Abstract
BACKGROUND
Because urothelial carcinoma (UC) is associated with a significant high risk of recurrence and progression, patients with UC require long-term surveillance. Fluorescence in situ hybridization (FISH) has been shown to be more sensitive than cytology in the detection of UC. This study evaluated the use of FISH for detecting UC.
METHODS
We used a pathology database to identify patients who had urine cytology and FISH performed at our institution between 2004 and 2006. Urinary specimens were analyzed using UroVysion FISH probes for abnormalities in centromeric chromosomes 3, 7 and 17 and locus specific 9p21. FISH results were correlated with cytologic findings and a minimal clinical follow-up of 24 months.
RESULTS
We identified 1006 consecutive urinary specimens from 600 patients (448 men and 152 women) who were monitored for recurrent UC (915 specimens) or evaluated for urinary symptoms (91 specimens). On FISH analysis, 669 specimens were negative for UC and 272 specimens were positive for UC. Sixty-five (6%) specimens were insufficient for FISH analysis. The sensitivity and specificity of FISH for UC were 58% and 66%, respectively, and 59% and 63% when FISH and cytology results were combined. Factors contributing to decreased FISH sensitivity included the paucity or absence of tumor cells, low-grade tumors, degenerated cells, method of specimen collection, type of specimen, and obscuring inflammatory cells or lubricant.
CONCLUSIONS
We found UroVysion FISH had good sensitivity and specificity for detecting UC in urinary specimens. It is important to correlate the FISH results with the cytologic findings.
Keywords: Urothelial carcinoma, fluorescence in situ hybridization, chromosomal abnormalities, urine, cytology, multitarget FISH, bladder neoplasms, UroVysion
INTRODUCTION
Bladder cancer is the fourth most common cancer among men in the United States. It is estimated that more than 70,000 patients will be diagnosed with bladder cancer in 2009.1 Urothelial carcinoma (UC) is the most common type of bladder cancer; the majority of these tumors are low-grade, noninvasive papillary carcinomas. Because up to 70% of these tumors recur, with 15-30% progressing to high-grade lesions including carcinoma in situ,2 patients with UC require long-term surveillance. Typically, UC patients are monitored with cystoscopy and cytology every 3 months for 2 years after their initial diagnosis and with decreased frequency thereafter if they are free of disease.3 However, in addition to being expensive and unpleasant, cystoscopy often fails to detect flat tumors and carcinoma in situ,4 and although cytology has high sensitivity for high-grade lesions, it has a low sensitivity for low-grade tumors, which are the most prevalent.
Several urine-based assays have been used in the surveillance of UC patients.4-11 In a comparative study, Halling et al.5 found that UroVysion fluorescence in situ hybridization (FISH) test had the highest sensitivity and specificity for UC. UroVysion is a commercially available FISH test that contains centromeric probes for chromosomes 3, 7, and 17 and a locus-specific probe to chromosome 9p21.12 In the current study, we evaluated the routine use of FISH analysis for the detection of UC in a cancer center and investigated the potential pitfalls associated with using FISH to detect UC.
METHODS AND MATERIALS
This retrospective study was approved by the Institutional Review Board, which waived the requirement for patient informed consent. We used the pathology database at The University of Texas M. D. Anderson Cancer Center to identify patients with a history of UC or hematuria for whom urine cytology and UroVysion FISH analysis had been performed between January 1, 2004, and December 31, 2006. Clinical follow-up data, including cystoscopy, biopsy, and cytology findings, were obtained from patients’ medical records. The biopsy results were considered the standard.
During the study period, FISH analysis was done only when requested by the urologists. In the laboratory, the test was performed twice a week. If a specimen had less than 25 well preserved, nonoverlapping epithelial cells, then it was considered insufficient for FISH evaluation. All FISH results were e-mailed to the cytopathologist, who incorporated the resulting data into a final cytology report. Specifically, the comment section of the cytology report stated the number of abnormal cells that had an abnormal signal pattern out of the 25 cells analyzed using UroVysion FISH kit, if it was a positive or negative FISH result, and how the FISH result correlated with the cytologic findings. Furthermore, an addendum report was issued using the International System for Human Cytogenetic Nomenclature.
