Abstract
5-HT7 receptors in the dorsal raphe nucleus (DRN) influence circadian rhythms, sleep, and serotonin release. Because interactions between 5-HT7 receptors and glutamatergic and GABAergic neurons have been demonstrated previously, the current studies tested the hypothesis that GABAergic and/or glutamatergic neurons mediate phase shifts induced by activation of DRN 5-HT7 receptors. Hamsters were fitted with guide cannulae aimed at the DRN, housed in cages with running wheels, and exposed to 14 h light (L):10 h dark (D). In Experiment 1, hamsters received DRN pretreatment with muscimol (87.6 picomoles) or vehicle before DRN 8-OH-DPAT (6 picomoles) microinjections at ZT6. After exposure to constant darkness (10 days), phase shifts were calculated and animals were re-exposed to 14L:10D. The procedure was repeated to give each animal the alternate pretreatment. In Experiment 2, hamsters received DRN pretreatment with NMDA (20 picomoles) or vehicle before 8-OH-DPAT at ZT 6. Other experiments tested the effects of single DRN microinjections of muscimol, bicuculline (136 picomoles), NMDA, MK-801 (10 picomoles) or vehicle. Phase shifts (mean±S.E.M., h) in muscimol/8-OH-DPAT-microinjected hamsters (1.02±0.30) were not different (P=0.11) from those in vehicle/8-OH-DPAT-microinjected hamsters (1.34±0.30), while those in NMDA/8-OH-DPAT-microinjected hamsters (0.67±0.17) were smaller (P<0.05) than those in vehicle/8-OH-DPAT-microinjected hamsters (0.97±0.10). DRN single microinjections of bicuculline, but not muscimol, NMDA, or MK-801 induced phase advances. Bicuculline also potentiated 8-OH-DPAT-induced phase advances (P<0.05). These finding suggest that the mechanism mediating DRN 5-HT7 receptor induction of phase advances involves decreased glutamatergic neurotransmission, and furthermore, that inhibition of DRN GABAergic neurotransmission causes a phase advance.
Keywords: 5-HT7 receptors, circadian rhythms, phase shift, GABA receptors, glutamate receptors, dorsal raphe
1. Introduction
The 5-HT7 receptors in the central nervous system modulate a range of physiological and cognitive functions, including circadian timekeeping, paradoxical (rapid eye movement [REM]) sleep, thermoregulation, memory, and affective state (Bonaventure et al., 2007; Duncan et al., 2004; Ehlen et al., 2001; Guscott et al., 2005; Hedlund et al., 2003; Monti and Jantos, 2006; Roberts et al., 2004a; Thomas et al., 2003). Electrophysiological studies have shown that activation of 5-HT7 receptors can either potentiate neuronal excitability (e.g., in the hippocampus and thalamus) (Bacon and Beck, 2000; Tokarski et al., 2003; Tokarski et al., 2005) or attenuate the responsiveness to excitatory stimuli (e.g., in the hypothalamic suprachiasmatic nucleus (SCN), the site of the master mammalian circadian pacemaker) (Bacon and Beck, 2000; Quintero and McMahon, 1999; Smith et al., 2001). Although the highest densities of 5-HT7 receptor expression have been detected in hypothalamic, thalamic and limbic regions, studies in rodents and humans have revealed that these receptors are also present, albeit at lower levels, in the dorsal raphe nucleus (DRN) (Duncan and Franklin, 2007; To et al., 1995; Varnas et al., 2004), a major site of serotonergic neurons that innervate the forebrain (Jacobs and Azmitia, 1992; Molliver, 1987). Within the DRN, 5-HT7 receptors regulate serotonin release, REM sleep, and circadian rhythms (Duncan et al., 2004; Monti and Jantos, 2006; Roberts et al., 2004b).
We have previously demonstrated that activation of DRN 5-HT7 receptors alters the phase of circadian locomotor rhythms (Duncan et al., 2004; Duncan and Davis, 2005). For example, local microinjection of 5-HT1A/7 receptor agonists, e.g., (±)-8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT) or 5-carboxyimidotryptamine (5-CT), to the hamster DRN during the mid-subjective day induces advances of the circadian locomotor activity rhythm (Duncan et al., 2004; Mintz et al., 1997). This treatment mimics the circadian phase shifting effects of electrical stimulation of the DRN (Meyer-Bernstein and Morin, 1999) and other nonphotic stimuli such as systemic administration of serotonergic drugs or benzodiazepines, and behavioral conditions, including presentation of a novel wheel or sleep deprivation (Antle and Mistlberger, 2000; Turek and Losee-Olson, 1986; Van Reeth et al., 1994). The 5-HT7 receptors mediate the phase advances induced by DRN microinjection of serotonergic drugs because these phase advances can be blocked by DRN co-administration of the selective 5-HT7 receptor antagonist SB-269970 (at doses as low as 50 nanomolar) or by RP-cAMP, an antagonist to cyclic AMP, which is the intracellular second messenger to which 5-HT7 receptors are positively coupled (Bard et al., 1993; Duncan et al., 2004; Duncan and Davis, 2005; Plassat et al., 1993; Ruat et al., 1993). DRN microinjections of 8-bromo-cAMP also induce circadian phase advances (Duncan and Davis, 2005). Furthermore, DRN microinjection of another 5-HT7 receptor antagonist, DR4004, attenuates phase advances stimulated by presentation of a novel running wheel (Glass et al., 2003). Thus, activation of DRN 5-HT7 receptors is sufficient to induce nonphotic phase shifts, and these receptors are necessary for at least one type of nonphotically-induced phase shift.
In contrast to the identification of cAMP as the intracellular signal linked to 5-HT7 receptor activation, the neural mechanisms mediating the effect of DRN 5-HT7 receptor activation on circadian phase shifts or other functions are not well understood. A role for GABA neurons is suggested by findings that the activation of DRN GABA-A receptors attenuates novel wheel induced phase shifts (Glass et al., 2003). Also, changes in either GABAergic or glutamatergic neurotransmission have been reported to mediate the effect of 5-HT7 receptor activation on serotonin release in the DRN in vitro (Harsing et al., 2004; Roberts et al., 2004b). Therefore, we tested the hypotheses that circadian phase shifts induced by activation of DRN 5-HT7 receptors are mediated by 1) GABAergic neurotransmission or 2) glutamatergic neurotransmission. We also investigated whether alterations in GABAergic or glutamatergic neurotransmission alone would alter circadian phase.
2. Results
General results
There was variability among the experiments in the mean phase shifting effects of vehicle (ranging from 0.57 h [Expt. 1b & 2b] to 0.88 h [Expt. 2c] ) or 8-OH-DPAT (ranging from 0.97 h [Expt. 1c] to 1.34 h [Expt. 1a]). Based on the within-subjects, randomized order design, the data from each experiment were analyzed using a paired t-test to identify significant drug effects. Acute behavioral effects of the drug administration were observed in a few cases, as noted for individual experiments.
