Abstract
O-linked-β-N-acetylglucosamine (O-GlcNAc) modification is a regulatory, nuclear and cytoplasmic post-translational glycosylation of proteins associated with age-related diseases such as Alzheimer's, Parkinson's, and type II diabetes. Global elevation of O-GlcNAc levels on intracellular proteins can induce insulin resistance, the hallmark of type II diabetes, in mammalian systems. In C. elegans, attenuation of the insulin-like signal transduction pathway increases adult lifespan of the nematode. We demonstrate that the O-GlcNAc cycling enzymes OGT and OGA, which add and remove O-GlcNAc respectively, modulate lifespan in C. elegans. Median adult lifespan is increased in an oga-1 deletion strain while median adult life span is decreased upon ogt-1 deletion. The O-GlcNAc-mediated effect on nematode lifespan is dependent on the FoxO transcription factor DAF-16. DAF-16 is a key factor in the insulin-like signal transduction pathway to regulate reproductive development, lifespan, stress tolerance, and dauer formation in C. elegans. Our data indicates that O-GlcNAc cycling selectively influences only a subset of DAF-16 mediated phenotypes, including lifespan and oxidative stress resistance. We performed an affinity purification of O-GlcNAc-modified proteins and observed that a high percentage of these proteins are regulated by insulin signaling and/or impact insulin pathway functional outcomes, suggesting that the O-GlcNAc modification may control downstream effectors to modulate insulin pathway mediated cellular processes.
Keywords: C. elegans, lifespan, O-GlcNAc, OGT, OGA
INTRODUCTION
Insulin resistance precedes and is a hallmark of type II diabetes [1]. Despite decades of progress in under-standing insulin-mediated signal transduction, the molecular mechanisms underlying insulin resistance are complex, and are not fully explored. The onset of insulin resistance is correlated with increased flux through the hexosamine biosynthetic pathway that leads to elevated UDP-N-acetylglucosamine (GlcNAc) from a glycolysis intermediate in mammalian cells [2]. UDP-GlcNAc is the donor for the post-translational modification of nuclear and cytosolic proteins through the addition of an O-linked GlcNAc molecule to serine and threonine residues [3]. Metazoa have conserved O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) enzymes that catalyze the addition and removal of O-GlcNAc, respectively. O-GlcNAc cycling occurs in both the nucleus and cytoplasm, and numerous proteins have been reported to be O-GlcNAc modified including transcription factors, metabolic enzymes, and kinases (reviewed in [4]). Elevation of O-GlcNAc levels, either pharmacologically or genetically, has been demonstrated in mammalian systems to induce insulin resistance. Aberrant O-GlcNAc modification is implicated in human diabetes, and age related neurodegenerative diseases [5-7].
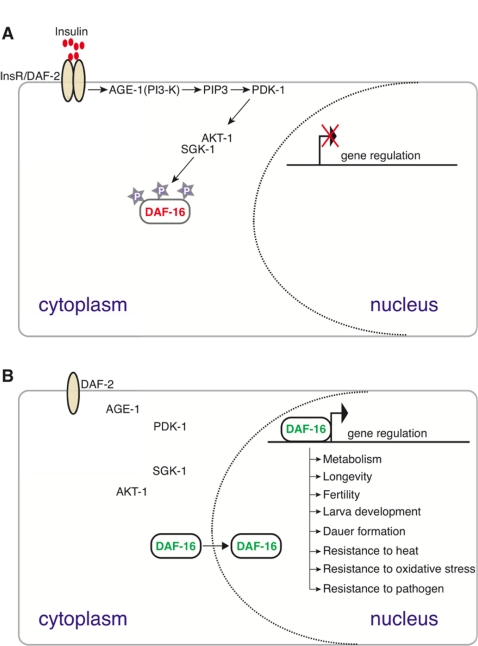
In C. elegans, the conserved insulin-like signaling pathway regulates lifespan as well as reproductive development, stress response, and dauer formation [8,9]. Upon insulin binding, the insulin receptor (DAF-2) catalyzes receptor tyrosine phosphorylation events to activate phosphatidylinositol-3 kinase (PI3K/AGE-1) [10]. AGE-1 activates the effector kinases, AKT-1 and SGK-1, which antagonize the forkhead box O (FoxO) transcription factor DAF-16 by preventing its nuclear localization (see Figure 6). A reduction in DAF-2-mediated signaling allows DAF-16 to localize to the nucleus where it regulates a large number of genes essential for metabolism, energy storage, reproductive development, immunity, stress resistance, and other novel processes which collectively modulate lifespan in C. elegans [11-13]. Adult lifespan extension upon reduction in insulin signaling has also been reported in Drosophila, mice, and recently in a centenarian human population [14,15]. DAF-16 function is controlled by additional mechanisms beyond its nuclear localization, as neither constitutive nuclear localization nor over-expression is sufficient to induce maximum lifespan extension [16]. The mechanism by which insulin signaling mediated through FoxO/DAF-16 produces distinct biological outcomes in response to varying levels of insulin signaling remains ambiguous [12,17].
Figure 6.
A conserved insulin signaling pathway in C. elegans regulates numerous functions including stress response, metabolism, dauer formation, and reproductive development by restricting the nuclear localization of the DAF-16/FoxO transcription factor upon nutrient availability [45]. Upon ligand binding (top panel), the insulin-like receptor DAF-2 activates the AGE-1 PI3 kinase that facilitates the activation of PDK-1 and AKT-1. AKT-1-mediated phosphorylation sequesters DAF-16 in the cytoplasm. In the absence of PI3K/AKT signaling (bottom panel) DAF-16 enters the nucleus and regulates the expression of target genes to mediate numerous DAF-16 dependent processes, including those listed.
The C. elegans genome contains a single OGT ortholog, ogt-1, and a single OGA ortholog, oga-1. In the current study we demonstrate that the O-GlcNAc cycling enzymes modulate median adult lifespan in C. elegans. Intriguingly, we delineate downstream functions of the insulin signaling pathway that are O-GlcNAc dependent, and others that appear to be O-GlcNAc independent. Finally we used a biochemical approach to identify putative O-GlcNAc target proteins, and found that the majority of these proteins are regulated by insulin signaling, and a significant percentage functionally impact lifespan. Our work therefore suggests a potential mechanism by which the post-translational O-GlcNAc modification of proteins downstream of the insulin pathway could differentially modulate insulin-mediated signaling outcomes.
