Abstract
Objective
To determine impact of parity on mechanical behavior of the vagina and correlate with alterations in collagen structure.
Study Design
Mechanical properties of 5 nulliparous and 6 parous rhesus macaques were derived from uniaxial tensile tests. Collagen ratios and alignment were quantified via quantitative fluorescent microscopy and picrosirius red staining. Outcomes were compared via Student's t-test or Mann Whitney U (p<0.05), and Spearman's rho for correlation coefficients.
Results
Mechanical properties were inferior in parous vs nulliparous vagina with decreased tangent modulus (p=0.03), tensile strength (p<0.001), and strain energy density (p=0.006). While no difference in collagen ratios (p=0.26) were observed, collagen alignment decreased with parity (p=0.06). Worsening pelvic organ support negatively correlated with decreasing collagen alignment (r2 = −0.66) and mechanical properties (r2 = −0.67).
Conclusions
Vaginal parity is associated with inferior tissue mechanics and loss of collagen alignment. Such behavior likely predisposes to the development of pelvic organ prolapse.
Keywords: Prolapse, pregnancy, mechanical properties, rhesus macaques
Introduction
A well supported vagina is essential for normal pelvic organ support. The vagina, in turn, is supported by the physiologically complex interactions between the levator ani muscles and the connective tissue attachments between the vagina and the pelvic sidewall (1). Thus, with transient increases in intra-abdominal pressure, the vagina and its supportive tissues orchestrate an equally distributed counter pressure to maintain pelvic organ support. Failure of any of the components of this intricate system can lead to loss of support by the vagina and the development of pelvic organ prolapse (POP) (2, 3). Up to 11% percent of all women in the United States will undergo a major surgery to repair prolapse or related conditions by age 80 (4) with a direct cost of surgery greater than $1 billion per year.
Unfortunately the etiology of POP is unknown. Injury to the vagina and it supportive tissues at the time of vaginal birth is considered a major risk factor (2) since most parous women have mild asymptomatic prolapse (5) and vaginal delivery has been shown to result in a quantifiable deterioration of vaginal support as measured by the Pelvic Organ Prolapse Exam (POP Q; 6). However, surprisingly little is known of the mechanism by which vaginal birth alters the supportive function of the vagina and predisposes to POP.
Mechanically, it is well known that all supportive tissues require a minimum stiffness and strength in order to meet the demands of physiological loading. The primary load bearing protein of supportive tissues is collagen. Indeed, collagen composition and alignment have been shown to be related to the mechanical integrity of soft tissues. For example, a higher collagen I/(III+V) ratio and/or increased alignment of collagen in the direction of applied forces is associated with superior mechanical properties. Collagen III is increased in the vagina of women with advanced prolapse relative to those without prolapse but whether this is a cause or an effect of prolapse is not clear (7).
One of the primary limitations in establishing a link between vaginal parity and the development of prolapse is access to tissue. Indeed, the small quantity of tissues obtained from biopsies of women precludes conventional mechanical testing. Thus, studies investigating how changes in the structural proteins such as collagen and their impact on the mechanical properties of the vagina and supportive tissues subsequent to vaginal delivery, relate to the pathogenesis of pelvic organ prolapse have been limited. To overcome these issues, we have turned to animal models; however, choosing the appropriate model for the research question at hand is an important factor to consider. Previously, we have utilized a rodent model to assess changes that occur during pregnancy and postpartum. These studies have proved to be invaluable in our understanding of how the vagina supportive tissue complex adapts during pregnancy, but have been limited in illustrating the long-term effects of vaginal delivery on vaginal mechanical properties as the rat does not sustain a large degree of maternal birth trauma and completely recovers after a vaginal birth (8,9,10). In contrast, the rhesus macaque is an established model to study the impact of vaginal delivery on vaginal tissue behavior because it has similar reproductive physiology to humans and spontaneously develops prolapse. More importantly, the size of the fetal head relative to the vaginal diameter places the mothers at a high risk to sustain an injury at the time of vaginal delivery, similar to humans (5).
In this study, we hypothesized that vaginal delivery results in long term changes in the vagina that predispose to the development of POP in the context of specific risk factors. Specifically, we believe that parity induced changes manifest as inferior mechanical properties associated with altered collagen structure. To test this hypothesis, we compared collagen alignment, the amount of the fibrillar collagens I, III and V, and mechanical properties (tangent modulus or stiffness and tensile strength) in nulliparous and vaginally parous rhesus macaques. We focused our studies on the vagina because of the relative ease of access to this tissue as well as our contention that any loss of pelvic organ support ultimately manifests itself in the vagina.