Investigations of the role of GABA neurotransmission on phase shifts induced by DRN microinjection of 8-OH-DPAT
Extp. 1a
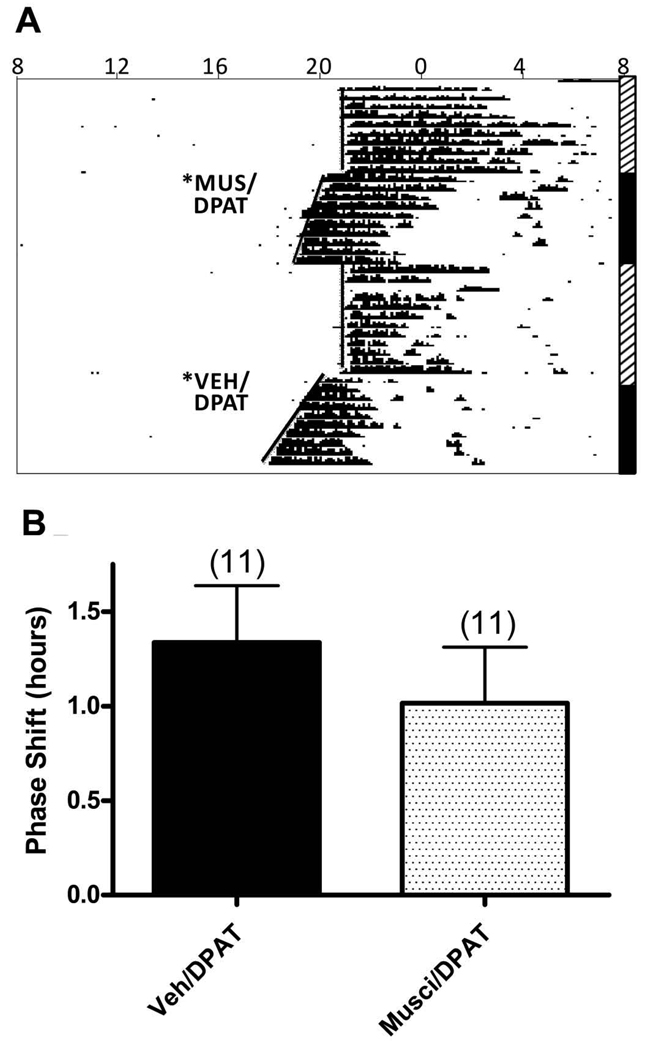
DRN microinjection of 8-OH-DPAT (6 picomoles) at zeitgeber time 6 (ZT 6, i.e., 6 hours before normal time of lights-off) induced robust phase advances (∼ 1.3 h on average) as reported previously (Duncan et al., 2004; Duncan and Davis, 2005), that were not significantly affected by pretreatment of the DRN with the GABA-A receptor agonist, 5-aminomethyl-3-hydroxyisoaxozole (muscimol, 87.6 picomoles) (P=0.110) (Figure 1).
Figure 1. Pretreatment with muscimol did not alter phase shifts induced by microinjections of 8-OH-DPAT in the dorsal raphe.
A. Representative actogram of circadian wheel running rhythms showing phase shifts induced by 8-OH-DPAT (6 picomoles), preceded by muscimol (87.6 picomoles) or vehicle, microinjected into the dorsal raphe at ZT6. The 24-h day is represented horizontally, from left to right. Black marks show the time and relative intensity of wheel running activity. The vertical axis represents successive days, from top to bottom. The vertical bars on the right indicate exposure to a light:dark cycle (14L:10D, striped bars) or constant darkness (black bars). The times of microinjections are represented by asterisks. The phase shift after microinjection of muscimol and 8-OH-DPAT was 1.36 h, as compared to a 1.42 h phase shift after microinjection of vehicle and 8-OH-DPAT. B. Bars represent the mean+S.E.M. for each treatment. Numbers in parentheses represent sample size.
Expt. 1b
Phase shifts induced by DRN microinjection of muscimol alone were not significantly different from those induce by vehicle alone (Mean±S.E.M., muscimol: 0.41±0.29 h; vehicle: 0.85±0.20 h; P=0.14).
Expt. 1c
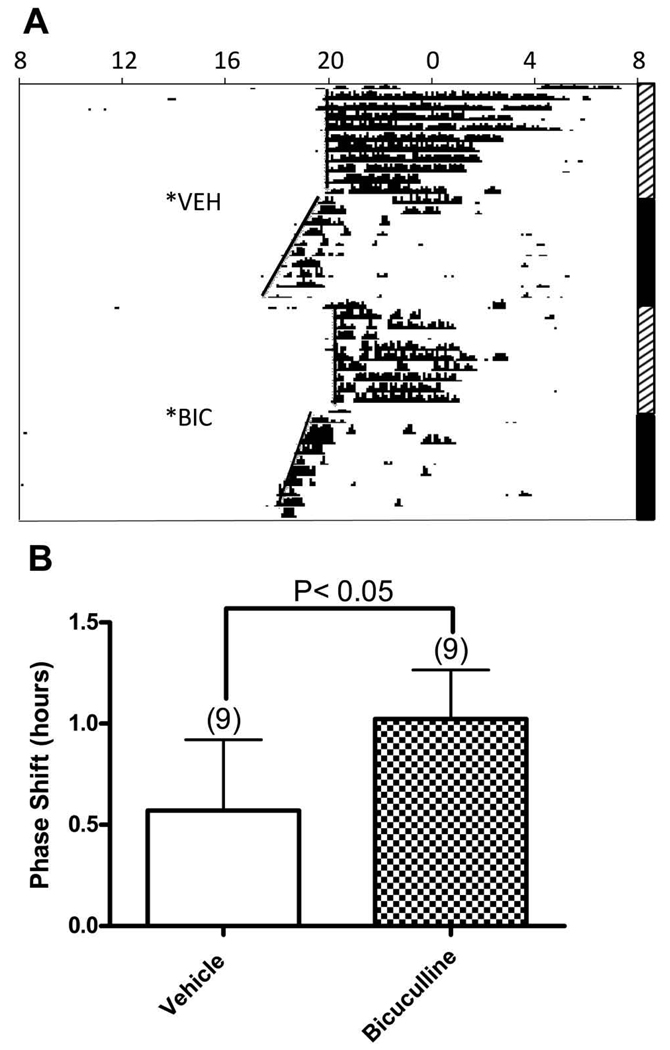
DRN microinjections of the GABA-A receptor antagonist, R-(R*S*)-5–6-(6,8-dihydro-8-oxofuro[3,4,e-]-1,3-benxodioxol-6-yl)-5,6,7,8-tetrahydro-6,6-dimethyl-1,3-dioxolo[4,5-g]isoqinolinium chloride, (bicuculline, 136 picomoles) induced phase advances that were nearly twice as large as those induced by vehicle (P<0.05) (Figure 2). Five of the nine hamsters that received a microinjection of bicuculline exhibited immediate behavioral effects, e.g., vocalization, running around the cage, and jumping, that lasted for up to ten minutes, but their phase shifts were not different from those of the other four bicuculline-injected hamsters (phase shifts [h]: behavioral expression, 0.87±0.32 [N=5]; no behavioral expression, 1.16±0.39 [N=4]; P=0.293).
Figure 2. Microinjection of bicuculline in the dorsal raphe induced a phase shift.
A. Representative actogram of circadian wheel running rhythms from a hamster that received dorsal raphe microinjections of vehicle or bicuculline (136 picomoles) at ZT6. Microinjection of bicuculline led to a phase advance of 1.29 h, in contrast to microinjection of vehicle which led to a 0.87 h phase shift. See legend for Figure 1 for detailed description of actograms. B. Bars represent the mean+S.E.M. for each treatment. Numbers in parentheses represent sample size.
