Summary
The thymus is an organ vital to proper T cell development, and the regulation of cell survival and death contributes significantly to its efficient function. Vital to many of the developmental processes that occur in the thymus, control over cell survival and death is orchestrated by several signaling processes. In this review, we focus on the regulation of death in early thymocytes known as CD4/CD8 double negative cells, including the roles of interleukin-7 and Bcl-2 family members in this developmental stage. We next consider the survival and death of later thymocytes that express both CD4 and CD8, the “double-positive” thymocytes. These findings are discussed within the context of recent studies demonstrating the existence of caspase-independent cell death pathways.
Keywords: thymocytes, T cells, apoptosis, necroptosis, programmed necrosis, homeostasis, immunological tolerance
Introduction
Among the first cell types to be characterized as undergoing steady state cell death, thymocytes offer valuable insight into the regulation of organ system development and tissue homeostasis. The thymus itself directs the process of early T cell differentiation, and a number of developmental stages depend on life vs. death choices that are intricately programmed. Perhaps unique to the genesis of lymphocytes, the essentially stochastic process involved in the rearrangement of T and B cell antigen receptor genes requires subsequent selective mechanisms that ensure that antigen receptors are minimally functional without being autoreactive. Indeed, the deletion of autoreactive lymphocytes is one of the key means by which “immunological tolerance,” the prevention of autoreactivity is achieved. This elimination of autoreactive lymphocytes during their development is termed “central tolerance,” and is the primary means of elimination of lymphocytes that would otherwise potentiate autoimmune disease. Programmed mechanisms that modulate cell survival vs. cell death are essential for many of these selective processes. Once functional lymphocytes are produced and released to the periphery, additional cell death mechanisms control lymphoid homeostasis, and provide a secondary layer of self-tolerance termed “peripheral tolerance.” Our focus in this review is specifically directed toward the cell death processes that occur within the thymus. For those interested in peripheral tolerance and lymphoid homeostasis, the reader is directed to other reviews on the subject.
Survival and death of early thymocytes, the “double-negative” sub-populations
A thymocyte is first generated from the interaction of a hematopoietic stem cell with a thymic stromal cell. In order for a thymocyte to differentiate into a mature T lymphocyte it must go through maturational stages. The first stage is termed the double negative (DN) stage because thymocytes in this stage lack the CD4 or CD8 co-receptors. The double negative stage can be broken down even further into four successive developmental subsets based on the cell surface expression of the CD117, CD44, and CD25 receptors [1]. Therefore the double negative subsets are as follows: DN1 (CD117+CD44+CD25−), DN2 (CD117+,CD44+,CD25+) DN3 (CD117lo/−,CD44−,CD25+), and DN4 (CD117−,CD44−,CD25−) [Fig. 1]. DN1 cells are not yet committed T cell precursors since they have been shown to be able to develop into other thymus-derived lineages such as dendritic cells and natural killer cells. Transition to the DN2 subset occurs when DN1 cells begin expressing CD25. Transition from DN1 to DN2 also triggers the developmental program leading to a diverse T cell receptor (TCR) repertoire. This is accomplished by distinct α and β TCR chains along with their variable region encoded by several variable (V), joining (J), and diversity (D) gene segments. Besides expressing CD25, the DN2 subset contains joined D and J segments of T cell receptor β chain (TCRβ). The DN2 subset progresses to the DN3 subset by joining of the V gene segment to the DJ segments of the TCRβ chain and losing expression of CD117 and CD44. In order to continue to the DN4 subset, the DN3 subsets must express a functional TCRβ chain and a pre-TCRα chain on the cell surface, a process known as β-selection.
Figure 1.
Development of T cells at the double negative stage. Factors that lead to double negative T cell survival are listed above the cells and factors that lead to cell death are below the cells. The cell-surface phenotype of each subpopulation is shown. The purple boxes indicate gene rearrangements of the TCRβ receptor. The thymic stromal cells are represented in orange. Please see text for more details.
