Abstract
Recent evidence suggests that the sodium calcium exchanger (NCX) may contribute to the etiology of pentylenetetrazol-induced seizures. Here we further investigated the role of NCX in the etiology of seizures by quantifying the effects of KB-R7943 and SN-6, potent inhibitors of the reverse mode of NCX subtypes 3 (NCX3) and 1 (NCX1), respectively, on the occurrence of acute seizures and status epilepticus induced by intraperitoneal administration of pilocarpine, a muscarinic acetylcholine receptor agonist. Pretreatment with KB-R7943 significantly reduced the incidence of pilocarpine-induced seizures and status epilepticus in 22–56% of treated animals. In the remaining animals that exhibited seizures, KB-R7943 pretreatment delayed the onset of seizures and status epilepticus, and reduced seizure severity. Delayed onset of seizures and reduced seizure severity also were seen following pretreatment with SN-6. These findings suggest that altered NCX activity may contribute to the pathophysiology of pilocarpine-induced seizures and status epilepticus.
Keywords: KB-7943, SN-6, status epilepticus
1. Introduction
Epilepsy is a chronic neurological disorder characterized by spontaneous recurrent seizures. Approximately 1–2% of the global population suffers from various epileptic syndromes, and about 30% of patients with epilepsy live with uncontrolled seizures (French 2007). Evidence indicates that temporal lobe epilepsy is the most common type of refractory epilepsy in humans (Semah et al., 1998), and therefore, novel antiepileptic treatments based on new underlying mechanisms for temporal lobe seizures are needed. Experimental evidence indicates that altered levels of intracellular Ca2+ in hippocampal CA1 neurons play an important role in the underlying mechanisms of neuronal hyperexcitability that leads to pilocarpine-induced seizures, a validated model of temporal lobe epilepsy (DeLorenzo et al. 2005; Turski et al., 1983). The levels of intracellular Ca2+ are highly regulated by Ca2+ binding proteins and Ca2+ extrusion through transporters/exchangers. One transporter/exchanger of interest is the sodium/calcium exchanger (NCX), a bidirectional membrane ion transporter that couples the influx/efflux of Ca2+ to the efflux/influx of Na+ to regulate levels of intracellular Ca2+ in neurons (Blaustein and Lederer, 1999; Annunziato et al., 2004). Under physiological conditions, NCX transports one Ca2+ out of the cell and three Na+ into the cell. The Ca2+ exit is known as the “forward” mode of NCX (Blaustein and Lederer, 1999; Annunziato et al., 2004). However, under certain conditions such as during membrane depolarization, the exchanger can reverse function and transport Na+ out of the cell and Ca2+ into the cell. The Ca2+ entry represents the “reverse” mode of NCX (Baker and McNaughton, 1976; Annunziato et al., 2004). Three different isoforms of NCX including NCX1, NCX2, and NCX3 have been characterized, cloned, and found in the brain, with multiple splice variants of NCX1 and NCX3 (Kofuji et al., 1994; Papa et al., 2003; Quednau et al., 1997). Despite the fact that reverse mode of NCX allows Ca2+ entry into the cell and possibly thereby altering Ca2+ homeostatic mechanisms, the role of NCX in the pathophysiology of limbic epilepsy remains poorly understood (Keele et al., 2000; Ketelaars et al. 2004). Nevertheless, a recent study reports that genetic deletion of NCX1 confers resistance to pentylenetrazole-induced tonic flexion, indicative of the important role this NCX isotope has in the etiology of these seizures (Saito et al., 2009). Because dysregulation of Ca2+ homeostatic mechanisms is an important feature of limbic epileptogenesis and the reverse mode of NCX contributes to Ca2+ influx, we sought to determine the extent to which inhibition of the reverse mode of NCX may affect acute pilocarpine-induced seizures and status epilepticus (SE) in rats.
2. Results
The incidence of pilocarpine-induced motor limbic seizures and SE were first evaluated using two doses of intraperitoneally (i.p.) administered pilocarpine (280 and 380 mg/kg). Although no difference was found in seizure severity between the two groups (280 mg/kg: 4.7±0.3, n=11; 380 mg/kg: 5±0, n=9; H=0.8, P=0.4), the onset of motor limbic seizures and SE was significantly delayed in the 280 mg/kg group (motor limbic seizures: 19±3 min, n=10, F=7.4, P=0.01; SE: 30±1 min, n=10, F=10.4, P=0.005) compared to the 380 mg/kg group (motor limbic seizures: 10±1 min, n=9; SE: 23±2 min, n=9). Furthermore, higher mortality rates were found in the 380 mg/kg group (9/9) compared to those the 280 mg/kg group (1/11; Chi-square: 163, P=0.0001). Therefore, we used the 280 mg/kg dose of pilocarpine for pharmacological studies.