Expt. 1d
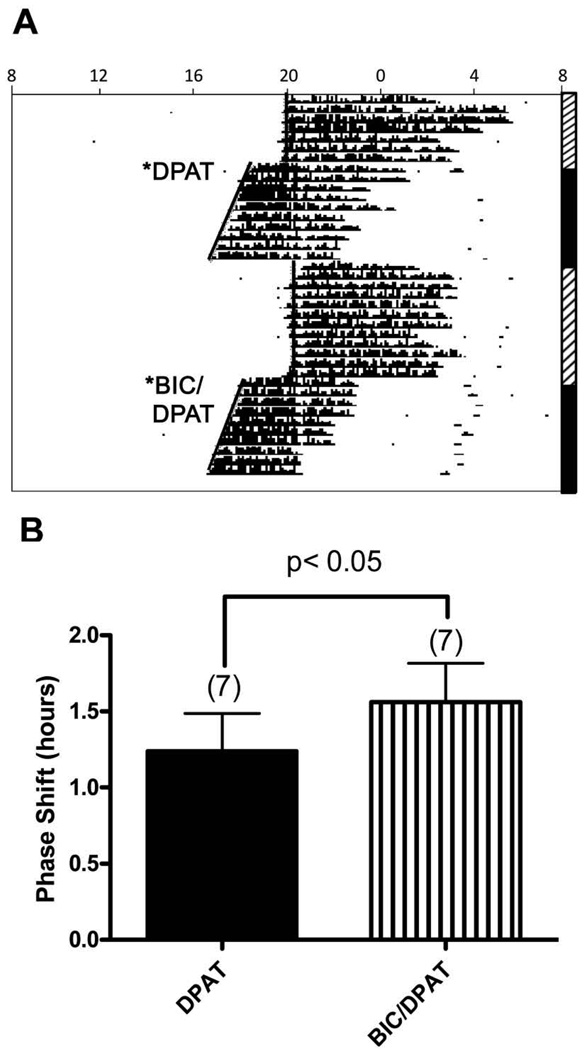
DRN microinjections of bicuculline mixed with 8-OH-DPAT induced phase shifts that were significantly larger than those induced by 8-OH-DPAT administered alone (p<0.05, Figure 3). Of the animals that received the bicuculline and 8-OH-DPAT co-injection, almost half exhibited behavioral activation similar to that observed in Expt. 1c, but had phase shifts of similar magnitude to those not showing activation (phase shifts [h]: behavioral expression, 1.42±0.51 [N=3]; no behavioral expression, 1.67±0.59 [N=4]; P=0.336).
Figure 3. Microinjection of bicuculline in the dorsal raphe potentiates the phase shift induced by 8-OH-DPAT.
A. Representative actogram of circadian wheel running rhythms from a hamster that received dorsal raphe microinjections of 8-OH-DPAT (6 picomoles) alone or bicuculline (136 picomoles) and 8-OH-DPAT (6 picomoles) together at ZT6. Microinjection of 8-OH-DPAT alone led to a phase advance of 1.42 h, in contrast to microinjection of bicuculline and 8-OH-DPAT, which led to a 1.96 h phase advance. See legend for Figure 1 for detailed description of actograms. B. Bars represent the mean+S.E.M. for each treatment. Numbers in parentheses represent sample size.
Investigations of the role of glutamatergic neurotransmission on phase shifts induced by DRN microinjection of 8-OH-DPAT
Expt. 2a
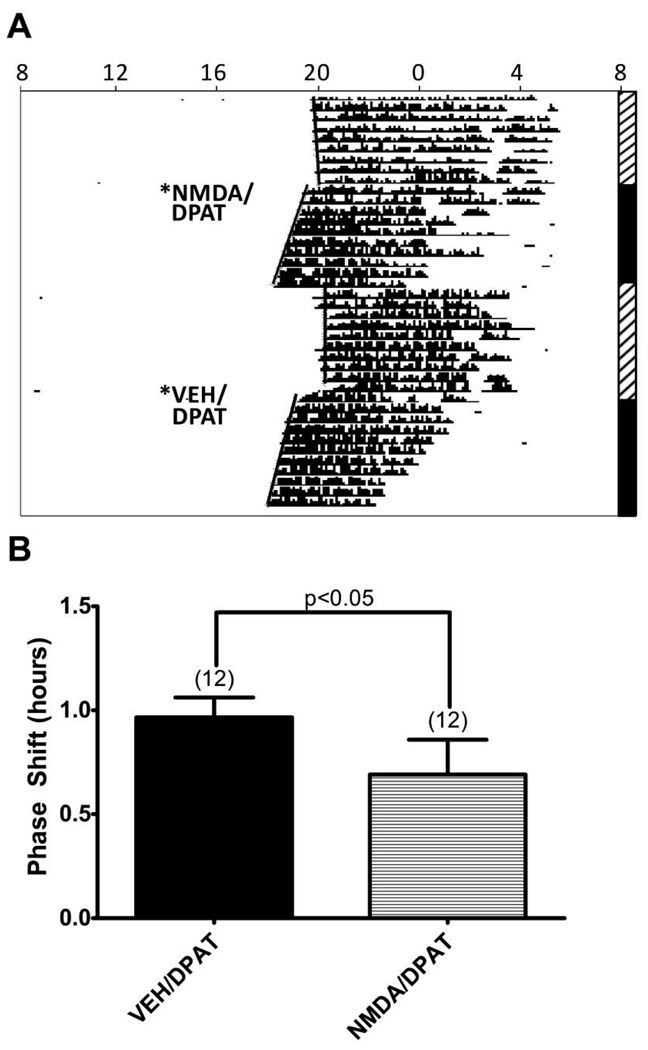
Pretreatment of the DRN with N-methyl-D-aspartic acid (NMDA, 20 picomoles) decreased the magnitude of 8-OH-DPAT-induced phase shifts by ∼ 29% (P<0.05) (Figure 4).
Figure 4. Pretreatment with NMDA attenuated the phase shift induced by 8-OH-DPAT in the dorsal raphe.
A. Representative actogram circadian wheel running rhythms from a hamster that received dorsal raphe microinjections of vehicle or NMDA (20 picomoles) 15 minutes before 8-OH-DPAT at ZT6. See legend for Figure 1 for detailed description of the actogram. Microinjection of NMDA and 8-OH-DPAT induced a 0.87 h phase shift, while microinjection of vehicle and 8-OH-DPAT induced a 1.53 h phase shift. B. Bars represent the mean+S.E.M. for each treatment. Numbers in parentheses represent sample size.
Expt. 2b
Microinjections of NMDA alone had no significant effect as compared with vehicle (NMDA: 0.76±0.17 h; Veh: 0.57±0.21 h, N=5, P=0.122).
