vadlamudi et al., 2005). Notably, several lines of evidence implicate CKAP1 in the modulation of microtuble dynamics and axonal growth. For example, over-expression of murine CKAP1 promotes microtubule depolymerization (Kortazar et al., 2007) and high CKAP1 levels in macrophage/microglia are associated with low microtubule densities (Fanarraga et al., 2009). Similarly, mutations in gigaxonin, a CKAP1-binding protein that targets CKAP1 for degradation induce giant axonal neurophaty (GAN) disorder. This disease features high levels of neuronal CKAP1, microtubule depolymerization, growth cone retraction, axonal damage and neuronal degeneration (Wang et al., 2005). Conversely, reducing CKAP1 levels in cultured neurons enhances axonal growth (Lopez-Fanarraga et al., 2007) and the PAK-mediated phosphorylation of CKAP1 modulates microtubule growth (Vadlamudi et al., 2005). We isolated mouse CKAP1 in a yeast two-hybrid screen for proteins that bind to the cytosolic tail of murine PlxnD1 (Carl M. Gay, Brant M. Weinstein, and Jesús Torres-Vázquez; unpublished results). CKAP1 was also found in a similar screen using PlxnA1’s C1 region as bait. This screen yielded other microtubule-regulating proteins, implicating the C1 region of Plxns in microtubule regulation (Togashi et al., 2006). The potential involvement of CKAP1 in PlxnD1 and/or PlxnA1 signaling is consistent with other observations, including the role of PAK, whose activity is antagonized by Sema-Plxn signaling (Driessens et al., 2001; Rohm et al., 2000; Vikis et al., 2000) in modulating CKAP1 function (Vadlamudi et al., 2005), the inverse correlation between Sema and tubulin abundance in human ovarian adenocarcinoma cell lines (Prislei et al., 2008) and the prominent expression of ckap1 in zebrafish embryonic tissues where PlxnA1 and/or PlxnD1 are also expressed (Carl M. Gay and Jesús Torres-Vázquez; unpublished results).
Overall, the identification of intracellular PlxnD1-binding proteins represents the first step towards a molecular understanding how PlxnD1 transforms Sema cues into intracellular signals. Future studies will elucidate the role that these proteins play during PlxnD1 signaling in vivo and uncover the significance of other candidate PlxnD1 effectors/modulators (see Box 3).
Hence, unraveling the role of these proteins as well as those of other candidate intracellular effectors/modulators of PlxnD1 such as the MICALs, MICAL-like proteins, CRMPs and the vertebrate homologs of Drosophila Nervy (see Box 2) will likely provide key insights into the molecular mechanisms by which chemotactic Sema-PlxnD1/Plxn signaling modulates cytoskeletal dynamics to guide cell migration and induce cell shape changes.
Post-translational Plxn modifications: Phosphorylation
Plxns are tyrosine phosphorylated at their cytosolic tails
Although Plxns lack intrinsic kinase activity they are phosphorylated at cytosolic tyrosine residues in vivo (Tamagnone et al., 1999). Tyrosine phosphorylation is a key regulatory protein modification made by both receptor and non-receptor tyrosine kinases. It creates docking sites for proteins with SH2 (Src Homology 2) or PTB (PhosphoTyrosine Binding) domains for the downstream activation of the Ras/ERK MAP and the PI3K cascades. It also leads to the recruitment of proteins with PTP (Protein Tyrosine Phospatase) domains. PTP-containing proteins that are catalytically active dephosphorylate regulatory tyrosine phosphates while those that are catalytically dead engage in phosphotyrosine-recognition to mediate scaffolding and localization functions (reviewed in (Hunter, 2009; Moorhead et al., 2009).
PlxnD1 is also tyrosine phosphorylated and contains unique tyrosine residues
Members of the four Plxn subfamilies share thirteen conserved intracellular tyrosines. Except for the fourth one, which is in the RBD, all of them are found in the SP domain. The corresponding residues in human PlxnD1 (hPlxnD1) are listed in Table 1 and shown in a sequence context in Fig. 5A–D (Franco and Tamagnone, 2008).
Table 1.
Conservation of tyrosine residues in the cytoplasmic tail of PlxnD1 proteins
| Y | Sequence | Phosphorylation | Substrate motifs for | pY context matches binding motif for/Comments | ||
|---|---|---|---|---|---|---|
| ScoreN | In vivo | Kinases | Phosphatases | |||
| 1303 | RAERYWQKT | 0.839 | Unknown | ALKK, FGFRK, RETK | SHP1P | No hit |
| 1351 | PFLEYKHFV | 0.068 | Unknown | EGFRP, FGRK, RETK, VEGFRK | TC-PTPP | No hit |
| 1367 | CSSLYEERY | 0.797 | Yes (PlxnD1) | ALKK, BTKK, FYNK, SrcP | No hit | SH2 domain of: FYNS |
| 1371 | YEERYVLPS | 0.981 | Unknown | ABLP, ALKP, EGFRS, K, EPHAK, FGFRK, FMSK, JAKK, LCKK, METK, PDGFRK, RETK, SRCK, SYKK, VEGFRK, ZAP-70K | SHP1P | SH2 domains of: CrkP ItkP, PLCg (N-terminal)S, RasGAP (C-terminal)P |
| 1458 | GKLEYYTSI | 0.106 | Unknown | EGFRP, ZAP-70K | PTP1BP, SHP1P, TC-PTPP | No hit |
| 1459 | KLEYYTSIM | 0.598 | Unknown | JAK2P, METK, RETK, SrcP | PTP1BP, SHP1P | No hit. Precedes RasGAP1 motif. |
| 1503 | SICMYSCLR | 0.036 | Unknown | JAK2P, FGFRK, LCKK, RETK, SrcP | No hit |
No hit. After the RasGAP1 motif. |
| 1539 | GKARYTLNE | 0.220 | Yes (PlxnB1) | FMSK, JAKK, RETK, SrcP | No hit | PlxnB1’s pY binds to PLCg’s (N-terminal) SH2 (Swiercz et al., 2009a) |
| 1597 | KNVPYSQWP | 0.689 | Unknown | ABLP, ALKK, FGFRK, FGRK, LCKK, RETK, RLKK, SrcP | No hit | PTB domain of: FRIPP |
| 1618 | STQSYILRD | 0.332 | Unknown | FMSK, JAKK, RETK | No hit | No hit |
| 1642 | TLAHYKIPE | 0.065 | Yes (PlxnD1) | FGFRK, EPHAK, | No hit | SH2 domains of: CrkS, P, RasGAP (C-terminal)P. Y1642 is at RBD. |
| 1673 | DTEKYFHLV | 0.509 | Unknown | EGFRS, FGRK, JAKK, JAK2P, PDGFRK, RETK, SRCK, SYKK, VEGFRK, ZAP-70K | SHP1P | SH2 domain of: SHP1P |
| 1703 | LPEIYLTRL | 0.054 | Unknown | No hit | SHP1P | No hit |
| 1739 | LAVKYFFDF | 0.009 | Unknown | ALKK | No hit |
No hit. Precedes RasGAP2 motif. |
| 1824 | NKLLYAKEI | 0.205 | Unknown | RETK, SrcP, TIE2K | No hit |
No hit. After the RasGAP2 motif. |
| 1831 | EIPEYRKIV | 0.216 | Unknown | ALKK, EGFRP, FGFRK, FGRK, JAK2P, RETK, SRCK, VEGFRK, ZAP-70K | TC-PTPP | SH2 domains of: AblP, CrkP, FgrP, LckP, NckP, SrcP |
