Abstract
The cone snail Conus pulicarius from the Philippines provides a specific habitat for actinomycetes and other bacteria. A phenotypic screen using primary cultures of mouse dorsal root ganglion neurons revealed that one C. pulicarius associate, Streptomyces sp. CP32, produces a series of natural products that enhance or diminish whole-cell Ca2+ flux. These compounds include known thiazoline compounds and a series of new derivatives, pulicatins A-E (6-10). Individual compounds were shown to bind to a series of human receptors, with selective binding to the human serotonin 5-HT2B receptor. Here, we report the structure elucidation of the new compounds and results of the neurological assays.
Symbiotic bacteria living with animals have been implicated in the synthesis of defensive and other natural products of importance to drug discovery.1-5 In the marine environment, most studies of symbiont-derived natural products have focused on soft-bodied, benthic invertebrates that are otherwise vulnerable to predation. By contrast, cone snail mollusks are well defended by their shells and their arsenal of extremely diverse, snail-derived peptide toxins that act on channels and receptors.6 We sought to investigate whether this type of well-defended organism would also house symbiotic bacteria involved in the synthesis of allelochemicals, potentially including venom components, antibacterial defenses, and other small-molecule natural products. In addition to investigating this basic question, we reasoned that exploration of an untapped bacterial niche could lead to the discovery of new bioactive natural products.
We recently reported that at least some cone snails are associated with diverse actinomycetes and other bacteria, and that extracts of some of these bacteria exhibit neurological activity.7 In order to maximize the discovery of new active bacterial metabolites from these new niches, we applied a broad-scope assay involving primary cultures of mouse dorsal root ganglion (DRG) neurons.7-9 The dorsal horn contains many types of neurons, largely involved in pain and other sensory perception, which express different types and combinations of channels and receptors. In this phenotypic assay different responses are observed in individual cells representing different types of neurons when active compounds or extracts are applied. Using this broadly-targeted biological assay as a primary screening strategy, we tested extracts from a total of 254 cone snail-associated bacteria, which yielded 29 isolates that either increase or decrease Ca2+ influx into DRG cells. One of the active isolates, Streptomyces sp. CP32 from C. pulicarius, was investigated by assay-guided fractionation, leading to the isolation of five new metabolites, pulicatins A-E (6-10), together with five known analogs (1-5).
Results and Discussion
Extracts of pilot-scale cultures of strain CP32 were strongly active in the DRG assay, resulting in K+-stimulated Ca2+ influx. CP32 was fermented in 2.8 L Fernbach flasks for eight days. In an assay-guided procedure, the culture broth was centrifuged and subjected to HP20 adsorption chromatography, followed by C18 flash chromatography and HPLC to yield compounds 1-10.

A known compound, aerugine (1) was isolated as a pale yellow solid.10, 11 The NMR and low resolution MS data also exactly matched a siderophore compound (1a) isolated from a bacterium associated with a marine sponge,12 leading us to initially misassign the structures of this compound series. High-resolution electrospray mass spectrometry (HRESIMS) revealed ions at m/z 210.0583 ([M+H]+), indicating a molecular formula of C10H11NO2S, allowing us to reassign the structure as 1. The difference between the reported structures of these two molecules is the presence of a thiazoline group in 1 or a hydroxamic acid group in 1a. However, in the structure elucidation of the siderophore compound, no evidence was provided for either the phenolic ether bond or the hydroxamic acid group. Comparison of the spectroscopic data of 1 and 1a suggests that they may be identical. The 4-bromobenzoyl derivative 1b was synthesized from 1, and a shift of C-7’ from 3.85 to 4.58 ppm indicated a primary alcohol group at C-7’. All of these data, as well as the HMBC correlations (Figure 1), were compatible with the structure for aerugine. Other known compounds, pulicatins F (2) and G (3) have been synthesized but are not previously known as natural products.13, 14 They are included in this series because of their structural similarities with the remainder of the compounds. Watasemycins A (4) and B (5), were identified by comparison of NMR and MS data with the literature.15
Figure 1.

Key HMBC, 1H-1H COSY and NOESY Correlations of Compounds 1 and 6-10.
