Abstract
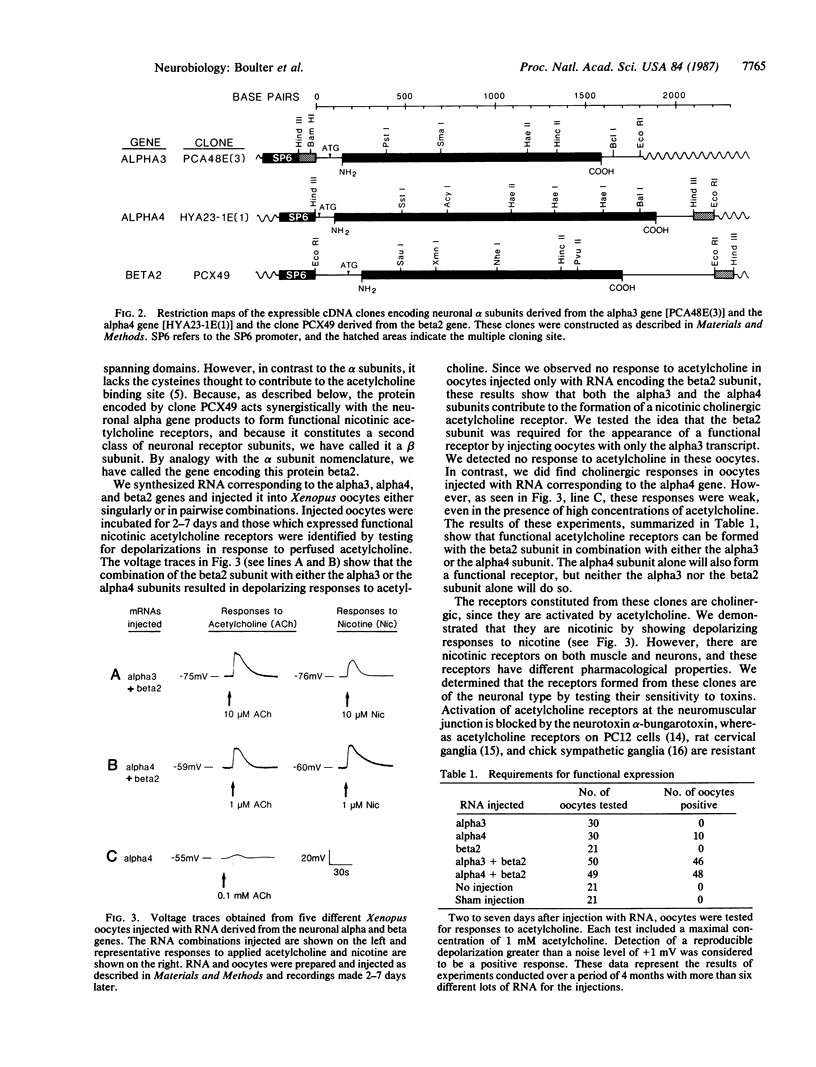
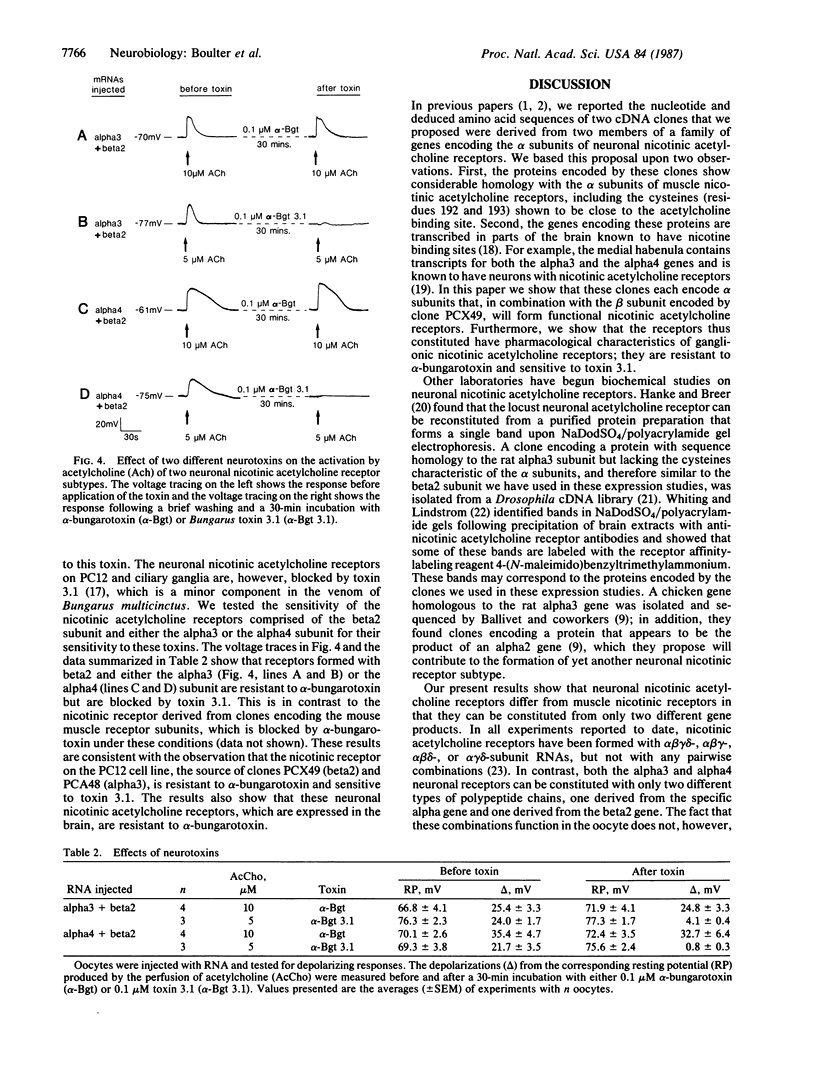
A family of genes coding for proteins homologous to the alpha subunit of the muscle nicotinic acetylcholine receptor has been identified in the rat genome. These genes are transcribed in the central and peripheral nervous systems in areas known to contain functional nicotinic receptors. In this paper, we demonstrate that three of these genes, which we call alpha 3, alpha 4, and beta 2, encode proteins that form functional nicotinic acetylcholine receptors when expressed in Xenopus oocytes. Oocytes expressing either alpha 3 or alpha 4 protein in combination with the beta 2 protein produced a strong response to acetylcholine. Oocytes expressing only the alpha 4 protein gave a weak response to acetylcholine. These receptors are activated by acetylcholine and nicotine and are blocked by Bungarus toxin 3.1. They are not blocked by alpha-bungarotoxin, which blocks the muscle nicotinic acetylcholine receptor. Thus, the receptors formed by the alpha 3, alpha 4, and beta 2 subunits are pharmacologically similar to the ganglionic-type neuronal nicotinic acetylcholine receptor. These results indicate that the alpha 3, alpha 4, and beta 2 genes encode functional nicotinic acetylcholine receptor subunits that are expressed in the brain and peripheral nervous system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Fumagalli L. Department of Pharmacology, The School of Pharmacy, University of London, London, Great Britain. Brain Res. 1977 Jun 24;129(1):165–168. doi: 10.1016/0006-8993(77)90981-7. [DOI] [PubMed] [Google Scholar]
- Carbonetto S. T., Fambrough D. M., Muller K. J. Nonequivalence of alpha-bungarotoxin receptors and acetylcholine receptors in chick sympathetic neurons. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1016–1020. doi: 10.1073/pnas.75.2.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. B., Schwartz R. D., Paul S. M., Pert C. B., Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985 May;5(5):1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio T., Ballivet M., Patrick J., Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1111–1115. doi: 10.1073/pnas.80.