RESULTS
Cellular O-GlcNAc levels modulate adult lifespan in C. elegans
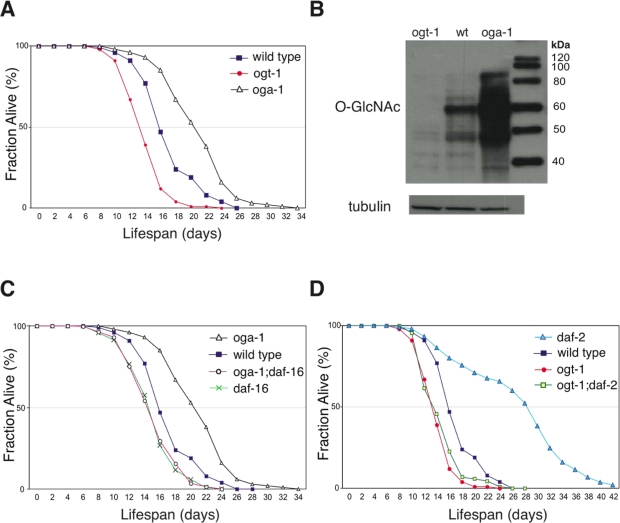
In C. elegans, O-GlcNAcase (oga-1) and O-GlcNAc transferase (ogt-1) homozygous null mutants are viable and appear overtly wild type [18,19]. However, we observed that oga-1(ok1207) null mutant adults live ~33% longer than the wild-type animals, while ogt-1(ok1474) null mutants have a lifespan ~20% shorter than the wild type at 20°C (Figure 1A). In C. elegans, movement ability is used as a general index of vitality [20]. The ogt-1 mutant animals appear to age prematurely, as exemplified by markedly slower movement at day 12 of adulthood relative to still-active wild-type and oga-1 mutant adults (Suppl. Movie 1). ogt-1 null mutant animals have normal developmental timing (43 ± 3.9 hrs vs. 41 ± 0.7 hrs for ogt-1 and wild type, respectively, p = not significant, >0.5), and generate fertile progeny numbers similar to wild-type animals (263 ± 80 vs. 289 ±32 for ogt-1 and wild type, respectively, p = ns), suggesting that the reduced movement and earlier death of ogt-1 mutant adults is not due to a general ‘sickness’. The oga-1(ok1207) mutant demonstrates a substantial increase in O-GlcNAc-modified protein levels as determined by immunoblot of whole animal lysate, while the ogt-1(ok1474) mutant has only residual staining (Figure 1B). To determine whether adult lifespan extension in oga-1 mutants arises from excessive O-GlcNAc-modifications, we analyzed the lifespan of the oga-1; ogt-1 double mutant which has a markedly reduced level of O-GlcNAc-modified proteins similar to the ogt-1 mutant (data not shown). The adult lifespan of theoga-1; ogt-1 double mutant is similar to that of wild type (Table 1), confirming that lifespan extension observed in oga-1 mutants is dependent on excessive O-GlcNAc-modified protein levels.
Figure 1. Elevated O-GlcNAc levels extend adult lifespan in C. elegans.
(A) The oga-1(ok1207) mutant has an extended adult lifespan while the ogt-1(ok1474) mutant has a reduced lifespan relative to wild-type animals, assayed at 20°C. Lifespan curves are based on pooled data from multiple replicates described in Table 1. (B) OGA-1 and OGT-1 regulate cellular O-GlcNAc-modified protein levels. Western blot of total animal extract from ogt-1(ok1474), wild-type, and oga-1(ok1207) animals probed with antibodies against O-GlcNAc and tubulin showing marked differences in total O-GlcNAcylated protein levels. (C) Lifespan extension in the oga-1 mutant is DAF-16-dependent, as seen in the reduced lifespan of the oga-1(ok1207); daf-16(mu86) double mutant relative to the oga-1(ok1207) single mutant. (D) Loss of O-GlcNAc modification, in the daf-2(e1370); ogt-1(ok1474) double mutant, reduces the extended lifespan of the daf-2(e1370) mutant. Control curves are re-plotted to facilitate comparisons in panels A, C, and D.
Table 1. Adult Lifespan Analysis.