Pulicatin A (6) was isolated as a pale yellow solid and assigned the molecular formula C11H13NO2S on the basis of HRESIMS analysis (m/z 224.0741 for [M+H]+) and NMR experiments (Table 1). Compound 6 had a higher mass than 1 by 14 Da, indicating that an additional CH2 was present. The 1H NMR and 13C NMR spectra of 6 were very similar to that of 1 except that they have signals corresponding to a methyl group at δH 1.35. Compound 6 contained a methine group (δH 4.04 m; δC 45.9 CH) instead of the methylene group in 1. A COSY experiment (Figure 1) led to the identification of the CH3CHCHCH2OH fragment. The HMBC (Figure 1) cross-peaks observed from both H-4’ and H-5’ to C-2’ confirmed the planar structure of 6. The NOESY correlations between H3-6’ and H2-7’ indicated a 4’S*,5’R* relative configuration of 6.
Table 1.
NMR Spectroscopic Data (500 MHz for 1H, 125 MHz for 13C, CDCl3) for Pulicatins A-E (6-10).
| Pulicatin A (6) |
Pulicatin B (7) |
Pulicatin C (8) |
Pulicatin D (9) |
Pulicatin E (10) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| position | δC, mult | δH (J in Hz) | δC, mult | δH (J in Hz) | δC, mult | δH (J in Hz) | δC, mult | δH (J in Hz) | δC, mult | δH (J in Hz) |
| 1 | 116.6, C | 116.3, C | 115.4, C | 116.3, C | 116.5, C | |||||
| 2 | 159.2, C | 159.2, C | 156.6, C | 157.1, C | 156.5, C | |||||
| 2-OH | 12.25, brs | 12.4, brs | 11.90, brs | 11.64, brs | 11.16, s | |||||
| 3 | 133.3, CH | 7.44, d (9.0) | 133.1, CH | 7.39, d (8.3) | 126.8, CH | 7.53, d (8.5) | 127.6, CH | 7.51, d (8.5) | 127.8, CH | 7.55, d (7.0) |
| 4 | 119.1, CH | 6.88, dd (9.0, 8.5) | 118.9, CH | 6.88, dd (8.3, 8.2) | 119.2, CH | 6.89, dd (8.5, 7.3) | 119.9, CH | 6.92, dd (8.5, 7.5) | 120.1, CH | 6.94, dd (7.7, 7.5) |
| 5 | 130.8, CH | 7.36, dd (8.5, 8.5) | 130.6, CH | 7.36, dd (8.3, 8.2) | 131.2, CH | 7.29, dd (8.2,7.3) | 132.5, CH | 7.35, dd (8.5,7.3) | 132.4, CH | 7.35, dd (7.4, 7.4) |
| 6 | 117.3, CH | 7.00, d (8.5) | 117.1, CH | 6.99, d (8.3) | 117.5, CH | 7.03, d (8.2) | 118.2, CH | 7.07, d (8.3) | 117.9, CH | 7.06, d (8.1) |
| 2’ | 173.0, C | 173.0, C | 166.3, C | 166.3, C | 165.5, C | |||||
| 4’ | 45.9, CH | 4.04, m | 45.2, CH | 3.96, dq (6.8, 6.8) | 128.6, C | 143.5, C | 121.6, C | |||
| 5’ | 79.0, CH | 4.56, ddd (6.8, 6.8, 6.7) | 84.2, CH | 4.45, ddd (6.8, 5.5, 4.8) | 149.5, C | 147.3, C | 141.3, C | |||
| 6’ | 16.7, CH3 | 1.35, d (7.1) | 21.7, CH3 | 1.49, d (6.8) | 10.9, CH3 | 2.52, s | 12.5, CH3 | 2.86, s | 13.1, CH3 | 2.88, s |
| 7’ | 61.7, CH2 | 4.04m, 4.19, dd (10.4, 6.1) | 63.2, CH2 | 3.85, dd (11.2, 5.5); 3.78, dd (11.2, 4.8) | 58.5, CH2 | 4.73, s | 185.4, C | 10.15, s | 164.9, C | |
Pulicatin B (7) was isolated as a pale yellow solid. Its HRESIMS analysis (m/z 224.0741 for [M+H]+) indicated a molecular formula of C11H13NO2S, which was identical to that of 6. Its MS and NMR data (Table 1) were almost identical to those of 6 except that the chemical shifts of C-5’, C-6’ and C-7’ were observed slightly downfield of those in 6 (Table 1), indicating it was isomeric with 6. The NOESY correlations (Figure 2) between H3-6’ and H-5’ indicated 7 was a diastereoisomer of 6 with a 4’S*,5’S* relative configuration.