Besides successful β selection, double negative thymocytes must be able to respond to cytokines released from thymic stromal cells in order to survive and transition to the double positive stage [2]. IL-7 and the ligand for CD117, stem cell factor (SCF) are crucial for the survival of double negative thymocytes (Fig. 1). CD117 is expressed on HSCs, and on DN1 through DN2 subsets. Mice deficient in CD117 or SCF contain a 40-fold reduction in DN1 cellularity and no defects in thymocyte development [3]. IL-7 receptor (IL-7R) is expressed on DN2 and DN3 cells and mice deficient in IL-7 or the IL- 7R α chain, CD127, have reduced numbers of thymic cells and such thymocytes fail to proceed past the DN2 subset [4,5].
The basis for the deleterious effects of an IL-7 or IL-7R deficiency is due to the ability of IL-7R signaling to regulate B cell lymphoma 2 (Bcl-2) family members. The Bcl-2 family is composed of pro-apoptotic (Bim, Bax, Bak, Bik, Puma, Noxa, Bad, etc.) and anti-apoptotic (Bcl-2, Bcl-xL, Bcl2-A1, Bcl-w, Mcl-1) proteins. A balance of the Bcl-2 family members is crucial in maintaining mitochondrial outer membrane integrity, which is necessary for cell survival. The link between IL-7 signaling and the ratio in expression of individual Bcl-2 family members that promotes survival of DN cells has been shown in numerous studies. T cell development in IL7Rα−/− mice was restored by overexpressing Bcl-2 in the T cell lineage [6], and through germline deletion of Bim [7]. Conditional deletion of Mcl-1 in developing thymocytes results in increased cell death within the DN2 subset, consequently preventing the development of the DN3 subset [8]. Failure of a DN2 cell line, D1, to receive IL-7 caused translocation of Bad to mitochondria, which resulted in the inhibition of the survival functions of Bcl-2 [9]. In a similar study, loss of IL-7R signaling in D1 cells caused translocation of Bax to mitochondria, leading to apoptotic cell death [10]. Recently, Bak deletion was shown to rescue the survival defect of Mcl-1−/− double negative cells [11]. Thus, a balance in expression of the Bcl-2 family members via IL-7R signaling prevents the demise of the double negative population.
During differentiation of DN cells up to and including the DN2 subset, cell survival is controlled primarily by cytokines secreted from thymic stromal cells, and IL-7 is vital for this stage of thymopoiesis. In the DN3 subset, cell survival is dependent on the ability to generate a pre-TCR composed of a successfully rearranged β chain and the surrogate TCR α chain called “pre-Tα”. Failure of a DN3 cell to productively rearrange a TCR β chain, e.g. in a Rag1- or pre-Tα-deficient thymus, results in the inability of the cell to move beyond the DN3 subset, with most cells dying prior to transition to the DN4 stage [12]. Properly formation of a pre-TCR (e.g. a rearranged β chain a in association with pre-Tα) leads to allelic exclusion of the TCR β loci, thereby blocking further attempts at β chain rearrangement. Surface exposure of the pre-Tα also induces a proliferative burst and expression of a pro-survival factor allowing the cell to proceed to the double positive stage. Bcl2-A1 may be the pro-survival factor since its expression is upregulated by the pre-TCR during β-selection [13] and does not inhibit proliferation, which distinguishes it from its anti-apoptotic family members. Another potential candidate is Akt since it has the ability to protect thymocytes from cell death at the pre-TCR or β-selection checkpoint [14]. Specifically, loss of Akt at the DN3 stage results in cell death following TCR stimulation in vitro due to the cells decreased ability to uptake glucose. Recently, the chemokine receptor CXCR4 has been shown to be necessary for survival following β selection since it has been shown to associate with the pre-TCR and conditional deletion of CXCR4 results in impairment of DN3 maturation [15].