| Genotype | Median LS ±STDEV | p-value vs. wild type | Sample size n(censored) | Replicates N | |
|---|---|---|---|---|---|
| Log-rank | Wilcoxon | ||||
| wild type | 15.9±3.6 | - | - | 404(59) | 8 |
| oga-1(ok1207) | 20.9±4.1 | <0.0001 | <0.0001 | 317(43) | 6 |
| ogt-1(ok1474) | 12.9±1.9 | <0.0001 | <0.0001 | 306(64) | 6 |
| oga-1(ok1207); ogt-1(ok1474) | 15.5±4.9 | 0.030 | 0.040 | 174(35) | 2 |
| daf-2(e1370) | 28.6±3.5 | <0.0001 | <0.0001 | 235(26) | 3 |
| oga-1(ok1207); daf-2(e1370) | 21.6±0.6 | <0.0001 | <0.0001 | 201(20) | 3 |
| ogt-1(ok1474); daf-2(e1370) | 13.2±1.9 | <0.0001 | <0.0001 | 218(28) | 3 |
| daf-16(mu86) | 14.3±2.5 | 0.0001 | <0.0001 | 228(60) | 3 |
| oga-1(ok1207); daf-16(mu86) | 14.8±1.8 | 0.019 | 0.009 | 173(25) | 2 |
| ogt-1(ok1474); daf-16(mu86) | 12.8±0.4 | <0.0001 | <0.0001 | 150(24) | 2 |
| age-1(hx546) | 25.6±4.1 | <0.0001 | <0.0001 | 216(18) | 3 |
| oga-1(ok1207); age-1(hx546) | 24.7±6.8 | <0.0001 | <0.0001 | 241(17) | 3 |
| ogt-1(ok1474); age-1(hx546) | 15.9±1.6 | 0.424 | 0.590 | 258(31) | 3 |
| sgk-1(ok538) | 29.8±3.2 | <0.0001 | <0.0001 | 235(16) | 3 |
| oga-1(ok1207); sgk-1(ok538) | 26.2±2.1 | <0.0001 | <0.0001 | 209(30) | 3 |
| ogt-1(ok1474); sgk-1(ok538) | 16.6±2.3 | 0.343 | 0.375 | 214(39) | 3 |
| pdk-1(mg142)gf | 14.8±1.7 | 0.013 | 0.007 | 334(84) | 5 |
| oga-1(ok1207); pdk-1(mg142) | 18.7±8.1a | <0.0001 | <0.0001 | 284(43) | 5 |
| akt-1(mg144)gf | 11.5±2.1 | <0.0001 | <0.0001 | 205(32) | 2 |
| oga-1(ok1207); akt-1(mg144) | 19.8±4.2b | 0.0002 | 0.0002 | 229(22) | 2 |
pdk-1 vs. oga-1;pdk-1 p-value <0.0001
akt-1 vs. oga-1;akt-1 p-value <0.0001
N = number of replicates; n = pooled animals from N replicate experiments (censored animals were included in the Log-rank/Wilcoxon analysis)
O-GlcNAc cycling enzymes modulate insulin-mediated signaling in C. elegans
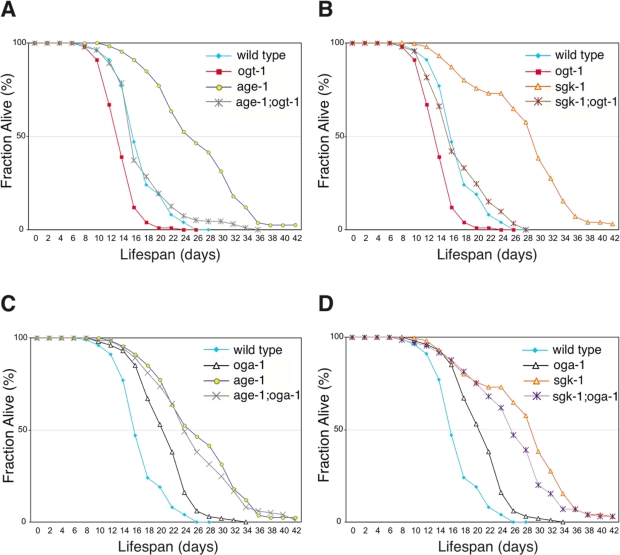
Inactivation of insulin-mediated signaling increases C. elegans adult lifespan in a DAF-16-dependent manner [21]. We observed that the lifespan extension in the oga-1(ok1207) mutant is also dependent on intact DAF-16 (Figure 1C). Inactivation of OGT-1 function in daf-2(e1370) mutants dramatically reduces its adult lifespan arguing that O-GlcNAc modification of cellular proteins is a requirement for the daf-2 mutant long lifespan (Figure 1D). Lifespan extension regulated by insulin-mediated signaling in C. elegans is mainly achieved through its downstream effector kinases, such as AGE-1 and SGK-1 (Figure 6). Inactivation of the effector kinases AGE-1 and SGK-1 also results in significant adult lifespan extension [22,23]. To further test whether O-GlcNAc modification is essential for insulin signaling mediated adult lifespan regulation, we inactivated O-GlcNAc transferase function in both the long-lived age-1(hx546) and sgk-1(ok538) mutants. We observed that adult lifespan extension in age-1 and sgk-1 mutants are completely suppressed to wild-type levels upon combination with the short-lived ogt-1(ok1474) mutant allele (Figure 2A and 2B).
Figure 2. Elevated O-GlcNAc modification of proteins does not increase adult lifespan further in long-lived insulin signaling pathway mutant animals.
(A and B) Lifespan of age-1(hx546) and sgk-1(ok538) mutants is dependent on protein O-GlcNAc modification as seen in age-1; ogt-1 (A) and sgk-1; ogt-1 (B) double mutants. (C and D) The adult lifespan extension associated with the oga-1(ok1207) mutant is not synergistic or additive with mutations of the long-lived insulin signaling pathway kinase age-1 (C) or sgk-1 (D). Lifespan curves (A-D) are based on pooled data from multiple replicates as described in Table 1. Control curves are re-plotted to facilitate comparisons in panels A-D.
In a conventional genetic analysis, genes that function in parallel pathways with the same functional output generally demonstrate additive interactions. However, combining the long-lived oga-1(ok1207) mutant allele with long-lived insulin signaling pathway mutants, daf-2, age-1, or sgk-1, did not extend lifespan further, suggesting that O-GlcNAc-modified proteins function in the same genetic pathway (Figure 2C and 2D, Table 1). To further investigate where O-GlcNAc cycling impinges on the insulin signaling pathway, we analyzed both akt-1 and pdk-1 gain-of-function (gf) mutant alleles that constitutively activate the insulin signaling pathway [24,25]. Both akt-1(mg144)gf and pdk-1(mg142)gf mutant lifespans are shorter than that of wild type (Table 1). The combination of akt-1(mg144)gf or pdk-1(mg142)gf mutant alleles with the long-lived oga-1(ok1207) allele failed to suppress the lifespan extension associated with the oga-1 mutant (Table 1). This data suggests that O-GlcNAc-modified proteins act downstream of the insulin pathway effector kinases to regulate insulin signaling outcomes. However both mg144 and mg142 alleles also do not suppress the extended lifespan of the age-1(hx546) mutant [24]; and thus this conclusion is not definitive.