| 1838 | IVQRYYKQI | 0.007 | Unknown | ALKK, VEGFRK | No hit | SH2 domain of: STAT3P |
| 1839 | VQRYYKQIQ | 0.097 | Unknown | ALKK, FESK, FGFRK, JAKK, JAK2P, LCKK, METK, VEGFRK, ZAP-70K | TC-PTPP | SH2 domains of: AblP, CrkP, FgrP, LckP, NckP, SrcP |
| 1864 | ESRKYQNEF | 0.531 | Unknown | ALKK, EPHAK, FYNK, PDGFRK, RETK, VEGFRK, ZAP-70K | No hit | SH2 domain of: Grb2P |
| 1878 | MAEIYKYAK | 0.603 | Unknown | ALKK, BTKK, LCKK | SHP1P | No hit. |
| 1880 | EIYKYAKRY | 0.132 | Unknown | RETK, SrcP | No hit | SH2 domain of: CskP |
| 1884 | YAKRYRPQI | 0.007 | Unknown | ALKK | No hit | SH2 domain of: STAT3P |
| 1919 | EDNIYECYS | 0.941 | Unknown | ABLP, ALKP, BTKK, EGFRK, FESK, FGRK, FYNK, FAKK, JAKK, LCKK, METK, PDGFRK, RETK, RLKK, SrcK,P, SYKK, ZAP-70K | No hit | PTB domain of: CblP. At T-segment. |
| 1922 | IYECYSEA* | 0.052 | Unknown | SrcP | SHP1P |
No hit. At T-segment. Y1922 precedes the PDZ-binding motif; thus phosphorylation might modulate GIPC1-binding(as in (Sulka et al., 2009)) |
NOTES: In the first column (Y) residues highlighted as follows: red (conserved across Plxn subfamilies), blue (PlxnD1-specific), black (shared with another Plxn subfamily) or grey (shared with two Plxn subfamilies). In the Score column, scores above the prediction threshold (0.5) are in bold. Superscripts refer to the database used for predictions as follows: K (PPSP, Prediction of Protein kinase-Specific Phosphorylation site; http://ppsp.biocuckoo.org/), N (NetPhos 2.0; http://www.cbs.dtu.dk/services/NetPhos/), P (PhosphoMotif Finder; http://www.hprd.org/PhosphoMotif_finder) and S (Scansite; http://scansite.mit.edu). Kinases: ABL (c-abl Abelson murine leukemia viral oncogene homolog 1), ALK (anaplastic lymphoma receptor tyrosine kinase), BTK (Bruton agammaglobulinemia tyrosine kinase), EGFR (epidermal growth factor receptor), EPHA (Eph receptor A), FAK (focal adhesion kinase), FES (feline sarcoma oncogene), FGFR (fibrobalst growth receptor), FGR (Gardner-Rasheed feline sarcoma viral oncogene homolog), FMS (Friend murine leukemia virus integration site 2 homolog/colony-sttimulating factor 1 receptor or CSF1R) FYN (proto-oncogene tyrosine-protein kinase fyn), JAK (Janus Kinase or TYK2, tyrosine kinase 2), JAK2 (Janus kinase 2), MET (met proto-oncogene/hepatocyte growth factor receptor), PDGFR (platelet-derived growth factor receptor), RET (ret proto-oncogene), Src (v-src sarcoma viral oncogene homolog), SYK (spleen tyrosine kinase), TIE2 (TEK tyrosine kinase) and VEGFR (vascular endothelial growth factor receptor). Phosphatases: PTP1B (Ptpn1/protein tyrosine phosphatase, non-receptor type 1), SHP1 (src homology region 2 domain-containing phosphatase 1 or HCP; hematopoietic cell phosphatase) and TC-PTP (PTPN2/protein tyrosine phosphatase, non-receptor type 2). pY context matches binding motif for: SH2 (Src homology 2) or PTB (phosphotyrosine binding) domains of ABL (c-abl Abelson murine leukemia viral oncogene homolog 1), Cbl (Casitas B-lineage lymphoma), Crk (v-crk sarcoma virus CT10 oncogene homolog), Csk (c-src tyrosine kinase), FRIP (IL-Four Receptor Interacting Protein’s phosphotyrosine-binding domain), FYN (proto-oncogene tyrosine-protein kinase fyn), FGR (Gardner-Rasheed feline sarcoma viral oncogene homolog), Grb2 (growth factor receptor-bound protein 2), SHP1 HCP (src homology region 2 domain-containing phosphatase 1 or HCP; hematopoietic cell phosphatase), Itk (IL2-inducible T-cell kinase), Lck (lymphocyte-specific protein tyrosine kinase), Nck (non-catalytic region of tyrosine kinase adaptor protein), PLCg (phospholipase C gamma), RasGAP (Ras GTPase-activating protein), Src (v-src sarcoma viral oncogene homolog) and STAT3 (Signal transducer and activator of transcription 3).
In addition, eleven tyrosine residues are conserved across the PlxnD1 proteins of mammals, birds, amphibians and fish (Table 1 and Fig. 5A–D). Six of them are PlxnD1-specific (in hPlxnD1: Y1367, Y1371, Y1618, Y1673, Y1919 and Y1922). The latter two are in the T-segment; Y1371 is absent from fishes. The remaining five tyrosines are shared with one (Y1303, Y1459, Y1597, Y1864) or two (Y1503) Plxn subfamilies (hPlxnD1 positions). Conversely, PlxnD1 proteins lack tyrosine residues that are highly conserved in other Plxn subfamilies. For example Y1708, which appears to be specific for B Plxns (see (Franco and Tamagnone, 2008; Swiercz et al., 2009b).
Overall, many of the conserved tyrosine residues in PlxnD1 proteins reside within sequence contexts predicted to be substrates for tyrosine kinases (both receptor and non-receptor) and phosphatases (Amanchy et al., 2007). Some of these sequences also match the consensus binding sites for known protein-protein interaction modules, such as those of adaptor proteins, which might enable cross talk and/or integration between Sema-PlxnD1 signaling and other cascades (see Table 1). Importantly, tyrosine phosphorylation of PlxnD1 has been experimentally confirmed in the human and mouse proteins for two residues: the PlxnD1-specific Y1367 and Y1642, which is located at the RBD and conserved across Plxn subfamilies (http://www.phosphosite.org),(http://www.abgent.com/products/catalog_no/AP3584a/specification), (Franco and Tamagnone, 2008).
Plxns associate with tyrosine kinases
Plxns lack kinase activity but are phosphotyrosine proteins. Accordingly, they form complexes with both receptor and non-receptor tyrosine kinases (reviewed in (Franco and Tamagnone, 2008). For example, ErbB2 phosphorylates PlxnB1 at both Y1708 (a PlxnB-specific residue) and at Y1732 (one of the thirteen tyrosines conserved across Plxns), while c-Met phosphorylates other PlxnB1 tyrosine residues. In particular, the phosphotyrosines Y1708 and Y1732 serve as docking sites for the SH2 domains of phospholipase C (PLC ), which in a lipase-independent manner activates RhoA to promote growth cone collapse. Interestingly, ErbB2 and PLC are not required for PlxnB1-mediated R-Ras inactivation (Swiercz et al., 2009b). Together, these results suggest that different tyrosine kinases are involved in Plxn phosphorylation and that each kinase is likely to modulate distinct Plxn-mediated events.