In addition to the differential regulation of Bcl-2 family members, a potential involvement of death receptor (DR) signaling molecules in β-selection has been demonstrated in mice expressing a dominantly interfering form of FADD (FADDdd) in thymocytes [16]. In such mice, expression of FADDdd leads to diminished thymic cellularity, with reduced numbers of DN4 cells. In other studies, Newton et. al. found that expression of FADDdd led to rescue of DN4 and double-positive thymocyte populations [17]. Given recent studies that have demonstrated that peripheral FADDdd T cells become hyper-autophagic and die through a programmed necrotic pathway [18], such results suggest that a similar process may take place during the proliferative phase following β-selection. Alternatively, it may be that DR signaling is engaged in cells failing β-selection, and that such cells are subject to DR-induced cell death. Identification of other candidate genes and signaling pathways that may play roles in the survival or death of thymocyte subsets following β selection are currently underway in several research laboratories.
Death at the CD4/CD8 Double Positive developmental stage of thymopoiesis
During the DN4 precursor stage of thymocyte development, TCR α chain rearrangement ensues, resulting in display of a fully assembled TCR. As thymocytes transit to the double positive (DP) stage, these thymocytes enter a period of quiescence that lasts an average of 3–4 days. Several survival factors are expressed that allow the opportunity to test the newly displayed TCR. This “TCR selection” is imperative, as it will lead to three potential fates as dictated by the strength of the TCR interaction with major histocompatability complex (MHC) molecules expressed on resident antigen presenting cells (APC): a) death by neglect if the TCR fails to be stimulated by APC, b) death by negative selection if the TCR:MHC interaction is very strong and c) positive selection to become either CD4+ T helper or CD8+ cytotoxic T cell precursors (Fig. 2). Upregulation of antiapoptotic Bcl-xL at the DP stage is thus a key event that maintains DP thymocytes prior to TCR selection [19–21]. More recently, Mcl-1 has been demonstrated to promote thymocyte survival through its ability to interfere with proapoptotic Bak [11]. Proapoptotic Bim plays a central role in both death by neglect and negative selection [22,23], and the downregulation of Bcl-2 likely sensitizes DP cells to Bim-mediated apoptosis. At this stage in which most thymocytes express a TCR that cannot recognize self-MHC-peptide, up to 90% of DPs are sensitized to and undergo death by neglect, a process that is at least in part dependent on signals from steroid hormones [24,25].
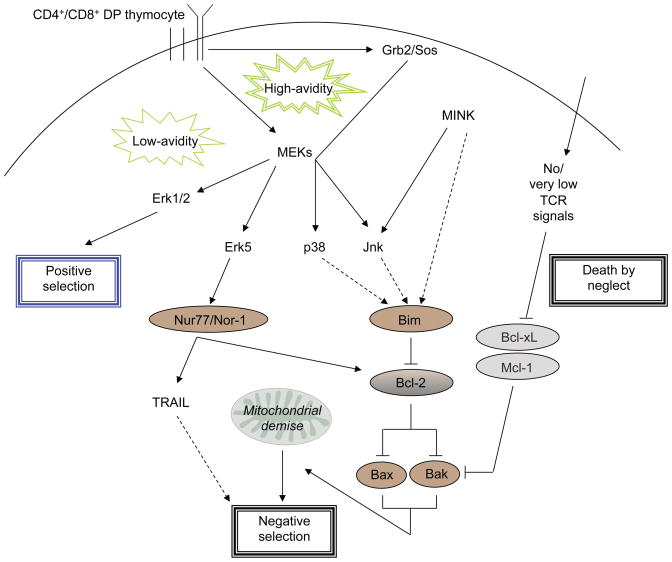
Figure 2.
Pathways involved in thymocyte development during the DP stage. The discrimination of MAPK pathways following TCR ligation results in different selection outcomes. Low-avidity TCR interactions with MHC-peptide (not shown) lead to positive selection, dependent on Erk1/2, whereas high-avidity interactions lead to negative selection, dependent on the recruitment of Grb2/Sos and activation of p38 and Jnk. Negative selecting ligands also result in ERK5 and MINK activation. The convergence of these pathways leads to activation of the Nur77 family and Bim death effectors. (Dotted lines represent potential signaling outcomes). Bim antagonizes Bcl-2 to allow Bax and Bak to effect mitochondrial dysfunction, while Nur77 family members have been shown to turn Bcl-2 into an apotosis promoting protein at the mitochondria. Mcl-1 and Bcl-xL antagonize the function of Bax/Bak, but in the absence of TCR signals, cannot protect cells from apoptosis. TRAIL is induced by Nur77, although the role of this DR in thymocyte apoptosis remains to be fully clarified.