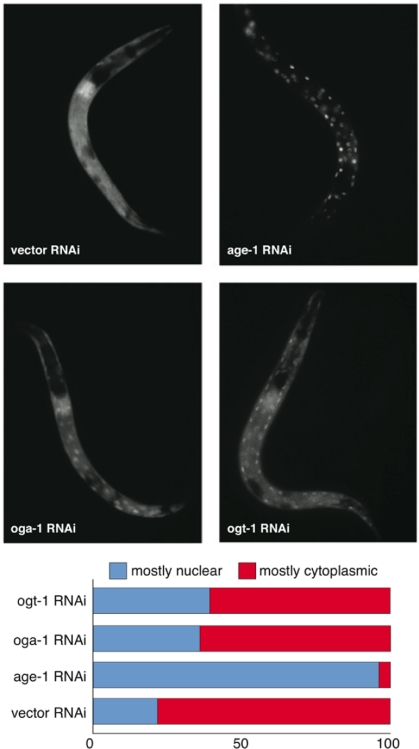
In C. elegans, the insulin signaling pathway regulates numerous biological functions through DAF-16 by restricting its nuclear localization including, dauer entry, longevity, stress resistance, fertility, and reproductive development [12]. We sought to determine whether perturbing the O-GlcNAc cycling enzymes OGT-1 or OGA-1 would affect DAF-16 subcellular localization. Inactivation of the insulin signaling pathway effector kinase AGE-1 (by RNAi) produced virtually complete nuclear localization of DAF-16 (Figure 3). Interestingly, inactivation of either OGA-1 or OGT-1 produced a similar but modest increase in DAF-16 nuclear localization (Figure 3). The observation that inactivating either OGA-1 or OGT-1 produces similar effects on DAF-16 nuclear localization but opposite effects on insulin pathway outcomes, suggests that O-GlcNAc cycling does not functionally modulate the insulin pathway at the level of DAF-16 nuclear localization.
Figure 3. Aberrant protein O-GlcNAc cycling modestly enhances DAF-16 nuclear localization.
DAF-16::GFP (translational fusion) nuclear localization in oga-1(RNAi) and ogt-1(RNAi) animals is modestly increased relative to control (vector) RNAi animals but much less than observed in age-1(RNAi) animals (upper panel). Lower panel is the quantification of DAF-16::GFP levels in intestinal cells upon RNAi inactivation of ogt-1, oga-1, age-1, and vector control.
Perturbation in O-GlcNAc cycling affects only a subset of insulin-mediated functions
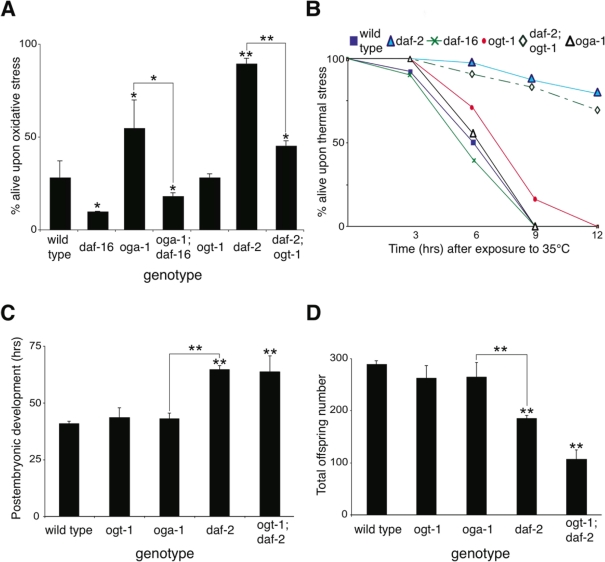
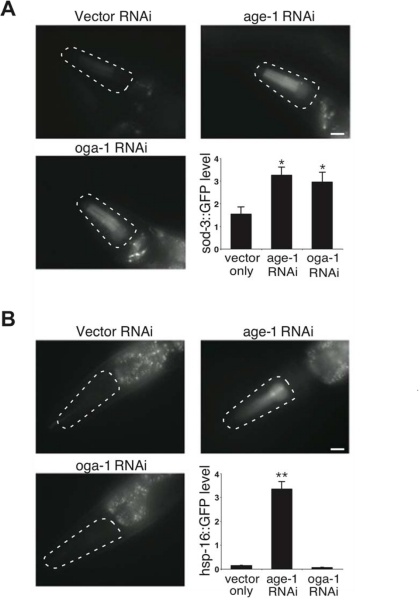
Lifespan extension in insulin pathway mutants is correlated with oxidative and thermal stress resistance as well as changes in animal development [26,27]. We observed that the long-lived oga-1 mutant is resistant to oxidative stress in a DAF-16-dependent manner similar to that observed with the daf-2 mutant (Figure 4A). Co-inactivation of OGT-1 and DAF-2 in daf-2(e1370);ogt-1(ok1474) double mutant animals significantly reduced the oxidative stress resistance associated with the daf-2 mutant (Figure 4A). This data implies that protein O-GlcNAc modification contributes to the oxidative stress tolerance that is prominent in insulin pathway mutants. In contrast to oxidative stress resistance, the long-lived oga-1 mutant differs from long-lived daf-2 mutants in that the oga-1 mutant does not exhibit increased thermal stress tolerance, delayed developmental timing defects, or reduced fecundity, which are prominent in daf-2 mutants (Figure 4B, 4C, and 4D, respectively) [16,26,28,29]. Oxidative stress resistance in the insulin pathway mutants arises from the DAF-16-mediated expression of several genes including superoxide dismutase SOD-3, while the increased thermotolerance is correlated with the induction of several heat-shock proteins, such as HSP-16 [27,30,31]. We observed that the inactivation of OGA-1 increased the expression of a sod-3::GFP reporter but not a hsp-16.2::GFP reporter (Figure 5A and 5B, respectively). Our data indicates that O-GlcNAc modification of proteins is critical for only a subset of the insulin pathway functional outcomes that are downstream of DAF-16.
Figure 4. Inactivation of O-GlcNAc cycling enzymes only affects a subset of functions regulated by the insulin-like signaling pathway.