Materials and Methods
Animals
The animals used in this study were maintained and treated according to experimental protocols approved by the Institutional Animal Care Utilization Committee of the University of Pittsburgh (IACUC # 0603434), and in adherence to the National Institutes of Health Guidelines on the use of Laboratory Animals. Accordingly, a minimum number of rhesus macaques (macacca mulatta) were utilized to answer our research question (see power analysis below) comprised of 14 cycling animals between 9 and 19 years of age. Parous animals (N=7) were a minimum of 12 months after their last delivery to allow adequate time to recover from short term birth injuries. Seven nulliparous control nonhuman primates (NHPs) were used for comparison. Routine laboratory tests and regular examinations by veterinarians during a quarantine period were used to certify that these experimental animals were pathogen-free and in good physical condition. Animals were maintained in standard cages with ad libitum water, and scheduled monkey chow supplemented with fresh fruit, vegetables, and multiple vitamins daily. A 12-h light/dark cycle (7am to 7 pm) was used and menstrual cycle patterns were recorded daily.
Available demographic data was collected including age, body mass index, gravidity, and parity of each NHP. A modified Pelvic Organ Prolapse Quantification (POP Q) exam was developed to take into account the smaller vaginal size in sedated NHP – rhesus macaque. Our values are based on 10 previously examined nulliparous NHPs. Utilizing these exams, we calculated the urethrovesical junction to be 2 cm proximal to the vaginal introitus. Thus, in the fully supported vagina this position corresponding to point Aa of the POP-Q exam is −2 cm. The corresponding position on the posterior vagina wall (Ap) is −2 cm from the introitus. The gh, pb, Ba, Bp, C, D and TVL are measured similar to humans. In addition, the descent of the organs in all compartments is reported relative to the vaginal introitus as the hymen is less prominent in these animals. Prior to harvesting the vagina, a POP-Q exam was performed as described using a pediatric speculum. A crede maneuver was employed to mimic valsalva as a proxy for strain.
Following a POP Q exam, the vagina and supportive tissues were excised en bloc using a transabdominal approach. After the vaginal tissue had been isolated, the middle 2/3 was divided for (1) histomorphology, (2) biochemical, and (3) biomechanical analyses. Tissue samples were divided for each analysis immediately to minimize the number of freeze-thaw cycles, and stored for an average of 1 month prior to analysis as to maximize the number of specimens per assay insuring that conditions were as consistent as possible. Tissue samples obtained for all analyses avoided the urethra to reduce the influence on collagen alignment, composition, and its affect on the mechanical properties. Due to limited vaginal sizes, of several animals were unable to be examined using all described experimental protocols. Each section of this study was performed by one of the researchers who were blinded to the identification of the samples.
Trichrome Staining
For histomorphological imaging, tissue was embedded and frozen in optimal cutting temperature compound (OCT, Sakura, Tokyo). Sections of the vaginal cross-section were cut onto slides roughly 5-8 μm thick. Trichrome slides were used to ensure full thickness samples of the vaginal cross-section (epithelium, sub-epithelium, muscularis, and adventitia). These images were used to qualitatively assess differences between the vaginal cross-sections that may be associated with vaginal parity. Blinded researchers examined each slide for any large changes in cross-sectional area composition, e.g. increases in smooth muscle or collagen composition.
Immunofluorescence
To assess the relative amounts of collagen I, III and V and the ratio of collagen I/(III+V), we followed an established protocol (11). Briefly, sections used for immunofluorescence were also oriented along the longitudinal axis of the vagina, consistent with trichrome staining. All animals had three separate serial sections (5-7 μm) analyzed per assay, with 5-7 sites were quantified per section. Blocks were fixed, rehydrated, and placed into normal donkey serum for 45 minutes. Primary antibodies for collagen type I (mouse anti-Human 1:200;Biodesign, Saco, ME), collagen type III (goat anti-Human 1:500;Biodesign, Saco, Me), and collagen V (rabbit anti-Human 1:1000; Biodesign, Saco, Me) were first applied followed by incubation with secondary antibodies for 60 minutes. Each sample was imaged using an Olympus Fluoview microscope (DSS Imagetech, New Dehli, India). Fluorescence signals were digitalized to form a pixel based image displayed on a monitor and quantitated using Metamorph (v5.0, Universal Imaging Corporation) and represented as a relative collagen I/(III+V) ratio for each specimen. Three slides per animal were analyzed in five to seven randomly selected sites within the subepithelium (dense connective tissue layer of the vagina). Results for each collagen sub-type (I, III, and V) were represented as a threshold area for that particular collagen per total area, along with the collagen I/(III+V) ratio.
Collagen Alignment
Picrosirius red staining was performed to assess the overall alignment of the vaginal collagen matrix within the cross-section (12,13). Circularly polarized light and image-analysis software, was utilized to quantify collagen alignment by assessing fiber hue. To do this, 5 to 10 mm full thickness mid-vagina sections from 4 nulliparous and 6 parous animals were oriented along the longitudinal axis, embedded in OCT, and placed in liquid nitrogen. Blocks of tissue were then sectioned (7μm), placed on glass slides and stored at −70°C. Seven to eight frozen sections per animal were hydrated briefly in dH2O and imaged using a BX51 Olympus microscope. Five polarized light images per sample were taken at 20X in both the vaginal subepithelium and the muscularis. An area analysis was performed using Metamorph 5.0, where the hue component of the resulting image was obtained, and the number of red, orange, yellow, and green (the colors of collagen fibers in order of decreasing thickness and organization) pixels were calculated.