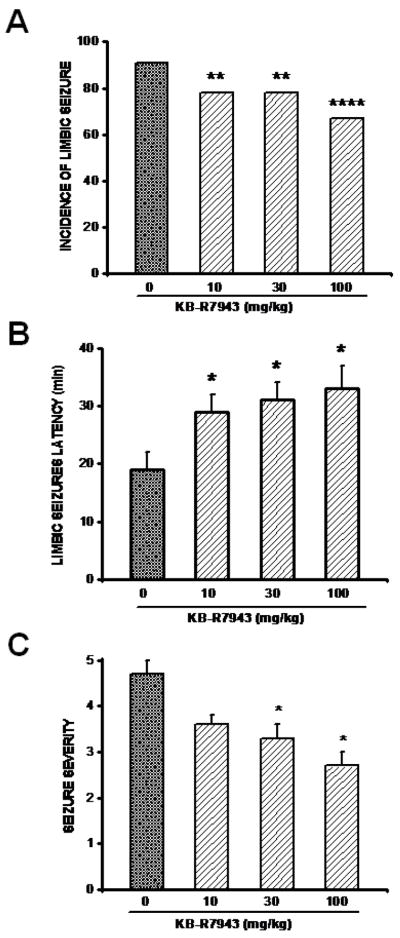
Orally administered (p.o.) pretreatment with KB-R7943 at the doses of 10, 30, and 100 mg/kg significantly suppressed the occurrence of motor limbic seizures in 22% (2/9, Chi-square=5.5; P=0.02), 22% (2/9, Chi-square=5.5; P=0.02), and 33% (3/9, Chi-square=17; P=0.0001) of tested animals, respectively, compared to 91% (10/11) in the control group (Fig. 1A). In the remaining animals that exhibited seizures, KB-R7943 pretreatment significantly (F=4, P=0.02) delayed the onset of motor limbic seizures. This effect was observed at all doses tested (10 mg/kg: 29±3 min, n=7; 30 mg/kg: 31±3 min, n=7; 100 mg/kg: 33±4 min, n=6; P<0.05, Fig. 1B) as compared to the control group (19±2 min, n=10; Fig. 1B). Pretreatment with KB-R7943 also significantly reduced the severity of pilocarpine-induced seizures (H=16, P=0.001); this effect was observed with both 30 mg/kg (3.3±0.3, n=7, P<0.05; Fig. 1C) and 100 mg/kg (2.8±0.3, n=5, P<0.05; Fig. 1C) compared to the control group (4.7±0.3, n=11). Similarly, pretreatment with SN-6 (10 mg/kg; p.o.) also reduced the severity of pilocarpine-induced seizures (control group: 4.7±0.3, n=11; SN-6: 2.8±0.6, n=5; H=7, P=0.01) and delayed the onset of motor limbic seizures (control group: 19±2 min, n=11; SN-6: 34±3 min, n=5; F=10, P=0.01). However, SN-6 (10 mg/kg) pretreatment did not affect the incidence of motor limbic seizures (5/6 compared to the control group 11/12).
Figure 1.
KB-R7943 pretreatment alters the expression of pilocarpine-induced seizures. A. KB-R7943 pretreatment (10, 30, or 100 mg/kg; p.o.) reduced the incidence of motor limbic seizures. B. KB-R7943 pretreatment (10, 30, or 100 mg/kg; p.o.) delayed the onset of motor limbic seizures. C. KB-R7943 pretreatment (10 or 30 mg/kg; p.o.) reduced the severity of pilocarpine-induced seizures. Data represent mean ± S.E.M. *P<0.05, **P<0.01, ****P<0.0001.