(A) The oga-1(ok1207) mutant is resistant to oxidative stress (100mM paraquat for 9 hrs). Notably, the oxidative stress resistance in daf-2(e1370) mutant is significantly reduced in the daf-2(e1370); ogt-1(ok1474) double mutant, suggesting that protein O-GlcNAc modification contributes to the oxidative resistance of the daf-2 mutant. (B) The long-lived oga-1(ok1207) mutant is sensitive to heat stress (35°C), unlike the long-lived daf-2 (e1370) mutant. (C) oga-1 and ogt-1 mutants have normal developmental timing [from hatch to the fourth larval molt] at 20°C. The daf-2 mutant has delayed post-embryonic development, and this delay is unaffected by loss of O-GlcNAc modification in the daf-2; ogt-1 double mutant. (D) oga-1 and ogt-1 mutants generate normal numbers of offspring while the daf-2 mutant has reduced fecundity at 20°C. The reduced fecundity in the daf-2 mutant is not rescued by loss of O-GlcNAc modification in the daf-2; ogt-1 double mutant. Asterisks above bars denote statistically significant differences from wild type (* p < 0.05 and ** p < 0.01); while asterisks above solid lines connecting two genotypes denote statistically significant differences between those genotypes.
Figure 5. Aberrant protein O-GlcNAc cycling differentially affects distinct stress response pathways in C. elegans.
(A) Images and quantification of sod-3::GFP reporter expression upon inactivation of OGA-1 and the insulin pathway effector kinase AGE-1. (B) Images and quantification of hsp-16-2::GFP reporter expression upon inactivation of OGA-1 and AGE-1. Note that inactivation of AGE-1 by RNAi induces both SOD-3 and HSP-16 expression, while OGA-1 inactivation only induces SOD-3 expression. Asterisks denote statistically significant differences from vector RNAi controls (* p < 0.05 and ** p < 0.01).
Identification of putative O-GlcNAc target proteins through affinity purification
In an effort to identify the proteins that are modified by O-GlcNAc in C. elegans, we affinity purified O-GlcNAc-modified proteins from oga-1(ok1207) and ogt-1(ok1474) mutant adults, and then identified the affinity purified proteins by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Non-specific proteins would be expected to be present in both purifications, while O-GlcNAc-modified proteins (or proteins tightly-associated with O-GlcNAc-modified proteins) would be specific for the oga-1 mutant lysate. Proteins that were identified from affinity purifications of both oga-1 and ogt-1 mutant lysates were censored as non-specific. Using this approach, we identified 13 distinct proteins that were specific for the O-GlcNAc affinity purification from oga-1 mutant lysate (Table 2). The 13 proteins encompass a total of 21 proteins when including paralogs with identical or nearly identical protein sequences that could not be distinguished by mass spectrometry. The majority of the putative O-GlcNAc target proteins are regulated by the insulin signaling pathway and a substantial percentage functionally modulate lifespan or oxidative stress (Table 2 and discussion below), suggesting that OGT-1 and OGA-1 may control a subset of insulin signaling functional outcomes by directly targeting the O-GlcNAc modification of proteins that act downstream of the insulin signal transduction pathway.
Table 2. Summary of putative C. elegans O-GlcNAc targets.
| Proteins | Molecular Identity | Expression in daf-2 mutant | O-GlcNAc modified in vertebrate homologs |
|---|---|---|---|
| PHI-37 | F0F1-type ATP synthase | Downregulated [46] | NR |
| ATP-2 | F0F1-type ATP synthase | Downregulated [46] | NR |
| MDH-1 | Malate dehydrogenase | Downregulated [47] | MDH1 [7] |
| GPD-2, GPD-3 | Glyceraldehyde-3-phosphate dehydrogenase, major isozyme | Upregulated [48,49] | GAPDH [7,50] |
| GPD-1, GPD-4 | Glyceraldehyde-3-phosphate dehydrogenase, minor isozyme | Downregulated [46] | GAPDH [7,50] |
| EFT-3, EFT-4 | Translation elongation factor 1 alpha | Downregulated [46] | EEF1AO [50] |
| ACT-1, ACT-2, ACT-3, ACT-4 | Actin | Downregulated [46] | ACTG1 [7] |
| TBA-1, TBA-2, TBA-4 | Alpha tubulin | Downregulated [46] | TUBA1A [7] |
| VIT-6 | Vitellogenin | Downregulated [46] | NR |
| VHA-13 | Vacuolar protein translocating ATPase | NR | NR |
| CDC-48.2 | AAA-type ATPase | NR | NR |
| F27D4.1 | Electron transfer flavoprotein | NR | NR |
| C16A3.10 | Ornithine aminotransferase | Downregulated [48] | NR |
EFT-3 and EFT-4 have identical protein sequences, as do GPD-2 and GPD-3. The peptides identified by LC-MS/MS did not distinguish between the almost identical ACT-1, ACT-2, ACT-3, and ACT-4; TBA-1, TBA-2, and TBA-4; or GPD-2 and GPD-3, respectively. NR, not reported.
DISCUSSION
In C. elegans, O-GlcNAc cycling enzyme mutants are viable and therefore provide a useful model to elucidate the role of O-GlcNAc protein modifications, which have been implicated in numerous cellular processes. In our current study we demonstrated that a loss of O-GlcNAc transferase (OGT-1) activity reduces adult lifespan in the nematode C. elegans. Conversely, inactivation of O-GlcNAcase (OGA-1) leads to a moderate increase in adult lifespan. While the accumulation of excessive levels of O-GlcNAc-modified proteins in oga-1 mutant animals is associated with lifespan extension, it is not associated with obvious abnormalities in reproductive development, as oga-1 mutants have an overtly wild-type appearance, develop normally into adults, and generate normal numbers of fertile offspring. Similarly, lifespan reduction in ogt-1 null mutants does not appear to be due to a general ‘sickness’ of the mutant animals, as ogt-1 mutants also develop normally into fertile adults, and generate normal numbers of offspring (Figure 4).
Love et al. recently reported similar results that OGT-1 inactivation shortens both wild-type and daf-2 mutant lifespans [32]. However, their findings differ from ours in that they reported that OGA-1 inactivation did not significantly affect lifespan in a wild-type background but slightly extended lifespan in a daf-2 mutant background [32]. We observed that oga-1 mutant lifespan was significantly longer than that of wild type (three independent experiments showed significant differences; p<0.0001, n=66-89 for each experiment); and double mutant analysis of oga-1 with each of three separate components of the insulin signal transduction pathway, daf-2, age-1, and sgk-1, did not show additional lifespan increases in our experiments.