Engagement of the TCR leads to a fate similarly dependent on the balance of pro- and anti-apoptotic members of the Bcl-2 family. Binding avidity between self-peptide:MHC and a thymocyte TCR determines the outcome of selection, where peptides with low-affinity and relatively rare expression lead to positive selection and the inhibition of apoptosis, while stronger signals through the TCR result in negative selection. The signaling mechanisms, particularly proximal TCR events, that achieve the shift in balance of pro- and anti-apoptotic effectors is still under interrogation, with the role of MAPKs being a focal point in this process [26–29]. TCR crosslinking leads to activation of the MAPKs ERK1/2, JNK1/2 and p38, as well as ERK5. Deletion of Erk1 or Erk2 indicated the requirement of this pathway for positive selection, while Erk1/2−/− mice displayed no defect in thymic deletion in vivo [30]. In contrast, inhibition of the JNK pathway [31], or deletion of either Jnk1 or Jnk2 [32,33], disrupts thymocyte apoptosis but not positive selection. The protein adaptor Grb2, through recruitment of Sos, plays a central role in differentiating between positively and negatively selecting ligands through its ability to selectively trigger the JNK and p38 pathways, but not ERK1/2 [34]. MINK, a serine/threonine kinase highly upregulated at the DP stage, has also implicated in mediating negative selection, potentially through its induction of JNK activity, and subsequent upregulation of Bim [35]. Whether or not MINK mediates Bim upregulation through JNK has not been directly shown, and the control of Bim expression remains an unresolved issue.
ERK5 activation, dependent on MEK5, correlates with the levels of Nur77 and Nor-1, nuclear steroid receptors belonging to a family of DNA binding proteins [36]. The Nur77 family and Bim represent the two main known effector pathways of thymocyte deletion. Induction of apoptosis through the mitochondrial pathway by Bim has been well characterized, and antagonism by Bcl-2 of Bax/Bak is instrumental for protection against Bim-mediated apoptosis. However, while Bim−/− mice display inefficient thymic deletion, over-expression of Bcl-2 does not block negative selection [37]. Other Bim-dependent death pathways are blocked in Bcl-2 transgenic mice including death by neglect, suggesting utilization of a pathway(s) other than Bim during negative selection or interference of the anti-apoptotic function of Bcl-2. Nur77 is required for negative selection as expression of a dominant-negative form achieves a blockade in negative selection, and its constitutive expression in thymocytes leads to massive apoptosis [38]. While its transcriptional activity has been correlated with its role in apoptosis, recent work has identified its ability to translocate to mitochondria and expose the BH3 domain of Bcl-2, thereby converting it a proapototic form [39]. This work may explain why Bcl-2 overexpression fails to rescue Bim-mediated apoptosis exclusively during negative selection. It will be interesting to further determine how Nur77’s transcriptional activity versus its effector function towards mitochondria contribute overall to its induction of thymocyte apoptosis.