Specimen Preparation
Urinary specimens were centrifuged in 50-ml tubes at 600 g for 10 minutes at room temperature. Enough supernatant was removed to leave 1–2 ml in the tube with the cell pellet. Glacial acetic acid (2-3 ml) was added, and the specimen was vortexed and diluted with normal saline. The diluted specimen was centrifuged at 600 g for 5 minutes at room temperature, and then the supernatant was removed. Six to ten drops of the remaining mixture were added to a cytospin chamber (Shandon Cytospin 3, Shandon Inc., Pittsburgh, PA) and centrifuged at 750 rpm for 3 minutes, leaving a button of concentrated cells on a frosted-tip, silane-coated slide. Four slides were prepared for each urine specimen; 2 slides were fixed in 95% alcohol for cytology, and 2 slides were fixed in Carnoy’s fixative at a 3:1 methanol-to-acetic acid ratio for 30 minutes for FISH. The latter slides were air-dried and stored at −20°C until pretreatment and hybridization for FISH.
Cytologic Evaluation
Cytology slides were stained using the Papanicolaou method, screened, and a diagnosis rendered of negative for malignancy, atypia, suspicious for malignancy, or positive for malignancy.
Denauration and Hybridization
The FISH procedure is preformed in accordance to the manufacturer’s recommendations. The slides used for FISH analysis were immersed in 2X saline sodium citrate (SSC) buffer for 2 minutes at 73°C, immersed in protease solution for 10 minutes at 37°C, washed in 1X phosphate buffered saline (PBS) for 5 minutes at room temperature, fixed in 1% formaldehyde for 5 minutes at room temperature, and then washed again in 1X PBS for 5 minutes at room temperature. The slides were then dehydrated in a series of 70%, 85%, and 100% ethanol solutions for 1 minute each at room temperature and allowed to dry completely. After 3 μl of FISH probe (Abbott Laboratories) was applied to the slide, a 13-mm round glass coverslip was immediately placed over the probe solution and sealed with rubber cement. The samples were placed in the HYBrite Hybridization System (Abbott Laboratories, Abbott Park, Illinois) denatured at 73°C for 5 minutes, and then hybridized at 37°C for 16 hours.
Following hybridization, the rubber cement and coverslip were removed. The slides were then placed in 0.4X SSC/0.3% nonyl phenoxylpolyethoxylethanol (NP)-40 and incubated for 2 minutes at 73±1°C. After 2 minutes, the slides were washed in 2X SSC/0.1% NP-40 at room temperature for 1 minute and dried. Then 10 μl of 4’,6-diamidine-2’-phenylindole dihydrochloride (DAPI) II solution (Abbott Laboratories) was applied to the target area, and the slides were coverslipped. The slides were stored in the dark at −20°C until signal enumeration.
Analysis of FISH Signals
An epi-fluorescence microscope equipped with a 100-W mercury lamp (Leica DMLB) was used to enumerate the hybridization signals. UroVysion probe signals and DAPI counterstain were viewed using the following filters: DAPI single bandpass, red/green dual bandpass (chromosomes 3 and 7), aqua single bandpass (chromosome 17), and yellow (gold) single bandpass (9p21 locus). The slides were scanned from left to right and top to bottom, without overlapping the same areas. The following criteria were used to select 25 of the most abnormal cells for signal enumeration: large nuclear size, irregular nuclear shape, “patchy” DAPI staining, and cell clusters. The cells are evaluated using the DAPI filter by focusing on different planes to identify the most abnormal cells and avoid counting squamous cells, umbrella cells, neutrophils and other inflammatory cells. Only non-overlapping cells and cells with distinct signals were scored. The number of signals for all 4 probes was determined and recorded. If chromosomes 3, 7, or 17 showed the loss of both chromosomal signals, the cell was considered to be uninterpretable owing to hybridization failure. A cell was considered abnormal if it contained abnormal signals for at least 2 chromosomes. Specimens were considered FISH positive if there were 4 or more cells with polysomy for the probed areas on at least 2 chromosomes (3, 7, or 17) and/or 12 cells with no signal for chromosome 9p21.