Biomechanics
For biomechanical analysis, vaginal tissue samples from each NHP were carefully isolated from the surrounding connective tissue and wrapped in saline soaked gauze, placed in a plastic bag, and stored at −20°C (14,15). On the day of testing, the tissue was thawed and a longitudinal section of tissue was isolated (18.2 ± 3.1 mm). Each sample was then gripped using custom designed soft-tissue clamps and further dissected to ensure the desired aspect ratio (length/width) of five within the mid-substance of the tissue (16). Subsequently, tissue cross-sectional area and geometry was measured using a laser micrometer system in three areas along the length of the sample's trimmed midsubstance (17). Throughout this protocol the sample was kept moist with 0.9% saline. Contrast markers were placed on the tissue near the mid-line of the sample at a distance of 1 cm apart for strain measurements using a camera system (Keyence CV-2600) and motion analysis software (Spicatek, Inc. Maui, HI). The specimen-clamp complex was then placed into a 37°C saline bath and attached to a uniaxial tensile testing machine (Instron™ 5565). The clamp securing the proximal end of the vagina was contiguous with a 50 lb load cell (Honeywell Model 31) with 0.1 N resolution, and the distal end was attached to the base of the material testing machine. The specimen was allowed to equilibrate unloaded in the bath for 30 minutes prior to testing. Next, a small 0.5 N preload was applied to the tissue and ten cycles of preconditioning to 7% clamp-to-clamp strain were performed at a rate of 10 mm/min. A load to failure test was carried out immediately after the preconditioning along the specimens longitudinal axis (16). The load (force, N) and elongation (mm) of the tissue were recorded and used to generate a load-elongation relationship.
The load-elongation relationship was then converted to a stress-strain relationship from which the parameters describing the mechanical properties of the tissue were determined. Stress was defined as the load per cross-sectional area and was normalized to the average of the three cross-sectional area measurements obtained from the laser micrometer. Strain was calculated from the motion of the contrast markers on the midsubstance of the tissue and is defined as the change in marker position relative to the original marker position (Δl/lo). The slope of the linear region of the resulting stress-strain curve was defined as the tangent modulus which describes the stiffness on a per unit of tissue basis, while the tensile strength (maximum stress) and ultimate strain (strain in the tissue corresponding to the maximum stress) were recorded at failure. The strain energy density was calculated by measuring the area underneath the stress-strain curve until failure and is a measure of the “toughness” of a tissue in material science jargon. Each of these mechanical properties were calculated from the stress-strain relationship as previously described (18). Importantly, the loading conditions used in these experiments were not meant to recapitulate the physiology of vaginal delivery but rather to provide insight into fundamental differences in tissue behavior between nulliparous and parous animals.
Statistics
All statistical analyses were performed using SPSS (SPSS Inc, Chicago, Ill version 12.0.1). All data was examined to determine if it was normally distributed using a one-sample Kolmogorov-Smirnov test. A Student's t-test was used to compare all demographic data (age, height, weight, BMI, and total vaginal length (TVL)), collagen subtypes (collagen I, collagen III, collagen V, and collagen I/(III+V)) , collagen orientation (red:green ratio), and biomechanical properties (tangent modulus, tensile strength, maximum strain, and strain-energy density).
Statistics were aimed to answer our primary hypothesis which states that the mechanical properties of the tangent modulus and tensile strength would be inferior in parous NHP compared to nulliparous animals. Based on a preliminary study done within our lab on the biomechanical properties in the rodent model 7 animals would be required per group to detect at least a 30% difference between nulliparous and parous animals with an 80% power. Significant differences were found between these parameters with five and six samples in the nulliparous and parous groups, respectively, which indicates that we had reached 100% power for our primary outcomes as discussed below. The non-parametric Mann-Whitney test was used to compare POP-Q measurements. A Spearman's Rho rank correlation was performed to assess if the number of vaginal deliveries in the parous group correlated with any changes in collagen ratios, collagen subtypes, alignment, or biomechanical properties. All statistical tests were evaluated at a significance level of 0.05. For normally distributed parameters, data is represented as mean (standard deviation) and for non-normally distributed data as median (interquartile range).
Results
Of the 12 animals used in this study, two were part of a shared study and had sufficient tissue for only a single analysis leaving one animal in each group without combined biomechanical and biochemical data. The demographical data of all 12 animals is shown in Table 1. Age in the nulliparous group had a mean of 11.0 ± 1.2 years which was significantly lower (p=0.04) than the parous group (13.0 ± 3.1 years). Parous animals had a median parity of 7 (range 1-8) which is relatively low for this species. The height (m) and weight (kg) of each animal was collected and used to calculate individual BMI values. There was no statistical difference found between the BMI of each group (p=0.37, Table 1).