Nur77 regulates expression of members of the TNF family, including TRAIL and Fas ligand, indicating that its transcriptional activity may lead to induction of death receptor (DR) mediated death pathways. Whether or not this is the case in thymocytes is unclear, and reports suggesting an involvement of extrinsic apoptotic (e.g. DR-mediated) pathways in death by neglect or negative selection remain controversial. Mice expressing FADDdd, an inhibitor of DR signaling that prevents caspase activation by multiple death receptors, had no defect in thymocyte deletion [16]. Surprisingly, these mice exhibited proliferative defects [40]. Similarly, caspase-8 and FADD, and the non-catalytic caspase-8 homolog cFLIP, have all been found to be essential for the proliferation and survival of mature T cells following their activation [41–43]. Such mutant T cells succumb to a non-apoptotic/caspase-independent form of programmed necrosis termed “necroptosis,” a form of death that requires the kinase activity of RIPK1, and in the case of TNF-alpha, RIPK3 [44–48]. RIPK1 activity is specifically required for induction of necroptosis in T cells lacking the capacity to activate caspase-8, as blockade RIPK1 catalytic activity with Necrostatin-1, or knockdown or RIPK1 expression led to recovery of these mutant T cells [18,48]. Given that T cell clonal expansion requires caspase-8 catalytic activity [49], these findings support the hypothesis that caspase-8 catalytic activity may be directed toward the inactivation of RIPK1 and RIPK3 following stimulation of naïve T cells, thereby preventing the induction of necroptosis. It is possible that developmental death during thymopoiesis may also involve both apoptosis and necroptosis. Supporting this, thymocytes from TRAIL−/− mice were described to possess defects in negative selection in multiple model systems [50]. Yet this work has been challenged by work on TRAIL-R−/− mice [51,52], as well as by others using distinct TRAIL−/− mice and blocking antibodies to TRAIL [53–55]. Currently, it is thought that thymocytes are destined for demise through the intrinsic pathway unless they are tuned in to low-avidity TCR signals that promote positive selection or regulatory T cell development. It will be interesting to determine if caspase-independent pathways may also contribute during thymocyte selection.
Conclusions
The thymus is an essential organ system that is imbued with the significant task of orchestrating the development of functional T cells, and the prevention of systemic autoimmunity. As described in this review, the proper regulation of cell death is vital to the function of the thymus. Although the function of the thymus wanes as humans reach adulthood, it remains active throughout one’s lifetime. The intricate control over myriad cell survival and death processes in the thymus underscores the need to understand more about how the signaling pathways contribute to key stages of thymopoiesis. While much has been discovered, many mysteries are yet left to be uncovered. Recent studies have uncovered alternative forms of cell death, many of which may play physiological roles in developmental and homeostatic pathways. As described, necroptosis occurs in cells in a caspase-independent manner [47,56]. It is likely that such caspase-independent pathways are vital to cell death stages during thymopoiesis. Supporting this hypothesis, expression of the baculovirus pan-caspase inhibitor p35 was shown to block caspase-dependent apoptosis in thymocytes, but did not interfere with physiological negative selection when expressed at high levels in DP thymocytes [57]. The potential exists that cellular macroautophagy may facilitate both survival and death signaling within thymocytes at distinct stages of development, in analogy to peripheral T cell subsets. Studies addressing these hypotheses are very much warranted, as they may reveal important new functions for distinct extrinsic and intrinsic (mitochondria-dependent) death pathways that have heretofore gone unappreciated.