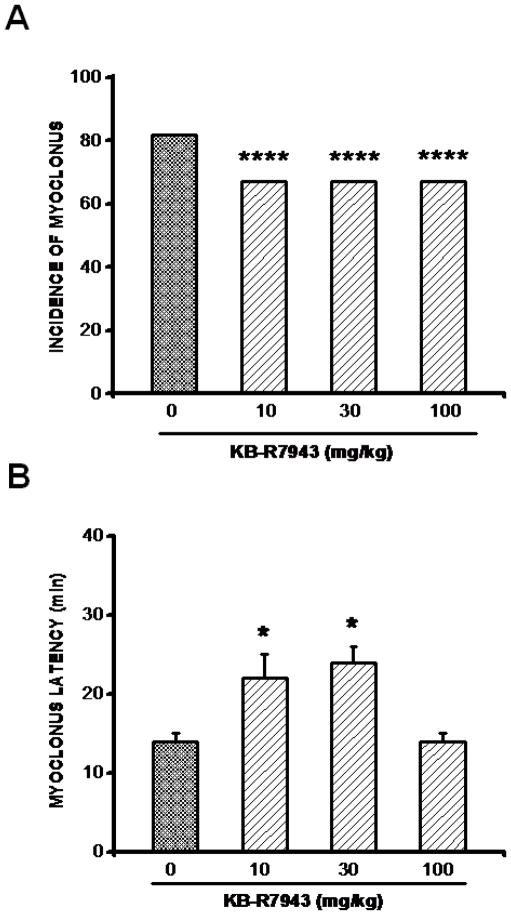
In addition to limbic seizures, pilocarpine can also trigger myoclonic seizures reminiscent of brainstem bouncing seizures. Such seizures were seen in 9 of 11 (82%) of tested animals in the control group. Pretreatment with KB-R7943 at all doses tested suppressed the occurrence of myoclonic seizures in 3 of 9 (33%) tested animals (Chi-square=14, P=0.0001; Fig. 2A). KB-R7943 pretreatment also significantly delayed the onset of myoclonic seizures (F=7, P=0.002). This effect was observed at 10 mg/kg (22±3 min, n=6; P<0.05; Fig. 2B) and 30 mg/kg (24±2 min, n=6; P<0.05; Fig. 2B), but not at 100 mg/kg (14±1, n=6; P>0.05; Fig. 2B), compared to the control group (14±1 min, n=9). Pretreatment with SN-6 (10 mg/kg) did not alter the incidence of myoclonic seizures (control group: 9/11; SN-6: 5/6) but significantly delayed the onset of these seizures (control group: 14±1 min, n=9; SN-6: 20±3 min, n=5; F=6; P=0.04).
Figure 2.
Effects of various doses of KB-R7943 on the expression of pilocarpine-induced myoclonic seizures. A. KB-R7943 pretreatment (10, 30, or 100 mg/kg; p.o.) reduced the incidence of myoclonic seizures. B. KB-R7943 pretreatment (10 or 30 mg/kg; p.o.) delayed the onset of pilocarpine-induced myoclonic seizures. Data represent mean ± S.E.M. *P<0.05, ****P<0.0001.
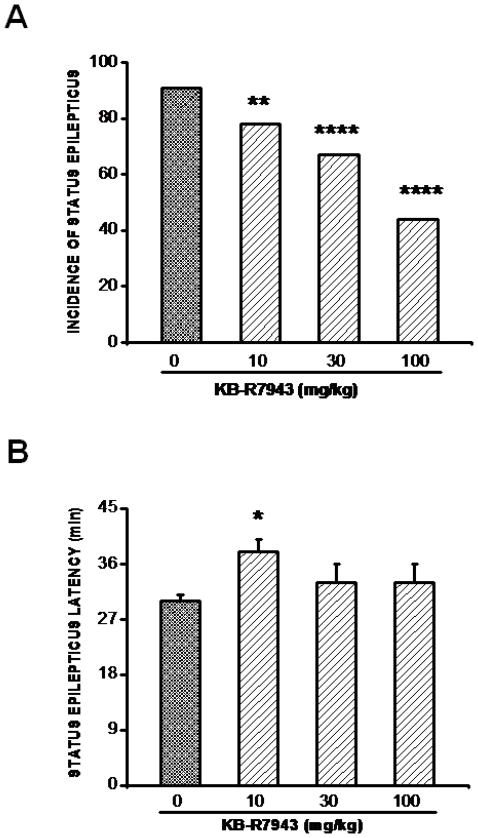
Motor limbic seizures progressively developed into SE in 30±3 min after pilocarpine administration in 91% (10/11) of tested animals in the control group. Pretreatment with KB-R7943 at the doses of 10, 30, and 100 mg/kg significantly suppressed the occurrence of pilocarpine-induced SE in 22% (2/9, Chi-square:5, P=0.02; Fig. 3A), 33% (3/9, Chi-square:17, P=0.0001; Fig. 3A), and 56% (5/9; Chi-square:48, P=0.0001; Fig. 3A) of tested rats, respectively. In the remaining rats in which continuous seizures were not suppressed, KB-R7943 pretreatment significantly (F=3; P=0.04) delayed the onset of SE; an effect that was only observed at 10 mg/kg (38±2 min, n=7; P<0.05) compared to the control group (30±1 min, n=10; Fig. 3B). No significant