The failure to observe additive lifespan extension when combining oga-1 and insulin pathway mutations is consistent with O-GlcNAc-modified proteins working in the same genetic pathway as insulin signaling. In C. elegans, insulin signaling regulates gene expression by restricting DAF-16, whose activity is essential for the extended lifespan that is observed in insulin pathway mutants [11,12]. We found that DAF-16 is required for the lifespan extension in oga-1 mutant animals, which is also consistent with O-GlcNAc cycling functioning in the same genetic pathway as insulin signaling.
The observation that elevated O-GlcNAc levels (in the oga-1 mutant) mimics only a subset of insulin pathway mutant phenotypes (lifespan extension and resistance to oxidative stress) but not others (delayed developmental timing, reduced fecundity, and increased thermotolerance) suggests that O-GlcNAc cycling only alters a subset of functional outcomes under the control of insulin-mediated signaling. The insulin signaling pathway regulates DAF-16 nuclear localization. If OGA-1 and OGT-1 impacted the insulin pathway upstream of DAF-16, it would be expected that oga-1 and ogt-1 mutants would have opposite effects on DAF-16 nuclear localization. However, we observed that both mutants have the same modest enhancement of DAF-16 nuclear localization, suggesting that O-GlcNAc cycling modulates insulin signaling outcomes downstream of DAF-16.
We performed an affinity purification of O-GlcNAc-modified proteins that identified 13 proteins (Table 2). The mammalian counterparts of 6 of the 13 proteins are known to be O-GlcNAc modified, suggesting that these are conserved targets of O-GlcNAc transferase (Table 2).
Strikingly, the expression of 10 of the 13 putative O-GlcNAc target proteins is controlled by the insulin signaling pathway (Table 2). More significantly, 4 of the 13 putative O-GlcNAc target proteins have been reported to modulate lifespan in C. elegans. Inactivation of either of the F0F1-type ATP synthases ATP-2 or H28O16.1 is observed to increase adult lifespan and increase the expression of a sod-3::GFP reporter [33]. Inactivation of the glyceraldehyde-3-phosphate dehydrogenase GPD-2 further extends the lifespan of daf-2 mutants [34]; while inactivation of the malate dehydrogenase MDH-1 shortens daf-2 mutant lifespan [35]. Additionally, inactivation of GPD-2 and GPD-3 allows the daf-2(e1370) mutant to escape dauer entry, thereby demonstrating a critical role for GPDs in regulating one of the functional outcomes under the control of the insulin signaling pathway [36]. Our work therefore demonstrates that downstream targets of the insulin pathway are O-GlcNAc modified. This provides the possibility that O-GlcNAc cycling enzymes act downstream of DAF-16 to directly functionally modulate specific insulin-regulated processes.
In mammals, increasing O-GlcNAc levels by reducing O-GlcNAcase (OGA) activity or increasing O-GlcNAc transferase (OGT) activity leads to insulin resistance [37]. Increased O-GlcNAc levels inhibit insulin stimulated activation of AKT, and disrupt insulin-mediated glucose transport in adipocytes demonstrating a direct involvement of O-GlcNAc cycling in the attenua- tion of insulin-mediated signaling functional outcomes. A reduced ability of insulin to activate glucose transport into cells, i.e., insulin resistance, is marked by high glucose and high insulin levels in circulating blood, and aberrant insulin-regulated gene functions [10]. Abnormalities in carbohydrate and fat metabolism are characteristic of insulin-resistant tissues, and are implicated in elevated plasma sugar and fatty acid levels in patients with type II diabetes [38]. In C. elegans, similar disarray in carbohydrate and fat storage is observed in insulin pathway mutant animals [9]. Previous studies also demonstrated abnormal carbohydrate and fat storage upon disruption of O-GlcNAc cycling [18]. Therefore, O-GlcNAc deregulation in C. elegans is strikingly similar to the insulin-resistant phenotype previously described in mammalian studies despite the absence of a blood circulation system and the consequent need for blood glucose homeostasis in the nematode.
Future studies will be required to establish the effects that O-GlcNAc modifications have on the target proteins that were identified in this study, and the functional significance of the modifications on specific insulin pathway outcomes. Many of the O-GlcNAc targets appear to be conserved between nematodes and vertebrates, and it will be interesting in the future to determine whether O-GlcNAc cycling acts downstream of the insulin pathway in vertebrates to control subsets of FoxO-dependent processes. The use of C. elegans as an amenable genetic model will allow a fuller exploration of the complexities of O-GlcNAc modification and FoxO-mediated gene expression, with implications for human neuro-degenerative diseases, diabetes, and developmental processes including aging.
METHODS
Nematode strains
The following nematode strains were used in this study: N2 wild type Bristol, RB1342 ogt-1(ok1474), RB1169 oga-1(ok1207), CF1041 daf-2(e1370), CF1380 daf-16(mu86), TJ1052 age-1(hx546), VC345 sgk-1(ok538), GR1310 akt-1(mg144), GR1318 pdk-1(mg142), and ET422 muIs109[Pdaf-16::GFP::DAF-16 cDNA + Podr-1::RFP], CF1553 muIs84[pAD76 (sod-3::GFP)], CL2070 dvls70[integrat-edhsp-16-2::GFP+pRF4], ET434 oga-1(ok1207), ET435 ogt-1(ok1474), ET436 oga-1(ok1207); ogt-1(ok1474), ET439 oga-1(ok1207); daf-2(e1370), ET438 ogt-1(ok1474); daf-2(e1370), ET437 oga-1(ok1207); daf-16(mu86), ET468 ogt-1(ok1474); daf-16(mu86), ET469 oga-1(ok1207); age-1(hx546), ET393 ogt-1(ok1474); age-1(hx546), ET471 oga-1(ok1207); sgk-1(ok538), ET470 ogt-1(ok1474); sgk-1(ok538), ET472 oga-1(ok1207); pdk-1(mg142), and ET445 oga-1(ok1207); akt-1(mg144). The oga-1(ok1207) and ogt-1(ok1474) mutants in strains ET434 and ET435 were outcrossed 6 times against N2 in our laboratory with the mutations followed by PCR. The mutation in ogt-1(ok1474) and oga-1(ok1207) alleles were verified using PCR primers provided by the C. elegans Gene Knockout Project at OMRF, which is part of the International C. elegans Gene Knockout Consortium. The mutations in double mutant strains were followed by PCR and restriction enzyme analysis or by DNA sequencing spanning the region corresponding to the mutations, where appropriate.