Expt. 2c
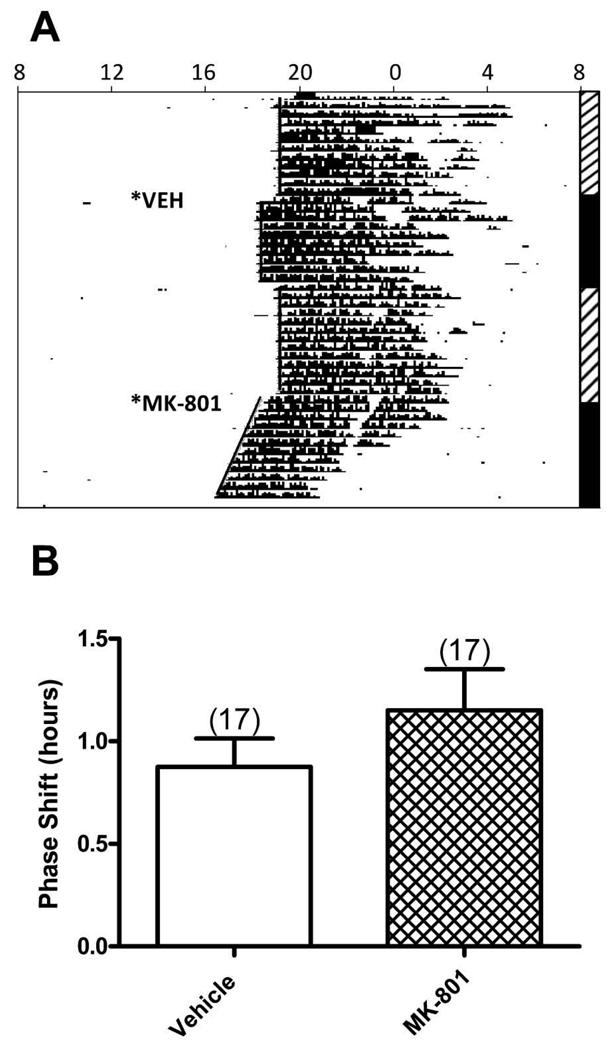
Microinjections of the NMDA glutamate receptor antagonist, (5S,10R)-(+)-5-Methyl-10,11-dihydro-5H–dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801, 10 picomoles) induced phase shifts (1.15±0.20 h, N=17) that were not significantly different from those observed after vehicle microinjections (0.88±0.14 h, N=17; P=0.103) (Figure 5). It is possible that the large vehicle-induced phase shift may have hampered detection of a significant effect of MK-801.
Figure 5. Microinjection of MK-801 into the DRN did not induce phase shifts.
A. Representative actogram circadian wheel running rhythms from a hamster that received dorsal raphe microinjections of vehicle or MK-801 (10 picomoles) at ZT 6. See legend for Figure 1 for detailed description of the actogram. In this hamster, the phase shifts after microinjections of vehicle or MK-801 were 0.78 h and 1.03 h, respectively. B. Bars represent the mean+S.E.M. for each treatment. Numbers in parentheses represent sample size.
3. Discussion
5-HT7 receptors in the DRN have been shown to modulate several functions, including serotonin release, REM sleep, and circadian rhythms (Duncan et al., 2004; Glass et al., 2003; Monti and Jantos, 2006; Roberts et al., 2004b). For example, previous studies have shown that activation of 5-HT7 receptors in the DRN is sufficient for induction of circadian phase advances in the mid-day (Duncan et al., 2004; Duncan and Davis, 2005), and necessary for induction of these phase advances by at least one nonphotic stimulus, running on a novel wheel (Glass et al., 2003). In order to elucidate the neural mechanisms underlying DRN 5-HT7 receptor-dependent circadian phase advances, the current studies tested the hypotheses these phase advances are mediated by: 1) GABAergic neurotransmission or 2) glutamatergic neurotransmission. The findings lend support to the second but not the first hypothesis, and also suggest that GABAergic neurotransmission may affect circadian phase in a manner independent of 5-HT7 receptor activation.
This finding is consistent with previous reports that 5-HT7 receptor activation decreases glutamate release (Harsing et al., 2004) (Harsing, 2006). As shown in the rat DRN in vitro, administration of the 5-HT1A/1B/7 agonist, 5-carboximidotryptamine (5-CT), inhibits electrically-evoked release of [3H]serotonin and [3H]glutamate (Harsing et al., 2004; Harsing, 2006). Simultaneous administration of the 5-HT7 receptor-selective antagonist, SB-258729, blocks these inhibitory effects of 5-CT (Harsing et al., 2004; Harsing, 2006). Furthermore, the inhibition of [3H]serotonin release by 5-CT (in the presence of antagonists to the 5-HT1A and 5-HT1B receptors) was blocked by tetrodotoxin, suggesting that this effect requires sodium-dependent action potentials, and therefore, that the 5-HT7 receptors mediating this effect are not located on serotonin neurons (Harsing et al., 2004). Also, co-administation of MK-801, an antagonist of NMDA-type glutamate receptors, attenuates the inhibition of [3H]serotonin release by 5-CT (Harsing et al., 2004). Based on these findings, Harsing and colleagues suggested that activation of 5-HT7 receptors on glutamatergic terminals in the rat DRN attenuates glutamate release, leading to decreased serotonin release (Harsing et al., 2004). In the current studies, although NMDA administration in the DRN attenuated the 8-OH-DPAT-induced phase shift, DRN administration of MK-801 alone was not sufficient to stimulate a phase advance. It is possible that the unexpected and unusually large vehicle-induced phase shift in this experiment may have hindered detection of a specific effect of MK-801. Alternatively, induction of phase advances may require not only blockade of NMDA receptors, but also some other event(s).
Attenuation of glutamatergic neurotransmission by 5-HT7 receptors has been demonstrated not only in the DRN, but also in the SCN in vitro. For example, activation of 5-HT7 receptors reduces the amplitude of optic nerve-evoked glutamatergic excitatory postsynaptic potentials in the mouse SCN and inhibits glutamate-induced increases in intracellular Ca2+ levels in dissociated rat SCN neurons, suggesting modulation of photic input to the SCN (Quintero and McMahon, 1999; Smith et al., 2001). 5-HT7 receptor-induced attenuation of glutamatergic neurotransmission in the SCN also modulates nonphotic phase shifts. Activation of glutamate receptors with either NMDA or AMPA blocks 8-OH-DPAT-induced phase shifts of the SCN electrical activity rhythms in vitro (Prosser, 2001). In contrast to these findings that 5-HT7 receptors reduce glutamatergic excitatory neurotransmission in the DRN and SCN, other reports indicate that 5-HT7 receptors potentiate neuronal firing in some brain regions. For example, activation of 5-HT7 receptors induces depolarization in the thalamic anterodorsal nucleus and decreases the slow afterhyperpolarization potential and increases bursting frequency in the rat hippocampal CA1 andCA3 subfields (Bacon and Beck, 2000; Chapin and Andrade, 2001; Tokarski et al., 2003; Tokarski et al., 2005). The various effects of 5-HT7 receptor activation may depend on their neuroanatomical location, including the neurochemical phenotype of the cells with which they are associated or the subcellular locus of the receptors.