increase in seizure latency was observed at the doses of 30 mg/kg (33±3 min, n=6) and 100 mg/kg (33±3 min, n=4) compared to the control group (30±1 min, n=10; Fig. 3B). SN-6 pretreatment also significantly (F=83, P=0.0001) delayed the onset of SE (control group: 30±1 min, n=10; SN-6: 48±2 min, n=5) but did not alter its incidence (control group: 10/11; SN-6: 5/6).
Figure 3.
KB-R7943 pretreatment alters the expression of pilocarpine-induced status epilepticus (SE). A. KB-R7943 pretreatment (10, 30, or 100 mg/kg; p.o.) reduced the incidence of SE. B. KB-R7943 pretreatment (10 mg/kg; p.o.) delayed the onset of pilocarpine-induced SE. Data represent mean ± S.E.M. *P<0.05, **P<0.01, ****P<0.0001.
3. Discussion
In the present study, we used a relatively low dose of pilocarpine to induce seizures because higher doses (300–380 mg/kg) can cause a massive seizure-induced brain damage syndrome (Fabene et al., 2003, 2007; Fujikawa, 1996; Marchi et al., 2007). The main finding of this study is that blockade of the reverse mode of NCX significantly reduced the incidence of acute pilocarpine-induced seizures and SE. In the remaining animals that exhibited seizures, inhibition of the reverse mode of NCX reduced seizure severity and delayed the onset of motor limbic seizures, myoclonus, and SE. These findings suggest that inhibition of the reverse mode of NCX may have anticonvulsant activity in an acute model of pilocarpine-induced seizures and SE.
Evidence shows that levels of KB-R7943 plasma concentration at 5, 15 and 100 minutes following its intravenous administration were 31, 15, and 0.6 nmol/L, respectively, (Miyata et al. 2002). These findings suggested that KB-R7943 is rapidly cleared from the plasma and its brain penetration may be excellent. Interestingly, brain concentration of 2-[4-[2,5-difluorophenyl)methoxyl]-phenoxyl]-5-ethoxyaniline (SEA400), another NCX inhibitor was 3 and 7 μM after its intravenous injection at the doses of 1 and 3 mg/kg, respectively (Matsuda et al., 2001). Such brain concentration of KB-R7943 and SN-6 also may be found following their administration.