Lifespan analysis
All lifespan assays were performed using 85-125 animals for each genotype per assay at 20°C on NGM plates seeded with OP50 bacteria following established procedures [8]. Adults were transferred to fresh plates every alternate day during the fertile period, and later when necessary. Adults were counted as dead when they failed to respond to repeated head and tail prodding. Multiple assays were pooled for final analysis.
Statistical analyses
Survival curves were analyzed by the Log-rank (Mantel-Cox) and Wilcoxon tests using GraphPad Prism (version 5.0) to determine the significance between mutant and wild-type controls as indicated in Table 1. In other experiments, statistical significance was determined with the unpaired two-tailed Student's t-test.
Stress tolerance assay
Oxidative stress resistance assays were performed in multi-well plates by immersing adults in M9 media containing 100 mM paraquat (N,N'-dimethyl-4,4'-bipyridinium dichloride) using 10-20 animals for each genotype per assay at 20°C [39,40]. A complete absence of swimming movement was scored as death, and also reconfirmed by transferred animals not moving upon prodding on agar plates. Death was scored every 3, 6, 9, and 12 hrs. Experimental data from multiple assays were pooled together for final analysis. Thermal resistance assays were performed on NGM plates pre-heated at 35°C using at least 50 animals for each genotype per assay [29,41]. Adults were incubated at 35°C, and were scored every hour by response to touch until all animals were dead. Experimental data from multiple assays were pooled together for final analysis.
Developmental timing and fecundity assays
Postembryonic developmental timing was assayed by collecting comma-stage embryos from animals maintained at 20°C. The exact time of hatching was recorded, and then animals were transferred onto separate OP50-seeded NGM plates. After the third molt, animals were inspected more frequently (every 15 mins) to record the exact time of the fourth molt. The assay was repeated several times using at least 10 animals for each genotype per assay, and multiple experimental data were pooled together for final analysis. For fecundity analysis, individual larval stage animals were placed on OP50-seeded NGM plates at 20°C. Offspring were counted by moving them onto a fresh plate one-by-one. The assay was repeated multiple times with at least 10 animals per genotype in each assay, and data were pooled together for final analysis.
Western immunoblotting
Adult animals were flash frozen in liquid nitrogen in lysis buffer (50mM HEPES pH 7.8 and 300mM NaCl). Frozen worms were crushed in a pestle pre-chilled with liquid nitrogen. The frozen ground worms were re-suspended in cold lysis buffer (with DNaseI and protease inhibitor cocktail from Roche) and subjected to sonication for 6-8 seconds followed by centrifugation at 16,000 rpm for 1 hour at 4°C. The middle portion (not including the pellet or floating lipids) of each tube was collected for analysis. An equal amount of whole animal lysate was separated on a 10% NuPAGE Bis-Tris gel (Invitrogen) followed by transfer onto a PVDF membrane (Millipore). The membrane was probed with anti-O-GlcNAc antibody from Affinity Bioreagents (1:2000 in 3% BSA dissolved in 1XTBS + 0.05% Tween20). The immunoblot was visualized using a chemiluminescent substrate (Super Signal West Pico from Pierce). The same membrane was re-probed with anti-α-tubulin antibody (Sigma) to normalize protein loading.
RNAi experiments
All RNAi experiments were performed at 20°C unless otherwise mentioned using E. coli strain HT115 transformed with pPD129.36 clones from an RNAi library provided by Julie Ahringer for gene inactivation experiments, as previously described [42,43].
GFP reporter assays
Experimental animals for analysis were grown on feeding RNAi for more than one generation. All animals were imaged at single shutter setting, and equal nuclear and cytoplasmic areas were chosen for light intensity measurement using OpenLab 5.5 software. The average of three mean light intensity measurement values per cell (in arbitrary units) for both nuclear and cytoplasmic regions were recorded for multiple cells in each treated and untreated animals. The assay was repeated several times with at least 10 animals per genotype in each assay at 20°C. Data from multiple experiments were pooled for final analysis. Bothsod-3::gfp and hsp-16-2::gfp reporters were assayed in 10-day-old adults.
Affinity purification and identification of O-GlcNAc-modified proteins
Adult animals were collected and flash frozen in liquid nitrogen in 50mM HEPES pH 7.8 and 50mM NaCl buffer. Frozen worms were crushed with a mortar and pestle pre-chilled with liquid nitrogen. The frozen ground worms were re-suspended in the above buffer (with DNaseI and protease inhibitor cocktail from Roche), and disrupted by sonication followed by centrifugation at 16,000 rpm for 1 hour at 4°C. Clear supernatant was collected and O-GlcNAc-modified proteins were identified as previously described [44]. Briefly, affinity enrichment with mAb14 to O-GlcNAc-modified proteins was conducted followed by the enriched proteins being reduced, alkylated, and trypsin digested. Protein assignments were made following tandem mass spectrometry on a linear ion trap using the C. elegans protein database from NCBI with filtering for a false-discovery rate of less than 1%, as previously described [44]. The number of peptides leading to the identification of each protein described in Table 2 are as follows: PHI-37 (8); MDH-1 (7); ATP-2 (3); GPD-1 (2); GPD-2 (4); ACT-1 (5); TBA-2 (3); EFT-3 (6); VIT-6 (2); VHA 13 (2); CDC-48.2 (2); F27D4.1 (2); and C16A3.10 (3).
SUPPLEMENTAL MATERIAL
Three 10-second movie recordings, from left to right, oga-1(ok1207), wild type (N2), and ogt-1(ok1474) animals at day 12 of adulthood. Note that, while oga-1 mutant and wild-type animals are highly active, the ogt-1 mutant animal is considerably slower, a phenotype similar to that of older (day 18) wild-type animals (data not shown). At an earlier time point (day 6) all three genotypes had indistinguishable movement (data not shown).
