Abstract
Coordinated Nodal-related signals and Bozozok (Boz) activity are critical for the initial specification of dorsal mesoderm and anterior neuroectoderm during zebrafish embryogenesis. Overexpression of Boz expands gsc expression into the ventro-lateral marginal blastomeres where Nodal signaling is active, but is insufficient to induce ectopic gsc expression in the animal region. We found that overexpression of Boz together with depletion of Lnx-2b (previously named Lnx-like, Lnx-l), but not each manipulation alone, causes robust gsc expression in all blastomeres. Furthermore, Nodal-related signals are required for gsc expression in embryos with elevated Boz activity. Through targeted injection into single cells at the 128-cell stage we illustrate the role of maternally deposited Lnx-2b to restrict the expansion of gsc expression into the presumptive ectodermal region. This report provides a novel mechanism for limiting dorsal organizer specification to a defined region of the early zebrafish embryo.
Keywords: Lnx-2b, Nodal, Bozozok, goosecoid, dorsal organizer, dorsal mesoderm
1. Introduction
During early zebrafish development, multiple signaling molecules pattern the embryonic body axis [1,2]. The dorsal organizer, a region that is a source of inductive signals and a major regulator of axis establishment, is formed under the influence of the Wnt and Nodal signaling pathways [1,2]. An early consequence of Wnt signaling is the induction of the homeobox gene bozozok/dharma/nieuwkoid (boz) in a region that presages formation of the dorsal organizer [1–4]. Among other activities, Boz activates gsc expression in the dorsal organizer [5–7] in cooperation with Nodal-related factors Ndr1 (Squint) and Ndr2 (Cyclops). Overexpression of Boz can expand gsc expression into the lateral-marginal tissue, where Ndr1 and Ndr2 are present, but fails to induce gsc when Nodal signaling is inhibited [8,9]. In contrast, overexpression of Ndr1 induced ectopic gsc expression in regions where boz is not expressed [5,6,8]. Thus Nodal signaling is absolutely required for gsc expression, and enforced Nodal activity appears to be sufficient to induce ectopic gsc expression without contribution from Boz, although gsc expression in the animal pole region was activated only in cells expressing high levels of Ndr1 [9]. Collectively these observations indicate that gsc expression in the marginal blastomeres can be induced by the cooperative activities of Boz and Ndr1. The gsc expression domain based on cooperative induction by Boz and Ndr1 appears to be restricted to prospective mesendoderm, even though Ndr1 acts as a long-range morphogen and Boz, even though it is a nuclear protein, is capable of acting in a non-cell autonomous manner [9,10]. Thus a repressing mechanism should exist in the presumptive ectoderm to prevent expansion of the gsc expression domain towards the animal pole.
Recently we reported that Lnx-2b (previously named Lnx-like), an E3 ubiquitin ligase containing an amino terminal RING finger and four PDZ domains, restricts the organizer domain by negatively regulating Boz stability [11]. Depletion of Lnx-2b could expand gsc expression into the lateral and even ventral margin, but not into the animal region containing future ectoderm. In the present study, we start from the hypothesis that maternally deposited Lnx-2b counteracts Boz and Ndr1-dependent ectodermal expansion of gsc expression. We show that overexpression of Boz together with the depletion of Lnx-2b can induce gsc expression beyond the marginal blastomeres into the future ectodermal region of the embryo. Further we show that the ectopic gsc expression caused by the Boz overexpression in the absence of Lnx-2b is strictly depended on Nodal signaling. Finally, we observed radial expansion of gsc expression in cells surrounding a source of Boz and Ndr1 protein under conditions where Lnx-2b translation is inhibited.
2. Material and methods
2.1. Fish embryos
Embryos were obtained from natural spawnings of wild type (AB*). Embryos were raised and harvested according to Kimmel et al. [12].