Acknowledgments
This work was supported by grants from the NIH (CMW: R01 AI63419, AI50506; RHN: immunology training grant T32-AI60573), the National Multiple Sclerosis Foundation, the Arthritis National Research Foundation, and the Juvenile Diabetes Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* = significant
** = of special significance
- 1.Godfrey D, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150(10):4244–4252. [PubMed] [Google Scholar]
- 2.Ciofani M, Zuniga-Pflucker JC. A survival guide to early T cell development. Immunol Res. 2006;34:117–132. doi: 10.1385/IR:34:2:117. [DOI] [PubMed] [Google Scholar]
- 3.Rodewald HR, Kretzschmar K, Swat W, Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3:313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 4.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 9.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–29166. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 10.Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci U S A. 1999;96:14476–14481. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11.Dunkle A, Dzhagalov I, He YW. Mcl-1 promotes survival of thymocytes by inhibition of Bak in a pathway separate from Bcl-2. Cell Death Differ. 2010;17:994–1002. doi: 10.1038/cdd.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates an essential role for Mcl-1 in preventing thymocyte death that occurs independent of Bcl-2 activity, clarifying previous studies that failed to show Bcl-2 blockade of Bim-mediated thymocyte death
- 12.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 13.Mandal M, Borowski C, Palomero T, Ferrando AA, Oberdoerffer P, Meng F, Ruiz-Vela A, Ciofani M, Zuniga-Pflucker JC, Screpanti I, et al. The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med. 2005;201:603–614. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc Natl Acad Sci U S A. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Trampont PC, Tosello-Trampont AC, Shen Y, Duley AK, Sutherland AE, Bender TP, Littman DR, Ravichandran KS. CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol. 2010;11:162–170. doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that the chemokine receptor CXCR4 mediates survival signaling during early thymocyte development at the beta-selection stage
- 16.Walsh C, Wen B, Chinnaiyan A, O'Rourke K, Dixit V, Hedrick S. A role for FADD in T cell activation and development. Immunity. 1998;8(4):439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 17.Newton K, Harris AW, Strasser A. FADD/MORT1 regulates the pre-TCR checkpoint and can function as a tumour suppressor. Embo J. 2000;19:931–941. doi: 10.1093/emboj/19.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **18.Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA, Morrissette NS, Walsh CM. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that T cells expressing FADDdd or lacking caspase-8 succumb to hyper-active autophagy and necroptosis, processes dependent on RIPK1 activity
- 19.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- *21.Thompson J, Burger ML, Whang H, Winoto A. Protein kinase C regulates mitochondrial targeting of Nur77 and its family member Nor-1 in thymocytes undergoing apoptosis. Eur J Immunol. 2010;40:2041–2049. doi: 10.1002/eji.200940231. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that protein kinase C promotes cell death during negative selection through alteration of Nur77 targeting to mitochondrial membranes
- 22.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 23.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 24.Lu FW, Yasutomo K, Goodman GB, McHeyzer-Williams LJ, McHeyzer-Williams MG, Germain RN, Ashwell JD. Thymocyte resistance to glucocorticoids leads to antigen-specific unresponsiveness due to "holes" in the T cell repertoire. Immunity. 2000;12:183–192. doi: 10.1016/s1074-7613(00)80171-5. [DOI] [PubMed] [Google Scholar]
- 25.Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J Immunol. 2002;169:1837–1843. doi: 10.4049/jimmunol.169.4.1837. [DOI] [PubMed] [Google Scholar]
- 26.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- **27.Gallo EM, Winslow MM, Cante-Barrett K, Radermacher AN, Ho L, McGinnis L, Iritani B, Neilson JR, Crabtree GR. Calcineurin sets the bandwidth for discrimination of signals during thymocyte development. Nature. 2007;450:731–735. doi: 10.1038/nature06305. [DOI] [PMC free article] [PubMed] [Google Scholar]; Underscores the role of calcium and calcineurin in setting the threshold for thymocyte death vs. positive selection