Although KB-R7943 and SN-6 are potent inhibitors of the three NCX isotopes, evidence indicates that these compounds preferentially block NCX3 and NCX1, respectively, indicative of the important role of these NCX isotopes as molecular targets for seizure suppression in the acute pilocarpine model (Iwamoto and Shigekawa, 1998; Iwamoto 2004). The role of NCX in the pathophysiology of epilepsy remains poorly understood. Nevertheless, a recent report indicates genetic deletion of NCX1 subtype suppresses the expression of pentylenetetrazol-induced tonic flexion (Saito et al., 2009). Consistent with these studies, we found that inhibition of NCX3 and NCX1 significantly reduced the incidence of acute pilocarpine-induced seizures and SE. The underlying mechanisms of how blockade of the reverse mode of NCX by KB-R7943 and SN-6 suppresses pilocarpine-induced seizures and SE are not yet fully understood. Although KB-R7943 and SN-6 are potent inhibitors of the reverse mode of NCX, multiple lines of evidence indicate they also block (to some degree) various voltage- and ligand-gated channels, including L-type Ca2+ channels, N-methyl-D-aspartate (NMDA) receptors, canonical transient receptor potential channels (TRPCs), and nicotinic acetylcholine receptors (Birinyi et al., 2005; Kraft 2007; Ouardouz et al., 2005; Pintado et al., 2000; Sobolevsky and Khodorov, 1999). Evidence shows that nimodipine, a potent blocker of L-type Ca2+ channels that can also block T-channels, suppresses pilocarpine-induced seizures and SE (Akaike et al., 1989; Becker et al., 2008; Takahashi and Akaike, 1991; Su et al., 2002). Multiple lines of evidence indicate that MK-801, a noncompetitive NMDA receptor blocker as well as clinically used anticonvulsant phenytoin and carbamazepine thought to block voltage-gated Na+ channels channels, failed to prevent pilocarpine-induced seizures (Leite and Cavalheiro, 1995; Ormandy et al., 1989; Turski et al. 1989). Evidence also shows that KB-R7934 potently blocks the TRPCs that also play an important role in Ca2+ homeostasis in the brain (Kraft, 2007). However, the role of TRPCs in the pathophysiology of epilepsy remains unknown. Nicotine can also induce seizures in rodents; however, the role nicotinic receptors play in pilocarpine epileptogenesis remains unknown (Newman et al., 2001). Thus, KB-R7943 or SN-6 pretreatment likely suppresses the occurrence of pilocarpine-induced seizures and SE via inhibition of the reverse mode of NCX.
In conclusion, blockade of the reverse mode of NCX has anticonvulsant potential against pilocarpine-induced acute seizures. Understanding how inhibition of the reverse mode of NCX affects neuronal hyperexcitability that leads to seizures can provide a framework for the development of clinically useful NCX blockers.
4. Experimental Procedures
Sprague-Dawley rats (male, 150–200g, Taconic, Germantown NY) were used. All experiments were performed with the approval of the Georgetown Animal Care and Use Committee. Effort was made to minimize the number of animals used as well as animal suffering. Pilocarpine hydrochloride (280 or 380 mg/kg, Sigma Chemical, St. Louis, MO) was dissolved in 0.9% saline and i.p. injected to induce seizures. Methyl scopolamine (1 mg/kg in 0.9% saline) was i.p. administered 30 min before pilocarpine administration to minimize the peripheral effects of pilocarpine (Turski et al., 1983). Rats were closely observed for the occurrence of limbic seizures and SE during 120 min following pilocarpine administration. The convulsive behavior of seizures were classified as follows (Racine 1972, modified): stage 0, no response; stage 1, mouth and facial automatisms; stage 2, myoclonus (clonic seizures while the animal is lying on its belly); stage 3, bilateral forelimb clonic seizures without rearing; stage 4, forelimb clonic seizures and rearing; stage 5, rearing and loss of posture. SE was defined as an uninterrupted seizure class 3–5 for 30 min. The pilocarpine model was chosen because seizures induced by this method exhibit a gradual development over a period of about 10 min as compared to seizures initiated by electrical stimulation, characterized by an abrupt onset. Inhibitors of the reverse mode of NCX including 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea methanesulfonate (KB-R7943; 10, 30, and 100 mg/kg; Tocris, Ellisville, MO) and SN-6 (2-[[4-[(4-Nitrophenyl)methoxy]phenyl]methyl]-4-thiazoli dinecarboxylic acid ethyl ester; 10 mg/kg; Tocris, Ellisville, MO) were dissolved in sterile water, filtered, and administered 90 min before pilocarpine injections. KB-R7934 and SN-6 were given orally by gastric intubation in a volume of 0.2 ml/100 g body weight using an 18-gauge stainless steel feeding needle with round tips (ball diameter 3 mm). The initial dose of 10 mg/kg KB-R7943 was based on previous in vivo pharmacological studies (Blokin et al., 2008). Subsequent doses of KB-R7943 were progressively increased by ~3-fold until significant protection against pilocarpine-induced seizures was observed. To validate the effects of KB-R7943, we also tested and quantified the effects of SN-6 (10 mg/kg), another potent blocker of NCX. The 90 min time interval was the most effective in our preliminary tests (data not shown). Animals that did not display class 1 seizures within the 2-hour observation period were considered protected. Time intervals from the end of pilocarpine injection to the appearance of the first episode of forelimb clonic seizures, myoclonus, and SE were recorded, and referred to as limbic seizure latency, myoclonus latency, and SE latency, respectively. For each animal, the seizure severity score and seizure latency were recorded. For each group, the occurrence of myoclonus, limbic seizures, and SE were recorded. Data analysis of seizure latency and SE were performed using one-way ANOVA with Dunn’s post hoc test. Before using ANOVA, data were submitted to the normality test (Shapiro-Wilk test) and the test for homogeneity of variances (Levene’s test). Comparison of seizure severity scores was performed with the Kruskal-Wallis rank test and Dunn’s post hoc test. The incidence of seizures and SE was analyzed using the Chi-square test. Significance level was set at P<0.05. Data are presented as mean ± S.E.M.