2.2. Microinjection
cDNAs encoding ndr1 and boz open reading frames were subcloned into the modified pcGlobin2 vector [13]. mRNAs were synthesized using the mMESSAGE mMACHINE kit (Ambion Inc.). Indicated RNAs or morpholinos (MO) were injected into the yolk of 1–4 cell stage of embryos. MOs were supplied from Gene Tools, LLC. 1 ng of p53 MO was co-injected with other MOs to inhibit off-target effects [14]. lnx-2b MO, 5’-CCTACGCCTCTTTCACAGCTCACAA-3’; p53 MO, 5’-GCGCCATTGCTTTGCAAGAATTG-3’.
2.3. Single cell injection
A mixture of 2 pg of ndr1 and 5 pg of boz mRNA was injected together with tracers, rhodamine dextran (3%, 10,000 MW) and biotinylated dextran (Molecular Probes), into a single cell of 128 cell wild-type (WT) or lnx-2b MO (5 ng) injected embryos. lacZ mRNA (7 pg) together with the tracers was injected as a negative control. Injected embryos were raised for 3 hours and fixed with 4% paraformaldehyde at the germ ring stage for whole mount in situ hybridization. Biotin dextran was detected with NovaRed substrate staining (Vector) using standard ABC protocol (Vectastain).
2.4. Whole mount in situ hybridization
Antisense gsc riboprobe was generated using T7 RNA polymerase following the manufacture’s instructions (Roche). Proteinase K treatment (10µg/ml) was performed for 1 min at RT. The hybridized probe was detected using pre-absorbed anti-digoxigenin-AP Fab fragments (Roche) diluted (1:2000) in blocking solution (PBS, 0.1% Tween-20, 5% sheep serum, 0.2% Blocking reagent (Roche)).
3. Results and discussion
3.1. Lnx-2b represses ectopic gsc expression in the presumptive ectoderm
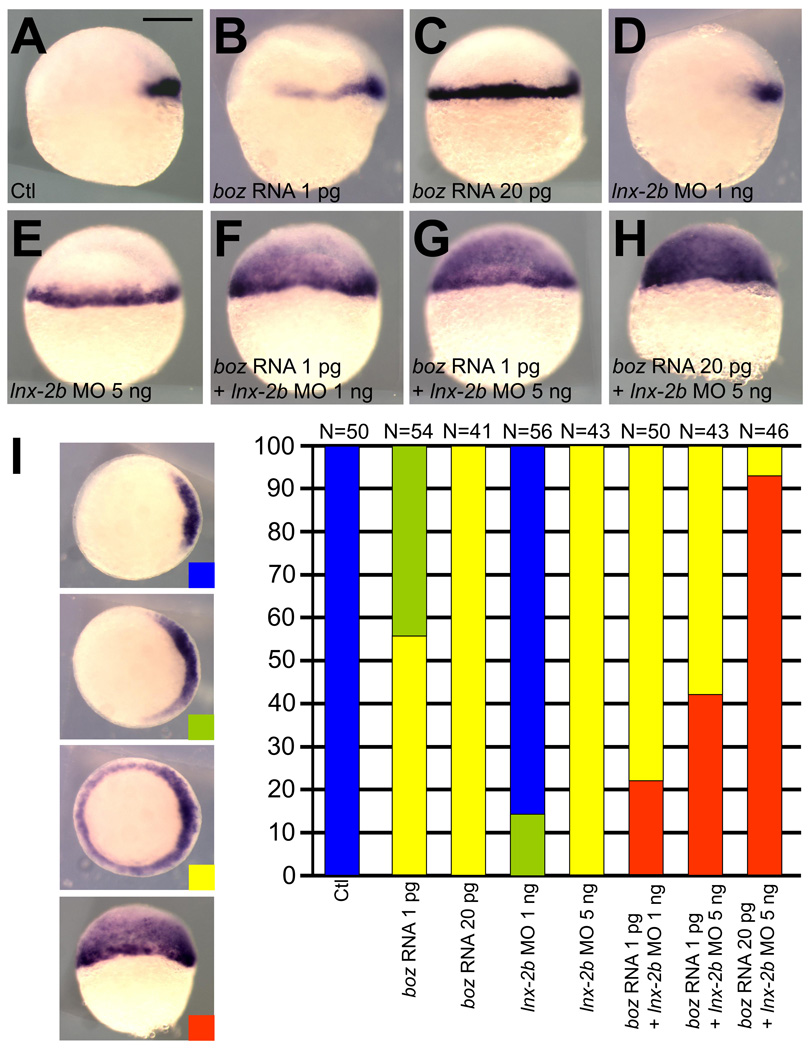
During early zebrafish development, boz expression induced by Wnt/β-catenin signaling mediates dorsal organizer formation by suppressing the expression of ventralizing genes such as bmp, vox (vega1), vent (vega2) and ved [15–20]. Thus overexpression of Boz, through counteracting ventralizing signals, expands gsc expression into the ventro-lateral marginal blastomeres in a dose-dependent manner (Fig. 1A–C). However, we have never observed ectopic gsc expression beyond the presumptive mesendodermal tissue even after injection of high amounts of boz mRNA. Similar to the overexpression of Boz, depletion of Lnx-2b by the injection of translation-blocking MO causes the expansion of gsc expression into the mesendoderm at the embryonic shield stage [11] (Fig. 1A, 1D, and 1E). The expanded gsc expression elicited by lnx-2b MO injection is due to stabilization of endogenously expressed Boz, which leads to gradually expanded expression of boz in the marginal blastomeres [11]. Interestingly, we observed strong gsc expression in the entire blastoderm including the ectodermal domain when Boz overexpression was coupled with depletion of Lnx-2b (Fig. 1F–H).