A role for GABA neurons in mediating the effects of 5-HT7 receptor activation has been suggested both by studies of serotonin release from the DRN in vitro and studies of circadian phase shifts in vivo. For example, in a study of the guinea pig DRN in vitro, the 5-HT7 receptor selective antagonist, SB-269970, inhibited electrically-evoked serotonin efflux; this effect was blocked by co-administration of the GABA-A receptor antagonist, bicuculline (Roberts et al., 2004b). This report suggested that tonic activation of 5-HT7 receptors on GABA neurons in the DRN inhibits GABA release, thus disinhibiting serotonin release (Roberts et al., 2004b). Participation of DRN GABA-A receptors in circadian phase shifts has been demonstrated by findings that microinjection of the GABA-A receptor agonist, muscimol, in the hamster DRN attenuates novel wheel-induced circadian phase shifts (Glass et al., 2003). Our studies showed that pretreatment of the DRN with the same dose of muscimol that blocks novel wheel-induced phase shifts (Glass et al., 2003), does not attenuate phase shifts induced by DRN microinjections of 8-OH-DPAT. This finding suggests that the phase shifts induced by DRN 5-HT7 receptor activation, unlike those induced by access to a novel wheel, may not depend on decreased GABAergic neurotransmission within the DRN. However, an alternative interpretation would be that pretreatment with muscimol led to GABA-A receptor down regulation or desensitization, such that GABA-A-ergic signaling was no longer effective when 8-OH-DPAT microinjections occurred fifteen minutes later.
In contrast to the lack of effect of the GABA-A receptor agonist, mid-day blockade of DRN GABA-A receptors by local administration of bicuculline is sufficient to induce circadian phase advances and to potentiate 8-OH-DPAT-induced phase advances. The phase shifting effects of DRN bicuculline administration may be caused by serotonin release in the SCN, which increases greatly in response to this treatment (Glass et al., 2003). Microdialysis of serotonin in the SCN during the midsubjective day induces circadian phase advances (Ehlen et al., 2001). Another possible mechanism that might be related to bicuculline induction of phase shifts was the intense behavioral arousal that sometimes accompanied this treatment, reminiscent of the report of hyperactivity in rats following DRN infusion of bicuculline (Tao and Auerbach, 2003). Interestingly, many phase shift-inducing nonphotic signals stimulate locomotor activity (e.g., novel wheels and Triazolam injections) or alertness/arousal (e.g., sleep deprivation), and in some cases, the resultant phase shift appears to depends on the expression of locomotor activity (Mistlberger et al., 2003; Van Reeth and Turek, 1989). However, in the present study, the magnitude of bicuculline-induced phase shifts was not associated with the expression of intense behavioral activation. This finding is similar to the report that DRN drug treatments (i.e., muscimol and the 5-HT7 receptor antagonist, DR 4004) that attenuate novel wheel-induced phase advances do not decrease the number of wheel revolutions, indicating that the phase shifts were not directly related to the amount of running (Glass et al., 2003).
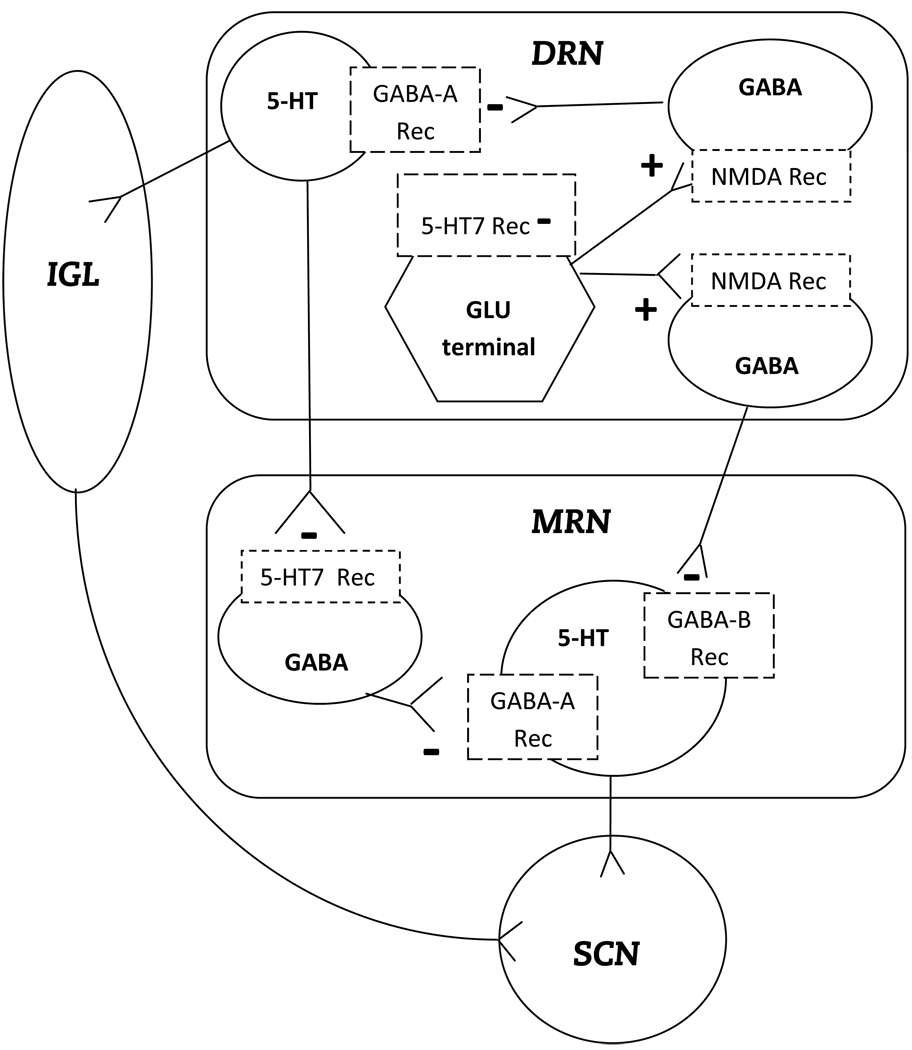
The current findings concerning the phase shifting effects of DRN 5-HT7 receptor activation and GABA-A receptor antagonism can be considered as extensions and modifications of a model designed to describe serotonergic signaling from the raphe nuclei to the SCN (Glass et al., 2003) (see Figure 6). That model proposed that behavioral input such as novel wheel running or sleep deprivation, occurring during the midsubjective day, stimulates serotonin release in the SCN due to inhibition of DRN GABAergic neurons. Reduction of DRN GABAergic tone disinhibits DRN serotonin neurons projecting either to the intergeniculate leaflet of the thalamus (IGL) or to the median raphe nucleus (MRN). Many studies have elucidated the roles of the IGL and MRN in conveying nonphotic signals to the SCN and the findings are summarized briefly as follows: Lesions of the IGL prevent phase shifts induced by a novel wheel or other nonphotic stimuli, demonstrating that the IGL is necessary for these nonphotically induced phase shifts (Janik and Mrosovsky, 1994; Schuhler et al., 1999; Wickland and Turek, 1994). Novel wheel running stimulates serotonin release in the IGL and NPY release in the SCN, and the latter is blocked by microdialysis of the IGL with muscimol or metergoline, a serotonergic antagonist (Glass et al., 2010; Grossman et al, 2003). Furthermore, SCN microinjection of NPY, the major neurotransmitter identified in IGL neurons projecting to the SCN, induces nonphotic phase shifts (Huhman and Albers, 1994). Concerning the MRN, electrical stimulation of this nucleus induces nonphotic phase shifts and serotonin release in the SCN (Glass et al., 2003; Meyer-Bernstein and Morin, 1999). Microdialysis of the MRN with metergoline or muscimol inhibits serotonin release in the SCN in response to electrical stimulation of the DRN (Glass et al., 2003). Thus, disihibition of DRN serotonin neurons innervating the MRN and IGL is a mechanism by which activation of 5-HT7 receptors in the DRN may mediate nonphotic circadian phase shifts.