Statistical Analysis
Clinical follow-up was considered positive if there was histologic verification of UC. Positive predictive value, negative predictive value, sensitivity and specificity for FISH, cytology, and FISH in conjunction with cytology were calculated based on the clinical findings at 12 and 24 months. The estimates were based on one observation per patient and only patients with measurements were analyzed for each time period. Patients with a biopsy with 365 days were included in the analysis for 12 months, and patients with a biopsy within 730 days were included in the analysis at 2 years. All the patients were followed up to 24 months. Negative or equivocal (atypical and suspicious) cytology for UC was considered negative for statistical analysis. The sensitivity of FISH and cytology were compared using the McNemar test. The FISH result was known at the time of the cytology diagnosis and may have influenced the diagnosis.
RESULTS
We identified 1006 consecutive urine specimens from 600 patients (448 men and 152 women) who were monitored for recurrent UC (915 specimens) or evaluated for urinary symptoms (91 specimens). The patients’ mean age was 66 years. There were 809 voided or not otherwise specified urinary specimens, 41 bladder washings, 28 urinary diversion specimens, 26 renal pelvic washing specimens, 19 ureteral washings/brushing specimens, 14 catheterized urinary specimens, 8 nephrostomy specimens and 1 urostomy specimen. On FISH analysis, 669 (67%) specimens had a negative result, 272 (27%) specimens had a positive result, and 65 (6%) specimens were insufficient for evaluation (Table 1). Of the 272 specimens with positive FISH results, 252 specimens were from patients who had a history of UC, and 20 specimens were from patients who were newly diagnosed with UC. Cytology revealed that, of the 272 positive FISH results, 58 specimens were negative for UC, 74 specimens were suspicious for malignancy (Figure 1), and 140 specimens were positive for malignancy (Figure 2). Clinical follow-up revealed that 170 of the 272 specimens were from patients who had UC, 92 specimens were from patients who did not have evidence of UC and 10 specimens were from patients who were lost to follow-up. Evaluation of the signal pattern of the 92 specimens from patients with false-positive FISH findings revealed that 40 specimens had tetrasomies and trisomies only and 52 specimens had polysomies, not exclusive of tetrasomies and trisomies. There were no positive FISH that contained only tetrasomies. Three positive FISH cases demonstrated only homozygous 9p21 deletions. Of these, 2 were confirmed positive for UC on biopsy and the other had no follow-up. Clinical follow-up in 57 of the 92 false-positive FISH specimens did reveal that 24 specimens were from patients who were receiving Bacillus Calmette-Guerin (BCG) treatment, 19 specimens were from patients for whom a second specimen showed positive FISH results, 5 specimens were from patients in whom cystoscopy revealed erythematous mucosa, 7 specimens were from patients in whom biopsy revealed denuded urothelium, 1 specimen was from a patient with hyperplastic epithelium and underlying chronic lymphocytic leukemia, and 1 specimen was from a patient with renal cell carcinoma.
Table 1.
Comparison of Urinary Specimens with Positive and Negative FISH Results for UC*
| FISH findings (no. of specimens) | Cytology | Clinical follow-up | ||||
|---|---|---|---|---|---|---|
| Neg/Aty | Susp | Pos | Neg | Pos | NF/U | |
| Negative (669) | 637 | 26 | 6 | 540 | 93 | 36 |
| Positive (272) | 58 | 74 | 140 | 92 | 170 | 10 |
Neg indicates negative; Aty, atypical; Susp, suspicious; Pos, positive; NF/U, no follow-up.
Insufficient specimens were excluded.
FIGURE 1.
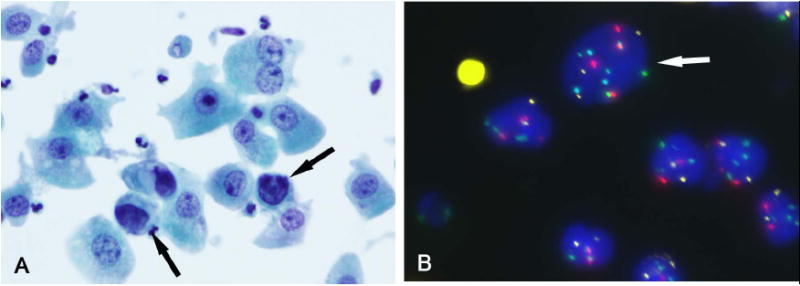
(A) A bladder washing specimen had rare atypical cells (arrow) with hyperchromatic irregular nuclei (Papanicolaou stain). (B) Atypical cell (arrow) with polysomies for chromosomes 3 (red), 7 (green), and 17 (aqua) compared to the other cells that are diploid (FISH). In this case, 6 of 25 cells analyzed showed an abnormal pattern, indicative of a positive FISH result. On clinical follow-up, the patient was found to have UC.
