Abstract
Objective
Emotion plays a strong role in the perception of risk information but is frequently underemphasized in the decision-making and communication literature. We sought to discuss and put into context several lines of research that have explored the links between emotion and risk perceptions,
Methods
In this article, we provide a focused, “state of the science” review of research revealing the ways that emotion, or affect, influences people’s cancer-related decisions. We identify illustrative experimental research studies that demonstrate the role of affect in people’s estimates of cancer risk, their decisions between different cancer treatments, their perceptions of the chance of cancer recurrence, and their reactions to different methods of presenting risk information.
Results
These studies show that people have strong affective reactions to cancer risk information and that the way risk information is presented often determines the emotional gist people take away from such communications.
Conclusion
Cancer researchers, educators and oncologists need to be aware that emotions are often more influential in decision making about cancer treatments and prevention behaviors than factual knowledge is.
Practice Implications
Anticipating and assessing affective reactions is an essential step in the evaluation and improvement of cancer risk communications.
1. Introduction
When the U.S. National Cancer Institute funded the initial Centers of Excellence for Cancer Communications Research (CECCR) in 2003, it sought to encourage research that would “produce new knowledge about and techniques for communicating complex health information to the public.”(1) One specific type of information had a particularly prominent place in the CECCR projects: information about cancer risks and the risks and benefits of cancer treatments. For example, CECCR-funded projects have examined cultural issues in the communication of colorectal cancer risk information,(2) communications about breast cancer risk,(3, 4) and media coverage of cancer risks.(5)
Each of the authors of this paper has been affiliated with the CECCR site based at the University of Michigan since its inception, and we have worked together to develop innovative techniques for visualizing cancer risks (6–10) and helping women at high risk of developing breast cancer to compare their cancer risk with the risks of cancer prevention medications.(3) Yet, our research has convinced us that simply increasing the public’s knowledge of cancer risks can often be insufficient. Even when people are presented with accurate and clear risk information in ways that support understanding and recall, they sometimes make medical decisions or perform health behaviors that are at odds with the situation. Even well-informed patients sometimes “go with their gut, instead of their head,” and choose options that appear to increase their risks or conflict with their own stated values.
Until recently, most research on both medical and non-medical decision making assumed that most biased or flawed decisions were the result of cognitive limitations.(11) In fact, over the past forty years, researchers in the field of judgment and decision making (JDM) have been documenting the many different ways that people’s judgments and decisions fall short of rational ideals. In particular, researchers have demonstrated that people are not good at generating accurate probability (risk) estimates. Their estimates are susceptible to numerous heuristics, including anchoring biases (e.g., by being pulled higher or lower if they are asked to state the last two digits of their social security number before making their estimates (12)) and availability biases (e.g., by providing higher estimates for occurrences that are “primed” to be more readily available in their minds (13)).
While traditional communications have focused on helping people overcome such cognitive limitations, emotions also play an important role in people’s cancer-related medical decisions. In healthcare contexts, especially those involving cancer, emotions often run high. When patients learn that they have cancer, for example, they often feel fear, alarm, anxiety, confusion, or dread. In the midst of such strong emotions, patients can have a hard time weighing the pros and cons of their treatment alternatives.
Even though medical professionals have long recognized that healthcare decisions can be influenced by people’s emotions, few recognize how central emotions are to all such decisions. Even decision making researchers are just beginning to grapple with a profound concept – that whenever people think their way through decisions, they feel their way, too.(14–18) As people think cognitively about the pros and cons of their decision alternatives, the affective centers of their brain also react to those same pros and cons.(19) Multiple theorists now argue that we use two parallel processes to process information and learn from it.(20–23) One process is generally seen as rational and analytical, but the other is described as intuitive, experiential, and/or emotional. Sometimes these two processes agree. When they do not, in many cases it is the affective centers that rule the day and determine people’s decisions and actions(14).
In this article, we provide a focused, “state of the science” review of research revealing the ways that emotion, or affect, influences people’s cancer-related decisions. (For the purposes of this article, we will use the terms emotion and affect interchangeably.) We do not attempt a systematic review of either the vast literature on decision making and risk perceptions or the many studies that have considered the interplay between affect and decisions. Instead, we familiarize readers with several lines of inquiry that we have pursued within the University of Michigan CECCR in our attempts to improve the ways patients make cancer treatment and prevention decisions. We discuss the progress that has been made in identifying the specific ways that affect can influence decisions by highlighting specific illustrative studies and placing them within the larger context of research in this area. In so doing, we provide evidence that anyone who wishes to inform patients about cancer risks needs to be cognizant of the determinants of patients’ emotional reactions to risk information. Only then, we argue, will clinicians and educators be able to craft their cancer risk communications and patient decision aids to not only transfer cancer risk information to patients but also to calibrate patients’ often-powerful “risky feelings.”
2. An Illustrative Story of Risky Feelings
To ground our discussion of the role of emotion in the public’s responses to cancer risk information, let us start by considering the story of a (hypothetical) woman who is contemplating breast cancer screening.