Figure 6. Working model of DRN 5-HT7 receptor induction of phase shifts.
The current findings are shown as a modification of nonphotic phase shifting mechanisms elucidated by Glass et al (2003). Activation of DRN 5-HT7 receptors by microinjection of 8-OH-DPAT inhibits glutamate release (Harsing et al, 2004), thus attenuating GABAergic neurotransmission. This leads to disinhibition of serotonin (5-HT) neurons projecting from the DRN to the IGL or from the MRN to the SCN, thus enhancing signaling to the SCN. NMDA in the DRN activates NMDA-type glutamate receptors, stimulating GABA neurons and inhibitory tone. DRN bicuculline microinjection blocks GABA-A receptors, disihibits IGL-projecting 5-HT neurons, and sends a phase shifting signal to the SCN. DRN muscimol, acting at GABA-A receptors, inhibits signaling from the IGL to the SCN, but does not prevent signaling from the DRN to MRN to SCN initiated by 8-OH-DPAT in the DRN.
Our present findings are consistent with this model. First of all, DRN administration of bicuculline induced a phase advance, as would be expected by its blockade of GABAA receptors and disinhibition of DRN serotonergic neurons. DRN microinjection of 8-OH-DPAT induced a phase advance, presumably by activating post-synaptic 5-HT7 receptors, located either on DRN GABAergic neurons projecting to the MRN, as previously suggested (Glass et al., 2003), or on glutamate neurons, thereby inhibiting glutamate release and decreasing tonic DRN GABAergic activity (Harsing et al., 2004; Harsing, 2006). Attenuation of the DRN 8-OH-DPAT-induced phase shift by DRN NMDA microinjection supports a role for attenuation of glutamate neurotransmission in these phase shifts. Pretreatment of the DRN with muscimol would overcome the disinhibition of DRN serotonergic neurons innervating the IGL brought about by DRN 8-OH-DPAT microinjection, but not the disihibition of MRN serotonergic neurons. This might explain why phase shifts induced by DRN 8-OH-DPAT microinjections were not attenuated by DRN muscimol administration.
As noted above, DRN 5-HT7 receptors not only influence the circadian pacemaker, but also regulate REM sleep and serotonin release in the DRN (Harsing et al., 2004; Harsing, 2006; Monti and Jantos, 2006). In view of these functions, it is important to note that 5-HT7 receptors in the DRN participate in a complex system of reciprocal interactions among serotonin neurons, glutamatergic axon terminals, GABAergic interneurons, and GABAergic axon terminals arising from other brain regions (Harsing et al., 2004; Harsing, 2006). Based on studies in rats, the glutamatergic innervations to the DRN arise mainly from the medial prefrontal cortex, the hypothalamic perifornical, lateral, and arcuate nuclei, and several medullary regions including the lateral and medial parabrachial nuclei, and the laterodorsal tegmental nucleus (Lee et al., 2003). Glutamate has an excitatory effect in the DRN. For example, reverse microdialysis studies of the rat DRN showed that administration of NMDA increases DRN extracellular serotonin levels (Tao and Auerbach, 1996), and that glutamate leads initially to increased DRN extracellular serotonin levels and then later to decreased levels (below baseline) for several hours (Mokler et al., 2009). As mentioned above, studies of the rat DRN in vitro have shown that activation of 5-HT7 receptors inhibits electrically-stimulated release of [3H]glutamate as well as [3H]serotonin (Harsing et al., 2004; Harsing, 2006). Conversely, activation of glutamate receptors with NMDA or AMPA stimulates release of both [3H]serotonin and [3H]GABA (Harsing et al., 2004; Harsing, 2006). Activation of GABA-A receptors or activation of presynaptic 5-HT1B/1D receptors inhibits electrically-stimulated [3H]serotonin and [3H]GABA release (Harsing, 2006). Therefore, drug treatments in the DRN are likely to alter a network of neural pathways affecting a variety of physiological processes and behaviors.
4. Conclusions
The present findings demonstrate that pretreatment of the DRN with NMDA but not muscimol attenuates the phase shifting effect of DRN microinjection of 8-OH-DPAT, previously shown to be mediated by 5-HT7 receptors (Duncan et al., 2004; Duncan and Davis, 2005). Thus, these findings suggest that the decreased glutamate release induced by activation of DRN 5-HT7 receptors, reported previously (Harsing et al., 2004; Harsing, 2006), plays a role in an overt biological response, i.e., resetting of the master circadian pacemaker by activation of DRN 5-HT7 receptors. Although attenuation of GABAergic neurotransmission within the DRN does not appear to be necessary for circadian phase advances induced by activation of DRN 5-HT7 receptors, blockade of DRN GABAA receptors with bicuculline is sufficient to induce a phase advance or to increase the magnitude of phase shifts induced by 8-OH-DPAT. These findings further describe the neural mechanisms within the midbrain raphe leading to nonphotic resetting of the master circadian pacemaker and may also help to elucidate the mechanisms mediating other physiological effects induced by activation of 5-HT7 receptors in the DRN.
5. Experimental Procedures
5.1 Animals, housing conditions, and surgical procedures
These studies used adult male Syrian hamsters (Mesocricetus auratus), 3–5 months old, obtained from Harlan labs (Harlan, HsdHan:AURA). The hamsters were housed individually in polycarbonate cages with rodent chow and water available continuously. Prior to experimentation, all hamsters were exposed to a 14 h light:10 h dark (14L:10D) photoperiod, with average light intensity of ∼ 250 lux, for at least 10 days. During experiments, the hamsters were exposed to either 14L:10D or constant darkness, as described below. The experimental procedures described below were approved by the Institutional Animal Care and Use Committees at the University of Kentucky and were consistent with AAALAC guidelines. Hamsters were deeply anesthetized with pentobarbital (∼95 mg/kg, i.p.) and fitted with indwelling guide cannulae targeting the DRN, using stereotaxic surgery under aseptic conditions, as we have described previously (Duncan et al., 2004; Duncan and Davis, 2005). The stereotaxic surgery, which was conducted with lamda and bregma level and the guide cannula at a 20° angle from the midline, used the following coordinates, from bregma: A–P= −4.2 mm, M–L= +1.9 mm, and D–V= −5 mm. The analgesic, meloxicam (0.1 ml, orally) was administered immediately before surgery and again 24 h later. After recovery from surgery, the hamsters were individually housed in cages equipped with running wheels electronically interfaced with a computer, such that circadian activity rhythms were monitored continuously using Chronobiology kit.