Figure 2.
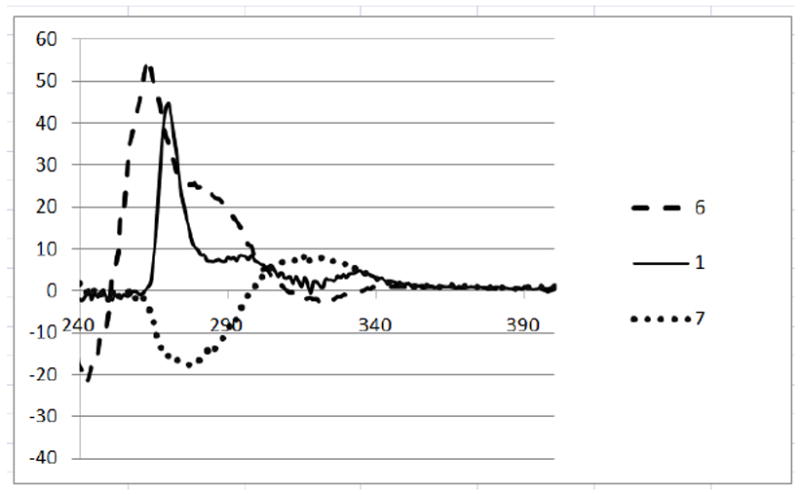
CD Spectra of Compounds 1, 6 and 7.
To determine the absolute configurations of 6 and 7, we employed the method of Yamaguchi and Yasuhara for determination of the absolute configurations of primary alcohols with the asymmetric center at the 2-position.16 In this approach, an acyclic alcohol is converted to the corresponding (R)-(+)-MTPA and (S)-(-)-MTPA esters. The magnitude of lanthanoid induced shift (LIS) by Eu(fod)3 for the methoxy group of the (R)-(+)-MTPA ester is larger than that of the (S)-(-)-MTPA ester when the asymmetric center is in R-configuration. Sugimoto applied this method to a primary alcohol in which a hydroxymethyl group is situated on the asymmetric ring carbon atom of the carbocycle.17 Len et al. assumed that complexation of europium salts with both oxygens of the ester and methoxy groups induced the existence of different conformers.18 Compounds 1, 6 and 7 were transformed to the corresponding (R)-(+)-MTPA and (S)-(-)-MTPA esters. The LIS values of the methoxy groups are given in Table 2. For 1 and 6, the LIS values for (R)-(+)-MTPA esters were larger than (S)-(-)-MTPA esters, indicating that the asymmetric centers at C-5’ of 1 and 6 were in the R-configurations. Since this is the known configuration of (+)-aerugine (1), this method is in good agreement with the literature.19 Compound 7 showed larger LIS values for the (S)-(-)-MTPA ester, indicating that the asymmetric center at C-5’ of 7 was in an S-configuration. These conclusions were consistent with the circular dichroism spectra of 1, 6 and 7: the CD spectra of 1 and 6 were very similar, but opposite to that of 7 (Figure 2).
Table 2.
LIS of Mosher Esters of 1, 6 and 7.
| MTPA esters | Δδ OCH3 |
Configuration of C5’ | |
|---|---|---|---|
| (S)- | (R)- | ||
| 1 | +0.01 | +0.05 | R |
| 6 | +0.05 | +0.34 | R |
| 7 | +0.16 | +0.13 | S |
Another pale yellow solid compound, pulicatin C (8), was assigned the molecular formula C11H10NO2S on the basis of HRESIMS analysis (m/z 222.0584 [M+H]+) and NMR experiments. In contrast to 6, 8 has NMR signals for a tetrasubstituted double bond (δC 128.6 C, 149.5 C) instead of the two sp3 hybridized carbons in 6. A singlet was assigned to a vinylic methyl (δH 2.52 s, δC 10.9 CH3). These data indicated that 8 was the 4’,5’-dehydro derivative of 6. This was also supported by the HMBC correlations (Figure 1) from H3-6’ to C-4’ and C-5’ and from H2-7’ to C-4’ and C-5’.
