Abstract
Background
Functional neuroimaging studies of autism spectrum disorders (ASD) have examined social and non-social paradigms, although rarely in the same study. Here, we provide an objective, unbiased survey of functional brain abnormalities in ASD, related to both social and non-social processing.
Methods
We conducted two separate voxel-wise activation likelihood estimation meta-analyses of 39 functional neuroimaging studies consisting of 24 studies examining social processes (e.g., theory of mind, face perception), and 15 studies examining non-social processes (e.g., attention control, working memory). Voxel-wise significance threshold was p< 0.05, corrected by false discovery rate.
Results
Compared to neurotypical controls (NC), ASD showed greater likelihood of hypoactivation in two medial wall regions: perigenual anterior cingulate cortex (ACC) in social tasks only, and dorsal ACC in non-social studies. Further, right anterior insula, recently linked to social cognition, was more likely to be hypoactivated in ASD in the analyses of social studies. In non-social studies, group comparisons showed greater likelihood of activation for the ASD group in the rostral ACC region that is typically suppressed during attentionally demanding tasks.
Conclusions
Despite substantial heterogeneity of tasks, the rapidly increasing functional imaging literature showed ASD-related patterns of hypofunction and aberrant activation that depended on the specific cognitive domain, i.e., social and versus non-social. These results provide a basis for targeted extensions of these findings with younger subjects and a range of paradigms, including analyses of default mode network regulation in ASD.
Keywords: autism, pervasive developmental disorders (PDD), anterior cingulate cortex, insula, social cognition, cognitive control, meta-analysis, functional magnetic resonance imaging (fMRI), positron emission tomography (PET), default mode network
Introduction
Recent functional neuroimaging studies focused on identifying the neural correlates of autism spectrum disorders (ASD) have generated several encouraging lines of investigation, albeit with varying degrees of replication. Since impairments in social and communicative skills are the hallmarks of ASD (1, 2, 3), most neuroimaging studies have used social cognition-based paradigms testing the ability to interpret and predict other’s beliefs, intentions and desires (i.e., theory of mind) as well as the perception of specific social stimuli such as human faces. Both processes have been found to be abnormal in early development of individuals with ASD and have been linked to the associated social and communicative impairments (4, 5, 3).
Based on models of the social brain (6, 7, 8, 9), studies have focused on a priori regions of interest typically implicated in mentalizing, including medial prefrontal cortex/paracingulate cortex, temporo-parietal junction, temporal pole, amygdala, and periamygdaloid cortices. Depending on the specific task employed, ASD-related abnormalities have been reported for each of these regions, with moderate degrees of agreement (10, 11, 12). For instance, hypoactivations of rostral anterior medial prefrontal cortex, adjacent anterior paracingulate cortex and perigenual anterior cingulate cortex (ACC) have been found in some studies using theory of mind paradigms (13, 14, 15) but not in others (16). Likewise, some studies of emotional processing (e.g., 16, 17) describe ASD-related amygdala hypoactivation, but not others (18, 19). An area of particular convergence is facial perception, with ASD-related hypoactivation of fusiform gyrus (FG) observed across both studies of facial form and facial expression perception (e.g., 20, 21, 22, 23). However, negative reports (18, 24, 19), have raised questions regarding the nature and specificity of FG dysfunction in ASD. In sum, studies of ASD based on social cognitive models have identified candidate regions of dysfunction, albeit with only moderate convergence across studies.
Although deviant development of the ability to engage in appropriate social interactions is the central dysfunction in ASD, additional cognitive and sensorimotor impairments often co-occur (e.g., 25, 1, 26). For instance, working memory, planning, cognitive flexibility, inhibitory control, and action monitoring are impaired in both children and adults with autism (e.g., 27, 28, 29, 30, 31, 32, 33, 34). Some authors hypothesize that such abnormalities underlie the pattern of restricted and stereotyped interests which complete the diagnostic triad of autism, along with social and communicative impairments (1). These observations have motivated parallel lines of investigation on the ASD- neuronal correlates of executive dysfunction. Brain correlates of other functions also found impaired in ASD, such as language, have been also examined (10).
Frontal cortical hypoactivation has emerged as one of the most consistent results across these studies. Specifically, reduced activation of dorsolateral prefrontal cortex (DLPFC) and dorsal ACC have been described in individuals with ASD performing working memory, inhibitory control, visuospatial attention, and embedded figure tasks (e.g., 35, 36, 37, 38, 39). Hypofunction of other frontal regions has been reported depending on the specific task employed (for reviews see 10, 40). Accompanying hypoactivation in task-targeted regions, increased function in areas implicated in more basic sensory processing such as primary visual cortex has been consistently described (41, 12, 10). Of note, such patterns of atypical recruitment have also been reported in studies examining social processes (e.g., 20, 22).
Despite broadly convergent findings, the neuronal correlates of ASD remain under-specified. Reasons include the use of generally small samples, with substantial heterogeneity with respect to age ranges, clinical presentation, tasks, and statistical methods. Most studies used fixed rather than random effects models, many lacked direct group comparisons, and most relied on region-of-interest analyses which limit generalizability and increase type I error rates. Overcoming these limitations definitively will require pooling larger samples and standardization of data collection methods across laboratories (42). Pending such a large-scale effort, a systematic assessment of current functional neuroimaging findings can inform the field and suggest priorities for future investigations.
Quantitative meta-analyses have emerged as useful methodological approaches to provide unbiased, objective measures of brain functioning in various clinical populations (43, 44, 45, 46), but none have been conducted in ASD. In contrast to qualitative syntheses of the current literature (10, 41, 11, 47, 48), a quantitative meta-analysis can lead to the identification of regions that might otherwise be over-looked, and is less likely to be driven by prominent theoretical models.
Here, we provide a voxel-wise quantitative meta-analysis using activation likelihood estimation (ALE) (49, 50, 51). The ALE meta-analysis produces voxel-wise formal estimates of probabilities of activation. Using the exact coordinates reported by each study instead of author-assigned anatomical labels, ALE provides better spatial resolution and reduces errors due to overly broad spatial designations or region mislabeling (52).
Given that ASD-related abnormalities extend across multiple cognitive domains, it is important to take into account the impact of domain-specificity. In other words, the ability of meta-analytic techniques to detect consistent ASD-related abnormalities in a given region likely depends on the specific processing domain examined. For example, an extensive literature in neurotypical subjects supports the hypothesis that studies examining social cognition would show ASD-related hypoactivation in the perigenual ACC/rostral medial prefrontal cortex (7, 9, 53, 54, 55, 56, 3, 57, 6, 58). As most studies of non-social cognition in ASD included components of executive functions, we anticipated ASD-related hypoactivation in dorsal ACC/pre-supplementary motor area (pre-SMA) and lateral prefrontal regions commonly identified in normative studies (e.g., 59, 60, 61, 62, 63, 64, 65). Fortunately, ALE allows comparing different task domains, even when not directly contrasted in the same studies. Accordingly, we conducted ALE meta-analyses of published functional neuroimaging studies of ASD in both social and non-social domains.