Fig. 1.
Overexpression of Boz and concomitant depletion of Lnx-2b cause ectopic gsc expression in the presumptive ectoderm. (A) Control uninjected embryo; gsc is expressed in the dorsal organizer. (B) Embryo injected with 1 pg of boz mRNA; the gsc expression domain is laterally expanded. (C) Embryo injected with 20 pg of boz mRNA; the gsc expression domain includes the entire margin. (D) Embryo injected with 1 ng of lnx-2b MO; slight lateral expansion of gsc. (E) Embryo injected with 5 ng lnx-2b MO showing similar gsc expansion to (C). (F) One pg of boz mRNA was co-injected with 1 ng of lnx-2b MO; 22% of embryos (N=50) showed ectopic expanded expression of gsc in animal (presumptive ectodermal) cells. (G) One pg of boz mRNA co-injected with 5 ng of lnx-2b MO; 42% of injected embryos (N=43) displayed graded expansion of gsc into the animal region. (H) Co-injection of 20 pg boz mRNA and 5 ng lnx-2b MO; 93% of embryos (N=46) showed strong ectopic gsc expression. (I) Dorsalization was determined by gsc expression at the germ ring stage, showing synergism between boz mRNA and lnx-2b MO. The classification is depicted by different colors (Blue, unaffected; Green, moderate expansion; Yellow, circumferential gsc expression; Red, ectopic gsc expression into animal region). (A–H) Shield stage. Lateral view, dorsal is right. Scale bar, 200 µm.
Quantitative aspects of the regulation of gsc expression by Boz and Lnx-2b are presented in Figure 1I. Classification of gsc domains into four categories allowed quantification of the effects. The data show dose dependence for the effect of Boz and of Lnx-2b MO, as well as for the cooperative action of increasing Boz levels while reducing Lnx-2b levels in the embryo. Collectively, our data indicate that Lnx-2b represses ectopic gsc expression in the presumptive ectodermal tissue by antagonizing Boz. The environment of the ectoderm is less favorable for gsc expression, possibly because it is more distant from the source of Nodal signals, as will be discussed below. In this environment, even the injection of high levels of boz RNA cannot induce gsc unless the level of Boz protein is increased sufficiently by reduction of Lnx-2b, thereby preventing the ubiquitylation-mediated destruction of Boz that otherwise limits its accumulation [11].