Pulicatin D (9) was isolated as a white solid. The molecular formula for 9, C11H8NO2S, was derived from NMR data and the HRESIMS ion at m/z 220.0427 ([M+H]+). The NMR data were very similar to that of 8, except that 9 had an aldehyde group (δC 185.4 CH) that was assigned to C-7’. The chemical shift of the aldehyde carbon indicated it was conjugated with the C-4’-C-5’ double bond. This was also supported by the HMBC correlations (Figure 1) from H3-6’ to C-4’ and C-5’ and from H2-7’ to C-4’ and C-5’.
Pulicatin E (10) was isolated as a pale yellow solid. It was assigned the molecular formula C11H10N2O2S on the basis of HRESIMS analysis (m/z 235.0546 [M+H]+) and NMR experiments. Analysis of the NMR spectra of 10 showed this compound to have a carbonyl instead of the aldehyde group present in 9. Based on the molecular formula, the carbonyl at was assigned to a carboxamide group located at C-5’.
In the DRG assay of purified compounds, two metabolites (1 and 3) observably increased Ca2+ influx at 20 μg / mL and were responsible for the activity of the extract of CP32, while compound 6 showed a decreased Ca2+ influx (see Supporting Information). This was of particular interest as 1 and 6 differ solely by a methyl group, yet they exhibit opposite effects on whole DRG cells. Although the activity seems modest, in our experience an observable effect in these neurons requires a relatively high dose that almost completely blocks the target. Therefore, to determine the potential molecular targets and potencies underlying these observed phenotypes, we further screened 1-5, 6, and 9 at the National Institute of Mental Health’s Psychoactive Drug Screening Program (PDSP).20 The remaining compounds were isolated in insufficient amounts for testing. A primary assay was performed binding 46 targets at a final concentration of 10 μM of each compound. Initial hits were obtained in radioligand displacement assays. For targets where significant binding avtivity was detected, secondary binding assays were performed, and Ki values were calculated using radioligand displacement with test compound concentrations from 1 to 10,000 nM. Ki values from secondary assays were >5,000 nM, with the exception of results reported below.
All of the seven tested compounds showed binding activity to the 5-HT2B receptor, with Ki values from 505 nM to 4,695 nM (Table 3). Compound 6 exhibited the strongest effect, while bisthiazolines 4 and 5 were weak in comparison to the single-thiazol(in)e compounds. Compounds were relatively selective for 5-HT2B versus other serotonin receptors, for which no significant binding was detected at 10 μM, indicating that 6 has at least 20-fold selectivity for 5-HT2B over related receptors. Only 2 and 3 showed selectivity for the histamine H1 receptor, at 517±28 nM and 678±46 nM, respectively, indicating the potential importance of the amide functionality for H1 receptor affinity. Only 3 exhibited significant binding to the kappa opioid receptor (3,708±145 nM).
Table 3.
Ligand Displacement Activity at the 5-HT2B Receptor.
| Compounds | Ki (nM) vs 5-HT2B |
|---|---|
| 1 | 1360±110 |
| 2 | 1031±76 |
| 3 | 1260±99 |
| 4 | 3922±465 |
| 5 | 4695±262 |
| 6 | 505±29 |
| 9 | 1541±134 |
The G protein-coupled receptor 5-HT2B has been extensively explored in drug discovery because of its diverse physiological effects, but undesired cardiac activity has limited development of drugs that target this receptor.21 Recently 5-HT2B has been shown to play a direct role in modulating neuropathic pain in the dorsal horn of rats, where selective 5-HT2B antagonism enhanced evoked potentials under certain conditions.22 However, the PDSP results and their connection to the DRG assay need to be interpreted with caution, especially because binding assays do not necessarily reflect physiological function.
Pulicatins are members of a large family of benzyl thiazole and thiazoline natural products, including aerugine,11, 19 aeruginoic acid,23 ulbactins, 19, 24 desferrithiocin,25 pyochelin,26 yersiniabactin,27 vibriobactin,28 micacocidins,29, 30 thiazostatins,31 watasemycins,13 and other unnamed small molecules.32 Gene clusters for new compounds in this group are commonly found in bacteria.33, 34 However, the 4’-methyl group is a feature that has only been found previously in 9 and 10 among natural products in this series and rarely elsewhere.
Neurological activity has not been previously reported for compounds in this series. Compounds from the aerugine family exhibited antimicrobial35 and anti-inflammatory activity36 at mid-micromolar levels. Aeruginoic acid, which is somewhat similar to compounds 2 and 8-10, exhibited antihypotensive activity,37 also in the micromolar range.