“I need to remember to schedule my mammogram,” Janice thought to herself as she drove to work that morning. Even though she had no family history of breast cancer, she had just celebrated her 40th birthday and had heard that you’re supposed to get a mammogram when you turn 40. As she thought about breast cancer and the friends she knew who had gotten it, she started to wonder what her chance of getting breast cancer was. 50/50? Probably not, but at least 25–35% or so. That number felt like a big chance to her, and she started to worry about what would happen to her family if she were to get cancer. She resolved to make the appointment that very morning.
Once at work, on the way to get some coffee, she ran into a friend and mentioned that she was thinking about scheduling a mammogram. Her friend said, “Oh that’s great! It’s so important. After all, something like 13% of women get breast cancer at some point.” Janice then asked where her friend went to get her mammogram done and chatted some more about how it went the last time her friend had hers done.
On the way back to her desk, however, she started reconsidering. “Thirteen percent?” she thought to herself. “Is that all? My risk of getting breast cancer in my lifetime is only 13%? That number doesn’t sound very high at all -- I thought it was much more likely. What a relief to know it’s that low! You know, no one in my family has been diagnosed with breast cancer in recent memory. And, this center that she recommended, it is way on the other side of town. What a hassle! Maybe I don’t need to do this right now – I’ll wait a few more years until I’m really at risk.”
3. Research on Risky Feelings
3.1 The Potential Emotional Hazards of Risk Education
How did Janice make her decision to postpone getting screened? She had heard the guidelines about mammography and the need for cancer screening and had a friend who reinforced the value of cancer screening in their conversations. Yet upon hearing a concrete estimate of the risk of developing breast cancer at some point in her life, her evaluation of the importance and urgency of mammography shifted dramatically. It is important, however, to note that the risk information that Janice received from her colleague didn’t just improve Janice’s factual knowledge. It also had a profound influence on Janice’s emotional state. She changed her decision about whether or not to get a mammogram not so much because of her understanding of the risk number but because of how that number made her feel.
If we look closer at Janice’s decision, there are two distinct issues at play. First, before she talked to her colleague, Janice substantially overestimated the likelihood that she would develop breast cancer. Such misestimates have been demonstrated in numerous studies (24–28). Concerned about this pattern, healthcare researchers have developed communication interventions, designed to improve people’s risk perceptions. In one test, however, although the intervention succeeded in making women’s risk perceptions more accurate, it also ended up making women less interested in having mammograms. (29)
This counterintuitive result can be explained by the second issue in Janice’s decision: the fact that when Janice received factual information about her cancer risk, she compared that statistic to her own internal estimate. Because her estimate was much higher than the true number, the comparison made the true 13% risk seem small and hence less worrisome. And, it was that feeling of relative security that prompted her to postpone her mammogram.
On a related note, the seemingly innocuous instruction to have patients estimate their risk of breast cancer as an introduction to risk communications can influence the “feel” of their actual risk. In one study (28), participants were randomized into one of two groups, one that was asked to estimate the average woman’s risk of breast cancer before receiving the 13% statistic and a second that received the 13% number without being asked to make any kind of estimate. Women’s reactions to the 13% statistic differed significantly across the two groups. Consistent with the other studies noted above, the women in the first group substantially overestimated the risk of breast of cancer (mean estimate: 41%). More importantly, however, they were also more likely to say that the 13% number made them feel “relieved” and more likely to say that the risk struck them as “low” (see Table 1 for details). By contrast, the second group was not particularly relieved by this information. In fact, collectively, they exhibited what is known as a “hindsight bias” (30) – roughly equal numbers of people felt that the 13% number was either higher or lower than they expected, and on average, they indicated that the 13% statistic was just about what they would have guessed it to be.
Table 1.
Effect of estimating the average woman’s lifetime breast cancer risk on reactions to actual risk information.
| Estimate Group | No Estimate Group | |
|---|---|---|
| Feel relieved about risk | 40% | 19% |
| Risk perceived as low | 43% | 16% |
From: Fagerlin A, Zikmund-Fisher BJ, Ubel PA. How making a risk estimate can change the feel of that risk: shifting attitudes toward breast cancer risk in a general public survey. Patient Educ Couns. 2005 Jun;57:294–9.
This study demonstrates two important findings. First, the seemingly simple act of guessing the risk influenced how women responded to the risk information. This raises important concerns for studies of communication interventions and decision aids. If researchers conduct pretests prior to their interventions, they may alter the way people perceive subsequent information because the pretests act as interventions themselves. Similarly, clinicians and cancer educators should be wary about asking patients to estimate their risks as a way to “break the ice” for a conversation about concrete risk statistics.
Second, the 41% figure wasn’t already in women’s heads when they were asked to make the estimates. If it had been, the act of guessing would not have influenced the first group’s subsequent reactions to the information, nor would the second group have been susceptible to hindsight bias. Instead, the pretest forced women to come up with a numerical estimate, and this estimate then influenced their subsequent reaction to the actual risk information.