PlxnD1 interacts physically with tyrosine kinases
PlxnD1 forms a complex with VEGFR2 within a ternary complex that also includes Nrp1. The PlxnD1/Nrp1/VEGFR2 complex promotes Sema3E-induced/VEGFR2-mediated growth of subicular axons. While this event does not appear to involve the phosphorylation of PlxnD1’s tyrosine residues (Bellon et al., 2010), it is possible that in other contexts VEGFR2 might phosphorylate PlxnD1 to modify its activity. It would be interesting to determine if this is the case in the endothelium, where all three proteins are expressed.
In addition, in human lung carcinoma cells p61Sema3E induces the formation of a PlxnD1-ErbB2 complex in which both partners are phosphotyrosinated. Remarkably, PlxnD1 phosphorylation requires ErbB2’s kinase activity. However, ErbB2’s activation usually involves its transphosphorylation in association with either EGFR (Epidermal Growth Factor Receptor) or ErbB3. Accordingly, p61Sema3E also induces EGFR tyrosine phosphorylation. Thus, it remains unclear if ErbB2, EGFR or both mediate PlxnD1’s tyrosine phosphorylation. In addition, the specific tyrosine residue(s) of PlxnD1 phosphorylated in carcinoma cells in response to p61Sema3E treatment have not been reported (Casazza et al., 2010).
Plxns are also Serine phosphorylated
Plxns are also phosphorylated at cytosolic Serine (S) residues, but the role of this modification remains to be characterized (see Phospho.ELM server) and (Collins et al., 2005; Dai et al., 2007; Trost et al., 2009). A candidate for this activity is the cAMP (cyclic adenosine monophosphate)-dependent protein kinase A (PKA) which phosphorylates S and Threonine (T) residues on its substrates and has been implicated as a negative regulator of Sema1a/PlxnA-induced axonal repulsion (see comments about Nervy on Box 3). The S/T phosphorylation of PlxnD1 has not been reported. However, PlxnD1 proteins contain six conserved predicted PKA phosphorylation sites (Table 2).
Table 2.
Conservation of predicted PKA phosphorylation sites (S/T) in PlxnD1’s intracellular tail
| Human | T1486 | S1488 | S1693 | S1709 | S1766 | T1901 |
| Mouse | T1486* | S1488 | S1693 | S1709 | S1766 | T1901 |
| Chicken | T1489 | S1491 | S1696 | S1712 | S1769 | T1889 |
| Xenopus | T1087 | S1089 | S1294 | S1310 | S1367 | T1502 |
| Zebrafish | T1441 | S1443 | S1648 | S1664 | S1721 | T1856 |
Notes: PKA phosphorylation sites were predicted using the pkaPS server (http://mendel.imp.ac.at/sat/pkaPS/). The accession numbers of PlxnD1 proteins are as follows: Human (), mouse (), chicken (), Xenopus (, partial sequence), zebrafish ().
Murine PlxnD1 residue T1486 is conserved but not a predicted PKA target.
CONCLUDING REMARKS
The biological roles of Sema-PlxnD1 signaling are being elucidated at a fast pace. The recent isolation of the first candidate modulator/effectors of PlxnD1 promises to illuminate the molecular mechanisms by which the Sema-PlxnD1 pathway orchestrates organ morphogenesis. Key future research directions in this exciting field include elucidation of the Sema/PlxnD1-specific functions of new cascade components such as GIPC1, GTPases (both R-Ras and Rho-like), cytoskeletal binding proteins and other Plxn effectors/modulators and defining if these molecules play similar or distinct roles in different tissues, determine how Nrp1 and VEGFR2, which are co-expressed with PlxnD1 in both the vascular and nervous systems, contribute to the differential effects -repulsion vs attraction- of Sema-PlxnD1 signaling in these tissues (Bellon et al., 2010; Chauvet et al., 2007; Christensen et al., 2005), understand how PlxnD1 signals are integrated with the inputs of other cascades to modulate the development of different organs (see (Alvarez et al., 2009; Childs et al., 2002; Choi et al., 2008; Eickholt, 2008; Lamont et al., 2009; Moriya et al., 2010) and uncovering the factors that regulate the expression patterns, levels, stability and activity of Sema-PlxnD1 signaling components (for example, see (Franco and Tamagnone, 2008; Liu et al., 2005; Parra and Zou, 2010). Finally, it is critical to explore the potential of Sema-PlxnD1 signaling as a therapeutic target for improving the health of patients suffering from ischemia, vascular disorders, cancer and nerve regeneration deficits (see (Casazza et al., 2010; Galamb et al., 2008; Moriya et al., 2010; Neufeld and Kessler, 2008; Pasterkamp and Giger, 2009; Roodink et al., 2005; Rozanov et al., 2008; Suzuki et al., 2003).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Lohrum M, Klostermann A, Betz H, Püschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Y, Astudillo O, Jensen L, Reynolds AL, Waghorne N, Brazil DP, Cao Y, O’Connor JJ, Kennedy BN. Selective inhibition of retinal angiogenesis by targeting PI3 kinase. PLoS ONE. 2009;4:e7867. doi: 10.1371/journal.pone.0007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Y, Cederlund ML, Cottell DC, Bill BR, Ekker SC, Torres-Vazquez J, Weinstein BM, Hyde DR, Vihtelic TS, Kennedy BN. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev Biol. 2007;7:114. doi: 10.1186/1471-213X-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanchy R, Periaswamy B, Mathivanan S, Reddy R, Tattikota SG, Pandey A. A curated compendium of phosphorylation motifs. Nat Biotechnol. 2007;25:285–286. doi: 10.1038/nbt0307-285. [DOI] [PubMed] [Google Scholar]
- Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal JK, Singh KK, Tessier-Lavigne M, Miller FD, Kaplan DR. Semaphorin 3F antagonizes neurotrophin-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase kinase signaling: a mechanism for growth cone collapse. J Neurosci. 2003;23:7602–7609. doi: 10.1523/JNEUROSCI.23-20-07602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci USA. 2002;99:12085–12090. doi: 10.1073/pnas.142433199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J. 2006;20:2150–2152. doi: 10.1096/fj.05-5698fje. [DOI] [PubMed] [Google Scholar]
- Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA. 2006;103:9017–9022. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi DE, Lopez De Cicco R, Mahloogi H, Zucker S, Thomas G, Klein-Szanto AJ. Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc Natl Acad Sci USA. 2001;98:10326–10331. doi: 10.1073/pnas.191199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R, Newgreen DF. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in thedeveloping brain. Neuron. 2010;66:205 –219. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Bernier G, Mathieu M, De Repentigny Y, Vidal SM, Kothary R. Cloning and characterization of mouse ACF7, a novel member of the dystonin subfamily of actin binding proteins. Genomics. 1996;38:19–29. doi: 10.1006/geno.1996.0587. [DOI] [PubMed] [Google Scholar]
- Bernier G, Pool M, Kilcup M, Alfoldi J, De Repentigny Y, Kothary R. Acf7 (MACF) is an actin and microtubule linker protein whose expression predominates in neural, muscle, and lung development. Dev Dyn. 2000;219:216–225. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1041>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bork P, Doerks T, Springer TA, Snel B. Domains in plexins: links to integrins and transcription factors. Trends Biochem Sci. 1999;24:261–263. doi: 10.1016/s0968-0004(99)01416-4. [DOI] [PubMed] [Google Scholar]