3.2. Nodal signaling is a pre-requisite for the expression of gsc
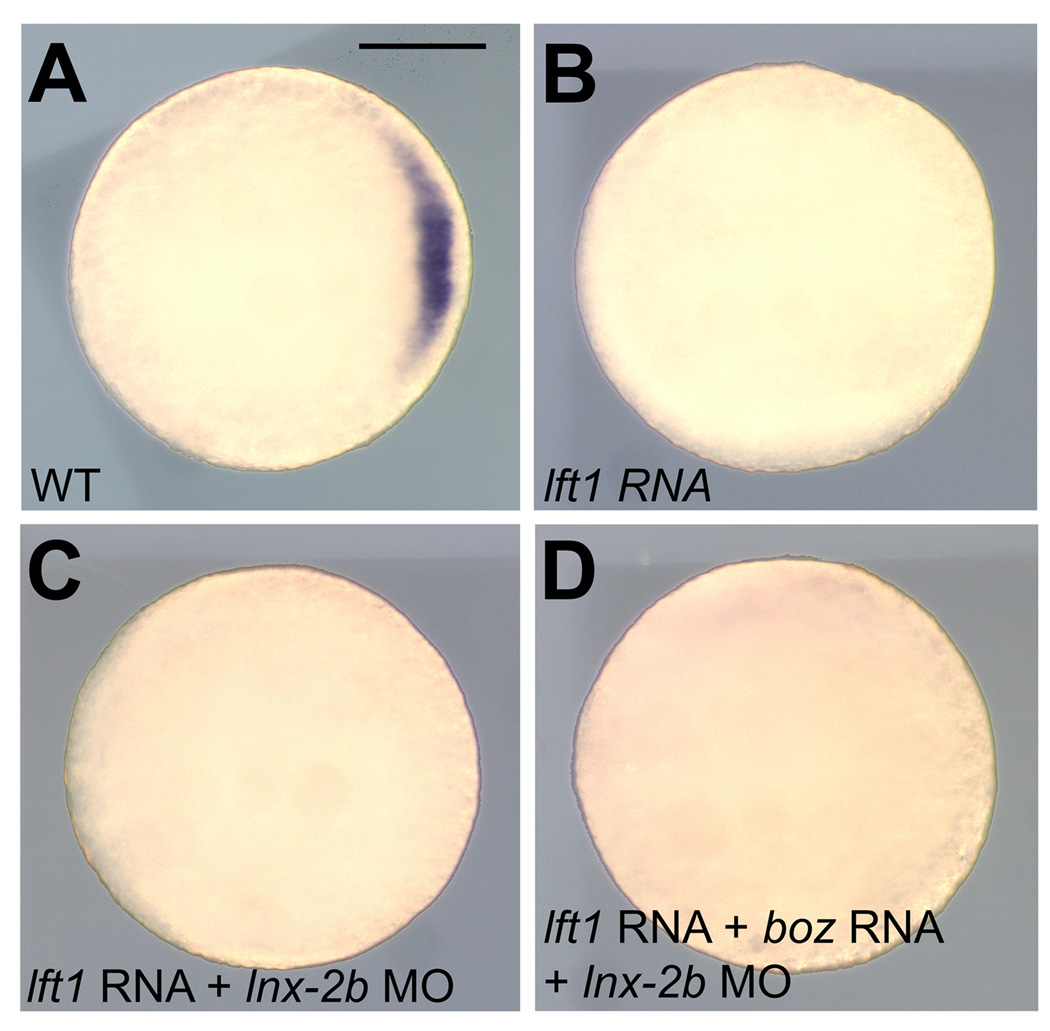
Boz and Nodal-related factors act in parallel to specify dorsal organizer and anterior neuroectoderm formation [6,7,21,22]. Ndr1 and Ndr2, members of TGF-β superfamily, bind to heteromeric type I and type II Activin receptors, which in turn act through the Smad2/Smad3/Smad4 signaling pathway to regulate the transcription of target genes [23]. Nodal signaling is negatively regulated by extracellular inhibitors, for example, Lefty1/Antivin (Lft1) and Lefty2 [24,25]. Because overexpression of Boz failed to induce gsc expression in the absence of Nodal signals, Nodal functions are required for the development of dorsal mesoderm [6,7,26]. To determine whether the depletion of Lnx-2b overcomes the suppression of gsc expression by the repression of Nodal signaling, we tested gsc expression in embryos in which both Nodal signaling and Lnx-2b expression were inhibited. In order to suppress the Nodal signaling we injected lft1 mRNA into one-two cell stage embryos. Confirming previous reports, Nodal inhibition by Lft1 completely eliminated gsc expression in the dorsal mesoderm at the germ ring stage [24,25] (Fig. 2B). Overexpression of Lft1 furthermore inhibited expanded gsc expression within the ventro-lateral marginal blastomeres as well as in the entire blastoderm when co-injected with lnx-2b MO and boz mRNA (Fig. 2C and 2D). Thus inhibition of nodal signaling by Lft1 suppressed all gsc expression, irrespective of any manipulation of the levels of Boz and Lnx-2b. These observations strongly support the view that gsc expression in the dorsal mesoderm is absolutely dependent on Nodal signaling. Boz and Lnx-2b modulate the induction of gsc in opposite directions, but ectopic expression of gsc through Boz stabilization via Lnx-2b repression [11] could be achieved only when Nodal signaling is active.