The gist message for anyone attempting to communicate breast cancer risk information is that treating 13% like it was simply a number, indicating that 13 out of 100 women develop breast cancer, is insufficient. There is an understandable tendency to assume that patients will always see 13% as being a lower risk than 15% and a higher risk than, say, 10%. One reason to question that assumption is the extensive evidence that many people lack the numeracy skills to understand what risk statistics mean.(31–34) But even these studies have not fully characterized how people think about risks, because they have placed too much emphasis on the cognitive meaning of 13% and underemphasized the importance of the affective or intuitive meaning of the number.(35)
3.2 Weighing Risks and Benefits vs. Weighing Feelings
The role of affect in decision making raises fundamental challenges for cancer risk communicators. In the absence of affect, for example, oncologists could involve cancer patients in their health care decisions by taking the time to communicate the risks and benefits of their treatment alternatives, checking to make sure patients understand the information and have time to integrate that information with their individual preferences. This would be no simple task, because oncologists would need to overcome many barriers to help the patients understand their situations. But in the presence of affect, this challenge becomes even larger.
Peters, Lipkus and Diefenbach have recently argued that affect serves four distinct functions in the context of health communications: affect is information, a spotlight, a motivator, and a common currency for comparing disparate outcomes.(36) As an example, people use their feelings about a risk to judge how large the risk must be. The “affect heuristic” leads us to presume that the risks are low for risks associated with things we like and that the reverse is true for things we don’t like.(15) In Janice’s case, affect both provided information (by defining the meanings of both her risk estimate and the actual risk statistic) and acted as a motivator (her worry motivated her desire to be screened while her relief undermined it).
Paul Windschitl has conducted numerous studies that have illustrated the distinction between what people believe about risks versus what they intuit about the risks (37, 38). He contends that people’s beliefs about the numeric probability of an event, such as the 13% lifetime risk of breast cancer, are only part of how people perceive the risk. There is also “a more intuitive and non-analytic component to uncertainty that is not necessarily well represented in a numeric subjective probability response but can be an important mediator of decisions and behavior.”(38).
For example, Denes-Raj, Epstein, and Cole (39) gave people a chance to win money by picking a jelly bean from one of two bowls, offering them $1 if they chose a red jelly bean. The first bowl contained 9 red beans out of 100 and was labeled (accurately) as having 9% red beans. The second bowl contained 1 red bean out of 10 and was labeled as having 10% red beans. Many people in this study reported knowing that the second bowl gave them the best chance of winning, but feeling like the first bowl gave them a better chance, because it contained a larger number of red beans. And many people were compelled by these feelings to choose from the first bowl.
Another recent study demonstrates how such feeling-based processes may be at play in cancer treatment decisions.(40) In this study, people were asked to imagine that they had been diagnosed with colon cancer, and that without treatment they would die. They were then shown information about the risks and benefits of two surgical treatment options that are shown in Table 2.
Table 2.
Hypothetical treatment options for colon cancer.
| Treatment Options | ||
|---|---|---|
| Possible outcome | Surgery 1 | Surgery 2 |
| Cure without complication | 80% | 80% |
| Cure with colostomy | 1% | |
| Cure with chronic diarrhea | 1% | |
| Cure with intermittent bowel obstruction | 1% | |
| Cure with wound infection | 1% | |
| No cure (death) | 16% | 20% |
From: Amsterlaw J, Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Can avoidance of complications lead to biased healthcare decisions? Judgment and Decision Making. 2006;1:64–75.
How should people decide between Surgery 1 and 2? The dominant view among decision making experts is that people ought to make decisions like this by weighing the risks and benefits of each option, by thinking about the probability of each possible outcome and the value they place on each of these outcomes. This view is the basis of decision analysis (41), the health belief model (42), and economic theories of rationality (43). As such, this view emphasizes explicit cognitive judgments – the rational weighing of pros and cons.
Returning to Table 2, the pros and cons of the two surgeries are clear. Both provide an 80% chance of surviving the cancer without complication. The cure rate of the two surgeries, however, differs. Surgery 2 yields a 20% death rate from cancer, whereas Surgery 1 yields only a 16% death rate. The remaining 4% of the people receiving Surgery 1 do not die of their cancer but, instead, survive with some kind of temporary or permanent surgical complication. The two treatments, in other words, involve a tradeoff between accepting a chance of these complications versus accepting a higher chance of death. The decision depends on what people think about dying from cancer versus living with either of these surgical complications.
As it turns out, most people have little difficulty saying what they think about this tradeoff. When faced with an explicit choice between dying or living with a colostomy, more than 90% of the people say they would choose to live with the colostomy.(40) People feel even stronger about their preference for the other three surgical outcomes, compared to death. In fact, in one study more than 90% preferred each of the four surgical complications to death.(40) Based on these values, Surgery 1 should be the best treatment for more than 90% of people. And yet, a majority of people still chose Surgery 2, the surgery that carried a higher risk of death.(40) Even when people’s own preferences (e.g., that preserving life was more important than avoiding complications) were made clear and explicit to them, their decisions did not reflect those values and preferences.