Methods
Article Selection
Using PubMed (http://www.pubmed.org), we searched for English-language, task-based, functional neuroimaging studies of ASD published between 1990 and January 2008 with the keywords “autism,” “fMRI,” “PET,” “neuroimaging,” “PDD-NOS,” and “Asperger.” Abstracts of initially identified articles were first reviewed as the basis for selecting papers for full-text review. References cited in the selected articles were also reviewed. We included studies where both ASD and NC groups were examined and within-group foci were available in standardized stereotaxic space (Talairach (66) or Montreal Neurological Institute (MNI) atlases). To preserve data interpretability, we included only task-based fMRI and blood flow PET studies. Likewise, because few papers reported deactivations, only foci of significant activations were included. When more than one paper was based on the same sample, we selected the first published study. Thirty-nine studies met inclusion criteria comprising 453 NC and 479 subjects with ASD. Most (79%) studies focused on adults (group mean age >18 years), the remaining on school-age children and adolescents (weighted mean ages 28.2 and 27.7 years for adults with ASD and NC, respectively, and 12.7 years for youths in both groups). Of the 39 studies included, 24 tested paradigms related to social cognition such as theory of mind, face processing, and emotional processing and were classified as the Social Study group. The remaining 15 studies mostly examined executive functions ranging from spatial attention, interference control, working memory and motor control. They were grouped as Non-Social studies. This distinction provided a sufficient number of foci within each diagnostic group per domain to yield reliable ALE results (Social: 290 and 220 foci; Non-Social: 189 and 139 foci, for NC and ASD, respectively). See Figure 1 for study selection flow diagram and Table 1 for study characteristics.
Figure 1.
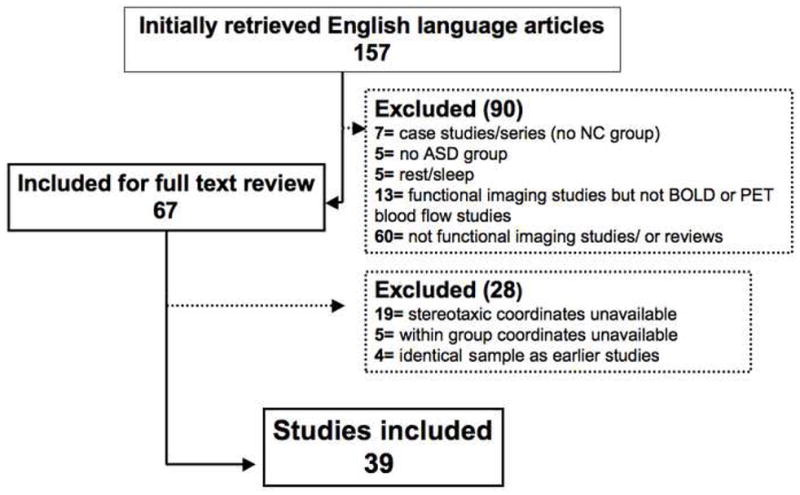
Article Selection Flow. Number of studies selected and reasons for exclusion.
Table I.
Characteristics of the included studies
| Article/Ref. # | Imaging modality | ASD | NC | Task | Contrast | # foci | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Age M (SD)* | n | Age M (SD) | NC | ASD | ||||
| Social (24) | |||||||||
| Ashwin et al., 2006** (144) | fMRI | 13 | 31.2 (9.1) | 13 | 25.6 (5.1) | Facial processing | Faces vs. scrambled faces | 5 | 1 |
| Hubl et al., 2003** (145) | fMRI | 10 | 27.7 (7.8) | 10 | 25.3 (6.9) | Facial processing | Faces vs. scrambled faces | 4 | 4 |
| Schultz et al., 2000** (20) | fMRI | 14 | 23.8 (12.4) | 14 | 21.7 (7.2) | Facial processing | Faces vs. objects | 1 | 1 |
| Bird et al., 2006** (146) | fMRI | 16 | 33.3 (12.1) | 16 | 35.3 | Facial processing | Faces vs. houses | 5 | 5 |
| Deeley et al., 2007** (147) | fMRI | 9 | 34 (10) | 9 | 27 (5) | Facial processing | Neutral faces vs. fixation | 21 | 25 |
| Kleinhans et al., 2008** (148) | fMRI | 19 | 23.5 (7.8) | 21 | 25.1 (7.6) | Facial processing- one back task | Neutral faces vs. houses | 1 | 3 |
| Koshino et al., 2007** (149) | fMRI | 11 | 24.5 (10.2) | 11 | 28.7 (10.9) | Facial processing n-back WM | n-back face task vs. fixation | 15 | 9 |
| Dichter et al., 2006** (150) | fMRI | 14 | 22.9 (5.2) | 15 | 23.2 (5.7) | Flanker gaze task | Congruent vs. Incongruent gaze | 8 | 2 |
| Dapretto et al., 2005# (151) | fMRI | 10 | 12.05 (2.5) | 10 | 12.38 (2.2) | Imitating facial expressions | Face Imitation vs. rest | 36 | 16 |
| Wang et al., 2004# (152) | fMRI | 12 | 12.2 (4.8) | 12 | 11.8 (2.5) | Facial emotion processing | Faces (angry/fearful) vs. geometric forms | 10 | 11 |
| Hall et al., 2003# (153) | PET | 8 | 20–33 | 8 | n.a | Facial emotion processing | Emotion vs. gender | 6 | 7 |
| Critchley et al., 2000# (22) | fMRI | 9 | 37 (7) | 9 | 27 (7) | Facial emotion processing | Emotional vs. neutral faces | 6 | 3 |
| Pelphrey et al., 2007# (154) | fMRI | 8 | 24.5 (11.5) | 8 | 24.1 (5.6) | Facial emotion processing | Dynamic vs. static emotional face | 6 | 1 |
| Pelphrey et al., 2005# (155) | fMRI | 10 | 23.2 (9.9) | 9 | 23.4 (5.8) | Intentional eye gaze processing | Congruent vs. incongruent eye gaze | 7 | 4 |
| Pierce et al., 2004# (156) | fMRI | 7 | 27.1 (9.2) | 9 | (16–40) | Facial processing | Familiar vs. stranger faces | 9 | 4 |
| Pinkham et al., 2008# (157) | fMRI | 10 | 24.08 (5.71) | 12 | 27.08 (3.99) | Trustworthiness face task | Trustworthy faces vs. baseline | 6 | 6 |
| Baron-Cohen et al., 1999# (16) | fMRI | 6 | 26.3 (2.1) | 12 | 25.5 (2.8) | Inferring mental states/eye | Emotion vs. gender | 53 | 29 |
| Castelli et al., 2002 (158) | PET | 10 | 33 (7.6) | 10 | 25 (4.8) | Inferring mental states/ animated shapes | ToM animation vs. random animation | 10 | 10 |
| Happe et al., 1996 (13) | PET | 5 | 24 | 6 | 38 | Story comprehension | Tom story vs. unconnected sentences | 4 | 4 |
| Gervais et al., 2004 (159) | fMRI | 5 | 25.8 (5.9) | 8 | 27.1 (2.9) | Voice processing | Vocal vs. non-vocal sounds | 6 | 0 |