Table 1. Demographics and Modified POP-Q Scores.
Demographical and modified POP-Q variables from nulliparous and parous NHP.. Statistical differences were determined using a Mann-Whitney or Student's t-test (normally distributed data indicated byn).
| Nulliparous (n=7) | Parous (n=7) | p-value | |
|---|---|---|---|
| Age (years) | 11.0 ± 1.2 | 13.0 ± 3.1 | 0.04n |
| BMI (kg/m2) | 16.0 ± 3.9 | 19.0 ±4.4 | 0.37n |
| Parity | 0 (0) | 7.0 (2.0-8.0) | - |
| GH (cm) | 0.5 (0.5 – 1.0) | 2.0 (0.8 – 2.3) | 0.12 |
| PB (cm) | 1.5 (1.0 – 1.5) | 1.0 (1.0 – 1,8) | 0.69 |
| C (cm) | −6.0 (−6.0 – −6.0) | −3.5 (−5.5 – 2.3) | 0.02 |
| D (cm) | −7.0 (−7.0 – −6.3) | −4.0 (−5.8 – −2.8) | 0.006 |
| Aa (cm) | −2.0 (−2.0 – −2.0) | −2.0 (−2.0 – −1.0) | 0.69 |
| Ba (cm) | −2.0 (−2.0 – −2.0) | −2.0 (−2.0 – −1.0) | 0.69 |
| Ap (cm) | −2.0 (−2.0 – −2.0) | −2.0 (−2.0 – −1.0) | 0.69 |
| Bp (cm) | −2.0 (−2.0 – −2.0) | −2.0 (−2.0 – −1.0) | 0.69 |
| TVL (cm) | 6.4 ± 0.79 | 6.1 ± 1.1 | 0.34n |
Non-parametric data is represented as median (interquartile range) while the remaining data is presented as mean ± S.D.
POP-Q scores were compared between nulliparous and parous animals based on a modified exam (see Methods). All POP-Q measurements can be seen within Table 1. Nulliparous animals were observed to have a well supported vagina, as reflected in their superior POP-Q scores. The distance from the hymen to the cervix (C, p=0.02) and the hymen to the most dependent portion of the posterior fornix (D, p=0.006) was greater in parous relative to nulliparous animals indicating greater descent. No statistical differences were found in the remaining POP-Q measurements: genital hiatus (GH), perineal body (PB), anterior wall (Aa and Ba), and posterior wall (Ap and Bp), or the total vaginal length (TVL) (Table 1).
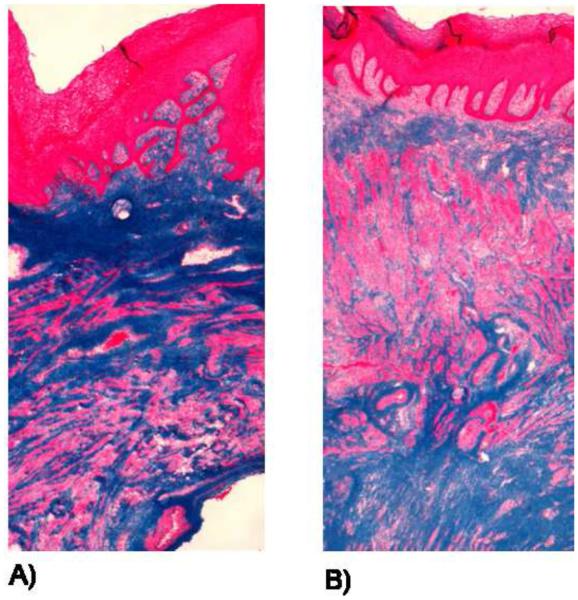
All specimens analyzed were corroborated to be full thickness vagina by trichrome staining. Similar to humans, the NHP vagina is comprised of 4 layers including a cellular epithelium (stained pink), sub-epithelium which consists of predominately collagen III (stained blue), a muscularis layer (stained pick) comprising 40-50% of the total volume, and an adventitia tissue layer comprised of loose connective tissue layer (stained blue). . There was not a gross difference in histological appearance between nulliparous (Figure 1A) and parous (Figure 1B) animals.
Figure 1.
Trichrome images of nulliparous (A) and parous (B) NHP. Stained bright pink is the epithelium layer, followed by the sub-epithelium layer (blue), which contains a majority of the collagen matrix of the vaginal cross-section. The muscularis bilayer consists of a layer of circumferentially oriented and a layer of longitudinally oriented muscle fibers, which are stained pink. The adventitia, which is a loose connective tissue layer stained blue.