Acknowledgments
Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). This work was supported by NIH grant R01DK075069 to L.W. and E.T.K.
Glossary
- O-GlcNAc
O-linked b-N-acetylglucosamine
- OGT
O-GlcNAc transferase
- OGA
O-GlcNAcase (neutral b-Nacetylglucosaminidase)
- PI3K
phosphatidylinositol-3-OH kinase
Footnotes
The authors of this manuscript have no conflict of interests to declare.
REFERENCES
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annual review of physiology. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. The Journal of biological chemistry. 1991;266:4706–4712. [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer's disease. Mol Biosyst. 2007;3:766–772. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- Wang Z, Park K, Comer F, Hsieh-Wilson LC, Saudek CD, Hart GW. Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes. Diabetes. 2009;58:309–317. doi: 10.2337/db08-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A. Tabtiang RAC. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Alessi DR. The insulin signalling pathway. Curr Biol. 2002;12:R236–238. doi: 10.1016/s0960-9822(02)00777-7. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Bonafe M, Johnson TE. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. 2006;61:444–460. doi: 10.1093/gerona/61.5.444. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, Ashwell G, Krause MW, Hanover JA. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11952–11957. doi: 10.1073/pnas.0601931103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, Ashwell G, Krause M. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11266–11271. doi: 10.1073/pnas.0408771102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhon SA, Johnson TE. Movement as an index of vitality: comparing wild type and the age-1 mutant of Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 1995;50:B254–261. doi: 10.1093/gerona/50a.5.b254. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nature genetics. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Hertweck M, Gobel C, Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Developmental cell. 2004;6:577–588. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes & development. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gami MS, Iser WB, Hanselman KB, Wolkow CA. Activated AKT/PKB signaling in C. elegans uncouples temporally distinct outputs of DAF-2/insulin-like signaling. BMC developmental biology. 2006;6:45. doi: 10.1186/1471-213X-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. Faseb J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol. 1994;49:B270–276. doi: 10.1093/geronj/49.6.b270. [DOI] [PubMed] [Google Scholar]
- Walker GA, White TM, McColl G, Jenkins NL, Babich S, Candido EP, Johnson TE, Lithgow GJ. Heat shock protein accumulation is upregulated in a long-lived mutant of Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2001;56:B281–287. doi: 10.1093/gerona/56.7.b281. [DOI] [PubMed] [Google Scholar]
- Link CD, Cypser JR, Johnson CJ, Johnson TE. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones. 1999;4:235–242. doi: 10.1379/1466-1268(1999)004<0235:doosri>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love DC, Ghosh S, Mondoux MA, Fukushige T, Wang P, Wilson MA, Iser WB, Wolkow CA, Krause MW, Hanover JA. Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7413–7418. doi: 10.1073/pnas.0911857107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS genetics. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong MQ, Venable JD, Au N, Xu T, Park SK, Cociorva D, Johnson JR, Dillin A, Yates JR., 3rd Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science. 2007;317:660–663. doi: 10.1126/science.1139952. [DOI] [PubMed] [Google Scholar]
- Samuelson AV, Carr CE, Ruvkun G. Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes & development. 2007;21:2976–2994. doi: 10.1101/gad.1588907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Kyung Kim Y, Cipriani PG, Kang M, Khersonsky SM, Walsh DP, Lee JY, Niessen S, Yates JR, 3rd, Gunsalus K, Piano F, Chang YT. Forward chemical genetic approach identifies new role for GAPDH in insulin signaling. Nat Chem Biol. 2007;3:55–59. doi: 10.1038/nchembio833. [DOI] [PubMed] [Google Scholar]
- Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53:921–930. doi: 10.2337/diabetes.53.4.921. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocrine reviews. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- Olsen A, Vantipalli MC, Lithgow GJ. Checkpoint proteins control survival of the postmitotic cells in Caenorhabditis elegans. Science. 2006;312:1381–1385. doi: 10.1126/science.1124981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro E, Hegi de Castro S, Johnson TE. Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Radic Biol Med. 2004;37:139–145. doi: 10.1016/j.freeradbiomed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Teo CF, Ingale S, Wolfert MA, Elsayed GA, Not LG, Chatham JC, Wells L, Boons GJ. Glycopeptide-specific monoclonal antibodies suggest new roles for O-GlcNAc. Nat Chem Biol. 2010;6:338–343. doi: 10.1038/nchembio.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Dixit BL, Raha T, Green MR, Tissenbaum HA. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nature genetics. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- Halaschek-Wiener J, Khattra JS, McKay S, Pouzyrev A, Stott JM, Yang GS, Holt RA, Jones SJ, Marra MA, Brooks-Wilson AR, Riddle DL. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. 2005;15:603–615. doi: 10.1101/gr.3274805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thornton J, Gems D. Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans. Mech Ageing Dev. 2006;127:458–472. doi: 10.1016/j.mad.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Mendenhall AR, LaRue B, Padilla PA. Glyceraldehyde-3-phosphate dehydrogenase mediates anoxia response and survival in Caenorhabditis elegans. Genetics. 2006;174:1173–1187. doi: 10.1534/genetics.106.061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehennaut V, Slomianny MC, Page A, Vercoutter-Edouart AS, Jessus C, Michalski JC, Vilain JP, Bodart JF, Lefebvre T. Identification of structural and functional O-linked N-acetylglucosamine-bearing proteins in Xenopus laevis oocyte. Mol Cell Proteomics. 2008;7:2229–2245. doi: 10.1074/mcp.M700494-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three 10-second movie recordings, from left to right, oga-1(ok1207), wild type (N2), and ogt-1(ok1474) animals at day 12 of adulthood. Note that, while oga-1 mutant and wild-type animals are highly active, the ogt-1 mutant animal is considerably slower, a phenotype similar to that of older (day 18) wild-type animals (data not shown). At an earlier time point (day 6) all three genotypes had indistinguishable movement (data not shown).