5.2 Investigations of the role of GABA neurotransmission on phase shifts induced by DRN microinjection of 8-OH-DPAT
After baseline activity recordings during exposure to a light:dark cycle for ∼7–10 days, the hamsters were given DRN microinjections of drugs or vehicle solutions at Zeitgeber time (ZT) 6, i.e., six hours before the normal time of lights off, which is considered ZT 12 by convention) or fifteen minutes earlier (as described below for each experiment). The microinjections for this experiment and all others consisted of a 200 nl volume administered at a rate of ∼ 50 nl/30 seconds. Immediately after microinjections, the animals were transferred to constant darkness for 10–14 days. The circadian phase shifts were calculated by linear regression analysis using Chronobiology Kit software. The hamsters were then re-entrained to the light:dark cycle for at least a week. The procedure was repeated such that hamsters in each group received the treatment originally administered to the other group. At the end of the study, the hamsters were anesthetized and diluted India ink was microinjected. The hamsters were euthanized by decapitation and the brains were dissected from the skulls and prepared for histological verification of the microinjection sites. Only hamsters with neuroanatomically accurate microinjection sites were included in the data analysis. The phase shifts were statistically analyzed by paired T-test, using Prism software, with significance defined as P<0.05.
Expt. 1a. The effect of pretreatment with muscimol on phase shifts induced by DRN microinjection of 8-OH-DPAT
The two experimental treatments consisted of sets of two microinjections in the DRN: 1) vehicle (0.9% saline) at ZT 5.75 and (+/−)-8-OH-DPAT (6 picomoles) at ZT 6, and 2) muscimol (87.6 picomoles) at ZT 5.75 and (+/−)-8-OH-DPAT (6 picomoles) at ZT 6. The dose of 8-OH-DPAT is equivalent to that used in our previous studies (Duncan et al., 2004; Duncan and Davis, 2005). The dose of muscimol chosen is the same as that demonstrated to inhibited novel wheel-induced phase shifts after microinjection into the hamster DRN (Glass et al., 2003) and is higher than the dose shown to inhibit serotonin release from the DRN in vitro (Roberts et al., 2004b).
Expt. 1b. The effect of DRN administration of muscimol alone on circadian phase shifts
In this study, hamsters received at ZT 6 DRN microinjections of either: 1) the GABA-A receptor agonist, muscimol (87.6 picomoles) or 2) vehicle (0.9% sterile saline).
Expt. 1c. The effect of DRN microinjections of bicuculline alone on circadian phase shifts
The drug treatments in this study consisted of DRN microinjections (at ZT 6) of: 1) the GABA-A receptor antagonist, bicuculline [(−)-bicuculline methochloride, 136 picomoles] or 2) vehicle (0.9% sterile saline). This dose of bicuculline was chosen because it was shown previously to stimulate serotonin release in the SCN after administration into the DRN (Glass et al., 2003).
Expt. 1d. The effect of DRN microinjections of bicuculline and 8-OH-DPAT together on circadian phase shifts
The drug treatments in this study consisted of DRN microinjections (at ZT 6) of: 1) (+/−)-8-OH-DPAT (6 picomoles) dissolved in sterile saline (0.9%) or 2) (+/−)-8-OH-DPAT (6 picomoles) and bicuculline (136 picomoles) dissolved in sterile saline, injected together.
5.3 Investigations of the role of glutamatergic neurotransmission on phase shifts induced by DRN microinjection of 8-OH-DPAT
The basic experimental design described for Experiment 1 was also used here. The drug treatments are listed below for each experiment.
Expt. 2a. The effect of pre-treatment with the glutamate receptor agonist, NMDA, on phase shifts induced by DRN microinjection of 8-OH-DPAT
The treatment groups in this study consisted of two sets of 2 DRN microinjections: 1) vehicle (0.9% saline) at ZT 5.75 and (+/−)-8-OH-DPAT (6 picomoles) at ZT 6, and 2) NMDA (20 picomoles) at ZT 5.75 and (+/−)-8-OH-DPAT (6 picmoles) at ZT 6.
Expt. 2b. The effect of DRN microinjection of the glutamate receptor antagonist, MK-801, on circadian phase shifts
The hamsters in this study received, at ZT 6, DRN microinjections of: 1) vehicle (sterile 0.9% saline) or 2) MK-801 [(+)MK 801 maleate, 10 picomoles]. This dose of MK-801, which is 1,300 times higher than the Kd value of MK-801 in receptor binding studies (Wong et al., 1986), is likely to block virtually all of the NMDA receptor binding sites.
Drugs and reagents
All drugs were purchased from Tocris (Ellisville, MO), with the exception of pentobarbital (Nembutal sodium solution, Ovation Pharmaceuticals, Deerfield, IL, USA) and meloxicam (Metacam, Boehringer Ingelheim, St. Joseph, MO, USA). The drug solutions for DRN microinjections were prepared 30–60 minutes before use by dissolving and diluting the drugs in sterile physiological saline. For microinjection of all drugs and vehicle, the volume was 200 nl.
Data analysis
Phase shifts were calculated by the linear regression analysis method using the computer program, Clocklab (Actimetrics, Wilmette, IL). Regression lines were fitted through the daily onsets of activity for 7–14 days before and after each treatment. A phase shift was defined as the difference between the onset of activity predicted by the pre-treatment regression line and the actual onset shown by the post-treatment regression line. Positive numbers represented phase advances while negative numbers represented phase delays. For each experiment, the phase shift data were analyzed by paired t-test (Prism 5.00, GraphPad Software, San Diego, CA). Significance was defined as P values less than 0.05.