FIGURE 2.
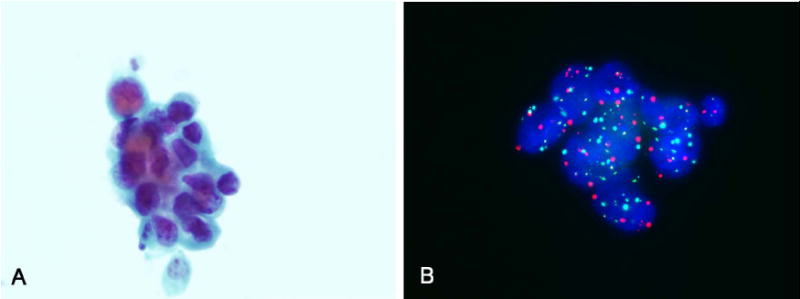
(A) A voided urine specimen contained few groups of tumor cells consistent with high-grade UC (Papanicolaou stain). (B) Atypical cells showed polysomies for chromosomes 3 (red), 7 (green), and 17 (aqua) (FISH). In this case, 16 of 25 cells analyzed showed an abnormal result, indicative of a positive FISH result. Clinical follow-up showed carcinoma in situ.
Of the 669 specimens that had negative FISH results, 608 specimens were from patients who had a history of UC, and 61 specimens were from patients who were evaluated for hematuria. For specimens with negative FISH results, cytology diagnoses were negative for malignancy in 456 specimens, atypical in 181 specimens (Figure 3), suspicious for malignancy in 26 specimens, and positive for malignancy in 6 specimens. Clinical follow-up of the negative FISH cases revealed that 540 specimens were from patients who did not have UC and 93 specimens were from patients who had UC. Thirty-six specimens were from patients who were lost to follow-up. Of the 93 specimens from patients who had UC, 61 (66%) were from patients with low-grade tumors, and 32 (34%) were from patients with high-grade tumors. The time to relapse was variable; 35 specimens (38%) were from patients whose UC relapsed within 90 days, and 58 specimens (62%) were from patients whose UC relapsed after 90 days (Table 2). The majority of these specimens were voided urine specimens. Eleven specimens with negative FISH results were from patients who were later diagnosed with renal cell carcinoma. A review of 50 negative FISH cases that were clinically positive for which cytology slides were available revealed that 12 specimens were primarily composed of mature squamous epithelial cells with sparse or no urothelial cells, 5 specimens were paucicellular, 5 specimens had numerous acute inflammatory cells, 2 specimens had abundant bacteria, 2 specimens had numerous crystals, and 1 specimen had abundant lubricant (Figure 4). The remaining specimens contained unremarkable urothelial cells.
FIGURE 3.
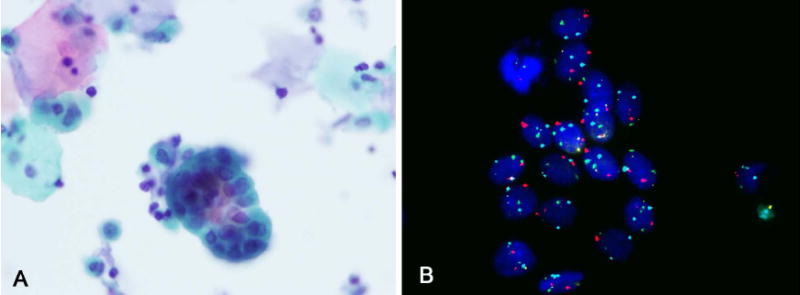
(A) Urine cytology showed groups in a urine specimen from a patient with a history of UC (Papanicoloau stain). (B) The cells showed 2 signals for centromeric chromosomes 3, 7, 17, and locus-specific probe 9p21, indicating a negative result (FISH). On clinical follow-up the patient was negative for UC.
Table 2.