A remaining question is whether the pulicatins are important to symbiosis or to the survival of C. pulicarius. Strain CP32 has a 16S gene that is very similar to genes identified in the uncultivated pool in this organism and is in fact identical to the published CP5 sequence (500 bp) and to the sequence from uncultivated bacteria associated with the cone snails. In addition, actinobacteria were identified in the foot margin and in the hepatopancreas by in situ hybridization.7 These results are at best suggestive of a role for the pulicatins and require further investigation. Finally, 6 was also identified as an active component of Streptomyces sp. strain CT8, cultivated from the hepatopancreas of the cone snail C. tribblei, possibly indicating a habitat-related function for the compounds.
Experimental Section
General Experimental Procedures
Optical rotations were measured on a Jasco DIP-370 polarimeter. UV spectra were obtained using a Perkin-Elmer Lambda2 UV/vis spectrometer. Circular dichroism spectra were obtained on a Jasco J720A spectropolarograph. NMR data were collected using either a Varian INOVA 500 (1H 500 MHz, 13C 125MHz) NMR spectrometer with a 3 mm Nalorac MDBG probe or a Varian INOVA 600 (1H 600 MHz, 13C 150 MHz) NMR spectrometer equipped with a 5 mm 1H[13C,15N] triple resonance cold probe with a z-axis gradient, utilizing residual solvent signals for referencing. High-resolution mass spectra (HRMS) were obtained using a Bruker (Billerica, MA) APEXII FTICR mass spectrometer equipped with an actively shielded 9.4 T superconducting magnet (Magnex Scientific Ltd., UK), an external Bruker APOLLO ESI source, and a Synrad 50W CO2 CW laser.
Bacterial Material
Streptomyces sp. CP32 was cultivated from C. pulicarius obtained by professional collectors near Mactan Island, Cebu, Philippines as previously described.7 The strain was cultured from dissected foot and body tissue, purified, and later the strain was recovered from a glycerol stock and used for further chemical analysis. The 16S gene was cloned using primers 27f and 1492r and submitted to GenBank, accession number HQ337089.
Fermentation and Extraction
Streptomyces sp. CP32 was cultured at 30 °C with shaking at 200 rpm in 8 2.8 L Fernbach flasks each containing 1 L of the medium ISP2 (0.2% yeast extract, 1% malt extract, 0.2% glucose, 2% NaCl). After 8 days, the broth was centrifuged and the supernatant was extracted with HP-20 resin for 4 h. The resin was filtered through cheesecloth and washed with H2O to remove salts. The filtered resin was eluted with MeOH to yield an extract, which was further purified by bioassay-guided (DRG) fractionation.
Purification
The extract (400 mg) was separated into 7 fractions (Fr1-Fr7) on a C18 column using step-gradient elution of MeOH in H2O (20%, 40%, 50%, 60%, 70%, 80%, 100%). Fr 3 eluting in 50% MeOH was further purified by C18 HPLC using 45% CH3CN in H2O to obtain compound 3 (1.5 mg). Fr 4 eluting in 60% MeOH was further purified by C18 HPLC using 65% MeOH in H2O to obtain compounds 1 (3.0 mg), 6 (1.0 mg), and 7 (0.4 mg). Fr 5 eluting in 70% MeOH was further purified by C18 HPLC using 68% CH3CN in H2O with 0.1% TFA to obtain compound 9 (5.0 mg) and subfraction Fr 5-1. Fr 5-1 was further purified by C18 HPLC using 48% CH3CN in H2O with 0.1% TFA to obtain compounds 8 (1.0 mg), 10 (0.3 mg), 2 (1.5 mg), 4 (10.0 mg) and 5 (2.0 mg).
Preparation of (S)- and (R)-MTPA Esters
Compound 1 (100 μg), 6 (500 μg) and 7 (100 μg) were separately transferred into clean reaction bottles and dried completely under vacuum. Pyridine (0.5 mL) and α-methoxy-α-(trifluoromethyl)phenylacetyl chloride (1 equiv) were added into the reaction bottle quickly under a N2 gas stream and then stirred for 12 h at room temperature. The organic layer was then washed with H2O and concentrated under reduced pressure to obtain the ester. Final purification was achieved by HPLC (90% MeOH, 4.0 mL/min).