| Wang et al., 2006 (160) | fMRI | 18 | 11.9 (2.8) | 18 | 11.9 (2.3) | Judging sentences sarcasm | Sarcastic sentences vs. rest | 16 | 19 |
| Wang et al., 2007 (15) | fMRI | 18 | 12.5 (2.9) | 18 | 11.8 (1.9) | Cartoon irony/sarcasm task | Ironic cartoons vs. non-ironic cartoons | 35 | 32 |
| Mason et al., 2008 (161) | fMRI | 18 | 26.5 | 18 | 27.4 | Inference reading comprehension task | intentional inference vs. fixation | 13 | 20 |
| Williams et al., 2006 (162) | fMRI | 16 | 15.4 (2.24) | 15 | 15.5 (1.6) | Action imitation task | Imitation vs. action execution | 7 | 4 |
| Non-social (15) | |||||||||
| Ring et al., 1999 (39) | fMRI | 6 | 26.3 (2.1) | 12 | 25.5 (2.8) | Embedded figure task | Task vs. fixation | 10 | 10 |
| Manjaly et al., 2007 (38) | fMRI | 12 | 14.4 (2.7) | 12 | 14.3 (2.7) | Embedded figure task | Embedded figure task vs. control task | 2 | 4 |
| Lee et al., 2007 (37) | fMRI | 12 | 13.5 (1.6) | 12 | 13.8 (1) | Embedded figure task | Embedded vs. matching task | 11 | 3 |
| Just et al., 2007 (131) | fMRI | 18 | 27.1 (11.9) | 18 | 24.5 (9.9) | Tower of London task | Hard vs. easy condition | 13 | 19 |
| Kennedy et al., 2006 (114) | fMRI | 15 | 25.5 (9.6) | 14 | 26.1 (8) | Stroop task | Number vs. rest | 8 | 8 |
| Koshino et al., 2005 (115) | fMRI | 14 | 25.7 | 13 | 29.8 | N-back working memory task | n-back task vs. fixation | 24 | 24 |
| Schmitz et al., 2006 (163) | fMRI | 10 | 38 (8) | 12 | 39 (9) | Go/No-Go | No-Go vs. go | 11 | 6 |
| Haist et al., 2005 (164) | fMRI | 8 | 23.4 (11.4) | 8 | 25.6 (3.8) | Spatial attention task | Spatial target vs. null | 40 | 1 |
| Belmonte et al., 2003 (165) | fMRI | 6 | 32.7 (9.8) | 6 | 27.2 (4.4) | Visual spatial attention task | Task vs. fixation | 7 | 4 |
| Schmitz et al., 2008 (116) | fMRI | 10 | 37.8 (7) | 10 | 38.2 (6) | CPT task with monetary incentive | Rewarded vs. non-rewarded stimuli | 5 | 4 |
| Gomot et al., 2006 (166) | fMRI | 17 | 10.37 (1.52) | 14 | 10.87 (1.47) | Auditory change detection | Novel vs. standard sound | 17 | 17 |
| Gaffrey et al., 2007 (72)$ | fMRI | 10 | 26.1 (10.5) | 10 | 25.3 (9.8) | Semantic category | Semantic vs. perceptual task | 14 | 13 |
| Harris et al., 2006 (71) $ | fMRI | 14 | 36 (12) | 22 | 31 (9) | Single word lexical semantic processing | Concrete vs. abstract words | 8 | 4 |
| Just et al., 2004 (70) $ | fMRI | 17 | n.a. | 17 | n.a. | Sentence comprehension | Sentence vs. fixation | 8 | 10 |
| fMRI | 8 | 28.4 (8.9) | 8 | 28.5 | Paced finger tapping | Tapping vs. rest | 11 | 12 | |
The 39 studies included in the meta-analysis were subdivided into a social and non-social task-class based on the paradigm used. Overall a total of 359 and 479 foci were included for the ASD and the NC group, respectively.
M age (SD) = mean age of subjects ± standard deviation are reported if available in published article. ASD =autism spectrum disorders, fMRI = functional magnetic resonance imaging, NC = neurotypical controls, n.a.= not available , PET = positron emission tomography, Ref = reference #; Tom = Theory of Mind.
grouped as face form perception study;
grouped as facial emotional/intentional expression studies; these two study groups formed the face processing study group for post-hoc analyses.
these studies were excluded in the secondary analysis of non-socials studies of executive processes only.
Meta-Analyses
For each study, statistically significant foci of activation from one contrast were included (see Table 1). When more than one contrast was reported (31% of studies), we selected the broadest comparison available in order to better detect common group differences across studies. MNI coordinates were converted to Talairach space using Brett’s transformation (67). Meta-analyses were conducted using ALE (51) implemented in BrainMap (50, 52). We first conducted a meta-analysis within each group (i.e., NC, ASD). Then, to directly compare the groups, we ran a subtraction meta-analysis. Specifically, for each group, ALE maps were generated by modeling each equally weighted activation peak using a 3-D Gaussian probability density function centered at the given coordinates. For any given voxel, meta-analytic significance results from the degree of spatial overlap of independently produced 3-D Gaussian probability density functions. In agreement with other ALE meta-analyses (50, 51, 44, 45, 43, 68), we set FWHM = 10mm to account for spatial resolution limitations and inter-individual differences in anatomic variability. Next, voxel-wise likelihoods of activation for the two ALE maps (ASD, NC) were calculated using permutation testing (5000 permutations), and corrected for multiple comparisons using false discovery rate (p<0.05, corrected), with a cluster extent threshold of eight voxels. As these foci were generated randomly, no assumption was made as to their spatial location or separation within the brain. The group subtraction meta-analysis yielded an ALE map of the regions in which the two groups differed significantly (43, 50). The difference ALE maps were then permuted and statistically corrected to generate voxel-wise statistical scores.
