Abstract
We demonstrate a robust procedure for the quantitative characterization of glial metabolism in human brain. In the past, the slope of the uptake and production of enriched label at steady state were used to determine metabolic rates, requiring the patient to be in the magnet for 120 – 160 minutes. In the present method, 13C cerebral metabolite profiles were acquired at steady state alone on a routine clinical MR scanner in 25.6 minutes. Results obtained from the new short method (SAGA) were comparable to those achieved in a conventional, long method and effective for determination of glial metabolic rate in posterior-parietal and frontal brain regions.
Keywords: Spectroscopy, Carbon-13 MRS, Brain, Bicarbonate, Glial metabolism
Introduction
Clinical applications of 13C MRS have been explored in a wide range of human brain diseases from the new born to advanced age [1–8]. 13C-enriched glucose and acetate have been used to distinguish neuronal from glial metabolism and, with low-power (noise) decoupling within the FDA limits of power deposition (SAR), previous no-go frontal areas of the brain have become accessible to 13C MRS examinations [9–10]. Data analyses based on complex, steady state multi-enzyme models have been replaced by simpler single step calculations, to distinguish between normal and diseased states of neurons [3, 6]. Recently, recognition of an important role for glia in brain diseases involving neuroinflammation would make a non-invasive 13C MR assay an important tool to study glial dysfunction in human brain [11–14]. A remaining hurdle preventing wider utilization of this 13C MRS for this purpose is the long data-acquisition, demanding two or more hours of MR examination time. This has two important drawbacks: First, poor patient tolerance results in a significant number of failures to complete the examination. Second, the high cost of MR scanner time means 13C MRS is not cost effective. We have developed a new approach [15] in which the subject (patient) receives the 13C substrate before entering the MR scanner and then is immediately positioned in the scanner where a brain 13C MRS profile is then acquired in 25.6 minutes. We call this new method of short 13C MRS brain examination Swift Acetate Glial Assay (SAGA). In this paper, we compare our SAGA method to a long 13C MRS brain examination in which the patient lies in the MR scanner and 13C brain spectra are acquired for 120–160 minutes. We found that our short method (SAGA) gives very similar results to the long method when bicarbonate production is calculated. We conclude the SAGA technique is an excellent way to determine the in vivo metabolic rate of human glial with an exam that takes less than 30 minutes of scanner time.
Results
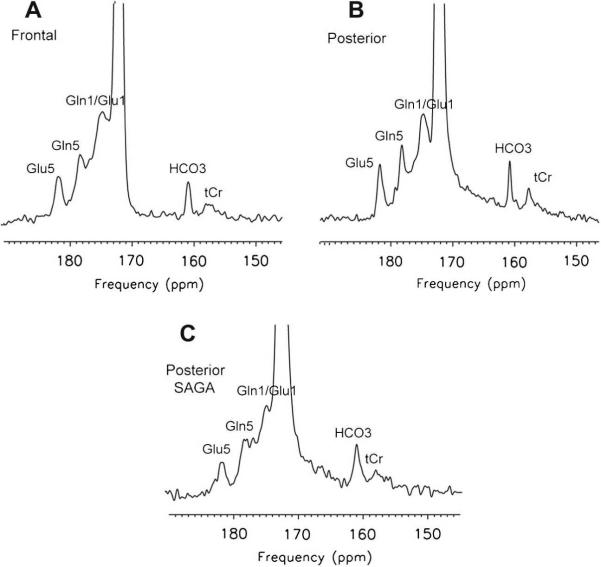
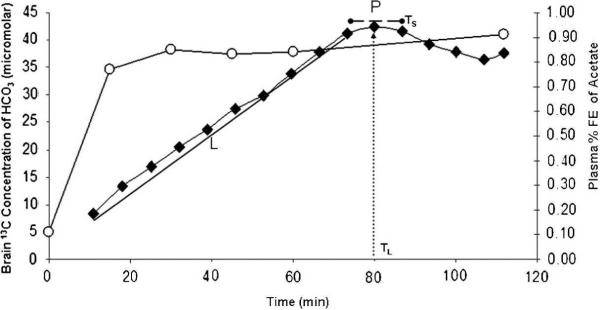
Intravenous infusion of [1-13C] acetate for 60 minutes with the subject within the MR scanner for 120 – 160 minutes (long method) resulted in the anticipated enrichment of cerebral glutamate (C5 + C1), glutamine (C5 + C1) and bicarbonate in 13C MR spectra of the frontal (Fig 1 A) and posterior parietal brain regions (Figure 1B). 13C bicarbonate enrichment in the posterior parietal brain was also readily measured in 25.6 minutes, using the SAGA technique when the intravenous infusion of [1-13C] acetate was completed before the subject entered the MR scanner (Figure 1C). Rates at which the precursor, [1-13C] acetate appeared in blood and the oxidation product, 13C- bicarbonate appeared in the brain are shown in Figure 2. H13CO3 was detected in the brain within 10 minutes of commencing [1-13C] acetate infusion. The H13CO3 peak within the brain increased linearly over 70 minutes (L) and reached a plateau (P) between 75 – 85 minutes, before declining. Cerebral bicarbonate enrichment was not limited by [1-13C] acetate fractional enrichment in blood which reached 80% at 15 minutes. The rate of cerebral 13C bicarbonate accumulation was higher in frontal than posterior parietal brain (Table 1). The midpoint and duration Ts of period “P” as defined in 13 `long-method' examinations was selected for SAGA examinations (see Methods). Glial metabolic rate measured with SAGA was 0.4 micromoles/g/min, 20% lower than that assayed in the long method (significant vs Method L but not significant vs Method P; Table 1). We demonstrate that, using data acquired from “steady state” rather than the “slope” of 13C enrichment, the rate of bicarbonate production from [1-13C] acetate in normal human brain can be determined in 25.6 minutes in an MR scanner using the proposed SAGA method.