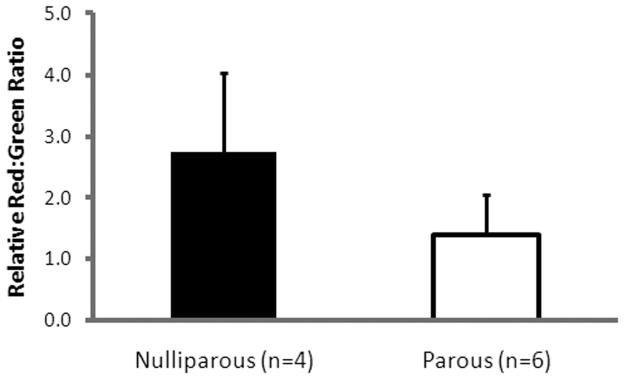
The ratio of collagen I/(III+V) was similar in the vaginal sub-epithelium between nulliparous and parous animals (Table 2; p=0.26). Individual collagen subtypes per area were also similar with nulliparous NHP having a collagen I (0.04 ± 0.02), collagen III (0.5 ± 0.04) and collagen V (0.2 ±0.06; see Table 2) and parous with collagen I (0.04 ± 0.03, p=0.83), collagen III (0.4 ± 0.2, p=0.11), and collagen V (0.07 ± 0.09, p=0.09). In contrast, quantitative analysis of picrosirius red stained sections showed an overall higher red:green ratio indicating more aligned collagen fibers in nulliparous vs parous animals. Indeed, nulliparous NHP had a red:green ratio of 2.7 ± 1.3 compared to parous NHP with a ratio of 1.4 ± 0.64 (p =0.06, Figure 2). Although these values were not statistically different, we found this was attributable to a single outlier in the parous group. After removing this single parous animal from the data analysis, the collagen alignment was significantly lower in parous compared to nulliparous NHPs (p=0.02).
Table 2. Collagen Ratio and Sub-type Data on Nulliparous and Parous Animals.
Relative collagen I/(III+V) ratio and individual collagen sub-types (I, III, and V) per area for nulliparous and parous NHP.
| Nulliparous (n=5) | Parous (n=6) | p-value | |
|---|---|---|---|
| Collagen I/(III+V) | 0.07 ± 0.03 | 0.09 ± 0.03 | 0.26 |
| Collagen I | 0.04 ± 0.02 | 0.04 ± 0.03 | 0.83 |
| Collagen III | 0.5 ± 0.04 | 0.3 ± 0.2 | 0.08 |
| Collagen V | 0.2 ± 0.06 | 0.07 ± 0.09 | 0.09 |
All data is represented as mean ± S.D. Significance was determined using a Student's t-test (p<0.05).
Figure 2.
Relative red:green ratio for nulliparous and parous animals. A higher red:green ratio indicates a higher degree of alignment within the cross-section. We observed a decrease in collagen alignment associated with parity.
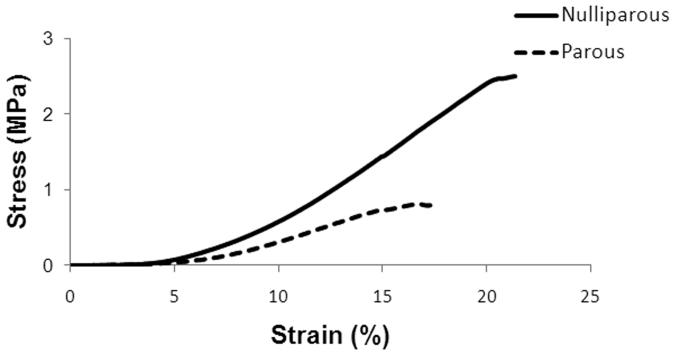
The parameters describing the mechanical properties of nulliparous versus parous primate vagina were determined from their stress-strain relationship (Figure 3). Both curves demonstrated nonlinear behavior with distinct toe, linear, and failure regions. However, the nulliparous group was more nonlinear beyond 3% strain, which is reflected by the higher tangent modulus (slope of the stress-strain curve corresponding to the stiffness of the specimen; p=0.03) with nulliparous NHP having 52% higher tangent modulus as compared to parous animals (Table 3). The nulliparous group also displayed a significantly higher tensile strength, which measured almost 3 times that of the parous group (Table 3; p<0.001). Correspondingly, it was found that the strain energy density of nulliparous samples was roughly 3 times greater than that of the parous group (Table 3, p=0.006). No statistical differences were detected between each group for ultimate strain (p=0.26). Thus, the data indicate that nulliparous tissue is stronger, more difficult to deform, and tougher than parous tissue.
Figure 3.
Representative stress-strain curves for nulliparous and parous animals from a uniaxial tensile test to failure. Both curves demonstrated nonlinear behavior with a distinct toe, linear, and failure regions. However, the nulliparous group was more nonlinear beyond 3-5% strain characterized by significant differences in the tensile strength, tangent modulus, and strain-energy density (Table 2).
Table 3. Uniaxial Tensile Biomechanical Properties of Vaginal Tissue.