Task-Domain Analyses
Prior to conducting group analyses, we verified the presence of significant differences in neural activation for the two processing domains (social, non-social), by conducting a subtraction ALE meta-analysis between social and non-social study foci across both groups (NC, ASD) combined. We then examined ASD-related differences in activation for each processing domain, by performing within- and between-group ALE comparisons (i.e., ASD > NC, NC >ASD) for the social and non-social domains separately. Given the prominence of studies examining face processing, we conducted post-hoc analyses on ASD-related differences across the 16 studies employing faces, as well as sub-groupings of face form perception (e.g., faces vs. scrambled faces; 8 studies), and facial emotional/intentional expression (e.g., emotion vs. gender; 9 studies).. Finally, to examine a more homogeneous group of Non-Social studies, we conducted a secondary meta-analysis of those papers focusing on executive functions, thus excluding works on language processing and paced finger tapping (69, 70, 71, 72). See Table 1
Results
Social versus Non-Social Studies: Both Groups Combined
As shown in Supplementary Figure 1, direct comparisons between social and non-social studies resulted in a broad functional distinction of the ACC (here defined as anterior cingulate gyrus proper and paracingulate gyrus (73, 74, 75, 76)). Specifically, consistent with the hypothesized linkage to mentalizing, person perception, joint attention, and self-knowledge (e.g., 7), a cluster centered at the perigenual ACC (pgACC; BA32) and extending anteriorly toward the rostral medial prefrontal cortex (MPFC; BA10), and a cluster in posterior cingulate cortex (PCC) showed greater probability of activation in social studies compared to non-social studies. In contrast, non-social studies displayed significantly larger likelihood of activation in a cluster extending from dorsal ACC (dACC; BA32) to neighboring pre-SMA (BA6). Other regions typically implicated in different aspects of social cognition, such as the right amygdala, the posterior cingulate, and a region extending from the right superior temporal gyrus deep to the anterior insula, showed significantly larger likelihood of activation in the social studies. Bilateral mid-FG was also highlighted, as expected given the high proportion of face processing studies.
Social Studies: NC versus ASD
Between-group comparisons of the individual group ALE maps for the social studies (see Supplementary Table 1 for within-group clusters of activation) showed that NC had significantly higher probability of activation in those clusters consistently activated in the combined group analysis for social studies. These included pgACC, right amygdala, and left FG. Accompanying these areas that are classically involved in social cognition (e.g., 7, 9, 57, 56) and that have also been implicated in ASD (2, 3, 77), NC also showed greater probability of activation in the right anterior insula (AI), related to the attribution of emotions to others and oneself (78), and in the posterior cingulate, implicated in attribution of emotional salience, episodic memory and self-referential processing (79, 80, 81). See Table 2, Figures 2 and 3. In contrast, the ASD group displayed greater probability of activation in somato-sensory regions, such as postcentral gyrus, posterior portions of the superior temporal gyrus, inferior occipital gyrus, and posterior-lateral FG, but not in medial wall areas, nor in the sublobar regions specifically related to social processing that were revealed in the comparison between social and non-social studies (See Table 2, Figure 2). Post-hoc analyses limited to the 17 face processing studies revealed ASD-related differences highly similar to those obtained in the primary social study analysis, with a notable difference in FG activation. When limited to face processing studies, ASD-hypoactivation of FG was noted bilaterally. In the primary meta-analysis including all social studies, FG hypoactivation in ASD reached significance only in the left hemisphere with subthreshold right-sided differences. When face processing studies were further divided into those requiring perception of facial forms and those focusing on facial expressions, ASD-related abnormalities in the medial wall (pgACC, PCC) were only detectable in the facial expression studies. Fusiform gyrus hypoactivations in face form processing studies were limited to the right FG and were located more posteriorly than the loci resulting from the facial expression studies. See Supplementary Figure 2.
Table 2.
Group Comparisons of Regions with Significantly Elevated Likelihood of Activation: Social Studies
| BA | Volume (mm3) | Talairach | ALE (X 10−2)* | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| NC > ASD | ||||||
| Precentral Gyrus R & L | 6 | 128 | 48 | −2 | 38 | 1.10 |
| 6 | 296 | −44 | −5 | 37 | 1.18 | |
| 44 | 248 | 54 | 11 | 8 | 1.22 | |
| Middle Frontal Gyrus L | 9 | 168 | −44 | 16 | 36 | 1.13 |
| Inferior Frontal Gyrus R & L | 46 | 872 | 49 | 20 | 23 | 1.61 |
| 47 | 112 | 40 | 22 | −13 | 1.03 | |
| 47 | 400 | −43 | 27 | 1 | 1.35 | |
| Anterior Cingulate | 32 | 600 | 0 | 47 | 6 | 1.43 |
| Subcallosal Gyrus | 34 | 176 | −24 | 5 | −14 | 1.19 |
| Cingulate Gyrus | 24 | 184 | 5 | 4 | 42 | 1.24 |
| Inferior Parietal Lobule /Angular Gyrus | 7 | 392 | −32 | −55 | 43 | 1.46 |
| Superior Temporal Gyrus R & L | 22 | 248 | 51 | −10 | 2 | 1.05 |
| 22 | 816 | −63 | −27 | 2 | 2.03 | |
| Insula/Superior Temporal Gyrus R | 13/38 | 488 | 47 | 11 | −6 | 1.54 |
| Posterior Cingulate | 30 | 176 | 0 | −49 | 18 | 1.19 |
| Parahippocampal Gyrus Amygdala R | 712 | 19 | −7 | −10 | 1.68 | |
| Fusifom Gyrus (middle) L | 208 | −35 | −57 | −11 | 1.21 | |
| Lingual Gyrus L | 248 | −17 | −78 | −13 | 1.30 | |
| Middle Occipital Gyrus L | 19 | 200 | −51 | −68 | 8 | 1.15 |
| Inferior Occipital Gyrus R | 1 | 200 | 23 | −88 | −9 | 1.12 |
| Thalamus R | 448 | 28 | −27 | 1 | 1.33 | |
| ASD > NC | ||||||
| Precentral Gyrus L | 9 | 328 | −43 | 6 | 31 | 1.21 |
| Postcentral Gyrus L | 3 | 136 | −37 | −32 | 54 | 1.12 |
| Middle Temporal Gyrus L | 22 | 208 | −48 | −44 | 9 | 1.20 |
| Superior Temporal Gyrus R | 22 | 616 | 56 | −28 | 5 | 1.42 |
| Inferior Temporal Gyrus R | 37 | 104 | 45 | −64 | −8 | 1.07 |
| 37 | 240 | 49 | −46 | −14 | 1.25 | |
| Fusiform Gyrus (posterior-lateral) L | 37 | 504 | −36 | −70 | −14 | 1.17 |
| Inferior Occipital Gyrus L | 18 | 224 | −27 | −86 | −4 | 1.16 |
Brain regions labels, their corresponding Brodmann area (BA), and Talairach coordinates of the weighted center for each cluster showing greater probability of activation resulting from group subtraction using only the social study foci. Anatomical labels are based on the Talairach atlas.
Each cluster was observed with a peak p value ≤ 0.01, corrected; Activation Likelihood Estimates (ALE) are reported. L: Left; R: Right.
Figure 2.