Enantioselective Analysis with Eu(fod)3
Each MTPA ester of 1, 6 and 7 was added to an NMR tube in CDCl3, and an equimolar mixture of Eu(fod)3 was added to each tube. The difference in chemical shift with and without the chiral reagent was recorded: Δδ OCH3 (ppm) = Δδ OCH3 (with Eu(fod)3) − Δδ OCH3 (without Eu(fod)3).
Preparation of p-Bromobenzoate (1a)
To a stirred suspension of 1 (1.0 mg) in dry CH2Cl2 (2 mL) was added Et3N (1 mL) and p-BrC6H4COCl (3 mg) at room temperature. Four hours later the reaction was quenched by adding H2O (2 mL). The mixture was extracted with EtOAc (3 × 5 mL), and the EtOAc solution was dried on anhydrous Na2SO4 and evaporated at reduced pressure. The residue was subjected to C18 HPLC (95% MeOH/H2O) to give 1b (1.1 mg). 1H NMR (CDCl3, 400MHz) δ: 7.36~7.99 (8H, m, ArH), 4.92 (1H, m, H-4’), 4.35, 4.28 (each 1H, m, H-7’), 3.46, 3.24 (each 1H, m, H-4’). ESI MS: m/z 392.0, 394.0 [M+H]+.
DRG Assay
For details of the isolation and culture of DRG neurons see Light et al.8 Briefly, dorsal root ganglia (DRG) cells from cervical and lumbar regions were obtained from mice and used in an assay with bacterial culture extracts and pure compounds. DRG cells were suspended in medium with additives and loaded with Fura-2 AM (Molecular Probes), a fluorescent dye used to measure intracellular calcium levels. Experiments were performed at room temperature (20 to 25°C) in a 24-well plate format using fluorescence microscopy. Individual cells were treated as single samples, so that the individual responses of diverse neuron subtypes from the DRG could be examined. After baseline measurements, the cells were treated with 25 mM KCl solution and then washed. After return to baseline, bacterial extracts, fractions, or pure compounds were applied. This solution was then later replaced with 25 mM KCl solution. The use of KCl permitted observation of direct, modulatory, as well as inhibitory and excitatory effects of the extracts.
Receptor Affinity Screen
Assays for the following receptors were performed by the PDSP/NIMH: (1) muscarinic receptors: M1, M2, M3, M4, M5; (2) Serotonin receptors: 5ht1a, 5ht1b, 5ht1d, 5ht1e, 5ht2a, 5ht2b, 5ht2c, 5ht3, 5ht4, 5ht5a, 5ht6, 5ht7; (3) GABA receptors: BZP (Rat Brain Site), GABA A, GABA B; (4) histaminergic receptors: H1, H2, H3, H4; (5) Dopamine receptors: D1, D2, D3, D4, D5; (6) Transporters: NET, SERT, DAT; (7) Opiate receptors: DOR, KOR, MOR; (8) Adrenergic receptors: Alpha1A, Alpha1B, Alpha1D, Alpha2A, Alpha2B, Alpha2C, Beta1, Beta2, Beta3; (9) others: Sigma 1, Sigma 2, Ca+Channel. Detailed on-line protocols are available for all assays at the PDSP/NIMH web site (http://pdsp.med.unc.edu/). The serotonin and histamine receptor protocols have been described.38-40
Pulicatin A (6): pale yellow solid (MeOH); [α] 25 D -53 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 211 (4.12), 250 (3.71), 312 (3.50) nm; CD (CHCl3)λmax (Δε) 263 (+52), 292 (+17), 327 (-1.3) nm; IR (film) νmax: 2925, 1700, 1670, 1565, 1535, 1510, 1310, 1265, 760 cm-1; 1H and 13C NMR (see Table 1); HRESIMS m/z 224.0741 [M+H]+ (calcd for C11H13NO2S, 224.0740).
Pulicatin B (7): pale yellow solid (MeOH); [α] 25 D -25 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 211 (4.11), 250 (3.69), 312 (3.47) nm; CD (CHCl3)λmax (Δε) 276 (-17), 324 (7.6) nm; IR (film) νmax: 2909, 1685, 1520, 1457, 1415, 1340, 1310, 1265, 770 cm-1; 1H and 13C NMR (see Table 1); HRESIMS m/z 224.0741 [M+H]+ (calcd for C11H13NO2S, 224.0740).