Figure 1. 13C MRS spectra (145–190ppm) acquired from normal human brain following enrichment with [1-13C] acetate.
Spectra are scaled to the carbonyl carbon of the unenriched lipid resonance at 172 ppm. All show enrichment of cerebral glutamate (Glu5 plus acetate C1, 182ppm), glutamine C5 (Gln5, 178.4ppm), glutamine C1 plus glutamate C1 (Gln1/Glu1, 175ppm), and bicarbonate (HCO3−, 161ppm) together with natural abundance creatine plus phosphocreatine (tCr, 158ppm). Long Method: A) Frontal brain (2176 averages). B) Posterior brain (2688 averages); SAGA Method: C) Posterior brain (512 averages). Spectrum C acquired in 25.6 minutes with the SAGA method shows lower SNR
Figure 2. Time course of enrichment of blood (13C acetate) and brain (13C HCO3−) during Long Method.
[1-13C] acetate 3mg/kg/min was administered intravenously over 60 minutes with the subject inside a GE 1.5T clinical MR scanner. 13C brain spectra were acquired for 120 minutes (Long Method). Blood 13C acetate rose rapidly to achieve 80% fractional enrichment within the first 15 minutes. Cerebral 13C HCO3− concentration increased slowly with slope L, during the period of infusion to level off to a plateau P, then decreased. The mid-point of P (TL) and its duration (Ts) (Figure 2) were established in 13 Long method examinations. This experimental data defined the short, SAGA method in which [1-13C] acetate infusion was completed while the subject was outside the MR scanner and inside the MR scanner only for 25.6 minutes. Glial metabolic rates in Table 1 were calculated from the slope L and the steady state, P of cerebral 13C bicarbonate in the Long method and the mean cerebral 13C bicarbonate in the short (SAGA) method.
Table 1.
Glial metabolic rate in human brain using SAGA 13C MRS
| Protocol (number of studies) | Long (N=13) | SAGA (N=3) | P Short vs Long | |
|---|---|---|---|---|
| Brain region | Frontal (N=7) | Posterior-Parietal (N=6) | Posterior-Parietal (N=3) | |
| 13C bicarbonate Produced (micromoles/g/min) | ||||
| Data Analysis (L) Slope | 0.73 ± 0.24 | 0.52 ± 0.07 | 0.02 | |
| Data Analysis (P) Plateau | 0.48 ± 0.11 | 0.49 ± 0.11 | 0.40 ± 0.09 | 0.45 |
| P value L vs. P | 0.01 | 0.30 | ||
| P value Frontal vs. Posterior | 0.03 (L) 0.44 (P) |
|||
13C bicarbonate production rates were compared in three subjects (three studies) who received intravenous [1-13C] acetate while outside the magnet (SAGA) and ten subjects (thirteen studies) who received intravenous [−13C] acetate infusion for 60 min while positioned within the bore of 1.5T GE clinical scanner and followed by continuous 13C MRS data acquisition (Long method = 160 min, SAGA = 25.6 min). Data acquired from frontal (N=7) or posterior parietal (N=6) brain locations in the long method, were analyzed based either on the slope (L in Figure 2) or upon the ′steady state′ plateau (P in Figure 2). Statistics were resulted of unpaired t-test. Values are mean ± standard deviation (N= number of examinations).