This colon cancer scenario is a clear example of a situation in which people’s feelings contradicted their cognitions, just as they did in the jellybean study. Many study participants reported that they knew that the first surgery was better than the second, but felt that they should still choose Surgery 2, so that they wouldn’t have to deal with the possibility of experiencing a surgical complication. After all, descriptions of things like having a colostomy or a wound infection are “affect-rich,” evoking strong feelings of fear or disgust.(17) It is likely that the prospect of experiencing these conditions evoked an avoidance reaction. In addition, affect acted as a spotlight,(36) leading people to put disproportionate weight on the complications risks. As a result, their avoidance reactions were strong enough to overcome a significantly higher risk of death with Surgery 2 and persisted even after participants had explicitly said that they preferred life with those conditions to death. Both the jellybean and the colon cancer surgery studies illustrate the fact that risk information is never received dispassionately but is always coded in affective and intuitive ways, too. Risks create feelings.
3.3 Risk: A Basis for Comparison, Not Just a Number
When people receive information about cancer or complication risks, they do not simply encode the numbers into a mathematical algorithm. They pull meaning out from the numbers, stamping the information with affective or intuitive labels such as “high versus low” or “something to be worried about versus something to be relieved about.” Which meaning people take away from risk information, however, can depend a lot on what other statistics they know.
Research by Hsee and others on “information evaluability” has consistently shown that people find quantitative data hard to evaluate (i.e., difficult to use in decision making) if they are both unfamiliar, as most risk statistics are, and presented in isolation.(44–46) When given other data to use as standards of comparison (e.g., the risk of another group), however, most people can interpret even unfamiliar numbers based on whether they are higher or lower than the standard. Contextual information fundamentally changes what risk information means to people.(37)
Context effects are common when discussing treatment options with cancer patients. For example, if a patient learns that a procedure has a 28% success rate, that information is initially very hard to evaluate. Is 28% good or bad? Most patients lack the domain-specific knowledge to know. But, if you tell them that an alternate procedure has, say a 35% success rate, all of a sudden the 28% rate doesn’t feel very good at all. In fact, providing such additional contextual statistics not only changes how people feel about their alternatives, it can change what they choose to do.(47)
To illustrate this point further, multiple studies have demonstrated that the way people encode information about risk can depend on whether they believe their own risk is higher or lower than average (48–50). In one such study,(48) women were asked to imagine that they had a 6% risk of developing breast cancer over the next 5 years. (The 6% figure was chosen because it was the average risk of women who had been enrolled in the P-1 Trial, a study which showed that tamoxifen can reduce the risk of experiencing a first breast cancer (51)). Participants were also told to imagine that they could take a pill that would cut their risk in half, to 3%. However, they were also informed of several potential side effects of this hypothetical pill, including risks of endometrial cancer, stroke, and hot flashes.
While every woman who participated in this study (48) received identical personal risk information, the study was designed to test whether the way women felt about both breast cancer and the prevention pill would change if they were given hypothetical information suggesting that their 6% risk was either above or below average. Some participants were told (counterfactually) that the average risk of breast cancer over 5 years was 3%, not 6%, while another group was told that the average risk was 12%.
Women’s perceptions of breast cancer and of the prevention pill were significantly influenced by this comparative information.(48) Those in the 3% group felt more worried about their own risk of breast cancer than the 12% group, because the comparative information made them perceive their own 6% figure as an above average risk, and therefore something to be worried about. The 3% group was also more interested in taking the pill than the 6% group, and more convinced about the effectiveness of the pill.
We contend that such comparative information should not influence people’s decisions. The real choice facing the women in this study was to decide whether a 3% absolute reduction in the risk of breast cancer is a large enough benefit to justify the risks of this pill. The comparative information did nothing to change either the risks or the benefits of the pill. Nor did it place the individual in an objectively meaningful category of being at “high risk” (since it is not uncommon for a patient to be at above average risk and yet not at “high risk” or the inverse). And yet, the comparative information, by itself, significantly influenced how women felt both about breast cancer and about the risks and benefits of the pill.
We should be clear that the effects of comparative data are not limited to hypothetical scenarios. For example, Lipkus et al. had 121 women age 40 and older estimate their risk of developing breast cancer and then gave them their personalized true risk.(52) Half of their participants, however, also received information about the lowest risk among women their age and race. While the factual information reduced perceived risk (by adjusting the common overestimates we discussed earlier), the change was much smaller among women who received the comparative risk data. Why? Because seeing the lowest risk level made these women’s own risk feel “high” by comparison.
Providing comparative risk statistics, however, is by no means the only way to change the intuitive meanings patients draw from risk statistics. For example, a study that examined prenatal genetic screening decisions showed that the seemingly innocuous practice of labeling a screening test result as “negative” or “positive” significantly changed both people’s risk perceptions and their decision making about amniocentesis as compared to simply providing the statistical risk information without additional interpretation.(53) The concept that evaluative labels can be particularly influential is also supported by recent work by Peters et al. that demonstrated that a manipulation of “evaluative mapping” that provided both verbal and visual categorizations of statistics into categories such as “fair” or “good” resulted in increased use of numerical information in a quality-of-care decision by a less numerate population.(54)
In the cancer domain, this finding is particularly relevant to discussions of tumor marker assays in the context of adjuvant therapy decisions. Assays such as Oncotype DX, which utilize multiple tumor characteristics to estimate future risk of cancer recurrence, generally provide interpretive classifications such as “low risk” or “intermediate risk” in addition to a continuous recurrence score (RS). Of course, the decision about whether borderline recurrence scores, such as an RS of 16, should be classified as “low” vs. “intermediate” is both somewhat arbitrary and open for debate. The prenatal screening study suggests that people don’t necessarily need help interpreting such statistics – they can derive appropriate meanings from risks or scores if appropriate standards of comparison are provided. Furthermore, if oncologists tell patients the interpretive labels provided by Oncotype DX, it will likely be those affect-laden labels, and not the more specific numerical results, which will be most influential on cancer patients’ decisions about how best to prevent cancer recurrence.