Evaluation of 93 Urinary Specimens with Negative FISH Results but Positive Clinical Findings for UC
| Tumor grade | No. of specimens | Days to relapse | Specimen type | |||
|---|---|---|---|---|---|---|
| <90 | 91-180 | >180 | Voided urine | Other* | ||
| 1 | 16 | 8 | 1 | 7 | 16 | 0 |
| 2 | 45 | 15 | 8 | 22 | 40 | 5 |
| 3/in situ | 32 | 12 | 6 | 14 | 19 | 13 |
Bladder washings, ureter washings/brushings, renal pelvic washings, conduit urine.
FIGURE 4.
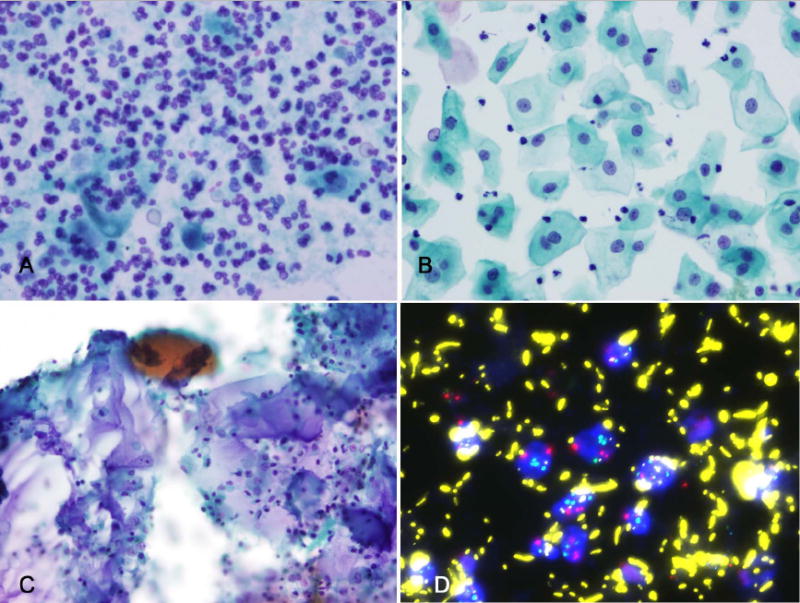
(A) Findings that may contribute to false-negative fluorescence in situ hybridization (FISH) results for urothelial carcinoma include rare degenerated cells and budding yeast forms in a background of marked acute inflammation (Papanicolaou stain); (B) voided urine containing only mature squamous cells (Papanicolaou stain); (C) bladder washings containing abundant lubricant (potentially obscuring FISH signals) (Papanicolaou stain); and (D) abundant autofluorescent bacteria (FISH).
Of the 65 urine specimens that had insufficient cells for FISH analysis, 44 were from voided or not otherwise specified urinary specimens, 10 were urinary diversion specimens, 5 were upper tract specimens, 5 were catheterized urines or bladder washing specimens, and 1 was a nephrostomy tube specimen. Among the different types of urine specimens, 5% of catheter or bladder washing specimens, 5% of voided urines, 26% of upper tract specimens, and 36% of urinary diversion specimens were considered unsatisfactory for FISH analysis because they had too few epithelial cells and numerous acute inflammatory cells with or without bacteria (24 specimens), too few or no epithelial cells (23 specimens), acellularity (9 specimens), degenerated cell nuclei (4 specimens), paucicellularity with abundant lubricant (2 specimens), overly thick preparation (1 specimen), and preparation on frosted slide (1 specimen).
The sensitivity and specificity of FISH for the detection of UC were 61% and 58% at 12 months and 58% and 66% at 24 months, respectively. The negative and positive predictive values of FISH for UC were 79% and 42%. The sensitivity and specificity for cytology alone for the detection of UC were 39% and 84%, respectively at 24 months. When FISH and cytology were combined the sensitivity and specificity were 59% and 63%, respectively at 24 months. Using the McNemar test, FISH was statistically more sensitive than cytology in detecting UC. Regarding gender, the sensitivity for FISH for the detection of UC was 62% for both men and women.