Discussion
Rates obtained with SAGA differed by about 20% (P = 0.02) from those measured by the 120 – 160 minute long 13C MRS method. Bicarbonate production rate in the anterior brain measured by 13C MRS was slightly higher but not statistically significantly, compared to the posterior parietal brain regions (P>0.1 in paired t-tests N=4). We noted that the SAGA method is vulnerable to a systematic underestimation of the glial metabolic rate, due at least in part to the gap (10 min maximum) when the infusion is discontinued and the MRS data acquisition begins. This underestimation will have a greater impact when the glial metabolism is faster as we have observed in the anterior brain. However, several techniques can be used to reduce this time gap, for example performing the infusion procedure with subject laying supine on a MRI table for swift transfer and positioning in the MRI scanner. Also, the lower signal intensities observed in the frontal brain could contribute to the discrepancy observed. Further studies are required to confirm these apparent differences. An improved dual tuned proton-carbon coil for the anterior brain is expected to provide the necessary signal-to-noise ratio (SNR) when SAGA is employed in practice for assays in the frontal brain.
Calculation was also performed to determine if a faster rate of plasma enrichment (for example 5 min to reach plateau rather than 15 min) with the precursor [1-13C] acetate would have any impact of the observed rate of glial TCA cycle. Using the kinetics parameters measured by Deelchand [16], the calculated cerebral metabolic rate of acetate (using equation 3 in [16]) was unchanged.
Because acetate is metabolized exclusively in astrocytes (glia) [17–18], the metabolic rate of 13C bicarbonate production as determined by SAGA reflects accurately the glial-metabolic cycle rate for posterior parietal brain. The mean bicarbonate production rate from [1-13C] acetate observed in this study, 0.62 ± 0.14 micromoles/g/min for the human brain (average of frontal and posterior-parietal; Table 1), is similar to that recently reported in rodents [16] (0.50 micromoles/g/min) and represents the maximum rate of acetate oxidation or glial metabolic rate under these infusion conditions. However, we note that in human studies the amounts of acetate infused are limited by concerns over possible toxicity, plasma 13C acetate never exceeded 1mM. For rat brain, the observed rate of acetate metabolism reached its maximum at plasma acetate concentrations = 2–3 mM [16].
Glial metabolic rate is reported here as bicarbonate production rate which represents 16% of total cerebral oxygen consumption (3.0 micromoles/g/min). This proportion of glial metabolism as a fraction of the total cerebral metabolic rate is comparable to that previously established for [1-13C] acetate by Bluml [5] or more recently from this Laboratory [19] and for [2-13C] acetate by Lebon [20]. In a recently completed study [19], we demonstrate that both the higher value for human glial metabolic rate and the lower value can be generated in the same subjects depending on the method of analysis employed [19, p.6]. While the present findings for SAGA (as for the Long method) do exceed the rates expressed as glial TCA cycle by Bluml [5] and others [20–21] all of those reports used a similar method of calculation based upon an earlier estimate of neuronal TCA rate in the human brain of 0.7 micromoles/g/min [22]. The source of the differences reported here remain to be established, but the discrepancy is most probably to be found in the alternative method of calculation rather than species difference.
There are limitations to the present approach: first our observed glial-TCA cycle rate reflects only the conversion of a specific substrate, acetate to its end-product and takes no account of endogenous 12C glial respiratory fuels. Second, it is possible that the glial TCA cycle rate is overestimated due to an unknown contribution of 13C bicarbonate signal from the blood in the brain that could be shuttling bicarbonate from extracerebral tissues that have metabolized 13C acetate. Finally, the advantage of brevity in the SAGA MRS procedure must be counterbalanced by the lower glial metabolic rate measured, compared to the rate assayed as L in long-method (Figure 2, Table 1). A possible explanation may lie in the shorter 13C MRS acquisition, since the difference becomes statistically insignificant when SAGA is compared with long method rate P. Alternatively, the difference may be the result of the delay, 5 minutes on average, in commencing 13C MRS brain scans after completion of the [1-13C] acetate infusion and could be overcome in future by prolonging a infusion.
Conclusion
Glial metabolic rate for the human frontal brain is established for the first time. These preliminary studies also establish a means of dramatically reducing the time currently needed to perform human 13C MRS studies and show promise for 13C MRS studies in patients who cannot tolerate 120–160 minutes of MR scanning. A higher rate for glial metabolism based upon bicarbonate production from acetate is consistent with previous estimates that glial metabolism represents 15 – 20% of the whole human brain oxygen consumption in vivo.