Pulicatin C (8): pale yellow solid (MeOH); UV (MeOH) λmax 204 (4.08), 296 (3.82), 326 (3.79) nm; IR (film) νmax: 2925, 1700, 1625, 1505, 1415, 1280, 1000, 775 cm-1; 1H and 13C NMR (see Table 1); HRESIMS m/z 222.0584 [M+H]+ (calcd for C11H11NO2S, 222.0583).
Pulicatin D (9): white solid (CHCl3); UV (MeOH) λmax 219 (4.10), 289 (3.84), 321 (3.77) nm; IR (film) νmax: 2835, 1700, 1670, 1535, 1415, 1430, 1295, 1250, 745 cm-1; 1H and 13C NMR (see Table 1); HRESIMS m/z 220.0427 [M+H]+ (calcd for C11H9NO2S, 220.0427).
Pulicatin E (10): pale yellow solid (MeOH); UV (MeOH) λmax 212 (4.11), 279 (3.87), 321 (3.79) nm; IR (film) νmax: 2924, 1670, 1595, 1490, 1475, 1415, 1360, 1150, 750 cm-1; 1H and 13C NMR (see Table 1); HRESIMS m/z 235.0546 [M+H]+ (calcd for C11H10N2O2S, 235.0536).
Supplementary Material
Acknowledgments
This work was funded by ICBG grant U01TW008163 from Fogarty (NIH). We thank the government of the Philippines and the community of Mactan Island for permission to conduct this study. Binding assay data was generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is directed by B. L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer J. Driscol at NIMH, Bethesda MD, USA.
Footnotes
Supporting Information Available: NMR data for pulicatins A-E (6-10), DRG assay results, application of LIS, 1H NMR of MTPA esters for compounds 1, 6 and 7, HRESIMS data of compounds 1 and 6-10. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Schmidt EW. Nat Chem Biol. 2008;4:466–473. doi: 10.1038/nchembio.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piel J. Nat Prod Rep. 2009;26:338–362. doi: 10.1039/b703499g. [DOI] [PubMed] [Google Scholar]
- 3.Piel J. Nat Prod Rep. 2004;21:519–538. doi: 10.1039/b310175b. [DOI] [PubMed] [Google Scholar]
- 4.White JF, Torres MS. CRC Press: New York. 2009 [Google Scholar]
- 5.Gil-Turnes MS, Hay ME, Fenical W. Science. 1989;246:116–118. doi: 10.1126/science.2781297. [DOI] [PubMed] [Google Scholar]
- 6.Terlau H, Olivera BM. Physiol Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- 7.Peraud O, Biggs JS, Hughen RW, Light AR, Concepcion GP, Olivera BM, Schmidt EW. Appl Environ Microbiol. 2009;75:6820–6826. doi: 10.1128/AEM.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, Pinnock RD, Scott RH. Neuropharmacology. 2002;42:353–366. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- 10.Zamri A, Abdallah MA. Tetrahedron. 1999;56:249–256. [Google Scholar]
- 11.Zunnundzhanov A, Bessonova IA, Abdullaev ND, Ogai DK. Khim Prir Soedin. 1987:553–558. [Google Scholar]
- 12.You M-X, Zhang H-P, Hu C-Q. Chinese J Chem. 2008;26:1332–1334. [Google Scholar]
- 13.Mathur KB, Iyer RN, Dhar ML. J Sci Ind Res, Sect B. 1962;21B:34–37. [Google Scholar]
- 14.Istanbullu I, Safak C, Sahin MF. Hacettepe Univ Eczacilik Fak Derg. 1986;6:21–28. [Google Scholar]
- 15.Sasaki O, Igarashi Y, Saito N, Furumai T. J Antibiot (Tokyo) 2002;55:249–255. doi: 10.7164/antibiotics.55.249. [DOI] [PubMed] [Google Scholar]
- 16.Yasuhara F, Yamaguchi S. Tetrahedron Lett. 1977;47:4085–4088. [Google Scholar]
- 17.Sugimoto Y, Tsuyuki T, Moriyama Y, Takahashi T. Bull Chem Soc Jpn. 1980;53:3723–3724. [Google Scholar]
- 18.Len C, Pilard S, Lipka E, Vaccher C, Dubois M-P, Shrinska Y, Tran V, Rabiller C. Tetrahedron. 2005;61:10583–10595. [Google Scholar]