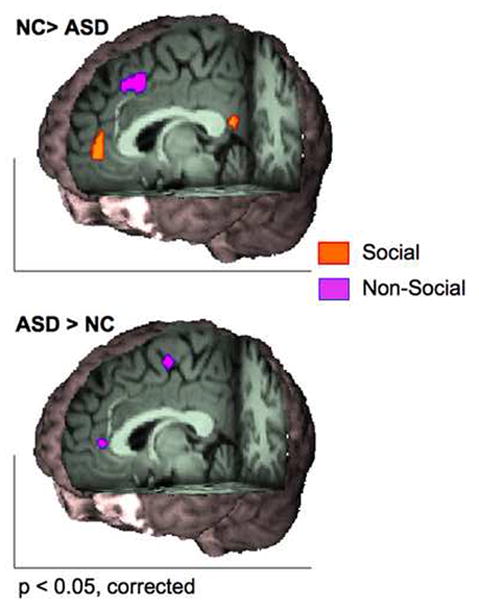
Medial-wall task-based group difference emerging from the separately conducted group subtractions including social studies only (orange) and non-social studies only (purple). The top panel shows greater probability of activation in neurotypical controls (NC) compared to autism spectrum disorders (ASD) in a cluster centered at the perigenual anterior cingulate cortex (ACC; x= 0, y= 47, z= 6) and posterior cingulate (x= 0, y= −49 , z= 18) for the analysis limited to social studies (red orange), while greater activation in a cluster centered at the pre-supplementary motor area (x= 0, y=19, z= 46) resulted from the analysis limited to non-social studies (blue-violet). The bottom panel shows ASD > NC activation likelihood estimate maps in a cluster centered in ventral ACC (x= 0, y= 40, z= 8), and in the supplementary motor area (x= 0, y= −10, z= 58) appearing only in the analysis of non-social studies. Images are displayed in neurological convention (right is right).
Figure 3.
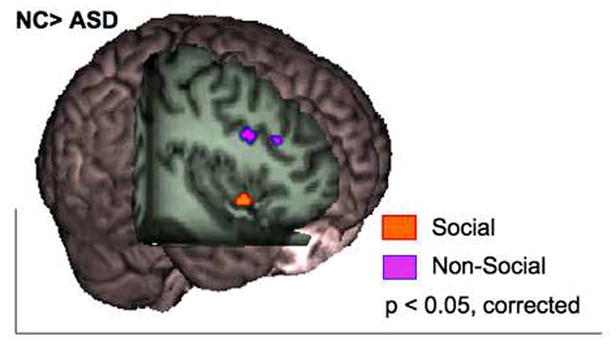
For social studies, neurotypical controls (NC) showed greater likelihood of activation in the right anterior insula when compared to participants with autism spectrum disorders (orange; x= 47, y= 11, z= −6). For non-social studies, NC exhibited greater activation in the middle frontal gyrus (purple; x = 40; y = 13; z = 27; x= 42; y=27; z= 26). Image displayed in neurological convention.
Non-Social Studies: NC versus ASD
Neurotypical controls showed a greater likelihood of activation in a cluster extending from pre-SMA to dACC, which is typically implicated in cognitive control (e.g., 82) (See Figure 2). Similar differences with the NC group showing greater likelihood of activation appeared in DLPFC (BA 9/10), and lateral parietal cortex such as supramarginal gyrus and inferior parietal lobule (BA40). This group subtraction also revealed atypical regional recruitment in ASD compared to NC. Specifically, in contrast to the pre-SMA/dACC region that was more likely to be activated in NC, the ASD group showed a greater probability of activation in the more posterior SMA proper which is typically related to lower-order motor planning (83). Similarly, meta-analysis of non-social studies revealed a greater likelihood of ASD-activation in the pgACC (BA32) which is typically implicated in social paradigms (See Table 3 and Figure 2). Secondary analyses, limited to non-social studies focusing on executive function revealed substantially unchanged ASD-related hypo-activations. By contrast, only hyperactivation of rostral ACC in ASD compared to NC in rostral ACC remained in this more restricted analysis (See Supplementary Figure 3 and Supplementary Table 2).
Table 3.
Group Comparisons of Regions with Significantly Elevated Likelihood of Activation: Non-Social Studies
| BA | Volume (mm3) | Talairach | ALE (X 10−2)* | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| NC > ASD | ||||||
| Medial Frontal Gyrus R | 6 | 1856 | 1 | 19 | 46 | 1.54 |
| Middle Frontal Gyrus R & L | 9 | 656 | 40 | 13 | 27 | 1.37 |
| 9 | 168 | 42 | 27 | 26 | 0.92 | |
| 10 | 256 | −33 | 57 | 7 | 1.04 | |
| Superior Parietal Lobule R | 7 | 168 | 22 | −67 | 46 | 0.94 |
| Inferior Parietal Lobule L | 40 | 472 | −49 | −48 | 38 | 1.19 |
| Supramarginal Gyrus R | 40 | 168 | 51 | −51 | 33 | 0.93 |
| Claustrum/Insula R | 13 | 256 | 30 | 14 | 11 | 1.14 |
| Superior Temporal Gyrus R | 22 | 256 | 57 | −47 | 13 | 1.08 |
| Lingual Gyrus L | 17 | 104 | −20 | −93 | 0 | 0.89 |
| Thalamus L | 336 | −12 | −1 | 10 | 1.27 | |
| ASD > NC | ||||||
| Medial Frontal Gyrus | 6 | 360 | 0 | −10 | 58 | 1.24 |
| Inferior Frontal Gyrus L | 9 | 296 | −51 | 20 | 20 | 1.19 |
| Anterior Cingulate | 32 | 200 | 0 | 40 | 8 | 1.03 |
| Superior Temporal Gyrus L | 39 | 208 | −52 | −52 | 12 | 1.09 |
| Middle Occipital Gyrus L | 18 | 352 | −23 | −93 | 15 | 1.15 |
| Lingual Gyrus L | 224 | −14 | −84 | −8 | 1.17 | |
| Lateral Geniculum Body R & L | 320 | 24 | −25 | −5 | 1.23 | |
| 136 | −22 | −27 | −2 | 0.96 | ||
| Declive R | 176 | 30 | −78 | −18 | 1.03 | |
Brain regions labels, their corresponding Brodmann area (BA), and Talairach coordinates of the weighted center for each cluster showing greater probability of activation resulting from group subtraction using only the non-social study foci. Anatomical labels are based on the Talairach atlas. Each cluster was observed with a peak p value ≤ 0.01, corrected; Activation Likelihood Estimates (ALE) are reported. L: Left; R: Right.
Discussion
This quantitative meta-analysis revealed ASD-related abnormalities (both decreases and increases) in probabilities of activation in distributed regions, which appeared largely domain-specific. The primary distinction is between studies focusing on social processing versus those examining non-social cognition, typically pertaining to executive function.