Fig. 2.
Nodal-related signals are a prerequisite for gsc induction. (A) Control uninjected embryo; gsc was detected in dorsal mesodermal cells. (B) Embryo injected with 25 pg lft1 mRNA; gsc expression was eliminated. (C) Embryo injected with 25 pg of lft1 mRNA and 5 ng lnx-2b MO. Depletion of Lnx-2b did not recover gsc expression. (D) Embryo injected with 25 pg of lft1 mRNA, 20 pg boz mRNA and 5 ng lnx-2b MO showed no gsc expression. (A–D) Germ ring stage. Animal pole view, dorsal is right. Scale bar, 200 µm.
3.3. Lnx-2b restricts the domain of non-cell autonomous action of Nodal and Boz
The cooperative function of Nodal-related factors and Boz seems to be critical for the correct spatio-temporal expression of gsc in the dorsal mesoderm [1,4]. The gsc gene is expressed only within or close to the domain in which Boz and Nodal factors are expressed. Even though the ndr1 and ndr2 genes are expressed, at least at blastula stages, beyond the range of the gsc expression domain, the failure of gsc induction in lateral marginal blastomeres is likely due to the lack of boz expression in this region. In addition, the expression levels of ndr1 and ndr2 in the lateral mesoderm might be below the threshold required for activation of gsc expression in the absence of Boz activity [6]. However, the gsc expression domain does not correspond exactly to the region in which boz and Nodal-related factors are co-expressed but extends to a region of neighboring cells in the dorsal mesendoderm. Thus Boz together with Nodal signaling may induce gsc expression in a non-cell autonomous manner [10]. As we reported previously, lnx-2b MO-dependent expansion of the gsc expression domain is due to Boz stabilization resulting in gradual expansion of the boz expression domain into the lateral marginal blastomeres [11]. Thus it is plausible that the combined injection of lnx-2b MO and boz mRNA induced ectopic gsc expression beyond the presumptive mesendoderm at the onset of gastrulation stage (Fig. 1F–H and 1I) through the long range effects of Ndr1 [9] and the enhanced level of Boz achieved by depletion of Lnx-2b [11].