The biomechanical properties (tensile strength, maximum strain, tangent modulus, and strain-energy density) of nulliparous and parous NHP are presented as mean ± S.D.
| Nulliparous (n=5) | Parous (n=6) | p-value | |
|---|---|---|---|
| Tangent Modulus (MPa) | 27.0 ± 11.5 | 12.8 ± 6.6 | 0.03 |
| Tensile Strength (MPa) | 2.4 ± 0.53 | 0.96 ± 0.26 | <0.001 |
| Maximum Strain (%) | 19.0 ± 5.4 | 16.0 ± 5.0 | 0.24 |
| Strain-Energy Density (MPa) | 0.18 ± 0.08 | 0.07 ± 0.02 | 0.006 |
A Student's t-test (p<0.05) was used to determine statistical significance between each group.
Further analysis demonstrated no significant correlations between the number of vaginal deliveries and collagen sub-types, collagen I/(III+V) ratios, alignment, and biomechanical properties. Animals were then separated into three categories: nulliparous, parous no prolapse, and parous prolapse. For inclusion in the parous prolapse group one of the following POPQ parameters were required: C ≥ −1, Aa,Ba >−1 or Ap, Bp >−1 with 3 animals meeting one of these criteria. By this method, we found significant negative correlations between prolapse and tangent modulus (r2= −0.53; p=0.01), tensile strength (r2= −0.67 p=0.002), strain-energy density (r2= −0.71; p=0.001), and collagen alignment (r2= −0.66; p=0.005; Table 4). Interestingly, the parous prolapse NHPs had the lowest red:green ratio (1.0 ± 0.07) compared to well supported parous NHP (1.8 ± 0.8) and nulliparous animals (2.7 ± 1.3), suggesting an association between a loss of collagen architecture and the development/progression of prolapse. This also correlated very well with the biomechanical properties. Animals with prolapse displayed a lower tangent modulus (9.5 ± 1.0 MPa), tensile strength (0.9 ± 0.2 MPa), and strain-energy density (0.06 ± 0.01 MPa) compared to normally supported parous animals (values = 1.0 ± 0.4 MPa, 16.0 ± 8.7 MPa, and 0.08 ± 0.03 respectively).
Table 4. Relationship of Prolapse to Mechanical Properties and Collagen Alignment.
Relationship between degree of pelvic organ support (categorized as nulliparous, parous supported, and parous prolapsed) and tangent modulus, tensile strength, strain-energy density, and collagen alignment.
| Nulliparous (n=5) |
Parous, Supported (n=3) |
Parous, Prolapse (n=3) |
Correlation (r2) |
p-value | |
|---|---|---|---|---|---|
|
Tangent Modulus (MPa) |
27 ± 11.5 | 16.0 ± 8.7 | 9.5 ± 1.0 | −0.53 | 0.01 |
|
Tensile Strength (MPa) |
2.4 ± 0.53 | 1.0 ± 0.4 | 0.9 ± 0.2 | −0.67 | 0.002 |
|
Strain-Energy Density (MPa) |
0.18 ± 0.08 | 0.08 ± 0.03 | 0.06 ± 0.01 | −0.71 | 0.001 |
| Collagen alignment | 2.7 ± 1.3 | 1.8 ± 0.08 | 1.0 ± 0.07 | −0.66 | 0.005 |
All data is represented as mean ± S.D. Significance correlations were assessed using a Spearmen Rho ranks test (p<0.05).
Comment
The vagina is central to pelvic organ support such that a normally supported vagina will resist downward descent of the uterus, urethra, bladder, and rectum. If the mechanical integrity of the vagina and/or the structures that support it are compromised, pelvic organ prolapse may occur. Maternal birth injury subsequent to vaginal delivery is considered the greatest risk factor for the development of prolapse. In this study, we therefore, examined the impact of vaginal delivery on the vagina using histomorphologic, biochemical, and biomechanical outcomes in a non-human primate (NHP) model. The most important findings of the study were that vaginal parity was associated with decreased collagen alignment and inferior biomechanical properties without impacting overall tissue histomorphology. Importantly, the loss of collagen alignment and progression to inferior mechanical properties was highly correlated with the presence of pelvic organ prolapse.
In this study, we assumed that the composition and structural organization of the dense connective tissue layer of the vagina was the major determinant of the overall tissue's biomechanical function. However, we found no differences in the relative amount of collagen subtypes of the subepithelium in nulliparous and parous animals. Collagen fibrils I, III, and V are common within tissues that are required to provide mechanical strength. The ratio of collagen subtypes within a fibril, specifically collagen I/(III+V), has been established as an indirect measure of the biomechanical properties of a tissue. For example, collagen I forms the largest fibril diameter and is associated with increased tensile strength for a tissue. Tissues that have to withstand high level of forces (e.g. ligaments and tendons) typically have a large amount of collagen I. Elevated collagen III to type I is associated with smaller fibrils and a larger degree of distensibility (e.g. skin and blood vessel). It has been found to be elevated immediately after injury only to return to normal levels in the long-term (19). Elevated collagen V to type I also contributes to the formation of small fibers and is elevated after injury, but does not return to normal levels in the long-term. Thus, it is thought to be a long-term marker of a prior injury (19).