3.4 The Emotional Salience of Cancer Recurrence Risks
Thus far, we have discussed multiple ways in which the feelings people have about cancer risks can change as a result of additional information, such as information about emotionally-salient complications or affectively powerful comparison data. But, does a 6% risk of developing cancer feel the same regardless of when or how people come to face that risk. Our research team has been gathering evidence that a risk of cancer recurrence may feel qualitatively different to people than a risk of developing exactly the same cancer for the first time.
In this previously unpublished study, we recruited a random sample of 921 U.S. adults from a demographically diverse panel of Internet users to answer questions about a short hypothetical scenario about cancer recurrence risks. We asked study participants to imagine that they had previously been diagnosed either with skin cancer or thyroid cancer (randomized), which was successfully treated into remission. They were then informed of two future risks: the risk of recurrence of their prior cancer and the risk of developing the alternate cancer. The order of which risk was discussed first was also randomized, with the first risk numerically defined (1%) and the second described as having a “very similar” likelihood. This resulted in a 2 (prior cancer type) x 2 (order/description) factorial design.
Our study participants were drawn from an Internet panel administered by Survey Sampling International (SSI), using a procedure that received Institutional Review Board exempt status approval. To ensure demographic diversity (but not representativeness) and offset variations in response rates, we stratified our sample by age, gender, and race/ethnicity. Participants were eligible to receive modest prizes from SSI in return for their participation. Sample mean age was 48 (range 18–89), 46% were male, and 27% reported a non-white race or ethnicity. While 32% reported having completed a Bachelor’s or higher degree of education, 20% reported having only a High School education or less.
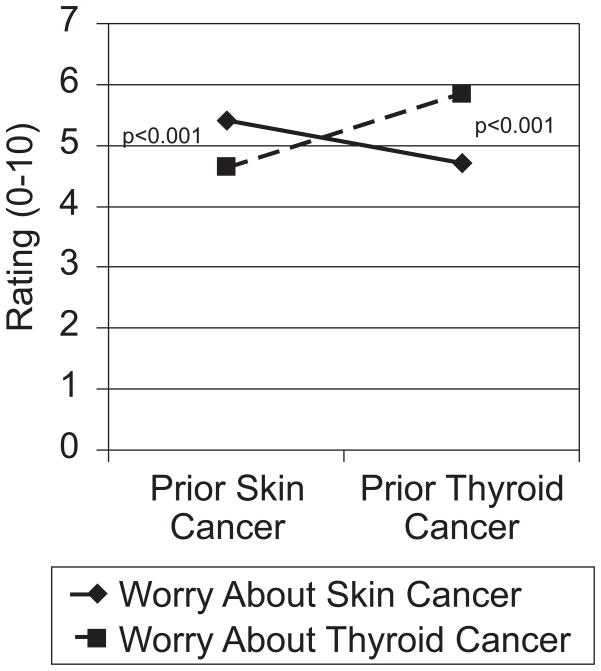
In describing the two cancers, we specifically told our study participants that both were equally likely to occur. But, to the people who read our scenario, they didn’t feel equally likely. In fact, almost half of participants (43% and 44%, depending on condition) stated that they believed that the recurrence risks were more likely than the new cancer risks, despite viewing specific information to the contrary and regardless of cancer type or order of presentation. In addition, as shown in Figure 1, ratings of worry about each type of cancer were significantly higher when it was described as a recurrence versus a new diagnosis.
Figure 1.
Cancer worry by imagined prior cancer experience
This study demonstrates that risks of cancer recurrence are particularly concerning for patients. Even when a cancer recurrence is fully treatable, no patient who has undergone arduous primary treatment regimens wants to face the prospect of doing so again. Yet, the fact that these effects occur in a carefully controlled experimental context demonstrates that it is the concept of recurrence itself, not just the details of particular cancers, which carries such emotional salience.
There are many ways that such emotional salience could manifest itself. For example, people may feel that recurrence risk statistics are more personally relevant. In fact, our results are particularly surprising given that our participants had none of the actual experiences of cancer survivors: They read a hypothetical scenario without receiving an actual diagnosis or undergoing invasive treatments. All of these factors likely increase cancer survivors’ emotional reactions to the possibility that malignant cancer cells remain and that the original cancer might return. Yet despite our affect-poor experimental situation, many of our survey respondents still appeared to confer special status to cancer recurrence risks.