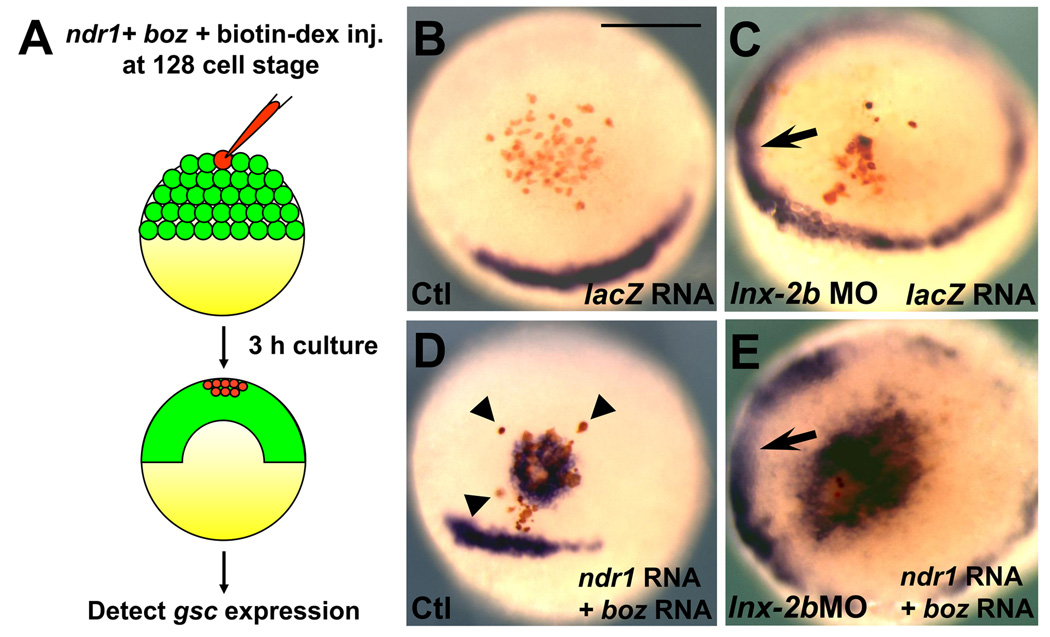
We therefore examined whether the capacity of Ndr1 and Boz to transform the presumptive ectoderm into dorsal mesoderm-like tissue is influenced by altered Lnx-2b levels. To provide a local source of Nodal and Boz for inducing gsc expression, we injected ndr1 and boz mRNA together with the lineage tracer fluorescein-dextran, into a single animal cell of 128-cell stage normal or lnx-2b morphant embryos. As a control, we injected lacZ mRNA together with fluorescein-dextran under identical experimental conditions. After 3 hours, the embryos were collected for in situ hybridization with gsc (Fig. 3A). lacZ injection failed to induce ectopic gsc expression in WT embryos (100%, N=11) as well as in lnx-2b morphants (100, N=9) (Fig. 3B and 3C). By contrast, ectopic gsc expression was detected in cells expressing Ndr1 and Boz (Fig. 3D, 62.5%, N=16). It should be noted that some of the isolated Ndr1 and Boz expressing cells could not drive ectopic gsc expression in their neighbors in WT embryos (arrowheads, Fig. 3D). These results suggest that only cell aggregates expressing Ndr1 and Boz may activate gsc expression in the presumptive ectoderm, an effect reminiscent of the “community effect” [27]. However, the gsc expressing domain became enlarged when ndr1 and boz mRNA were co-injected into a single cell of lnx-2b morphant embryos (Fig. 3E, 75%, N=16). In addition, in the morphant embryos we could not detect any isolated cells that express Ndr1 and Boz without inducing gsc (Fig. 3E). This observation suggests that every Ndr1/Boz overexpressing cell can induce gsc in its neighbors in the environment of Lnx-2b depletion.
Fig. 3.
Lnx-2b antagonizes Nodal/Boz-dependent gsc expansion. (A) Schematic representation of the experimental procedures. The indicated RNA(s) was co-injected with fluorescein-dextran (red) into a single cell at the 128-cell stage of control or lnx-2b morphants (embryos unjected with 5 ng lnx-2b MO at the 1–2 cell stage). Embryos were fixed 3h later for in situ hybridization. (B) lacZ mRNA injected control embryo. (C) lacZ mRNA injected lnx-2b morphant. Note that gsc was expressed in the ventro-lateral margin (arrow) but not in animal cells expressing LacZ. (D) Two pg of Ndr1 and 5 pg of boz mRNA was injected into a single cell, leading to a tight region of gsc expression and a few separate gsc-positive cells (arrowhead). (E) The same mix of Ndr1 and boz mRNA as in (D) was injected into one cell of lnx-2b morphants. A large gsc-positive region is seen in the animal hemisphere. Arrow indicates expanded gsc expression at the margin. (B–E) Germ ring stage. Animal pole view. Scale bar, 200 µm.