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Deneris E., Luyten W., Kochhar A., Patrick J., Heinemann S. Members of a nicotinic acetylcholine receptor gene family are expressed in different regions of the mammalian central nervous system. Cell. 1987 Mar 27;48(6):965–973. doi: 10.1016/0092-8674(87)90705-7. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanke W., Breer H. Channel properties of an insect neuronal acetylcholine receptor protein reconstituted in planar lipid bilayers. Nature. 1986 May 8;321(6066):171–174. doi: 10.1038/321171a0. [DOI] [PubMed] [Google Scholar]
- Hermans-Borgmeyer I., Zopf D., Ryseck R. P., Hovemann B., Betz H., Gundelfinger E. D. Primary structure of a developmentally regulated nicotinic acetylcholine receptor protein from Drosophila. EMBO J. 1986 Jul;5(7):1503–1508. doi: 10.1002/j.1460-2075.1986.tb04389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Fukuda K., Konno T., Mori Y., Tanaka K., Mishina M., Numa S. Functional properties of nicotinic acetylcholine receptor subunits expressed in various combinations. FEBS Lett. 1987 Apr 20;214(2):253–258. doi: 10.1016/0014-5793(87)80065-0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine causes rapid nicotinic excitation in the medial habenular nucleus of guinea pig, in vitro. J Neurosci. 1987 Mar;7(3):742–752. doi: 10.1523/JNEUROSCI.07-03-00742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Patrick J., Stallcup B. alpha-Bungarotoxin binding and cholinergic receptor function on a rat sympathetic nerve line. J Biol Chem. 1977 Dec 10;252(23):8629–8633. [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin P. M., Berg D. K. Inhibition of neuronal acetylcholine sensitivity by alpha-toxins from Bungarus multicinctus venom. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2072–2076. doi: 10.1073/pnas.76.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P., Lindstrom J. Affinity labelling of neuronal acetylcholine receptors localizes acetylcholine-binding sites to their beta-subunits. FEBS Lett. 1987 Mar 9;213(1):55–60. doi: 10.1016/0014-5793(87)81464-3. [DOI] [PubMed] [Google Scholar]