Our results suggest that cancer recurrence carries a unique emotional weight that directly affects both perceived likelihood and decision making. Actual cancer experience likely magnifies this effect and may influence a variety of decisions, including assessments of the risk-benefit tradeoff of chemoprevention and other adjuvant therapies (likely encouraging more invasive approaches), as well as prioritization of cancer prevention behaviors. Based on this finding, we suggest that anyone discussing future risks with cancer survivors or their caregivers should specifically draw attention to important non-recurrence risks in order to appropriately balance these risks versus the vivid risks of cancer recurrence.
4. Discussion and Conclusion
4.1 Discussion
In this article, we have summarized multiple lines of research that demonstrate that many biases in medical decision making result not from cognitive errors, per se, but from the influence of affect on how people perceive risks and benefits. This research is consistent with several recent theories of decision making formulated by Loewenstein, Slovic, Damasio and others (14–16, 18). More importantly, these studies have demonstrated many of the ways that emotion-based processing of risk information may alter, bias, or undermine efforts to inform the public about cancer risks and help cancer patients make informed, preference-congruent treatment choices.
It is important to recognize that none of these theories of emotion-based decision making hold that affect is either a negative or positive influence. Instead, each of these theories contends that affect is a strong determinant of most decisions, often working in parallel with “higher order” cognition. Sometimes emotions complement cognition. In such cases, tapping into affect-driven processing may be able to increase the salience or impact of informational cancer risk messages. Sometimes, however, emotions contradict people’s cognitions, leading to more difficult decision making at best and counterproductive patient choices at worst.
It is also important to clarify that the emotional component of risk perceptions is a universal phenomenon, not something limited to a particularly “emotional” subset of the population or “emotional” situations. We can neither sort people into emotional versus cognitive piles, nor segregate diseases into groups that generate affectively-determined decisions versus analytically-determined ones. Every risk communication is processed both cognitively and emotionally. Hence, every risk communicator must not only ask him- or herself the question, “what risk information do I want my audience to think about?” but also “what feelings will this message evoke?”
4.2 Conclusion
When presented with cancer risk statistics, people process the risk information affectively as well as cognitively. Many times, these emotional reactions, which exist side-by-side with cognition, can be even more influential in decision making about cancer treatments and prevention behaviors than factual knowledge is.
4.3 Practice Implications
Cancer researchers, educators, and oncologists need to be aware that whenever they communicate information about cancer-related risks and benefits, patients will respond affectively. As a result, how risk messages are presented can often matter more than what is being stated. Our research agenda at the University of Michigan CECCR site has reflected this philosophy. Our work has sought to identify the affect-based processes that influence risk beliefs and assess practical techniques that can either shape or offset such emotional influences. Yet we have only scratched the surface of the many ways that affect influences cancer risk perceptions as well as cancer treatment and prevention decisions. We therefore call upon the community of cancer communications researchers to be sure to measure emotional reactions to risk in their work, not just factual knowledge or recall, and assess to what degree these affect-based reactions are consistent with or in opposition to the gist of the intended message and how they affect behavior.
In the meantime, health educators need to recognize that success in a risk communication must be measured not only by what recipients know but by how they feel. In particular, educators should anticipate the influence of contextual statistics, interpretive labels, and other affect-moderating cues. Decisions about whether to include such information in cancer risk communications should be based on whether these elements will evoke emotional reactions consistent with the message goals. Clinicians should consider exploring how their patients are feeling about their risks at least as much as they seek to ensure comprehension. Only by acknowledging the key role of “risky feelings” in people’s responses to risk information can we design risk communications that support meaningful understanding and decision making about cancer risks.
Acknowledgments
Role of Funding Source
Financial support for this study was provided by grants from the U. S. National Institutes for Health (P50 CA101451 and R01 CA87595). Dr. Zikmund-Fisher is supported by a Mentored Research Scholar Grant from the American Cancer Society (MRSG-06-130-01-CPPB). The funding agreements ensured the authors’ independence in designing the studies, in the collection, analysis and interpretation of data, in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of interest – None declared.