Social-Related Abnormalities
The findings emerging from the meta-analyses of tasks examining social functioning (e.g., mentalizing, emotional processing) not only revealed hypofunction in regions classically associated with social impairments in ASD (i.e., pgACC, anterior rostral MPFC, and amygdala), but highlighted previously overlooked regions. In particular, the right anterior insula (AI), a recent focus of attention in the social cognition literature (78, 84, 85, 86, 87, 88), showed decreased likelihood of activation in ASD compared to NC. Insights into AI function have emerged from recent attempts to differentiate the neuronal correlates of mentalizing (i.e., attribution of other’s beliefs, desires and intentions) and empathizing (i.e., understanding and sharing other’s emotions) (78, 87, 89). Rather than being used interchangeably and/or simply being attributed to the MPFC, empathizing has been related to AI cortex, while mentalizing has been related to anterior rostral MPFC and adjacent ACC (78, 87, 89, 7). Consistent patterns of hypofunction in right AI cortex support an expanded focus on AI and on efforts to disentangle the relative contributions of ventral MPFC/ACC and AI in studies of ASD (90).
Our meta-analysis also revealed ASD-hypoactivation in PCC. ASD-related abnormalities in this region have been sporadically highlighted (e.g., 91), but its role in the pathophysiology of autism remains under-elaborated. Given recent work suggesting broad impairments in self-referential cognition in ASD (92), a possible link between PCC and ASD stems from studies implicating the PCC in various aspects of self-referential processing (e.g., representing self-mental states) (93, 81, 53). This intriguing hypothesis remains provisional as ASD-related deficits in self-referential cognition have yet to be extensively examined (94). The mirror neuron system (95) has been also implicated in ASD (96) based on its role in action understanding (97, 98, 99), awareness of the embodied-self (100), and of self-intentions and -emotions (93, 101, 102). We found ASD-hypoactivation in a mirror neuron system area (the right pars opercularis) but in both social and non-social studies. Further behavioral and neurological investigations are needed to determine the relevance of the mirror neuron system for ASD symptomatology.
Consistent with the amygdala theory of autism (77), we found right amygdala hypoactivation. Amygdala dysfunction was initially linked to ASD due to the region’s proposed role in evaluating facial expression and representing affective salience (57), and more recently, by work directly implicating it in the development of mentalizing (103). However, the nature of amygdala abnormalities in “autistic like” behaviors remains unclear. While our meta-analysis suggests that ASD is characterized by amygdala hypofunction, a recent fMRI study demonstrated amygdala hyperactivity in ASD, and related it to ASD-related phenomena such as diminished eye-gaze fixation (18). Similarly, amygdala lesions in monkeys produced increases in social interaction, not decreases (104). Such inconsistencies may reflect the functional and structural complexities of the amygdala, or possible differences in specific paradigms employed for amygdala activation.
Finally, consistent with the neurotypical literature (105), post-hoc analyses suggested that right hemisphere abnormalities in FG were limited to studies employing face processing tasks. Furthermore, they suggest that localization of FG abnormalities may be sensitive to the specific task employed, with hypoactivation observed for tasks assessing facial expression extending more ventrally than those examining facial form perception. However, the direct role of FG hypoactivation in the pathophysiology of ASD remains unclear in light of recent findings of lack of ASD differences in FG after controlling for fixation or time of eye-gaze, or during processing of familiar faces (24, 18, 19).
Non-Social Related Abnormalities
The analyses of non-social studies revealed ASD-related hypoactivation in regions typically implicated in top-down cognitive control processes (82, 62, 60, 63, 106, 107, 108). Such findings agree with empirical evidence of executive dysfunction in ASD (28, 36, 32, 109, 110, 111) and support the importance of examining broader cognitive functions beyond social cognition in ASD (112, 32, 113). Given the centrality of language abnormalities in autism, we examined whether findings for non-social studies depended on verbal paradigms. Only six of the 15 non-social studies employed verbal stimuli (114, 115, 116, 72, 71, 70); removing them from the non-social meta-analysis did not change the pattern of results appreciably (data not shown).
Atypical Activations in ASD
We found ASD-related patterns of hypofunction accompanied by abnormal recruitment of activity in lower-order processing systems. For example, while NC consistently activated mid-FG, commonly associated with face identity (117, 118, 119, 120), the ASD group exhibited consistent patterns of activation in the posterior lateral portion of the FG typically associated with physical aspects of face processing (117, 119). Similarly, for non-social tasks, NC consistently recruited activity in dACC/pre-SMA regions associated with attentional and motor control (82, 62, 60, 63, 106, 107, 108), while the ASD group recruited activity in the SMA proper, which is linked to more rote aspects of motor planning (83).
Finally, meta-analyses showed that the failure to activate dACC/pre-SMA regions during non-social tasks in the ASD group was accompanied by inappropriate recruitment of activity in the pgACC region activated during social tasks in NC. This finding is intriguing given recent studies highlighting the default mode network (DMN) as a novel locus of dysfunction in autism (121, 114). Motivated by reports that DMN deactivation facilitates performance of attentionally demanding tasks (122, 123, 124, 125), failure to suppress DMN was found in adults with ASD during a Stroop task and was related to clinical measures of autism severity (114). Parallel lines of research have demonstrated ASD-related compromises in both structural and functional integrity of long-range connections within the DMN (126, 127, 128). Such findings support the dysconnection model of autism (113, 129, 130, 131), emphasizing the potential importance of abnormalities in functional and structural connections between regions, rather than focal abnormalities alone. Future studies should characterize ASD-related abnormalities in both DMN integrity and the mechanisms by which the DMN and related networks (122, 125, 132) are regulated.
A Compelling Need for a Developmental Perspective
In discussing the neural basis of any childhood-onset disorder, an obvious concern is the degree to which group differences interact with development. This is especially true for ASD, where the nature of differences in gray and white matter volumes change during early development (133, 134, 135, 136). The current functional literature is dominated by studies in adults who are better able to minimize movement during scanning and comply with task demands, which limited our ability to detect age-related changes in the neural correlates of autism. Given the protracted development of the neocortical mentalizing areas BA10 and pgACC (137, 138, 139, 140), as well as dACC and its related cognitive functions (141, 142), it is likely that the pattern of ASD-activation in the ACC regions emphasized in this study will differ in pediatric samples. Future investigations will need to both place greater focus on examining young children with autism and provide direct comparisons of children and adults to clarify this issue.
Limitations
The present work has several limitations common to efforts to synthesize the psychiatric neuroimaging literature (143, 43). First, several studies did not report voxel-wise direct group comparisons. While ALE meta-analytic approaches allowed us to compare data provided by group, the paucity of direct between-group comparisons potentially limited our ability to detect more subtle group differences. Second, although the number of studies and the related number of foci included in the present work were substantial and comparable to other meta-analyses of clinical populations (43, 143), many studies which did not report stereotaxic coordinates were excluded, also limiting our power to detect more subtle differences. A priori regions-of-interest used by several investigators also limited our ability to discern novel regional differences. As for any meta-analyses, type II errors due to publication bias cannot be ruled out. Despite these limitations, we were able to detect meaningful consistent results, which future work can confirm through direct experimentation with sufficiently large samples. Finally, 70% of the studies within each study class used block designs, which though efficient, are potentially susceptible to the development of strategic processing and/or habituation which may differ between groups. Accordingly, event-related designs should be emphasized in future task-based approaches.