Acknowledgments
This publication was made possible by Public Health Service grant NS047193 (PN) and Short-Term Education Program for Underrepresented Persons (YM) from the National Institutes of Health (NIH). Contents are the responsibility of the authors and do not necessarily represent the official views of NIH.
Abbreviations
- NMDA
N-methyl-D-aspartate
- NCX
sodium calcium exchanger
- SE
status epilepticus
- TRPCs
transient receptor potential channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike N, Kostyuk PG, Osipchuk YV. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurons. J Physiol. 1989;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato L, Pignataro G, DiRengo GF. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- Baker PF, McNaughton PA. Kinetics and energetics of calcium efflux form intact squid giant axons. J. Physiol. 1976;259:103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y, Beck H. Transcriptional upregulation of CaV3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28:13341–13353. doi: 10.1523/JNEUROSCI.1421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birinyi P, Acsai K, Bányász T, Tóth A, Horváth B, Virág L, Szentandrássy N, Magyar J, Varró A, Fülöp F, Nánási PP. Effects of SEA0400 and KB-R7943 on Na+/Ca2+ exchange current and L-type Ca2+ current in canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:63–70. doi: 10.1007/s00210-005-1079-x. [DOI] [PubMed] [Google Scholar]
- Blaustein M, Lederer J. Sodium/calcium exchange: Its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Blokhin IO, Vlasov TD, Galagudza MM, Nifontov EM, Petrishchev NN. Role of sodium-calcium exchanger in the myocardial protection against ischemia-reperfusion injury. Ross Fiziol Zh Im I M Sechenova. 2008;94:284–92. [PubMed] [Google Scholar]
- DeLorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: The calcium hypothesis of the induction and maintenance of epilepsy. Pharmacol Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabene PF, Marzola P, Sbarbati A, Bentivoglio M. Magnetic resonance imaging of changes elicited by status epilepticus in the rat brain: diffusion-weighted and T2-weighted images, regional blood volume maps, and direct correlation with tissue and cell damage. Neuroimage. 2003;18:375–389. doi: 10.1016/s1053-8119(02)00025-3. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Marigo F, Galiè M, Benati D, Bernardi P, Farace P, Nicolato E, Marzola P, Sbarbati A. Pilocarpine-induced status epilepticus in rats involves ischemic and excitotoxic mechanisms. PloS One. 2007;2:21105. doi: 10.1371/journal.pone.0001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA. Refractory epilspy: clinical overview. Epilepsia. 2007;48(Suppl 1):3–7. doi: 10.1111/j.1528-1167.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res. 1996;725:11–22. doi: 10.1016/0006-8993(96)00203-x. [DOI] [PubMed] [Google Scholar]
- Iwamoto T. Forefront of Na+/Ca2+ exchanger studies: molecular pharmacology of Na+/Ca2+ exchange inhibitors. J Pharmacol Sci. 2004;96:27–32. doi: 10.1254/jphs.fmj04002x6. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Shigekawa M. Differential inhibition of Na+/Ca2+ exchanger isoforms by divalent cations and isothiourea derivative. Am J Physiol. 1998;275:C423–C430. doi: 10.1152/ajpcell.1998.275.2.C423. [DOI] [PubMed] [Google Scholar]
- Keele NB, Zinebi F, Neugebauer V, Shinnick-Gallagher P. Epileptogenesis up-regulates metabotropic glutamate receptor activation of sodium-calcium exchange current in the amygdala. J Neurophysiol. 2000;83:2458–62. doi: 10.1152/jn.2000.83.4.2458. [DOI] [PubMed] [Google Scholar]
- Ketelaars SO, Gorter JA, Aronica E, Wadman WJ. Calcium extrusion protein expression in the hippocampal formation of chronic epileptic rats after kainate-induced status epilepticus. Epilepsia. 2004;45:1189–1201. doi: 10.1111/j.0013-9580.2004.03304.x. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Lederer WJ, Schulze DH. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na/Ca exchanger. J Biol Chem. 1994;269:51145–51149. [PubMed] [Google Scholar]