DISCUSSION
This study evaluated the use of UroVysion FISH to detect UC in urinary specimens from patients with either a history of UC or hematuria. Overall, FISH analysis showed higher sensitivity in the detection of UC than urine cytology alone, 13-16 but it was lower than some of the other FISH studies.13, 17-20 When the FISH and cytology results were combined, the sensitivity did not increase significantly as seen in other studies.15, 19 Factors contributing to decreased sensitivity included the paucity or absence of tumor cells, low-grade tumors, degenerated cells, methods of collection, type of specimen, and obscuring inflammatory cells or lubricant.
Some investigators have hypothesized that because UCs of similar histologic grade and stage behave differently, other factors such as genetic alterations must account for these differences.11 Analyzing the genetic aberrations commonly associated with UC is thought to provide a more objective assessment of whether recurrent disease is present.17 While a number of chromosomal aberrations have been described in UC,12, 21 previous studies have shown that aberrations involving chromosomes 3, 7, 17, and locus specific p21 on chromosome 9 have a high sensitivity and specificity in the detection of UC in voided urine specimens.13 The FDA approved the UroVysion test for the surveillance of patients with a history of UC in 2001 and for evaluating specimens from patients with gross or microscopic hematuria in 2005 after studies demonstrated that the UroVysion test had higher sensitivity for all grades and stages of UC than conventional cytology alone.13, 22 Previous studies found that the sensitivity of FISH for UC ranged from 65% to 100%, depending on the grade and stage of the tumor.2, 13, 18, 22-25 As expected, FISH was more sensitive for pT2 stage tumors and higher than for pTa and pT1 tumors and was more sensitive for grade 3 tumors than for grade 1 and 2 tumors.23 Although the combination of FISH and cytology had higher sensitivity for UC than FISH alone,2, 15 the specificity of FISH plus cytology was lower in some instances.15, 25 The specificity for FISH in the detection of UC ranged from 77% to 97%.13, 22, 23, 25
In the current study, the overall sensitivity and specificity of FISH for UC were 58% and 66%, respectively. We attributed the decreased sensitivity of FISH compared to the majority of other studies primarily to the high number of low-grade tumors, which are often diploid or have relatively few chromosomal aberrations,12 as well as the lack of tumor cells in the urinary specimens. Other contributing factors included the predominance of mature squamous cells especially in voided urine specimens, the presence of degenerated cells seen in specimens from patients often undergoing treatment or with urinary diversions, and obscuring inflammatory cells or lubricant. Enumeration of the FISH signals can be hampered in degenerated specimens because they can have increased numbers of split signals and specimens containing abundant autofluorescent bacteria.26
Of the evaluable urinary specimens in the current study, 29% had positive FISH and 71% had negative FISH results. Of the specimens with positive FISH findings, 50% were from patients in whom clinical evidence of UC was detected within 6 months; 63% of the specimens were from patients in whom clinical evidence of UC was found with extended follow-up. Some of these specimens were from patients who were receiving the BCG treatment for their UC. Given the propensity of UC to be multifocal and/or recur, the positive FISH results in some specimens from patients without clinical evidence of recurrent disease may have in fact indicated occult disease. “Anticipatory positives” is the term used to describe patients without clinical evidence of UC who have positive FISH results and develop recurrent tumor on follow-up.2, 13, 18, 27-29 Yoder et al.30 found that 65% of patients who had no clinical evidence of UC but positive FISH results relapsed within 29 months. FISH in conjunction with urine cytology may be helpful in detecting residual or recurrent tumor which may be obscured by inflammation or difficult to evaluate because of degenerative changes in post-BCG specimens. Furthermore, FISH positive results may be valuable in predicting which patients will not respond to BCG therapy and are more likely to have recurrent bladder tumor. Savic et al.31 reported that both positive post-BCG cytology and positive post-BCG FISH were significantly associated with BCG failure compared to those with negative results. Similarly, Kipp et al.32 showed that patients with positive post-BCG FISH were significantly more likely to develop tumor recurrence and 9.4 times more likely to have muscle invasive cancer than those patients with negative post-therapy FISH results. Black et al. recommended closely following patients who have negative cystoscopy results but positive FISH findings.4
In addition to UC, adenocarcinoma, squamous carcinoma, small cell carcinoma of the bladder, and renal cell carcinoma have been found to have positive FISH results in urinary specimens.30 Therefore, a positive FISH test is not specific for UC. In our study, 1 patient with hematuria and positive FISH results was subsequently diagnosed with renal cell carcinoma.