- 28.Hogquist KA. Signal strength in thymic selection and lineage commitment. Curr Opin Immunol. 2001;13:225–231. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 29.Sohn SJ, Rajpal A, Winoto A. Apoptosis during lymphoid development. Curr Opin Immunol. 2003;15:209–216. doi: 10.1016/s0952-7915(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 30.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Rincon M, Whitmarsh A, Yang DD, Weiss L, Derijard B, Jayaraj P, Davis RJ, Flavell RA. The JNK pathway regulates the In vivo deletion of immature CD4(+)CD8(+) thymocytes. J Exp Med. 1998;188:1817–1830. doi: 10.1084/jem.188.10.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David JP, Jochum W, Wagner EF, Karin M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 33.Sabapathy K, Kallunki T, David JP, Graef I, Karin M, Wagner EF. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J Exp Med. 2001;193:317–328. doi: 10.1084/jem.193.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong Q, Cheng AM, Akk AM, Alberola-Ila J, Gong G, Pawson T, Chan AC. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 35.McCarty N, Paust S, Ikizawa K, Dan I, Li X, Cantor H. Signaling by the kinase MINK is essential in the negative selection of autoreactive thymocytes. Nat Immunol. 2005;6:65–72. doi: 10.1038/ni1145. [DOI] [PubMed] [Google Scholar]
- **36.Sohn SJ, Lewis GM, Winoto A. Non-redundant function of the MEK5-ERK5 pathway in thymocyte apoptosis. Embo J. 2008;27:1896–1906. doi: 10.1038/emboj.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Supports the hypothesis that, as opposed to ERK1/2, ERK5 signaling is required for thymocyte negative selection through its action on Nur77
- 37.Strasser A, Harris AW, von Boehmer H, Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci U S A. 1994;91:1376–1380. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calnan BJ, Szychowski S, Chan FK, Cado D, Winoto A. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity. 1995;3:273–282. doi: 10.1016/1074-7613(95)90113-2. [DOI] [PubMed] [Google Scholar]
- **39.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008;205:1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report shows that Nur77 mitochondrial localization promotes mitochondrial permeabilization and intrinsic apoptosis during negative selection
- 40.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 41.Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chau H, Wong V, Chen NJ, Huang HL, Lin WJ, Mirtsos C, Elford AR, Bonnard M, Wakeham A, You-Ten AI, et al. Cellular FLICE-inhibitory protein is required for T cell survival and cycling. J Exp Med. 2005;202:405–413. doi: 10.1084/jem.20050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J Exp Med. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **44.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an Energy Metabolism Regulator that Switches TNF-Induced Cell Death from Apoptosis to Necrosis. Science. 2009 doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]; Demonstrates that RIPK3 plays an essential role in controlling necroptosis induced by TNF alpha in cells lacking caspase activity; supports notion that RIPK3 promotes metabolic changes associated with programmed necrosis
- *45.He SW, Lai, Miao Lin, Wang Tao, Du Fenghe, Zhao Liping, Wang Xiaodong. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]; Identification of a RIPK1/RIPK3 necrotic complex that assembles and drives necroptosis in cells lacking caspase activity
- 46.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- **48.Ch'en IL, Beisner DR, Degterev A, Lynch C, Yuan J, Hoffmann A, Hedrick SM. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci U S A. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report shows that RIPK1 driven necroptosis promotes the premature demise of caspase-8 deficient T cells
- *49.Leverrier S, Salvesen GS, Walsh CM. Enzymatically active single chain caspase-8 maintains T-cell survival during clonal expansion. Cell Death Differ. 2010 doi: 10.1038/cdd.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates an essential role for the catalytic activity of caspase-8 during T cell clonal expansion
- 50.Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat Immunol. 2003;4:255–260. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- 51.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Corazza N, Brumatti G, Jakob S, Villunger A, Brunner T. TRAIL and thymocyte apoptosis. not so deadly? Cell Death Differ. 2004;11 (Suppl 2):S213–215. doi: 10.1038/sj.cdd.4401525. [DOI] [PubMed] [Google Scholar]
- 53.Simon AK, Williams O, Mongkolsapaya J, Jin B, Xu XN, Walczak H, Screaton GR. Tumor necrosis factor-related apoptosis-inducing ligand in T cell development: sensitivity of human thymocytes. Proc Natl Acad Sci U S A. 2001;98:5158–5163. doi: 10.1073/pnas.091100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cretney E, Uldrich AP, Berzins SP, Strasser A, Godfrey DI, Smyth MJ. Normal thymocyte negative selection in TRAIL-deficient mice. J Exp Med. 2003;198:491–496. doi: 10.1084/jem.20030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cretney E, Uldrich AP, McNab FW, Godfrey DI, Smyth MJ. No requirement for TRAIL in intrathymic negative selection. Int Immunol. 2008;20:267–276. doi: 10.1093/intimm/dxm144. [DOI] [PubMed] [Google Scholar]
- 56.Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- 57.Doerfler P, Forbush KA, Perlmutter RM. Caspase enzyme activity is not essential for apoptosis during thymocyte development. J Immunol. 2000;164:4071–4079. doi: 10.4049/jimmunol.164.8.4071. [DOI] [PubMed] [Google Scholar]