Methods
Human Subjects
Sixteen 13C brain MRS examinations were performed during or after intravenous infusion of [1-13C] acetate, in ten healthy subjects (8F/2M) aged 30 ± 5 years. Studies were approved by Internal Review Board of Huntington Memorial Hospital and subject gave their informed consent. All subjects were fasted for 6 hours prior to intravenous infusion of [1-13C] acetate.
[1-13C] acetate infusion
Intravenous [1-13C] acetate (Cambridge Isotope Laboratory, Andover, MA pyrogen free solution), as previously described [5], was infused over precisely 60 minutes. 0.4 M 13C enriched acetate solution (150 – 300 milliliter, according to body weight), was administered using an MRI-safe, automated infusion pump (MEDRAD Inc, Warrendale, PA) at a rate of 3 mg/kg/min. This dose of [1-13C] acetate had previously been established to be non-toxic for humans [22–23]. A total of six 2 ml blood samples were taken; one prior to the start of infusion, one at each 15 minute intervals, and a final sample at the end of both Long and SAGA methods.
13C MRS data acquisition
Brain
In seven subjects 13C MR spectra were acquired from the posterior parietal brain and, in six subjects from the frontal brain. Four subjects were scanned in both regions, allowing direct comparison of glial metabolism in frontal and parietal brain.
All studies were performed on a 1.5T clinical MR (GE Healthcare, Waukesha, Milwaukee) scanner equipped for multi-nuclear MRS with stand-alone decoupler hardware and dual tuned 1H/13C RF coils, a half head coil for posterior (parietal) brain [10] and half helmet coil for the anterior (frontal) brain [19]. For the posterior (parietal) brain examination, the Waltz-4 bi-level decoupling and NOE scheme was used with power level of 9W during decoupling and 1W during NOE period. For the anterior (frontal) brain exam, low power noise decoupling was used with maximum power of 1W throughout [9].
Long-method (MR scan duration = 160 minutes: N=13 studies): Subjects entered the MR scanner at time zero and fast gradient echo MRI (SPGR) TE = 4.2 ms, TR = 175 ms, flip angle = 60°, field of view = 24cm, slice thickness = 2mm, with 1 mm gap) MRI was acquired. Prior to the 13C acetate infusion, field homogeneity adjustment was performed using automatic single voxel shimming routine from which the voxel of interest was prescribed to include the active region of 13C coil. Water linewidth from the entire region was 25–30 Hz. Natural abundance 13C MRS were acquired. [1-13C] acetate infusion lasting 60 minutes was commenced and 13C enriched brain spectra were acquired for 120– 160 minutes, using pulse and acquire with spectral width = 5000 Hz, number of data point = 1024, RF pulse-width = 250 μs, TR=3s, number of excitation per block = 128. 20 – 25 spectra, each of 6.4 minutes acquisition were stored for analysis of Rate L. The 4 spectra acquired during the near-steady state plateau (P) of cerebral 13C-bicarbonate enrichment were analyzed separately (see Data Analysis; Rate P).
SAGA method (MR scan duration = 25.6 minutes: N=3): After completion of a 60 minute intravenous 13C acetate infusion identical to the Long protocol but with the subject outside MRI scanner, the subject was then rapidly positioned in the MR scanner. 13C MRS data acquisition commenced at 65 minutes was continued for the period Ts, defined by the steady state plateau (P). Four 13C brain spectra were acquired from the posterior (parietal) brain in 25.6 minutes.
Blood
Blood samples were centrifuged, the plasma deproteinized using 99.9% ethanol 2:1 volume ratio to plasma and the supernatant was dried. Dried ethanol extracts were resuspended in 0.5ml 99.9% D2O. Plasma 13C acetate fractional enrichment and total acetate (12C + 13C) were determined by proton NMR using a Varian 300 MHz spectrometer, as previously described [5].
Data Analysis
In each spectrum, cerebral 13C bicarbonate (mM) was determined from H13CO3− peak area relative to 13C creatine- plus phosphocreatine [Cr + PCr = 10 +/−1mM] not enriched from [1-13C] acetate [17] as internal reference. Glial metabolic rates were calculated as follows: Bicarbonate production rate in the Long-method, was obtained from the slope of increasing bicarbonate versus time (L), and 13C spectra were summed 4 × 6.5 min from the plateau (P). Bicarbonate production rate in SAGA was calculated from the 4 × 6.5 min 13C spectra acquired as mean 13C peak area / time. Results are expressed as micromoles bicarbonate. g brain −1. min−1 Brain volumes examined were approximately 140–200 cc in the posterior brain [10] and 100 cc in the frontal brain [9].
Statistics
Mean and standard deviation were compared in unpaired student t-tests; when the same subject (N=4) underwent both frontal and posterior 13C MRS brain examinations, paired student t-tests were applied. P < 0.05 was considered statistically significant.