Expertise in selecting and enumerating FISH signals is important for proper evaluation. Only the most atypical cells, which often have enlarged nuclei, irregular nuclear membranes, patchy DAPI staining, and cell clustering, should be scored.33 Counting umbrella cells may result in false-positive FISH findings, as these cells are often tetraploid. This is especially important in specimens taken from within the bladder, such as bladder washings, in which umbrella cells are commonly present. To avoid this pitfall, Zellweger et al.34 suggested that if the only abnormality revealed by all 4 probes is tetraploidy, at least 10 cells should be present for FISH findings to be considered positive. In our study, care was taken not to count umbrella cells at the time of signal enumeration and there were no FISH positive cases that demonstrated only a tetraploid signal pattern. Likewise, Bollman et al.27 urged caution when interpreting deletions of 9p21, because it is the weakest of the 4 probes and may contribute to false-positive results. The probe manufacturer recommends that, if only homozygous 9p21 deletions are present, at least 12 abnormal cells must be present for a specimen to be considered positive for UC. In our study, 3 cases were FISH positive based only on homozygous deletion of 9p21in 12 or more cells. Of these, 2 were confirmed positive for UC on biopsy and the other had no follow-up.
Polyoma virus infection is another potential source of false-positive FISH readings in urinary specimens. Kipp et al.35 noted that the specimens with the highest number of viral particles detected by polymerase chain reaction (PCR) were found to be positive by FISH. Previous DNA ploidy studies have found aneuploidy or polyploidy in urine specimens containing polyoma virus. Similarly, in our laboratory, we have seen positive FISH results in urine specimens from leukemic patients with urinary symptoms that contain polyoma virus with unequivocal intranuclear inclusions. Such findings emphasize the need to correlate cytologic features with FISH results. In equivocal cases, even after cytologic examination, PCR analysis or immunostaining with SV-40 may be helpful in confirming polyoma virus infection.36, 37 Analyzing seminal vesicle cells, which are aneuploid,38 is another potential caveat in FISH studies of urinary specimens. However, seminal vesicle cells are rarely seen in urine specimens; when present, they are usually sparse. Correlating FISH results with cytology findings, which show seminal vesicle cells as large cells with pleomorphic nuclei and brown granules in the cytoplasm, can help to correctly identify these cells.
In the current study, urinary diversion specimens and upper tract specimens were considered to be unsatisfactory for evaluation by FISH analysis more often than voided urine and bladder washing specimens. The urinary diversion specimens often contained sparse, poorly preserved epithelial cells, numerous acute inflammatory cells, abundant bacteria, and lubricant, making it difficult to score FISH signals.
Comparing urine studies evaluating FISH and cytology can sometimes be difficult since the endpoints used in the statistical analysis are not well defined or uniformly reported. One variable endpoint is the confirmation of tumor which may be based on either positive histologic or cystoscopic findings or only with histologic verification. The latter is more often reported;13, 18, 23, 30 however, the standard of comparison is not always described. One study required biopsy for a positive result, but also stated that if a lesion was fulgurated or ablated without histology it was considered positive when the cystoscopy was unequivocally positive.18 Another variable is the minimum follow-up time after the FISH test is performed which could range from12 months to 29 months.23, 30 Likewise, the categorization of atypical and suspicious for malignancy cytology diagnoses into positive and negative results is not consistently reported in the statistical analysis. These factors may have contributed to the range in sensitivities and specificities for FISH and cytology reported in the literature.
The limitations of this study include its retrospective nature and variability in test requisition by the urologists so that only a subset of patients with UC had the FISH analysis performed. In addition, the cytology and biopsy specimens were usually not concurrently acquired, the time was variable in specimen acquisition, and many cases had no follow-up biopsy. However, this study was undertaken to focus on the use of this multicolor FISH probe in a large cancer center with a high-risk population for recurrent UC and to identify potential pitfalls.
In conclusion, using FISH in conjunction with cytology increases the overall sensitivity for UC compared to cytology alone in patients with urinary symptoms and those under surveillance for recurrent disease. It is important to correlate cytologic findings with the FISH results and beware of the potential pitfalls that may be associated with different collection methods, the type of specimen such as a urinary diversion, and viral infections when interpreting FISH results.