Taken together our data suggest that Lnx-2b maintains the integrity of the ectodermal tissues via suppressing gsc expression which can be driven by the coordinated action of Nodal-related factors and Boz. We show that the depletion of Lnx-2b potentiates Boz activity to induce the dorsal mesodermal marker gsc. However, Boz alone cannot activate gsc expression in the absence of Nodal signaling. In contrast to the consequences of Lnx-2b depletion, gsc expression in the organizer was not affected by overexpression of Lnx-2b (data not shown). The failure of suppression of gsc expression in the dorsal mesoderm by Lnx-2b overexpression may suggest that co-expression of Nodal and Boz overrides the negative influence of Lnx-2b against gsc induction. Alternatively, it is possible that the maternally expressed Lnx-2b is already saturating, so that an additional increase of Lnx-2b does not influence gsc expression.
Recently Shih et al. showed that SoxB1 transcription factors, which are expressed in presumptive ectoderm, repress the expression of dorsal organizer markers in the animal pole [28]. This conclusion is supported by the evidence that soxB1 genes determine dorso-ventral patterning by controlling bmp2b and bmp7 expression [28]. Thus depletion of four soxB1 genes or overexpression of dominant negative SoxB1 induced similar hyperdorsalized phenotypes [28,29]. Since lnx-2b morphants became strongly dorsalized [11], it would be intriguing to test the possible molecular crosstalk between Lnx-2b and SoxB1 factors in their role to delimit the mesendoderm and dorsal organizer formation.
4. Conclusions
In this study we conclude that maternally deposited Lnx-2b is critical to repress the expansion of dorsal organizer genes as exemplified by gsc, and thereby delimits the tissues that can respond to Nodal signals and Boz activity.
Acknowledgements
This work has been supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Abbreviations
- Boz
Bozozok
- Gsc
Goosecoid
- Lft1
Lefty1
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schier AF, Talbot WS. Nodal signaling and the zebrafish organizer. Int. J. Dev. Biol. 2001;45:289–297. [PubMed] [Google Scholar]
- 2.Schier AF, Talbot WS. Molecular genetics of axis formation in Zebrafish. Annu. Rev. Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 3.Koos DS, Ho RK. The nieuwkoid gene characterizes and mediates a Nieuwkoop-center-like activity in the zebrafish. Curr. Biol. 1998;8:1199–1206. doi: 10.1016/s0960-9822(07)00509-x. [DOI] [PubMed] [Google Scholar]
- 4.Solnica-Krezel L, Driever W. The role of the homeodomain protein Bozozok in zebrafish axis formation. Int. J. Dev. Biol. 2001;45:299–310. [PubMed] [Google Scholar]
- 5.Toyama R, O'Connell ML, Wright CV, Kuehn MR, Dawid IB. Nodal induces ectopic goosecoid and lim1 expression and axis duplication in zebrafish. Development. 1995;121:383–391. doi: 10.1242/dev.121.2.383. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T, Yamanaka Y, Ryu SL, Hashimoto H, Yabe T, Hirata T, Bae YK, Hibi M, Hirano T. Cooperative roles of Bozozok/Dharma and Nodal-related proteins in the formation of the dorsal organizer in Zebrafish. Mech. Dev. 2000;91:293–303. doi: 10.1016/s0925-4773(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 7.Sirotkin HI, Dougan ST, Schier AF, Talbot WS. bozozok and squint act in parallel to specify dorsal mesoderm and anterior neuroectoderm in zebrafish. Development. 2000;127:2583–2592. doi: 10.1242/dev.127.12.2583. [DOI] [PubMed] [Google Scholar]
- 8.Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Schier AF. The zebrafish Nodal signal Squint functions as a morphogen. Nature. 2001;411:607–610. doi: 10.1038/35079121. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka Y, Mizuno T, Sasai Y, Kishi M, Takeda H, Kim CH, Hibi M, Hirano T. A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes. Dev. 1998;12:2345–2353. doi: 10.1101/gad.12.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ro H, Dawid IB. Organizer restriction through modulation of Bozozok stability by the E3 ubiquitin ligase Lnx-like. Nat. Cell Biol. 2009;11:1121–1127. doi: 10.1038/ncb1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the Zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 13.Ro H, Soun K, Kim EJ, Rhee M. Novel vector systems optimized for injecting in vitro-synthesized mRNA into zebrafish embryos. Mol. Cells. 2004;17:373–376. [PubMed] [Google Scholar]