Conflicts of Interest Notification
All authors substantially contributed to: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) giving final approval of the version submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Cancer Institute funds four Eenters of Excellence in Cancer Communications Research. National Cancer Institute; 2003. [September 18, 2009]; Available from: http://cancercontrol.cancer.gov/hcirb/ceccr/CECCR_Awards_Press_Announcement.pdf. [Google Scholar]
- 2.Nicholson RA, Kreuter MW, Lapka C, Wellborn R, Clark EM, Sanders-Thompson V, et al. Unintended effects of emphasizing disparities in cancer communication to African-Americans. Cancer Epidemiol Biomarkers Prev. 2008;17:2946–53. doi: 10.1158/1055-9965.EPI-08-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zikmund-Fisher BJ, Ubel PA, Smith DM, Derry HA, McClure JB, Stark A, et al. Communicating side effect risks in a tamoxifen prophylaxis decision aid: The debiasing influence of pictographs. Patient Educ Couns. 2008;73:209–14. doi: 10.1016/j.pec.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray SW, O'Grady C, Karp L, Smith D, Schwartz JS, Hornik RC, et al. Risk information exposure and direct-to-consumer genetic testing for BRCA mutations among women with a personal or family history of breast or ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1303–11. doi: 10.1158/1055-9965.EPI-08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stryker JE, Fishman J, Emmons KM, Viswanath K, Stryker JE, Fishman J, Emmons KM, Viswanath K. Cancer risk communication in mainstream and ethnic newspapers. Prev Chronic Dis [serial on the Internet] 2009;6(1) Available from: http://www.cdc.gov/pcd/issues/2009/jan/08_0006.htm. [PMC free article] [PubMed]
- 6.Hawley ST, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73:448–55. doi: 10.1016/j.pec.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Zikmund-Fisher BJ, Fagerlin A, Roberts TR, Derry HA, Ubel PA. Alternate methods of framing information about medication side effects: Incremental risk versus total risk occurence. J Health Commun. 2008;13:107–24. doi: 10.1080/10810730701854011. [DOI] [PubMed] [Google Scholar]
- 8.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Mortality versus survival graphs: Improving temporal consistency in perceptions of treatment effectiveness. Patient Educ Couns. 2007;66:100–7. doi: 10.1016/j.pec.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Improving understanding of adjuvant therapy options by using simpler risk graphics. Cancer. 2008;113:3382–90. doi: 10.1002/cncr.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. What's time got to do with it? Inattention to duration in interpretation of survival graphs. Risk Anal. 2005;25:589–95. doi: 10.1111/j.1539-6924.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 11.Kahneman D, Slovic P, Tversky A, editors. Judgment Under Uncertainty: Heuristics and Biases. Cambridge: Cambridge University Press; 1982. [DOI] [PubMed] [Google Scholar]
- 12.Tversky A, Kahneman D. Judgment under uncertainty: Heuristics and biases. Science. 1974;185:1124–31. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 13.Tversky A, Kahneman D. Availability: A heuristic for judging frequency and probability. Cognitive Psychology. 1973 September 5;:207–32. [Google Scholar]
- 14.Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychological Bulletin. 2001;127:267–86. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 15.Finucane ML, Alhakami A, Slovic P, Johnson SM. The affect of heuristic judgments of risks and benefits. Journal of Behavioral Decision Making. 2000;13:1–17. [Google Scholar]
- 16.Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. New York: G. P. Putnam's Sons; 1994. [Google Scholar]
- 17.Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001 May;12:185–90. doi: 10.1111/1467-9280.00334. [DOI] [PubMed] [Google Scholar]
- 18.Slovic P, Peters E, Finucane ML, MacGregor DG. Affect, risk and decision making. Health Psychol. 2005;24:S35–S40. doi: 10.1037/0278-6133.24.4.S35. [DOI] [PubMed] [Google Scholar]
- 19.LeDoux JE. The emotional brain: The mysterious underpinnings of emotional life. New York: Simon & Schuster; 1996. [Google Scholar]
- 20.Sloman SA. The empirical case for two systems of reasoning. Psychol Bull. 1996;119:3–22. [Google Scholar]
- 21.Smith ER, DeCoster J. Dual-process models in social and cognitive psychology: Conceptual integration and links to underlying memory systems. Personality and Social Psychology Review. 2000;4:108–31. [Google Scholar]
- 22.Kahneman D, Frederick S. Representativeness revisited: Attribute substitution in intuitive judgment. In: Gilovich T, Griffin D, Kahneman D, editors. Heuristics and Biases: The Psychology of Intuitive Judgment. New York: Cambridge University Press; 2002. pp. 49–81. [Google Scholar]
- 23.Reyna VF. How people make decisions that involve risk: a dual-processes approach. Curr Dir Psychol Sci. 2004;13:60–6. [Google Scholar]
- 24.Lerman C, Lustbader E, Rimer B, Daly M, Miller S, Sands C, et al. Effects of individualized breast cancer risk counseling: A randomized trial. Journal of the National Cancer Institute. 1995;87:286–92. doi: 10.1093/jnci/87.4.286. [DOI] [PubMed] [Google Scholar]
- 25.Croyle RT, Lerman C. Risk communication in genetic testing for cancer susceptibility. Journal of the National Cancer Institute Monographs. 1999;25:59–66. doi: 10.1093/oxfordjournals.jncimonographs.a024210. [DOI] [PubMed] [Google Scholar]
- 26.Mouchawar J, Byers T, Cutter G, Dignan M, Michael S. A study of the relationship between family history of breast cancer and knowledge of breast cancer genetic testing prerequisites. Cancer Detection & Prevention. 1999;23:22–30. doi: 10.1046/j.1525-1500.1999.00065.x. [DOI] [PubMed] [Google Scholar]