- Byers TJ, Beggs AH, McNally EM, Kunkel LM. Novel actin crosslinker superfamily member identified by a two step degenerate PCR procedure. FEBS Lett. 1995;368:500–504. doi: 10.1016/0014-5793(95)00722-l. [DOI] [PubMed] [Google Scholar]
- Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19:6519 –6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo JA. The Identification of Cooperating Mutations in TAL1-Mediated Leukemia in the Mouse: A Dissertation. 2005. pp. 1–177. [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Casazza A, Fazzari P, Tamagnone L. Semaphorin signals in cell adhesion and cell migration: functional role and molecular mechanisms. Adv Exp Med Biol. 2007;600:90–108. doi: 10.1007/978-0-387-70956-7_8. [DOI] [PubMed] [Google Scholar]
- Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest. 2010;120:2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano A, Lazzarini R, Di Nuzzo S, Orciari S, Procopio A. The plexin-A1 receptor activates vascular endothelial growth factor-receptor 2 and nuclear factor-kappaB to mediate survival and anchorage-independent growth of malignant mesothelioma cells. Cancer Res. 2009;69:1485–1493. doi: 10.1158/0008-5472.CAN-08-3659. [DOI] [PubMed] [Google Scholar]
- Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ. PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci. 2006;119:951–957. doi: 10.1242/jcs.02801. [DOI] [PubMed] [Google Scholar]
- Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, Mann F. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Lin CM, Lin CS, Perez-Olle R, Leung CL, Liem RKH. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev. 2006;20:1933–1945. doi: 10.1101/gad.1411206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M. Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron. 2001;32:249–263. doi: 10.1016/s0896-6273(01)00478-0. [DOI] [PubMed] [Google Scholar]
- Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129:973–982. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Dev Cell. 2006;10:783–795. doi: 10.1016/j.devcel.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Choi YI, Duke-Cohan JS, Ahmed WB, Handley MA, Mann F, Epstein JA, Clayton LK, Reinherz EL. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29:888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- Chu TJ, Peters DG. Serial analysis of the vascular endothelial transcriptome under static and shear stress conditions. Physiol Genomics. 2008;34:185–192. doi: 10.1152/physiolgenomics.90201.2008. [DOI] [PubMed] [Google Scholar]
- Chung L, Yang TL, Huang HR, Hsu SM, Cheng HJ, Huang PH. Semaphorin signaling facilitates cleft formation in the developing salivary gland. Development. 2007;134:2935–2945. doi: 10.1242/dev.005066. [DOI] [PubMed] [Google Scholar]
- Collins MO, Yu L, Coba MP, Husi H, Campuzano I, Blackstock WP, Choudhary JS, Grant SGN. Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem. 2005;280:5972–5982. doi: 10.1074/jbc.M411220200. [DOI] [PubMed] [Google Scholar]
- Comeau MR, Johnson R, DuBose RF, Petersen M, Gearing P, VandenBos T, Park L, Farrah T, Buller RM, Cohen JI, Strockbine LD, Rauch C, Spriggs MK. A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity. 1998;8:473–482. doi: 10.1016/s1074-7613(00)80552-x. [DOI] [PubMed] [Google Scholar]
- Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood. 2005;105:4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- D’Souza CA, Chopra V, Varhol R, Xie YY, Bohacec S, Zhao Y, Lee LLC, Bilenky M, Portales-Casamar E, He A, Wasserman WW, Goldowitz D, Marra MA, Holt RA, Simpson EM, Jones SJM. Identification of a set of genes showing regionally enriched expression in the mouse brain. BMC neuroscience. 2008;9:66. doi: 10.1186/1471-2202-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Dai J, Jin WH, Sheng QH, Shieh CH, Wu JR, Zeng R. Protein phosphorylation and expression profiling by Yin-yang multidimensional liquid chromatography (Yin-yang MDLC) mass spectrometry. J Proteome Res. 2007;6:250–262. doi: 10.1021/pr0604155. [DOI] [PubMed] [Google Scholar]
- De Vries L, Lou X, Zhao G, Zheng B, Farquhar MG. GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP. Proc Natl Acad Sci USA. 1998;95:12340–12345. doi: 10.1073/pnas.95.21.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11:339–344. doi: 10.1016/s0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- Driessens MHE, Olivo C, Nagata K-i, Inagaki M, Collard JG. B plexins activate Rho through PDZ-RhoGEF. FEBS Lett. 2002;529:168–172. doi: 10.1016/s0014-5793(02)03323-9. [DOI] [PubMed] [Google Scholar]
- Dunphy JL, Moravec R, Ly K, Lasell TK, Melancon P, Casanova JE. The Arf6 GEF GEP100/BRAG2 regulates cell adhesion by controlling endocytosis of beta1 integrins. Curr Biol. 2006;16:315–320. doi: 10.1016/j.cub.2005.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickholt BJ. Functional diversity and mechanisms of action of the semaphorins. Development. 2008;135:2689–2694. doi: 10.1242/dev.019968. [DOI] [PubMed] [Google Scholar]
- Fanarraga ML, Villegas JC, Carranza G, Castaño R, Zabala JC. Tubulin cofactor B regulates microtubule densities during microglia transition to the reactive states. Exp Cell Res. 2009;315:535–541. doi: 10.1016/j.yexcr.2008.10.045. [DOI] [PubMed] [Google Scholar]
- Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci USA. 2006;103:19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat CellBiol. 2004;6:1034 –1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- Fiedler SE, Schillace RV, Daniels CJ, Andrews SF, Carr DW. Myeloid translocation gene 16b is a dual A-kinase anchoring protein that interacts selectively with plexins in a phospho-regulated manner. FEBS Lett. 2010;584:873–877. doi: 10.1016/j.febslet.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R, Hu KQ, Lu Y, Nolan KM, Thissen J, Settleman J. Identification of a novel human Rho protein with unusual properties: GTPase deficiency and in vivo farnesylation. Mol Cell Biol. 1996;16:2689–2699. doi: 10.1128/mcb.16.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 2008;9:865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Katoh H, Ishikawa Y, Mori K, Negishi M. Rapostlin is a novel effector of Rnd2 GTPase inducing neurite branching. J Biol Chem. 2002;277:45428–45434. doi: 10.1074/jbc.M208090200. [DOI] [PubMed] [Google Scholar]
- Galamb O, Sipos F, Solymosi N, Spisak S, Krenacs T, Toth K, Tulassay Z, Molnar B. Diagnostic mRNA expression patterns of inflamed, benign, and malignant colorectal biopsy specimen and their correlation with peripheral blood results. Cancer Epidemiol Biomarkers Prev. 2008;17:2835–2845. doi: 10.1158/1055-9965.EPI-08-0231. [DOI] [PubMed] [Google Scholar]
- Gallo G. Semaphorin 3A inhibits ERM protein phosphorylation in growth cone filopodia through inactivation of PI3K. Dev Neurobiol. 2008;68:926–933. doi: 10.1002/dneu.20631. [DOI] [PubMed] [Google Scholar]
- Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11:31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Opin Struct Biol. 2004;14:669–678. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Gingrich JR, Ng D, Gore B, Tessier-Lavigne M, McInnes RR. The CUB domain protein Neto1 is a molecular switch that modulates Plexin D1 binding specificity for semaphorin axon guidance cues. The American Society of Human Genetics 59th Annual Meeting; October 20–24, 2009; Honolulu, Hawaii. 2009. POSTER ABSTRACT: 2405/T. [Google Scholar]
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovasculardevelopment. Dev Cell. 2004;7:107 –116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Grynberg M, Jaroszewski L, Godzik A. Domain analysis of the tubulin cofactor system: a model for tubulin folding and dimerization. BMC Bioinformatics. 2003;4:46. doi: 10.1186/1471-2105-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int. 2006;69:1564–1569. doi: 10.1038/sj.ki.5000313. [DOI] [PubMed] [Google Scholar]
- Guijosa MC. Caracterizacion biologica de la leucemia mieloide aguda con translocacion t(8;16)(p11;p13) y reordenamiento MYST3-CREBBP. 2007. pp. 1–55. [Google Scholar]
- Hartwig C, Veske A, Krejcova S, Rosenberger G, Finckh U. Plexin B3 promotes neurite outgrowth, interacts homophilically, and interacts with Rin. BMC neuroscience. 2005;6:53. doi: 10.1186/1471-2202-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Yang T, Terman JR, Zhang X. Crystal structure of the plexin A3 intracellular region reveals an autoinhibited conformation through active site sequestration. Proc Natl Acad Sci USA. 2009;106:15610–15615. doi: 10.1073/pnas.0906923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotani M, Ohoka Y, Yamamoto T, Nirasawa H, Furuyama T, Kogo M, Matsuya T, Inagaki S. Interaction of plexin-B1 with PDZ domain-containing Rho guanine nucleotide exchange factors. Biochem Biophys Res Commun. 2002;297:32–37. doi: 10.1016/s0006-291x(02)02122-8. [DOI] [PubMed] [Google Scholar]
- Horowitz A. Plexin D1 signals via the cytoskeletal scaffold protein filamin A. FASEB J. 2007;21:872–879. [Google Scholar]
- Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Hu LA, Chen W, Martin NP, Whalen EJ, Premont RT, Lefkowitz RJ. GIPC interacts with the beta1-adrenergic receptor and regulates beta1-adrenergic receptor-mediated ERK activation. J Biol Chem. 2003;278:26295–26301. doi: 10.1074/jbc.M212352200. [DOI] [PubMed] [Google Scholar]
- Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJH, Terman JR. Mical links semaphorins to F-actin disassembly. Nature. 2010;463:823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Tyrosine phosphorylation: thirty years and counting. Curr Opin Cell Biol. 2009;21:140–146. doi: 10.1016/j.ceb.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- Ithychanda SS, Hsu D, Li H, Yan L, Liu D, Das M, Plow EF, Qin J. Identification and characterization of multiple similar ligand-binding repeats in filamin: implication on filamin-mediated receptor clustering and cross-talk. J Biol Chem. 2009;284:35113–35121. doi: 10.1074/jbc.M109.060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Oinuma I, Katoh H, Kaibuchi K, Negishi M. Sema4D/plexin-B1 activates GSK-3beta through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep. 2006;7:704–709. doi: 10.1038/sj.embor.7400737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jeleń F, Oleksy A, Smietana K, Otlewski J. PDZ domains - common players in the cell signaling. Acta Biochim Pol. 2003;50:985–1017. [PubMed] [Google Scholar]
- Kameyama T, Murakami Y, Suto F, Kawakami A, Takagi S, Hirata T, Fujisawa H. Identification of a neuronal cell surface molecule, plexin, in mice. Biochem Biophys Res Commun. 1996a;226:524–529. doi: 10.1006/bbrc.1996.1388. [DOI] [PubMed] [Google Scholar]
- Kameyama T, Murakami Y, Suto F, Kawakami A, Takagi S, Hirata T, Fujisawa H. Identification of plexin family molecules in mice. Biochem Biophys Res Commun. 1996b;226:396–402. doi: 10.1006/bbrc.1996.1367. [DOI] [PubMed] [Google Scholar]
- Kanda I, Nishimura N, Nakatsuji H, Yamamura R, Nakanishi H, Sasaki T. Involvement of Rab13 and JRAB/MICAL-L2 in epithelial cell scattering. Oncogene. 2008;27:1687–1695. doi: 10.1038/sj.onc.1210812. [DOI] [PubMed] [Google Scholar]
- Kanda T, Yoshida Y, Izu Y, Nifuji A, Ezura Y, Nakashima K, Noda M. PlexinD1 deficiency induces defects in axial skeletal morphogenesis. J Cell Biochem. 2007;101:1329–1337. doi: 10.1002/jcb.21306. [DOI] [PubMed] [Google Scholar]
- Karakesisoglou I, Yang Y, Fuchs E. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J Cell Biol. 2000;149:195 –208. doi: 10.1083/jcb.149.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigel B, Varshavsky A, Kessler O, Neufeld G. Successful inhibition of tumor development by specific class-3 semaphorins is associated with expression of appropriate semaphorin receptors by tumor cells. PLoS ONE. 2008;3:e3287. doi: 10.1371/journal.pone.0003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman A, Tolkacheva T, Lorenzi MV, Osada M, Chan AM. Identification and characterization of R-ras3: a novel member of the RAS gene family with a non-ubiquitous pattern of tissue distribution. Oncogene. 1997;15:2675–2685. doi: 10.1038/sj.onc.1201674. [DOI] [PubMed] [Google Scholar]
- Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH. Ras GTPases: integrins’ friends or foes? Nat Rev Mol Cell Biol. 2003;4:767–776. doi: 10.1038/nrm1229. [DOI] [PubMed] [Google Scholar]
- Klostermann A, Lohrum M, Adams RH, Püschel AW. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J Biol Chem. 1998;273:7326–7331. doi: 10.1074/jbc.273.13.7326. [DOI] [PubMed] [Google Scholar]
- Kolk SM, Pasterkamp RJ. MICAL flavoprotein monooxygenases: structure, function and role in semaphorin signaling. Adv Exp Med Biol. 2007;600:38–51. doi: 10.1007/978-0-387-70956-7_4. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Ruoslahti E. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat Med. 2005;11:1346–1350. doi: 10.1038/nm1324. [DOI] [PubMed] [Google Scholar]
- Koppel AM, Raper JA. Collapsin-1 covalently dimerizes, and dimerization is necessary for collapsing activity. J Biol Chem. 1998;273:15708–15713. doi: 10.1074/jbc.273.25.15708. [DOI] [PubMed] [Google Scholar]
- Kortazar D, Fanarraga ML, Carranza G, Bellido J, Villegas JC, Avila J, Zabala JC. Role of cofactors B (TBCB) and E (TBCE) in tubulin heterodimer dissociation. Exp Cell Res. 2007;313:425–436. doi: 10.1016/j.yexcr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura SI, Tsutsui H, Yamamoto M, Takamatsu H, Ko-Mitamura EP, Takegahara N, Marukawa S, Ishida I, Morishita H, Prasad DVR, Tamura M, Mizui M, Toyofuku T, Akira S, Takeda K, Okabe M, Kikutani H. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22:305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Lalani SR, Safiullah AM, Molinari LM, Fernbach SD, Martin DM, Belmont JW. SEMA3E mutation in a patient with CHARGE syndrome. J Med Genet. 2004;41:e94. doi: 10.1136/jmg.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RE, Lamont EJ, Childs SJ. Antagonistic interactions among Plexins regulate the timing of intersegmental vessel formation. Dev Biol. 2009;331:199–209. doi: 10.1016/j.ydbio.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell. 2010;18:713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CL, Sun D, Zheng M, Knowles DR, Liem RK. Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J Cell Biol. 1999;147:1275–1286. doi: 10.1083/jcb.147.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhares Y, Gutkind JS. Plexin D1 signals to guide endothelial cells. 2005 Howard Hughes Medical Institute Meeting of Medical Fellows; 2005. pp. 51–51. (Poster 28) [Google Scholar]