Acknowledgement
The authors are grateful to Dr. Osama Abulseoud and Mr. Thomas Warren for their assistance. We thank Dr. Niki Zacharias for careful revision of the manuscript. The work was supported by NIH grant number: K25DA21112 (NS) and a Grant in Aid (BDR and TT) from Rudi Schulte Research Institute of Santa Barbara, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petroff OA, et al. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43(7):703–10. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- 2.Befroy DE, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56(5):1376–81. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluml S, et al. 1-(13)C glucose magnetic resonance spectroscopy of pediatric and adult brain disorders. NMR Biomed. 2001;14(1):19–32. doi: 10.1002/nbm.679. [DOI] [PubMed] [Google Scholar]
- 4.Lin AP, et al. Reduced glutamate neurotransmission in patients with Alzheimer's disease -- an in vivo (13)C magnetic resonance spectroscopy study. MAGMA. 2003;16(1):29–42. doi: 10.1007/s10334-003-0004-x. [DOI] [PubMed] [Google Scholar]
- 5.Bluml S, et al. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 2002;15(1):1–5. doi: 10.1002/nbm.725. [DOI] [PubMed] [Google Scholar]
- 6.Bluml S, Moreno-Torres A, Ross BD. [1-13C]glucose MRS in chronic hepatic encephalopathy in man. Magn Reson Med. 2001;45(6):981–93. doi: 10.1002/mrm.1131. [DOI] [PubMed] [Google Scholar]
- 7.Boumezbeur F, et al. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30(1):211–21. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otsuki T, et al. Carbon 13-labeled magnetic resonance spectroscopy observation of cerebral glucose metabolism: metabolism in MELAS: case report. Arch Neurol. 2005;62(3):485–7. doi: 10.1001/archneur.62.3.485. [DOI] [PubMed] [Google Scholar]
- 9.Sailasuta N, et al. Clinical NOE 13C MRS for neuropsychiatric disorders of the frontal lobe. J Magn Reson. 2008;195(2):219–25. doi: 10.1016/j.jmr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gropman AL, et al. Ornithine transcarbamylase deficiency with persistent abnormality in cerebral glutamate metabolism in adults. Radiology. 2009;252(3):833–41. doi: 10.1148/radiol.2523081878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esposito G, et al. Imaging neuroinflammation in Alzheimer's disease with radiolabeled arachidonic acid and PET. J Nucl Med. 2008;49(9):1414–21. doi: 10.2967/jnumed.107.049619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao JS, et al. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan EV, Zahr NM. Neuroinflammation as a neurotoxic mechanism in alcoholism: commentary on “Increased MCP-1 and microglia in various regions of human alcoholic brain”. Exp Neurol. 2008;213(1):10–7. doi: 10.1016/j.expneurol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Eldik LJ, et al. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int Rev Neurobiol. 2007;82:277–96. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- 15.Harris K, et al. SNAPSHOT: A rapid 13C MRS technique for clinical spectroscopy. Proc. Int. Soc. Magn. Reson. Med. 2004;12:452. [Google Scholar]
- 16.Deelchand DK, et al. Acetate transport and utilization in the rat brain. J Neurochem. 2009;109(Suppl 1):46–54. doi: 10.1111/j.1471-4159.2009.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18(14):5225–33. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassel B, Sonnewald U, Fonnum F. Glial-neuronal interactions as studied by cerebral metabolism of [2-13C]acetate and [1-13C]glucose: an ex vivo 13C NMR spectroscopic study. J Neurochem. 1995;64(6):2773–82. doi: 10.1046/j.1471-4159.1995.64062773.x. [DOI] [PubMed] [Google Scholar]
- 19.Sailasuta N, A. O, Harris K, Ross BD. Glial Dysfunction in Abstinent Methamphetami Abusers. J Cereb Blood Flow Metab. 2009;30:950–60. doi: 10.1038/jcbfm.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebon V, et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22(5):1523–31. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason GF, et al. Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J Cereb Blood Flow Metab. 1995;15(1):12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- 22.Burnier P, et al. Metabolic and respiratory effects of infused sodium acetate in healthy human subjects. Am J Physiol. 1992;263(6 Pt 2):R1271–6. doi: 10.1152/ajpregu.1992.263.6.R1271. [DOI] [PubMed] [Google Scholar]
- 23.Ward RA, et al. Comparative metabolic effects of acetate and dichloroacetate infusion in the anesthetized dog. Metabolism. 1985;34(7):680–7. doi: 10.1016/0026-0495(85)90098-8. [DOI] [PubMed] [Google Scholar]