- 14.Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fekany-Lee K, Gonzalez E, Miller-Bertoglio V, Solnica-Krezel L. The homeobox gene bozozok promotes anterior neuroectoderm formation in zebrafish through negative regulation of BMP2/4 and Wnt pathways. Development. 2000;127:2333–2345. doi: 10.1242/dev.127.11.2333. [DOI] [PubMed] [Google Scholar]
- 16.Kawahara A, Wilm T, Solnica-Krezel L, Dawid IB. Antagonistic role of vega1 and bozozok/dharma homeobox genes in organizer formation. Proc. Natl. Acad. Sci. U S A. 2000;97:12121–12126. doi: 10.1073/pnas.97.22.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawahara A, Wilm T, Solnica-Krezel L, Dawid IB. Functional interaction of vega2 and goosecoid homeobox genes in zebrafish. Genesis. 2000;28:58–67. doi: 10.1002/1526-968x(200010)28:2<58::aid-gene30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Melby AE, Beach C, Mullins M, Kimelman D. Patterning the early zebrafish by the opposing actions of bozozok and vox/vent. Dev. Biol. 2000;224:275–285. doi: 10.1006/dbio.2000.9780. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu T, Yamanaka Y, Nojima H, Yabe T, Hibi M, Hirano T. A novel repressor-type homeobox gene, ved, is involved in dharma/bozozok-mediated dorsal organizer formation in Zebrafish. Mech. Dev. 2002;118:125–138. doi: 10.1016/s0925-4773(02)00243-5. [DOI] [PubMed] [Google Scholar]
- 20.Leung T, Bischof J, Söll I, Niessing D, Zhang D, Ma J, Jäckle H, Driever W. bozozok directly represses bmp2b transcription and mediates the earliest dorsoventral asymmetry of bmp2b expression in zebrafish. Development. 2003;130:3639–3649. doi: 10.1242/dev.00558. [DOI] [PubMed] [Google Scholar]
- 21.Fekany K, Yamanaka Y, Leung T, Sirotkin HI, Topczewski J, Gates MA, Hibi M, Renucci A, Stemple D, Radbill A, Schier AF, Driever W, Hirano T, Talbot WS, Solnica-Krezel L. The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development. 1999;126:1427–1238. doi: 10.1242/dev.126.7.1427. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez EM, Fekany-Lee K, Carmany-Rampey A, Erter C, Topczewski J, Wright CV, Solnica-Krezel L. Head and trunk in zebrafish arise via coinhibition of BMP signaling by bozozok and chordino. Genes Dev. 2000;14:3087–3092. doi: 10.1101/gad.852400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schier AF. Nodal signaling in vertebrate development. Annu. Rev. Cell. Dev. Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 24.Bisgrove BW, Essner JJ, Yost HJ. Regulation of midline development by antagonism of lefty and nodal signaling. Development. 1999;126:3253–3262. doi: 10.1242/dev.126.14.3253. [DOI] [PubMed] [Google Scholar]
- 25.Thisse C, Thisse B. Antivin, a novel and divergent member of the TGF-β superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- 26.Dougan ST, Warga RM, Kane DA, Schier AF, Talbot WS. The role of the zebrafish nodal-related genes squint and Ndr2lops in patterning of mesendoderm. Development. 2003;130:1837–1851. doi: 10.1242/dev.00400. [DOI] [PubMed] [Google Scholar]
- 27.Gurdon JB. A community effect in animal development. Nature. 1988;336:772–774. doi: 10.1038/336772a0. [DOI] [PubMed] [Google Scholar]
- 28.Shih YH, Kuo CL, Hirst CS, Dee CT, Liu YR, Laghari ZA, Scotting PJ. SoxB1 transcription factors restrict organizer gene expression by repressing multiple events downstream of Wnt signaling. Development. 2010;137:2671–2681. doi: 10.1242/dev.054130. [DOI] [PubMed] [Google Scholar]
- 29.Okuda Y, Ogura E, Kondoh H, Kamachi Y. B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. PLoS Genet. 2010;6:e1000936. doi: 10.1371/journal.pgen.1000936. [DOI] [PMC free article] [PubMed] [Google Scholar]