- Liu T, Qian WJ, Gritsenko MA, Camp DG, Monroe ME, Moore RJ, Smith RD. Human plasma N-glycoproteome analysis by immunoaffinity subtraction, hydrazide chemistry, and mass spectrometry. J Proteome Res. 2005;4:2070–2080. doi: 10.1021/pr0502065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Fanarraga M, Carranza G, Bellido J, Kortazar D, Villegas JC, Zabala JC. Tubulin cofactor B plays a role in the neuronal growth cone. J Neurochem. 2007;100:1680–1687. doi: 10.1111/j.1471-4159.2006.04328.x. [DOI] [PubMed] [Google Scholar]
- Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol. 2003;10:843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- Lu J, Lian G, Lenkinski R, De Grand A, Vaid RR, Bryce T, Stasenko M, Boskey A, Walsh C, Sheen V. Filamin B mutations cause chondrocyte defects in skeletal development. Hum Mol Genet. 2007;16:1661–1675. doi: 10.1093/hmg/ddm114. [DOI] [PubMed] [Google Scholar]
- Maestrini E, Tamagnone L, Longati P, Cremona O, Gulisano M, Bione S, Tamanini F, Neel BG, Toniolo D, Comoglio PM. A family of transmembrane proteins with homology to the MET-hepatocyte growth factor receptor. Proc Natl Acad Sci USA. 1996;93:674–678. doi: 10.1073/pnas.93.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro GS, Shen K, Cheng HJ. Deal breaker: semaphorin and specificity in the spinal stretch reflex circuit. Neuron. 2009;63:8–11. doi: 10.1016/j.neuron.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Matthies AM, Low QEH, Lingen MW, DiPietro LA. Neuropilin-1 participates in wound angiogenesis. Am J Pathol. 2002;160:289–296. doi: 10.1016/S0002-9440(10)64372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004;32:W20–25. doi: 10.1093/nar/gkh435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettouchi A, Meneguzzi G. Distinct roles of beta1 integrins during angiogenesis. Eur J Cell Biol. 2006;85:243–247. doi: 10.1016/j.ejcb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita M, Ikeda T, Nakashiba T, Ogawa M, Tashiro K, Honjo T, Doi K, Itohara S, Endo S. A novel gene, Btcl1, encoding CUB and LDLa domains is expressed in restricted areas of mouse brain. Biochem Biophys Res Commun. 2003;306:680–686. doi: 10.1016/s0006-291x(03)01035-0. [DOI] [PubMed] [Google Scholar]
- Miyamori H, Hasegawa K, Kim KR, Sato H. Expression of metastasis-associated mts1 gene is co-induced with membrane type-1 matrix metalloproteinase (MT1-MMP) during oncogenic transformation and tubular formation of Madin Darby canine kidney (MDCK) epithelial cells. Clin Exp Metastasis. 2000;18:51–56. doi: 10.1023/a:1026523418456. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009a;29:12343–12354. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Fame RM, Macdonald JL, Macquarrie KL, Macklis JD. Novel Subtype-Specific Genes Identify Distinct Subpopulations of Callosal Projection Neurons. J Neurosci. 2009b;29:12343–12354. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead GBG, De Wever V, Templeton G, Kerk D. Evolution of protein phosphatases in plants and animals. Biochem J. 2009;417:401–409. doi: 10.1042/BJ20081986. [DOI] [PubMed] [Google Scholar]
- Moriya J, Minamino T, Tateno K, Okada S, Uemura A, Shimizu I, Yokoyama M, Nojima A, Okada M, Koga H, Komuro I. Inhibition of semaphorin as a novel strategy for therapeutic angiogenesis. Circ Res. 2010;106:391–398. doi: 10.1161/CIRCRESAHA.109.210815. [DOI] [PubMed] [Google Scholar]
- Morris JS, Stein T, Pringle MA, Davies CR, Weber-Hall S, Ferrier RK, Bell AK, Heath VJ, Gusterson BA. Involvement of axonal guidance proteins and their signaling partners in the developing mouse mammary gland. J Cell Physiol. 2006;206:16–24. doi: 10.1002/jcp.20427. [DOI] [PubMed] [Google Scholar]
- Nakatsuji H, Nishimura N, Yamamura R, Kanayama HO, Sasaki T. Involvement of actinin-4 in the recruitment of JRAB/MICAL-L2 to cell-cell junctions and the formation of functional tight junctions. Mol Cell Biol. 2008;28:3324–3335. doi: 10.1128/MCB.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Kikuno R, Ohara O. Protein-protein interactions between large proteins: two-hybrid screening using a functionally classified library composed of long cDNAs. Genome Res. 2002;12:1773–1784. doi: 10.1101/gr.406902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- Ng D, Pitcher GM, Szilard RK, Sertié A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, Cortez M, Roder JC, Salter MW, McInnes RR. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Lauritzen I, Mattei MG, Paris S, Hall A, Chardin P. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Mizutani A, Kawakami A, Murakami Y, Kasuya Y, Takagi S, Tanaka H, Fujisawa H. Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron. 1995;14:1189–1199. doi: 10.1016/0896-6273(95)90266-x. [DOI] [PubMed] [Google Scholar]
- Ohta K, Takagi S, Asou H, Fujisawa H. Involvement of neuronal cell surface molecule B2 in the formation of retinal plexiform layers. Neuron. 1992;9:151–161. doi: 10.1016/0896-6273(92)90230-b. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004a;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Harada A, Negishi M. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells. J Biol Chem. 2003;278:25671–25677. doi: 10.1074/jbc.M303047200. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Negishi M. Molecular dissection of the semaphorin 4D receptor plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampalneurons. J Neurosci. 2004b;24:11473 –11480. doi: 10.1523/JNEUROSCI.3257-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating beta(1) integrin activity. J Cell Biol. 2006;173:601–613. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova I, Silver L, Gallo G. Regulation of actomyosin contractility by PI3K in sensory axons. Dev Neurobiol. 2007;67:1843–1851. doi: 10.1002/dneu.20558. [DOI] [PubMed] [Google Scholar]
- Pagon RA, Graham JM, Zonana J, Yong SL. Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J Pediatr. 1981;99:223–227. doi: 10.1016/s0022-3476(81)80454-4. [DOI] [PubMed] [Google Scholar]
- Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ. R-Ras fills another GAP in semaphorin signalling. Trends Cell Biol. 2005;15:61–64. doi: 10.1016/j.tcb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot V, Vazquez-Prado J, Gutkind JS. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem. 2002;277:43115–43120. doi: 10.1074/jbc.M206005200. [DOI] [PubMed] [Google Scholar]
- Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411–419. doi: 10.1016/j.tibs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- Prislei S, Mozzetti S, Filippetti F, De Donato M, Raspaglio G, Cicchillitti L, Scambia G, Ferlini C. From plasma membrane to cytoskeleton: a novel function for semaphorin 6A. Mol Cancer Ther. 2008;7:233–241. doi: 10.1158/1535-7163.MCT-07-0390. [DOI] [PubMed] [Google Scholar]
- Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, Mulligan-Kehoe MJ, Byzova TV, Peterson RT, Simons M. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120:1217–1228. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SP. Filamin A: phenotypic diversity. Curr Opin Genet Dev. 2005;15:301–307. doi: 10.1016/j.gde.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Rohm B, Rahim B, Kleiber B, Hovatta I, Püschel AW. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett. 2000;486:68–72. doi: 10.1016/s0014-5793(00)02240-7. [DOI] [PubMed] [Google Scholar]
- Roodink I, Kats G, van Kempen L, Grunberg M, Maass C, Verrijp K, Raats J, Leenders W. Semaphorin 3E expression correlates inversely with Plexin D1 during tumor progression. Am J Pathol. 2008;173:1873–1881. doi: 10.2353/ajpath.2008.080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodink I, Raats J, van der Zwaag B, Verrijp K, Kusters B, van Bokhoven H, Linkels M, de Waal RMW, Leenders WPJ. Plexin D1 expression is induced on tumor vasculature and tumor cells: a novel target for diagnosis and therapy? Cancer Res. 2005;65:8317–8323. doi: 10.1158/0008-5472.CAN-04-4366. [DOI] [PubMed] [Google Scholar]
- Roodink I, Verrijp K, Raats J, Leenders WPJ. Plexin D1 is ubiquitously expressed on tumor vessels and tumor cells in solid malignancies. BMC Cancer. 2009;9:297. doi: 10.1186/1471-2407-9-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanov DV, Savinov AY, Williams R, Liu K, Golubkov VS, Krajewski S, Strongin AY. Molecular signature of MT1-MMP: transactivation of the downstream universal gene network in cancer. Cancer Res. 2008;68:4086–4096. doi: 10.1158/0008-5472.CAN-07-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane A, Honda K, Sasaki T. Rab13 regulates neurite outgrowth in PC12 cells through its effector protein, JRAB/MICAL-L2. Mol Cell Biol. 2010;30:1077 –1087. doi: 10.1128/MCB.01067-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, Weigert R, Gutkind JS. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoda M, Takagi S, Ohta K, Hirata T, Fujisawa H. Differential expression of two cell surface proteins, neuropilin and plexin, in Xenopus olfactory axon subclasses. J Neurosci. 1995;15:942–955. doi: 10.1523/JNEUROSCI.15-01-00942.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura D, Nomura K, Sugita Y, Mattei MG, Chu ML, Knowlton R, Uitto J. Bullous pemphigoid antigen (BPAG1): cDNA cloning and mapping of the gene to the short arm of human chromosome 6. Genomics. 1990;8:722–726. doi: 10.1016/0888-7543(90)90261-r. [DOI] [PubMed] [Google Scholar]
- Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Ruhrberg C. Neuropilin, you gotta let me know: Should I stay or should I go? Cell adhesion & migration. 2010:4. doi: 10.4161/cam.4.1.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Maione F, Giraudo E, Bussolino F. Semaphorins and tumor angiogenesis. Angiogenesis. 2009;12:187–193. doi: 10.1007/s10456-009-9138-4. [DOI] [PubMed] [Google Scholar]
- Sharma M, Giridharan SSP, Rahajeng J, Naslavsky N, Caplan S. MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol Biol Cell. 2009;20:5181–5194. doi: 10.1091/mbc.E09-06-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JY, Lee YCG, Yang SC, Hong TM, Huang CYF, Yang PC. Collapsin response mediator protein-1: a novel invasion-suppressor gene. Clin Exp Metastasis. 2003;20:69–76. doi: 10.1023/a:1022598604565. [DOI] [PubMed] [Google Scholar]
- Shim S, Ming G-l. Signaling of secreted semaphorins in growth cone steering. Adv Exp Med Biol. 2007;600:52–60. doi: 10.1007/978-0-387-70956-7_5. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Mammoto A, Italiano JE, Pravda E, Dudley AC, Ingber DE, Klagsbrun M. ABL2/ARG tyrosine kinase mediates SEMA3F-induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J Biol Chem. 2008;283:27230–27238. doi: 10.1074/jbc.M804520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji W, Isogai S, Sato-Maeda M, Obinata M, Kuwada JY. Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development. 2003;130:3227–3236. doi: 10.1242/dev.00516. [DOI] [PubMed] [Google Scholar]
- Shoji W, Yee CS, Kuwada JY. Zebrafish semaphorin Z1a collapses specific growth cones and alters their pathway in vivo. Development. 1998;125:1275–1283. doi: 10.1242/dev.125.7.1275. [DOI] [PubMed] [Google Scholar]
- Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM, Tamagnone L, Giordano S. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205:1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Kwon SK, Cho CG, Park SW. Skull base vascular anomaly in CHARGE syndrome: a case report and review. Int J Pediatr Otorhinolaryngol. 2008;72:535–539. doi: 10.1016/j.ijporl.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Steinmetz MO, Akhmanova A. Capturing protein tails by CAP-Gly domains. Trends Biochem Sci. 2008;33:535–545. doi: 10.1016/j.tibs.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Stöhr H, Berger C, Fröhlich S, Weber BHF. A novel gene encoding a putative transmembrane protein with two extracellular CUB domains and a low-density lipoprotein class A module: isolation of alternatively spliced isoforms in retina and brain. Gene. 2002;286:223–231. doi: 10.1016/s0378-1119(02)00438-9. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- Sulka B, Lortat-Jacob H, Terreux R, Letourneur F, Rousselle P. Tyrosine Dephosphorylation of the Syndecan-1 PDZ Binding Domain Regulates Syntenin-1 Recruitment. Journal of Biological Chemistry. 2009;284:10659–10671. doi: 10.1074/jbc.M807643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang J, Kraeft SK, Auclair D, Chang MS, Liu Y, Sutherland R, Salgia R, Griffin JD, Ferland LH, Chen LB. Molecular cloning and characterization of human trabeculin-alpha, a giant protein defining a new family of actin-binding proteins. J Biol Chem. 1999;274:33522–33530. doi: 10.1074/jbc.274.47.33522. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nakamoto T, Ogawa S, Seo S, Matsumura T, Tachibana K, Morimoto C, Hirai H. MICAL, a novel CasL interacting molecule, associates with vimentin. J Biol Chem. 2002;277:14933–14941. doi: 10.1074/jbc.M111842200. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nakagomi S, Namikawa K, Kiryu-Seo S, Inagaki N, Kaibuchi K, Aizawa H, Kikuchi K, Kiyama H. Collapsin response mediator protein-2 accelerates axon regeneration of nerve-injured motor neurons of rat. J Neurochem. 2003;86:1042–1050. doi: 10.1046/j.1471-4159.2003.01920.x. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol. 2004;165:869–880. doi: 10.1083/jcb.200312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem. 2008;283:1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Worzfeld T, Offermanns S. Semaphorin 4D Signaling Requires the Recruitment of Phospholipase C into the Plexin-B1 Receptor Complex. Mol Cell Biol. 2009a;29:6321–6334. doi: 10.1128/MCB.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Worzfeld T, Offermanns S. Semaphorin 4D signaling requires the recruitment of phospholipase C gamma into the plexin-B1 receptor complex. Mol Cell Biol. 2009b;29:6321–6334. doi: 10.1128/MCB.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Strittmatter SM. Plexina1 autoinhibition by the plexin sema domain. Neuron. 2001;29:429–439. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DVR, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Katoh H, Negishi M. Pragmin, a novel effector of Rnd2 GTPase, stimulates RhoA activity. J Biol Chem. 2006;281:10355–10364. doi: 10.1074/jbc.M511314200. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Maeda R, Shoji W, Wada H, Masai I, Shiraki T, Kobayashi M, Nakayama R, Okamoto H. Novel mutations affecting axon guidance in zebrafish and a role for plexin signalling in the guidance of trigeminal and facial nerve axons. Development. 2007;134:3259–3269. doi: 10.1242/dev.004267. [DOI] [PubMed] [Google Scholar]
- Terai T, Nishimura N, Kanda I, Yasui N, Sasaki T. JRAB/MICAL-L2 is a junctional Rab13-binding protein mediating the endocytic recycling of occludin. Mol Biol Cell. 2006;17:2465–2475. doi: 10.1091/mbc.E05-09-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman J, Kolodkin AL. Response to Comment on “Nervy Links Protein Kinase A to Plexin-Mediated Semaphorin Repulsion”. Science. 2005;309:558c. doi: 10.1126/science.1109259. [DOI] [PubMed] [Google Scholar]
- Terman JR, Kolodkin AL. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/s0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Togashi H, Schmidt EF, Strittmatter SM. RanBPM contributes to Semaphorin3A signaling through plexin-A receptors. J Neurosci. 2006;26:4961–4969. doi: 10.1523/JNEUROSCI.0704-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Buck M. 1H, 15N and 13C Resonance assignments and secondary structure determination reveal that the minimal Rac1 GTPase binding domain of plexin-B1 has a ubiquitinfold. J Biomol NMR. 2005;31:369 –370. doi: 10.1007/s10858-005-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park HW, Buck M. Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. J Biol Chem. 2007;282:37215–37224. doi: 10.1074/jbc.M703800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vázquez J, Gitler AD, Fraser SD, Berk JD, Pham VN, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 2007;26:1373–1384. doi: 10.1038/sj.emboj.7601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, Takegahara N, Suto F, Hori M, Fujisawa H, Kumanogoh A, Kikutani H. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol. 2008;321:251–262. doi: 10.1016/j.ydbio.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P. The phagosomal proteome in interferon-gamma-activated macrophages. Immunity. 2009;30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Turner LJ, Nicholls S, Hall A. The activity of the plexin-A1 receptor is regulated by Rac. J Biol Chem. 2004;279:33199–33205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- Uesugi K, Oinuma I, Katoh H, Negishi M. Different Requirement for Rnd GTPases of R-Ras GAP Activity of Plexin-C1 and Plexin-D1. Journal of Biological Chemistry. 2008;284:6743–6751. doi: 10.1074/jbc.M805213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Barnes CJ, Rayala S, Li F, Balasenthil S, Marcus S, Goodson HV, Sahin AA, Kumar R. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol Cell Biol. 2005;25:3726–3736. doi: 10.1128/MCB.25.9.3726-3736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaag B, Hellemons AJCGM, Leenders WPJ, Burbach JPH, Brunner HG, Padberg GW, van Bokhoven H. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Dev Dyn. 2002;225:336–343. doi: 10.1002/dvdy.10159. [DOI] [PubMed] [Google Scholar]
- Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for NRP1 ligands during neurovascular patterning. Development. 2007;134:1833–1843. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikis HG, Li W, He Z, Guan KL. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc Natl Acad Sci USA. 2000;97:12457–12462. doi: 10.1073/pnas.220421797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waimey KE, Cheng HJ. Axon pruning and synaptic development: how are they per-plexin? The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2006;12:398–409. doi: 10.1177/1073858406292631. [DOI] [PubMed] [Google Scholar]
- Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- Wang W, Ding J, Allen E, Zhu P, Zhang L, Vogel H, Yang Y. Gigaxonin interacts with tubulin folding cofactor B and controls its degradation through the ubiquitin-proteasome pathway. Curr Biol. 2005;15:2050–2055. doi: 10.1016/j.cub.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang W, Cheever T, Schwarz V, Opperman K, Hutter H, Koepp D, Chen L. The C. elegans L1CAM homologue LAD-2 functions as a coreceptor in MAB-20/Sema2 mediated axon guidance. J Cell Biol. 2008;180:233–246. doi: 10.1083/jcb.200704178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A, Ohsawa S, Hashikawa T, Yamamori T. Binding and complementary expression patterns of semaphorin 3E and plexin D1 in the mature neocortices of mice and monkeys. J Comp Neurol. 2006;499:258–273. doi: 10.1002/cne.21106. [DOI] [PubMed] [Google Scholar]
- Weber GJ, Choe SE, Dooley KA, Paffett-Lugassy NN, Zhou Y, Zon LI. Mutant-specific gene programs in the zebrafish. Blood. 2005;106:521–530. doi: 10.1182/blood-2004-11-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein BM. What guides early embryonic blood vessel formation? Dev Dyn. 1999;215:2–11. doi: 10.1002/(SICI)1097-0177(199905)215:1<2::AID-DVDY2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Wildonger J, Mann RS. Evidence that nervy, the Drosophila homolog of ETO/MTG8, promotes mechanosensory organ development by enhancing Notch signaling. Dev Biol. 2005;286:507–520. doi: 10.1016/j.ydbio.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Tamagnone L, Bai J, Comoglio PM, Montell D, Goodman CS. The transmembrane protein Off-track associates with Plexins and functions downstream of Semaphorin signaling during axon guidance. Neuron. 2001;32:53–62. doi: 10.1016/s0896-6273(01)00446-9. [DOI] [PubMed] [Google Scholar]
- Wu X, Kodama A, Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008;135:137–148. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan A, Lennarz WJ. Unraveling the mechanism of protein N-glycosylation. J Biol Chem. 2005;280:3121–3124. doi: 10.1074/jbc.R400036200. [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–788. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanata SM, Hovatta I, Rohm B, Püschel AW. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Singh M, Degenhardt K, Lu M, Bennett J, Yoshida Y, Epstein J. Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]