- 27.Durfy SJ, Bowen DJ, McTiernan A, Sporleder J, Burke W. Attitudes and interest in genetic testing for breast and ovarian cancer susceptibility in diverse groups of women in western Washington. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:369–75. [PubMed] [Google Scholar]
- 28.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. How making a risk estimate can change the feel of that risk: shifting attitudes toward breast cancer risk in a general public survey. Patient Educ Couns. 2005 Jun;57:294–9. doi: 10.1016/j.pec.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz MD, Rimer BK, Daly M, Sands C, Lerman C. A randomized trial of breast cancer risk counseling: The impact on self-reported mammography use. American Journal of Public Health. 1999;89:924–6. doi: 10.2105/ajph.89.6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischhoff B. Hindsight is not equal to foresight: The effect of outcome knowledge on judgement under uncertainty. Journal of Experimental Psychology: Human Perception and Performance. 1975;1:288–99. [Google Scholar]
- 31.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 32.Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff. 2007;26:741–8. doi: 10.1377/hlthaff.26.3.741. [DOI] [PubMed] [Google Scholar]
- 33.Peters E, Vastfjall D, Slovic P, Mertz CK, Mazzocco K, Dickert S. Numeracy and decision making. Psychol Sci. 2006;17:407–13. doi: 10.1111/j.1467-9280.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 34.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the subjective numeracy scale (SNS): Effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27:663–71. doi: 10.1177/0272989X07303824. [DOI] [PubMed] [Google Scholar]
- 35.Nelson W, Reyna VF, Fagerlin A, Lipkus IM, Peters E. Clinical implications of numeracy: theory and practice. Ann Behav Med. 2008;35:261–74. doi: 10.1007/s12160-008-9037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters E, Lipkus IM, Diefenbach MA. The functions of affect in health communications and the construction of health preferences. J Commun. 2006;56(Suppl 1):S140–S62. [Google Scholar]
- 37.Windschitl PD, Martin R, Flugstad AR. Context and the interpretation of likelihood information: The role of intergroup comparisons on perceived vulnerability. J Pers Soc Psychol. 2002;82:742–55. doi: 10.1037//0022-3514.82.5.742. [DOI] [PubMed] [Google Scholar]
- 38.Windschitl PD. Judging the accuracy of a likelihood judgment: the case of smoking risk. J Behav Dec Making. 2002;15:19–35. [Google Scholar]
- 39.Denes-Raj V, Epstein S, Cole J. The generality of the ratio-bias phenomenon. Personality and Social Psychology Bulletin. 1995;21:1083–92. [Google Scholar]
- 40.Amsterlaw J, Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Can avoidance of complications lead to biased healthcare decisions? Judgment and Decision Making. 2006;1:64–75. [Google Scholar]
- 41.Ubel PA, Loewenstein G. The role of decision analysis in informed consent: choosing between intuition and systematicity. Social Science & Medicine. 1997;44:647–56. doi: 10.1016/s0277-9536(96)00217-1. [DOI] [PubMed] [Google Scholar]
- 42.Strecher VJ, Rosenstock IM. The Health Belief Model. In: Glanz K, Lewis FM, Rimer BK, editors. Health Behavior and Education: Theory, Research, and Practice. 2. San Francisco: Jossey-Bass; 1997. p. 496. [Google Scholar]
- 43.Baron J. Thinking and Deciding. 2. New York: Cambridge University Press; 1994. [Google Scholar]
- 44.Hsee CK. The evaluability hypothesis: An explanation for preference reversals between joint and separate evaluations of alternatives. Organizational Behavior and Human Decision Processes. 1996;67:247–57. [Google Scholar]
- 45.Hsee CK. Less is better: When low-value options are valued more highly than high-value options. J Behav Decis Making. 1998;11:107–21. [Google Scholar]
- 46.Hsee CK, Blount S, Lowenstein GF, Bazerman MH. Preference reversals between joint and separate evaluations of options: A review and theoretical analysis. Psychol Bull. 1999;125:576–90. [Google Scholar]
- 47.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. “Is 28% good or bad?” Evaluability and preference reversals in health care decisions. Med Decis Making. 2004;24:142–8. doi: 10.1177/0272989X04263154. [DOI] [PubMed] [Google Scholar]
- 48.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. “If I'm better than average, then I'm OK?”: Comparative information influences beliefs about risk and benefits. Patient Educ Couns. 2007;69:140–4. doi: 10.1016/j.pec.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein WM. Objective standards are not enough: affective, self-evaluative and behavioral responses to social comparison information. J Pers Soc Psychol. 1997;72:763–74. doi: 10.1037//0022-3514.72.4.763. [DOI] [PubMed] [Google Scholar]
- 50.McCaul KD, Canevello AB, Mathwig JL, Klein WMP. Risk communication and worry about breast cancer. Psychology, Health & Medicine. 2003;8:379–89. doi: 10.1080/13548500310001604513. [DOI] [PubMed] [Google Scholar]
- 51.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 52.Lipkus IM, Biradavolu M, Fenn K, Keller P, Rimer BK. Informing women about their breast cancer risks: truth and consequences. Health Communication. 2001;13:205–26. doi: 10.1207/S15327027HC1302_5. [DOI] [PubMed] [Google Scholar]
- 53.Zikmund-Fisher BJ, Fagerlin A, Keeton K, Ubel PA. Does labeling prenatal screening test results as negative or positive affect a woman's responses? Am J Obstet Gynecol. 2007;197:528.e1–6. doi: 10.1016/j.ajog.2007.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters E, Dieckmann NF, Vastfjall D, Mertz CK, Slovic P, Hibbard JH. Bringing meaning to numbers: The impact of evaluative categories on decisions. J Exp Psychol Appl. 2009;15:213–27. doi: 10.1037/a0016978. [DOI] [PubMed] [Google Scholar]