Acknowledgements
These studies were supported by NIH AG13418. We thank Kelsey Lewis and Thomas Rogers for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antle MC, Mistlberger RE. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J. Neuroscience. 2000;20:9326–9332. doi: 10.1523/JNEUROSCI.20-24-09326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon WL, Beck SG. 5-Hydroxytryptamine(7) receptor activation decreases slow afterhyperpolarization amplitude in CA3 hippocampal pyramidal cells. J. Pharm. Exp. Ther. 2000;294:672–679. [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, Miller K, Lovenberg TW, Atack J, Dugovic C. Selective blockade of 5-HT7 receptors enhances 5-HT transmission, antidepressant-like behavior and REM sleep suppression induced by citalopram in rodents. J. Pharm. Exp. Ther. 2007;321:690–698. doi: 10.1124/jpet.107.119404. [DOI] [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT7 receptor-mediated depolarization in the anterodorsal thalamus. I. Pharmacological characterization. J. Pharm. Exp. Ther. 2001;297:395–402. [PubMed] [Google Scholar]
- Duncan MJ, Davis VA. Cyclic AMP mediates circadian phase shifts induced by microinjection of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 2005;1058:10–16. doi: 10.1016/j.brainres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Franklin KM. Expression of 5-HT7 receptor mRNA in the hamster brain: Effect of aging and association with calbindin-D28K expression. Brain Res. 2007;1143:70–77. doi: 10.1016/j.brainres.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Grear KE, Hoskins MA. Aging and SB-269970-A, a selective 5-HT7 receptor antagonist, attenuate circadian phase advances induced by microinjections of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 2004;1008:40–48. doi: 10.1016/j.brainres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Grossman GH, Glass JD. In vivo resetting of the hamster circadian clock by 5-HT7 receptors in the suprachiasmatic nucleus. J. Neurosci. 2001;21:5351–5357. doi: 10.1523/JNEUROSCI.21-14-05351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Guinn J, Kaur G, Francl JM. On the intrinsic regulation of neuropeptide Y release in the mammalian suprachiasmatic nucleus circadian clock. Eur J Neurosci. 2010;31:1117–1126. doi: 10.1111/j.1460-9568.2010.07139.x. [DOI] [PubMed] [Google Scholar]
- Glass JD, Grossman GH, Farnbauch L, DiNardo L. Midbrain Raphe Modulation of Nonphotic Circadian Clock Resetting and 5-HT Release in the Mammalian Suprachiasmatic Nucleus. J. Neurosci. 2003;23:7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman GH, Farnbauch L, Glass JD. Regulation of serotonin release in the hamster intergeniculate leaflet region. Neuroreport. 2004;15:103–106. doi: 10.1097/00001756-200401190-00021. [DOI] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacol. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Harsing LG. The pharmacology of the neurochemical transmission in the midbrain raphe nuclei of the rat. Curr Neuropharmacol. 2006;4:313–339. doi: 10.2174/157015906778520764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing LG, Prauda I, Barkoczy J, Matyus P, Juranyi Z. A 5-HT7 heteroreceptor-mediated inhibition of [3H]serotonin release in raphe nuclei slices of the rat: Evidence for a serotonergic-glutamatergic interaction. Neurochem. Res. 2004;29:1487–1497. doi: 10.1023/b:nere.0000029560.14262.39. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc. Natl. Acad. Sci. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Albers HE. Neuropeptide Y microinjected into the suprachiasmatic region phase shifts circadian rhythms in constant darkness. Peptides. 1994;15:1475–1478. doi: 10.1016/0196-9781(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Janik D, Mrosovsky N. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Res. 1994;651:174–182. doi: 10.1016/0006-8993(94)90695-5. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Morin LP. Electrical stimulation of the median or dorsal raphe nuclei reduces light-induced fos protein in the suprachiasmatic nucleus and causes circadian activity rhythm phase shifts. Neuroscience. 1999;92:267–279. doi: 10.1016/s0306-4522(98)00733-7. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Gillespie CF, Marvel CL, Huhman KL, Albers HE. Serotonergic regulation of circadian rhythms in Syrian hamsters. Neuroscience. 1997;79:563–569. doi: 10.1016/s0306-4522(96)00696-3. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC, Webb IC, Jones M, Weinberg J, Pollock MS. Circadian clock resetting by arousal in Syrian hamsters: the role of stress and activity. Am J Physiol Regul Integr Comp Physiol. 2003;285:R917–R925. doi: 10.1152/ajpregu.00222.2003. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Dugal JR, Hoffman JM, Morgane PJ. Functional interrelations between nucleus raphe dorsalis and nucleus raphe medianus: A dual probe microdialysis study of glutamate-stimulated serotonin release. Brain Res. Bull. 2009;78:132–138. doi: 10.1016/j.brainresbull.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J. Clin. Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- Monti JM, Jantos H. Effects of the 5-HT7 receptor antagonist SB-269970 microinjected into the dorsal raphe nucleus on REM sleep in the rat. Behav. Brain Research. 2006;167:245–250. doi: 10.1016/j.bbr.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Plassat J-L, Amlaiky N, Hen R. Molecular cloning, characterisation and localisation of a high-affinity serotonin receptor that activates adenylate cyclase. Mol. Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- Prosser RA. Glutamate blocks serotonergic phase advances of the mammalian circadian pacemaker through AMPA and NMDA receptors. J. Neurosci. 2001;21:7815–7822. doi: 10.1523/JNEUROSCI.21-19-07815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero JE, McMahon DG. Serotonin modulates glutamate responses in isolated suprachiasmatic nucleus neurons. J. Neurophysiol. 1999;82:533–539. doi: 10.1152/jn.1999.82.2.533. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT7 receptors show specific impairments in contextual learning. Eur J Neurosci. 2004a;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- Roberts C, Thomas DR, Bate ST, Kew JNC. GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guinea-pig dorsal raphe nucleus. Neuropharmacol. 2004b;46:935–941. doi: 10.1016/j.neuropharm.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang J-M, Schwartz J-C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. USA. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhler S, Pitrosky B, Saboureau M, Lakhdar-Ghazal N, Pevet P. Role of the thalamic intergeniculate leaflet and its 5-HT afferences in the chronobiological properties of 8-OH-DPAT and triazolam in Syrian hamsters. Brain Res. 1999;849:16–24. doi: 10.1016/s0006-8993(99)01914-9. [DOI] [PubMed] [Google Scholar]
- Smith BN, Sollars PJ, Dudek FE, Pickard GE. Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors. J. Biol. Rhythms. 2001;16:25–38. doi: 10.1177/074873040101600104. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Differential effect of NMDA on extracellular serotonin in rat midbrain raphe and forebrain sites. J. Neurochem. 1996;66:1067–1075. doi: 10.1046/j.1471-4159.1996.66031067.x. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Influence of inhibitory and excitatory inputs on serotonin efflux differs in the dorsal and median raphe nuclei. Brain Res. 2003;961:109–120. doi: 10.1016/s0006-8993(02)03851-9. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Melotto S, Massagrande M, Gribble AD, Jeffrey P, Stevens AJ, Deeks NJ, Eddershaw PJ, Fenwick SH, Riley G, Stean T, Scott CM, Hill MJ, Middlemiss DN, Hagan JJ, Price GW, Forbes IT. SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br. J. Pharmacol. 2003;139:705–714. doi: 10.1038/sj.bjp.0705290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To ZP, Bonhaus DW, Eglen RM, Jakeman LB. Characterization and distribution of putative 5-HT7 receptors in guinea pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Zahorodna A, Bobula B, Hess G. 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res. 2003;993:230–234. doi: 10.1016/j.brainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Tokarski K, Zahorodna A, Bobula B, Grzegorzewska M, Pitra P, Hess G. Repeated administration of citalopram and imipramine alters the responsiveness of rat hippocampal circuitry to the activation of 5-HT7 receptors. Eur. J. Pharmacol. 2005;524:60–66. doi: 10.1016/j.ejphar.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Turek F, Losee-Olson S. A benzodiazepine used in the treament of insomnia phase-shifts the mammalian circadian clock. Nature. 1986;321:167–168. doi: 10.1038/321167a0. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Tripathi B, Kirby J, Laartz B, Tecco J, Turek FW. Daily exposure to a nonphotic stimulus can alter photoperiodic response to short days in hamsters. Proc. Soc. Exp. Biol. Med. 1994;206:138–144. doi: 10.3181/00379727-206-43732. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Turek FW. Stimulated activity mediates phase shifts in the hamster circadian clock induced by dark pulses or benzodiazepines. Nature. 1989;339:49–51. doi: 10.1038/339049a0. [DOI] [PubMed] [Google Scholar]
- Varnas K, Thomas DR, Tupala E, Tiihonen J, Hall H. Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci. Lett. 2004;367:313–316. doi: 10.1016/j.neulet.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Wickland C, Turek FW. Lesions of the thalamic intergeniculate leaflet block activity- induced phase shifts in the circadian activity rhythm of the golden hamster. Brain Res. 1994;660:293–300. doi: 10.1016/0006-8993(94)91302-1. [DOI] [PubMed] [Google Scholar]
- Wong EHF, Kemp JA, Priestly T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK 801 is a potent NMDA antagonist. Proc. Natl. Acad. Sci. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]