- Kraft R. The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem Biophys Res Commun. 2007;361:230–236. doi: 10.1016/j.bbrc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Leite JP, Cavalheiro EA. Effect of conventional antiepileptic drugs in a model of spontaneous recurrent seizures in rats. Epilepsy Res. 1995;20:93–104. doi: 10.1016/0920-1211(94)00070-d. [DOI] [PubMed] [Google Scholar]
- Newman MB, Manresa JJ, Sanberg PR, Shytle RD. Nicotine induced seizures blocked by mecamylamine and its stereoisomers. Life Sci. 2001;69:2583–2591. doi: 10.1016/s0024-3205(01)01338-8. [DOI] [PubMed] [Google Scholar]
- Marchi N, Oby E, Batra A, Uva L, De Curtis M, Hernandez N, Van Boxel-Dezaire A, Najm I, Janigro D. In vivo and in vitro effects of pilocarpine: relevance to ictogenesis. Epilepsia. 2007;48:1934–1946. doi: 10.1111/j.1528-1167.2007.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Arakawa N, Takuma N, et al. SEA0400, a novel and selective inhibitor of Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Exp Pharmacol Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- Miyata A, Zipes DP, Hall S, Rubart M. KB-R7043 prevents acute, atrial fibrillation-induced shortening of atrial refractoriness in anesthetized dogs. Circulation. 2002;106:1410–1419. doi: 10.1161/01.cir.0000028587.85711.f6. [DOI] [PubMed] [Google Scholar]
- Ormandy GC, Jope RS, Snead OC., 3rd Anticonvulsant actions of MK-801 on the lithium-pilocarpine model of status epilepticus in rats. Exp Neurol. 1989;106:172–180. doi: 10.1016/0014-4886(89)90091-5. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Zamponi GW, Barr W, Kiedrowski L, Stys PK. Protection of ischemic rat spinal cord white matter: Dual action of KB-R7943 on Na+/Ca2+ exchange and L-type Ca2+ channels. Neuropharmacology. 2005;48:566–575. doi: 10.1016/j.neuropharm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Papa M, Canitano A, Boscia F, Castaldo P, Sellitti S, Prozig H, Taglialatela M, Annunziato L. Differential expression of Na2+-Ca2+ exchanger transcripts and proteins in rat brain regions. J Comp Neurol. 2003;461:31–48. doi: 10.1002/cne.10665. [DOI] [PubMed] [Google Scholar]
- Pintado AJ, Herrero CJ, García AG, Montiel C. The novel Na(+)/Ca(2+) exchange inhibitor KB-R7943 also blocks native and expressed neuronal nicotinic receptors. Br J Pharmacol. 2000;130:1893–1902. doi: 10.1038/sj.bjp.0703519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednau BT, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol. 1997;272 doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]; Cell Physiol. :C1250C1261. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Saito R, Kaneko E, Tanak Y, Honda K, Matsuda T, Baba A, Komuro I, Kita S, Iwamoto T, Tanako Y. Involvement of Na+/Ca2+ exchanger in pentylenetetrazole-induced convulsions by use of Na+/Ca2+ exchanger knockout mice. Biol Pharm Bull. 2009;32:1928–1930. doi: 10.1248/bpb.32.1928. [DOI] [PubMed] [Google Scholar]
- Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence. Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Khodorov BI. Blockade of NMDA channels in acutely isolated rat hippocampal neurons by the Na+/Ca2+ exchange inhibitor KB-R7943. Neuropharmacology. 1999;38:1235–1242. doi: 10.1016/s0028-3908(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Su H, Sochivko D, Becker A, Chen J, Jiang Y, Yaari Y, Beck H. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22:3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Akaike N. Calcium antagonist effects on low-threshold (T-type) calcium current in rat isolated hippocampal CA1 pyramidal neurons. J Pharmacol Exp Ther. 1991;256:169–175. [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioral, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomodou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]