Multiple limitations with respect to ALE should also be noted. First, the current version of ALE weights all studies equally, regardless of potential differences in sample sizes. However, NC and ASD samples were comparable across studies, so that this simplification did not likely impact the groups differentially. Second, ALE does not allow covarying potential confounders such as IQ, which is strongly associated with ASD. Fortunately, most studies group-matched patient and control groups for IQ, and mean IQ for NC and ASD groups did not differ across studies. Finally, to attain a sufficiently large number of foci as recommended for ALE (≥100), we heuristically divided domains into those corresponding to social and non-social studies. The non-social domain in particular could be characterized as arbitrary, as it mostly included an heterogeneous set of executive functions. Nevertheless the ability of ALE to demonstrate consistent findings which accorded with normative results suggests that this approach can be effective even in the face of such task heterogeneity. Future imaging studies of ASD may benefit from fractionating social and non-social domains into their major sub-components.
Conclusions
Meta-analyses of the existent ASD neuroimaging literature provided evidence of 1) the dependence of ASD-related patterns of hypofunction on the specific cognitive domains examined (e.g., dACC/preSMA for non-social, pgACC for social); 2) ASD-related abnormalities in regions commonly highlighted in neurobiological models (e.g., pgACC/MPFC, and amygdala), as well as regions only beginning to receive attention in relation to ASD (e.g., AI and PCC); 3) inappropriate recruitment of lower-order processing regions (e.g., SMA) in place of higher-order regions (e.g., dACC/pre-SMA); 4) abnormalities in the default-mode network (e.g., rostral ACC and PCC hypoactivation for social studies and abnormal activation in rostral ACC for non-social studies). Despite the limitations of the current neuroimaging literature on ASD, the remarkable overall coherence of these meta-analytical results appears to provide a solid basis for future work with even greater specificity.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Drs. Amy Krain Roy and A. M. Clare Kelly for their suggestions on an earlier version of this manuscript.
Support: This work was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award to ADM, and by grants from the Stavros S. Niarchos Foundation and NIMH (5T32MH0667762) to FXC.
Footnotes
Financial disclosure: All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 2.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- 4.Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- 5.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 6.Brothers L. The Social brain: a project for integrating primate behavior and neuropsychology a new domain. Concepts in Neuroscience. 1990;1(1):27–50. [Google Scholar]
- 7.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 8.Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:724–732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher HL, Frith CD. Functional imaging of 'theory of mind'. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 10.Williams DL, Minshew NJ. Understanding Autism and Related Disorders: What has Imaging Taught Us? Neuroimaging Clin N Am. 2007;17:495–509. doi: 10.1016/j.nic.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 12.Lainhart JE. Advances in autism neuroimaging research for the clinician and geneticist. Am J Med Genet C Semin Med Genet. 2006;142:33–39. doi: 10.1002/ajmg.c.30080. [DOI] [PubMed] [Google Scholar]
- 13.Happe F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, et al. 'Theory of mind' in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- 14.Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 15.Wang AT, Lee SS, Sigman M, Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 17.Ashwin C, Wheelwright S, Baron-Cohen S. Finding a face in the crowd: testing the anger superiority effect in Asperger Syndrome. Brain Cogn. 2006;61:78–95. doi: 10.1016/j.bandc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 20.Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 21.Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform 'face area' in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 22.Critchley HD, Daly EM, Bullmore ET, Williams SC, van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- 23.Piggot J, Kwon H, Mobbs D, Blasey C, Lotspeich L, Menon V, et al. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. J Am Acad Child Adolesc Psychiatry. 2004;43:473–480. doi: 10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Belmonte MK, Cook EH, Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- 26.Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Landa RJ, Goldberg MC. Language, Social, and Executive Functions in High Functioning Autism: A Continuum of Performance. J Autism Dev Disord. 2005:1–17. doi: 10.1007/s10803-005-0001-1. [DOI] [PubMed] [Google Scholar]
- 28.Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biol Psychiatry. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Russell J, Hill EL. Action-monitoring and intention reporting in children with autism. J Child Psychol Psychiatry. 2001;42:317–328. [PubMed] [Google Scholar]
- 30.Goldstein G, Johnson CR, Minshew NJ. Attentional processes in autism. J Autism Dev Disord. 2001;31:433–440. doi: 10.1023/a:1010620820786. [DOI] [PubMed] [Google Scholar]
- 31.Christ SE, Holt DD, White DA, Green L. Inhibitory control in children with autism spectrum disorder. J Autism Dev Disord. 2007;37:1155–1165. doi: 10.1007/s10803-006-0259-y. [DOI] [PubMed] [Google Scholar]
- 32.Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. Int J Dev Neurosci. 2008;26:239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liss M, Saulnier C, Fein D, Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10:155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- 34.Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, et al. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism network. J Autism Dev Disord. 2004;34:139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- 35.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, et al. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- 37.Lee PS, Foss-Feig J, Henderson JG, Kenworthy LE, Gilotty L, Gaillard WD, et al. Atypical neural substrates of Embedded Figures Task performance in children with Autism Spectrum Disorder. Neuroimage. 2007;38:184–193. doi: 10.1016/j.neuroimage.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manjaly ZM, Bruning N, Neufang S, Stephan KE, Brieber S, Marshall JC, et al. Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. Neuroimage. 2007;35:283–291. doi: 10.1016/j.neuroimage.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ring HA, Baron-Cohen S, Wheelwright S, Williams SC, Brammer M, Andrew C, et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain. 1999;122:1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- 40.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Muller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belmonte MK, Mazziotta JC, Minshew NJ, Evans AC, Courchesne E, Dager SR, et al. Offering to Share: How to Put Heads Together in Autism Neuroimaging. J Autism Dev Disord. 2007 doi: 10.1007/s10803-006-0352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2007;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Martino A, Castellanos FX. Functional neuroimaging of social cognition in pervasive developmental disorders: a brief review. Ann N Y Acad Sci. 2003;1008:256–260. doi: 10.1196/annals.1301.027. [DOI] [PubMed] [Google Scholar]
- 48.Cody H, Pelphrey K, Piven J. Structural and functional magnetic resonance imaging of autism. International Journal of Developmental Neuroscience. 2002;20:421–438. doi: 10.1016/s0736-5748(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 49.Lancaster JL, Laird AR, Fox PM, Glahn DE, Fox PT. Automated analysis of meta-analysis networks. Hum Brain Mapp. 2005;25:174–184. doi: 10.1002/hbm.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 52.Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, et al. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 54.Walter H, Adenzato M, Ciaramidaro A, Enrici I, Pia L, Bara BG. Understanding intentions in social interaction: the role of the anterior paracingulate cortex. J Cogn Neurosci. 2004;16:1854–1863. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- 55.Williams JH, Waiter GD, Perra O, Perrett DI, Whiten A. An fMRI study of joint attention experience. Neuroimage. 2005;25:133–140. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 56.Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 57.Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci. 2007;362:671–678. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 59.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 62.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 64.Posner MI, Digirolamo GJ, Fernandez-Duque D. Brain mechanisms of cognitive skills. Conscious Cogn. 1997;6:267–290. [PubMed] [Google Scholar]
- 65.Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Stuttgart: G. Thieme; 1988. [Google Scholar]
- 67.Brett M. The MNI brain and the Talaitach atlas. Cambridge Imagers; 1999. pp. 12–19. http://www.mrc.cbu.cam.ac.uk/Imaging/mnispace.html. [Google Scholar]