- 19.Kikuchi K, Chen C, Adachi K, Nishijima M, Araki M, Sano H. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu. 1996;38th:427–432. [Google Scholar]
- 20.http://pdsp.med.unc.edu/
- 21.Brea J, Castro-Palomino J, Yeste S, Cubero E, Parraga A, Dominguez E, Loza MI. Curr Top Med Chem. 2010;5:493–503. doi: 10.2174/156802610791111524. [DOI] [PubMed] [Google Scholar]
- 22.Aira Z, Buesa I, Salgueiro M, Bilbao J, Aguilera L, Zimmermann M, Azkue JJ. Neuroscience. 2010;168:831–841. doi: 10.1016/j.neuroscience.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y, Seki N, Kitahara T, Takahashi M, Matsui M. Agr Biol Chem. 1970;34:780–783. [Google Scholar]
- 24.Kikuchi K, Chen Y, Adachi K, Nishijima M, Nishida A, Takatera T, Sano H. Jpn Kokai Tokkyo Koho. 1998:6. [Google Scholar]
- 25.Naegeli HU, Zaehner H. Helv Chim Acta. 1980;63:1400–1406. [Google Scholar]
- 26.Cox CD, Rinehart KL, Jr, Moore ML, Cook JC., Jr Proc Natl Acad Sci U S A. 1981;78:4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drechsel H, Stephan H, Lotz R, Haag H, Zaehner H, Hantke K, Jung G. Liebigs Ann. 1995;10:1727–1733. [Google Scholar]
- 28.Griffiths GL, Sigel SP, Payne SM, Neilands JB. J Biol Chem. 1984;259:383–385. [PubMed] [Google Scholar]
- 29.Ino A, Kobayashi S, Hidaka S, Kawamura Y, Ozaki M, Hayase Y, Takeda R, Murabayashi A. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu. 1996;38th:121–126. [Google Scholar]
- 30.Kobayashi S, Hidaka S, Kawamura Y, Ozaki M, Kayase Y. J Antibiot. 1998;51:323–327. doi: 10.7164/antibiotics.51.323. [DOI] [PubMed] [Google Scholar]
- 31.Shindo K, Takenaka A, Noguchi T, Hayakawa Y, Seto H. J Antibiot. 1989;42:1526–1529. doi: 10.7164/antibiotics.42.1526. [DOI] [PubMed] [Google Scholar]
- 32.Bukovits GJ, Mohr N, Budzikiewicz H, Korth H, Pulverer GZ. Naturforsch, B: Anorg Chem, Org Chem. 1982;37B:877–880. [Google Scholar]
- 33.Waterfield NR, Sanchez-Contreras M, Eleftherianos I, Dowling A, Wilkinson P, Parkhill J, Thomson N, Reynolds SE, Bode HB, Dorus S, ffrench-Constant RH. Proc Natl Acad Sci U S A. 2008;105:15967–15972. doi: 10.1073/pnas.0711114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Udwary DW, Zeigler L, Asolkar RN, Singan V, Lapidus A, Fenical W, Jensen PR, Moore BS. Proc Natl Acad Sci U S A. 2007;104:10376–10381. doi: 10.1073/pnas.0700962104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmi R, Carmeli S. J Nat Prod. 1994;57:1200–1205. doi: 10.1021/np50111a002. [DOI] [PubMed] [Google Scholar]
- 36.Matsui M, Yamada Y, Takahashi M, Seki N. Ger Offen. 1971 DE 2045818 A 19710408. [Google Scholar]
- 37.Imai T, Takahashi M, Seki N, Irie Y. Ger Offen. 1971 DE 2045819 A 19710408. [Google Scholar]
- 38.Roth BL, Nakaki T, Chuang DM, Costa E. J Pharmacol Exp Ther. 1986;238:480–485. [PubMed] [Google Scholar]
- 39.Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Shen Y, Meltzer HY, Sibley DR. J Pharmacol Exp Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- 40.Moguilevsky N, Varsalona F, Noyer M, Gillard M, Guillaume JP, Lida Garcia L, Szpirer C, Szpirer J, Bollen A. Eur J Biochem. 1994;224:489–495. doi: 10.1111/j.1432-1033.1994.00489.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