Here, we proposed that a change in a collagen subtypes or the collagen I/III+V ratio would be a marker of injury to and/or remodeling of the vagina subsequent a maternal birth injury. Several explanations may account for our failure to observe such a change: 1) insufficient sample size as our study was not powered on changes in collagen subtypes but rather biomechanical parameters 2) a change in collagen subtypes is not a marker for maternal birth injury 3) altered collagen supermolecular structure (i.e. alignment) precedes changes in collagen subtypes in the course of prolapse progression. It is noteworthy that collagen is a highly insoluble protein that is notoriously difficult to quantify. We have chosen a quantitative fluorescence microscopy assay so that we can specifically quantitate changes in the dense connective tissue layer of the vagina. However, as is typical for collagen assays, ours shows significant variability and may not be sufficiently sensitive to detect changes that are actually present in a small sample size (type II error).
As an indication of altered molecular packing topology of collagen or its supermolecular structure, we measured collagen fiber alignment. We found that collagen fibers were less aligned in parous primates and that this correlated with a significant decrease in the parameters describing the mechanical properties of the tissue (tangent modulus, tensile strength, and strain-energy density). The packing topology or alignment of the collagen fibers within the extracellular matrix (ECM) is also an important contributor to the structural integrity of a tissue. In general, a more aligned matrix (ECM) has superior mechanical properties when compared to a disorganized unaligned matrix assuming similar collagen composition and the aligned specimens are tested along the direction of collagen alignment (20,21,22). The decrease in collagen alignment in parous animals provides tremendous insight into the biochemical basis for the inferior mechanical properties associated with parity. Moreover, we identified a correlation between the presence of prolapse, inferior biomechanical properties (tangent modulus, tensile strength, and strain-energy density), and a lower degree of collagen alignment in the vaginal cross-section. However, since only several of the NHP had prolapse we can only speculate how parity and the development of prolapse may be related, but it is an interesting finding that warrants further investigation in future studies. Indeed, as our understanding of the normal physiology of these processes matures, we become better able to construct testable hypotheses concerning the highly complex relationship between parity and the onset of prolapse.
The major strength of this study is that we utilized the non-human primate animal model to answer our research question. By using the NHP model, we were able to overcome some of the problems associated with obtaining tissue biopsies in humans including inconsistent tissue quality from manipulation during procurement and variable exposures including smoking, hormone use and physical/occupational activity. However, our study also has several limitations. First, age was lower in the nulliparous animals. As age is also cited as a risk factor for the development of a pelvic floor disorders, it is possible that the changes in collagen alignment and biomechanical properties occurred as a result of age independent of parity; however, the difference in mean age was only 2 years which is unlikely to be clinically significant. Moreover, no correlations were found between age and any biochemical or biomechanical properties examined in this study. It is noteworthy that NHPs are relatively difficult to obtain and due to the expense and limited quantity, studies with large sample sizes are virtually impossible to perform. A second limitation is that in the current study design, we are unable to distinguish changes due to an injury at the time of delivery from those incurred as a result of pregnancy tissue adaptations. Finally, as alluded to previously, we cannot provide a definitive relationship between the changes observed with parity and those seen in animals with prolapse. Therefore, we can speculate that the decrease in biomechanical properties (e.g. tangent modulus and tensile strength) would lead to the progression and development of prolapse over time. A decrease in the tangent modulus would render the vaginal wall and presumably its supportive tissues less resistant to repetitive deformations resulting in further remodeling and a loss of supportive function. Currently we are performing further studies to test this hypothesis.
In summary, parity has a significant long-term negative impact on collagen alignment and biomechanical properties of the vagina. The data provide a mechanism for the increased risk for prolapse in women after vaginal delivery due to altered collagen arrangement and mechanical properties.
Condensation.
Vaginal parity is associated with a loss of collagen alignment and inferior mechanical properties in the vagina.
Acknowledgements
We would like to acknowledge Christina Goldbach for her assistance on the imaging techniques. We would also like to acknowledge the financial support of the NIH R01HD061811 and K12HD043441) that allowed us to conduct this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no disclaimers or conflicts of interest for this study performed at Magee-Womens Research Institute and the Muscular Skeletal Research Center at the University of Pittsburgh
Contributor Information
Andrew Feola, Muscular Skeletal Research Center Department of Bioengineering, University of Pittsburgh. Pittsburgh, Pennsylvania..
Steven Abramowitch, Muscular Skeletal Research Center, Department of Bioengineering and, Magee-Womens Research Institute, University of Pittsburgh. Pittsburgh, Pennsylvania..
Keisha Jones, Division of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics, Gynecology, and Reproductive Sciences, Magee-Womens Hospital, Magee-Womens Research Institute, University of Pittsburgh. Pittsburgh, Pennsylvania..