- 68.Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 69.Muller RA, Cauich C, Rubio MA, Mizuno A, Courchesne E. Abnormal activity patterns in premotor cortex during sequence learning in autistic patients. Biol Psychiatry. 2004;56:323–332. doi: 10.1016/j.biopsych.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 71.Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 72.Gaffrey MS, Kleinhans NM, Haist F, Akshoomoff N, Campbell A, Courchesne E, et al. Atypical [corrected] participation of visual cortex during word processing in autism: an fMRI study of semantic decision. Neuropsychologia. 2007;45:1672–1684. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- 74.Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- 75.Steele JD, Lawrie SM. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. Neuroimage. 2004;21:868–875. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 76.Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- 77.Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 78.Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30:855–863. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 79.Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 80.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 81.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 83.Tanji J, Shima K. Supplementary motor cortex in organization of movement. Eur Neurol. 1996;36(Suppl 1):13–19. doi: 10.1159/000118878. [DOI] [PubMed] [Google Scholar]
- 84.Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci. 2005;8:955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- 85.deVignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 87.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 88.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 89.Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 90.Silani G, Bird G, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMR study. Social Neuroscience. 2008;3 (2):97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- 91.Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- 92.Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS ONE. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci. 2007;19:1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- 94.Happe F. Theory of mind and the self. Ann N Y Acad Sci. 2003;1001:134–144. doi: 10.1196/annals.1279.008. [DOI] [PubMed] [Google Scholar]
- 95.Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 96.Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- 97.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 98.Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. Journal of Neurophysiology. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- 99.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 100.Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal "mirror" network in the right hemisphere: an event-related fMRI study. Neuroimage. 2005;25:926–935. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 101.Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others' emotions relates to empathy and interpersonal competence in children. Neuroimage. 2008;39:2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS. The impact of early and late damage to the human amygdala on 'theory of mind' reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- 104.Amaral DG, Bauman MD, Capitanio JP, Lavenex P, Mason WA, Mauldin-Jourdain ML, et al. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- 105.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 106.Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, et al. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- 107.Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- 109.Steele SD, Minshew NJ, Luna B, Sweeney JA. Spatial working memory deficits in autism. J Autism Dev Disord. 2007;37:605–612. doi: 10.1007/s10803-006-0202-2. [DOI] [PubMed] [Google Scholar]
- 110.Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: further evidence for disordered complex information-processing. Child Neuropsychol. 2006;12:279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Solomon M, Ozonoff S, Carter C, Caplan R. Formal thought disorder and the autism spectrum: relationship with symptoms, executive control, and anxiety. J Autism Dev Disord. 2008;38:1474–1484. doi: 10.1007/s10803-007-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9:1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 113.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 116.Schmitz N, Rubia K, VAN AT, Daly E, Smith A, Murphy DG. Neural correlates of reward in autism. Br J Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- 117.Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- 119.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morris JP, Pelphrey KA, McCarthy G. Face processing without awareness in the right fusiform gyrus. Neuropsychologia. 2007;45:3087–3091. doi: 10.1016/j.neuropsychologia.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 122.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 123.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 124.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 126.Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 127.Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion Tensor Imaging of Frontal Lobe in Autism Spectrum Disorder. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62:270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frith C. Is autism a disconnection disorder? Lancet Neurol. 2004;3:577. doi: 10.1016/S1474-4422(04)00875-0. [DOI] [PubMed] [Google Scholar]
- 130.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 131.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Uddin LQ, Clare Kelly AM, Biswal BB, Xavier CF, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 134.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 135.Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol Psychiatry. 2006;59:1–6. doi: 10.1016/j.biopsych.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 136.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 137.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 138.Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc Cogn Affect Neurosci. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dumontheil I, Burgess PW, Blakemore SJ. Development of rostral prefrontal cortex and cognitive and behavioural disorders. Dev Med Child Neurol. 2008;50:168–181. doi: 10.1111/j.1469-8749.2008.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of Anterior Cingulate Functional Connectivity from Late Childhood to Early Adulthood. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn117. In Press. [DOI] [PubMed] [Google Scholar]
- 141.Velanova K, Wheeler ME, Luna B. Maturational Changes in Anterior Cingulate and Frontoparietal Recruitment Support the Development of Error Processing and Inhibitory Control. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev Sci. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- 143.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-Analysis of Structural Imaging Findings in Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2006;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 144.Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET. Differential activation of the amygdala and the 'social brain' during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 145.Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- 146.Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 147.Deeley Q, Daly EM, Surguladze S, Page L, Toal F, Robertson D, et al. An event related functional magnetic resonance imaging study of facial emotion processing in Asperger syndrome. Biol Psychiatry. 2007;62:207–217. doi: 10.1016/j.biopsych.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 148.Kleinhans NM, Schweinsburg BC, Cohen DN, Muller RA, Courchesne E. N-acetyl aspartate in autism spectrum disorders: Regional effects and relationship to fMRI activation. Brain Res. 2007;1162:85–97. doi: 10.1016/j.brainres.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI Investigation of Working Memory for Faces in Autism: Visual Coding and Underconnectivity with Frontal Areas. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35:1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 153.Hall GB, Szechtman H, Nahmias C. Enhanced salience and emotion recognition in Autism: a PET study. Am J Psychiatry. 2003;160:1439–1441. doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- 154.Pelphrey KA, Morris JP, McCarthy G, Labar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2007;2:140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- 156.Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004 doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 157.Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 159.Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, et al. Abnormal cortical voice processing in autism. Nat Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- 160.Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and 'mirror neuron' functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 163.Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 164.Haist F, Adamo M, Westerfield M, Courchesne E, Townsend J. The functional neuroanatomy of spatial attention in autism spectrum disorder. Dev Neuropsychol. 2005;27:425–458. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- 165.Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003;17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- 166.Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, et al. Change detection in children with autism: an auditory event-related fMRI study. Neuroimage. 2006;29:475–484. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 167.Muller RA, Pierce K, Ambrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biol Psychiatry. 2001;49:665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