Suzan Stein, Magee-Womens Research University of Pittsburgh. Pittsburgh, Pennsylvania..
Pamela Moalli, Division of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics, Gynecology, and Reproductive Sciences, Magee-Womens Hospital, Magee-Womens Research Institute, University of Pittsburgh. Pittsburgh, Pennsylvania..
References
- 1.Norton PA. Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol. 1993;36:926–38. doi: 10.1097/00003081-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 2.DeLancey JO. Pelvic organ prolapse: clinical management and scientific foundations. Clin Obstet Gynecol. 1993;36:895–896. [Google Scholar]
- 3.Norton PA. Prevalence and social impact of urinary incontinence in women. Clin Obstet Gynecol. 1990;33:295–297. doi: 10.1097/00003081-199006000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 5.Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol. 2000;183:277–285. doi: 10.1067/mob.2000.107583. [DOI] [PubMed] [Google Scholar]
- 6.Sze EHM, Sherard GB, III, Dolezal JM. Pregnancy, labor, delivery, and pelvic organ prolapse. Obstet Gynecol. 2002;100(5):981–986. doi: 10.1016/s0029-7844(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 7.Moalli PA, Shand SH, Zyczynski HM, Gordy SC, Meyn LA. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol. 2005;106(5 Pt 1):953–63. doi: 10.1097/01.AOG.0000182584.15087.dd. [DOI] [PubMed] [Google Scholar]
- 8.Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol, 2007;109(1):136–43. doi: 10.1097/01.AOG.0000250472.96672.6c. [DOI] [PubMed] [Google Scholar]
- 9.Daucher JA, Clark KA, Stolz DB, Meyn LA, Moalli PA. Adaptations of the rat vagina in pregnancy to accommodate delivery. Obstet Gynecol, 2007;109(1):128–35. doi: 10.1097/01.AOG.0000246798.78839.62. [DOI] [PubMed] [Google Scholar]
- 10.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979-1997. Am J Obstet Gynecol. 2003;188:108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 11.Moalli PA, Talarico LC, Sung VW, Klingensmith WL, Shand SH, Meyn LA, Watkins SC. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol. 2004;190(3):620–627. doi: 10.1016/j.ajog.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Junqueira LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 13.Arruda EM, Mondy K, Calve S, Baar K. Denervation does not change the ratio of collagen I and collagen III mRNA in the extracellular matrix of muscle. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R983–987. doi: 10.1152/ajpregu.00483.2006. [DOI] [PubMed] [Google Scholar]
- 14.Rubod C, Boukerrou M, Brieu M, Dubois P, Cosson M. Biomechanical Properties of Vaginal Tissue. Part 1: New Experimental Protocol. J Urol. 2007;178(1):320–325. doi: 10.1016/j.juro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Woo SL-Y, Orlando CA, Camp JF, Akeson WH. Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech. 1986;19(5):399–404. doi: 10.1016/0021-9290(86)90016-3. [DOI] [PubMed] [Google Scholar]
- 16.Abramowitch SD, Woo SL-Y, Clineff TD, Debski RE. An evaluation of the quasi-linear viscoelastic properties of the healing medial collateral ligament in a goat model. Ann Biomed Eng. 2004;32(3):329–335. doi: 10.1023/b:abme.0000017539.85245.6a. [DOI] [PubMed] [Google Scholar]
- 17.Lee TQ, Woo SL-Y. A new method for determining cross-sectional shape and area of soft tissues. J Biomech Eng. 1988;110(2):110–114. doi: 10.1115/1.3108414. [DOI] [PubMed] [Google Scholar]
- 18.Niyibizi C, Kavalkovich K, Yamaji T, Woo SL-Y. Type V collagen is increased during rabbit medial collateral ligament healing. Knee Surg Sports Traumatol Arthrosc. 8(5):281–285. doi: 10.1007/s001670000134. 200. [DOI] [PubMed] [Google Scholar]
- 19.Abramowitch SD, Feola AJ, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapsed: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144(Supp):S146–158. doi: 10.1016/j.ejogrb.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Musahl V, Abramowitch SD, Gilbert TW, Tsuda E, Wang JH-C, Badylak SF, Woo SL-Y. The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament - A functional tissue engineering study in rabbits. J Orthop Res. 2004;22(1):214–220. doi: 10.1016/S0736-0266(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 21.Woo SL-Y, Takakura Y, Liang R, Jia F, Moon DK. Treatment with bioscaffold enhances the the fibril morphology and the collagen composition of healing medial collateral ligament in rabbits. Tissue Eng. 2006;12(1):159–166. doi: 10.1089/ten.2006.12.159. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert TW, Wognum S, Joyce EM, Freytes DO, Sacks MS, Badylak SF. Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterial. 2008;29(36):4775–4782. doi: 10.1016/j.biomaterials.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]