Acknowledgments
The biostatistical research analysis is supported in part by the National Institutes of Health through M.D. Anderson Cancer Center Support Grant CA016672.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed]
- 2.Junker K, Fritsch T, Hartmann A, Schulze W, Schubert J. Multicolor fluorescence in situ hybridization (M-FISH) on cells from urine for the detection of bladder cancer. Cytogenet Genome Res. 2006;114(3-4):279–83. doi: 10.1159/000094214. [DOI] [PubMed]
- 3.Halling KC, Kipp BR. Bladder cancer detection using FISH (UroVysion assay) Adv Anat Pathol. 2008;15(5):279–86. doi: 10.1097/PAP.0b013e3181832320. [DOI] [PubMed]
- 4.Black PC, Brown GA, Dinney CP. Molecular markers of urothelial cancer and their use in the monitoring of superficial urothelial cancer. J Clin Oncol. 2006;24(35):5528–35. doi: 10.1200/JCO.2006.08.0895. [DOI] [PubMed]
- 5.Halling KC, King W, Sokolova IA, Karnes RJ, Meyer RG, Powell EL, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol. 2002;167(5):2001–6. [PubMed]
- 6.Glas AS, Roos D, Deutekom M, Zwinderman AH, Bossuyt PM, Kurth KH. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol. 2003;169(6):1975–82. doi: 10.1097/01.ju.0000067461.30468.6d. [DOI] [PubMed]
- 7.Budman LI, Kassouf W, Steinberg JR. Biomarkers for detection and surveillance of bladder cancer. Can Urol Assoc J. 2008;2(3):212–21. doi: 10.5489/cuaj.600. [DOI] [PMC free article] [PubMed]
- 8.Sharma S, Zippe CD, Pandrangi L, Nelson D, Agarwal A. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J Urol. 1999;162(1):53–7. doi: 10.1097/00005392-199907000-00014. [DOI] [PubMed]
- 9.Svatek RS, Sagalowsky AI, Lotan Y. Economic impact of screening for bladder cancer using bladder tumor markers: a decision analysis. Urol Oncol. 2006;24(4):338–43. doi: 10.1016/j.urolonc.2005.11.025. [DOI] [PubMed]
- 10.Grossman HB, Soloway M, Messing E, Katz G, Stein B, Kassabian V, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295(3):299–305. doi: 10.1001/jama.295.3.299. [DOI] [PubMed]
- 11.Baffa R, Letko J, McClung C, LeNoir J, Vecchione A, Gomella LG. Molecular genetics of bladder cancer: targets for diagnosis and therapy. J Exp Clin Cancer Res. 2006;25(2):145–60. [PubMed]
- 12.Sokolova IA, Halling KC, Jenkins RB, Burkhardt HM, Meyer RG, Seelig SA, et al. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn. 2000;2(3):116–23. doi: 10.1016/S1525-1578(10)60625-3. [DOI] [PMC free article] [PubMed]
- 13.Halling KC, King W, Sokolova IA, Meyer RG, Burkhardt HM, Halling AC, et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol. 2000;164(5):1768–75. [PubMed]
- 14.Sullivan PS, Nooraie F, Sanchez H, Hirschowitz S, Levin M, Rao PN, et al. Comparison of ImmunoCyt, UroVysion, and urine cytology in detection of recurrent urothelial carcinoma: a “split-sample” study. Cancer Cytopathol. 2009;117(3):167–73. doi: 10.1002/cncy.20026. [DOI] [PubMed]
- 15.Moonen PM, Merkx GF, Peelen P, Karthaus HF, Smeets DF, Witjes JA. UroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle-invasive bladder cancer. Eur Urol. 2007;51(5):1275–80. doi: 10.1016/j.eururo.2006.10.044. [DOI] [PubMed]
- 16.Landman J, Chang Y, Kavaler E, Droller MJ, Liu BC. Sensitivity and specificity of NMP-22, telomerase, and BTA in the detection of human bladder cancer. Urology. 1998;52(3):398–402. doi: 10.1016/s0090-4295(98)00219-2. [DOI] [PubMed]
- 17.Laudadio J, Keane TE, Reeves HM, Savage SJ, Hoda RS, Lage JM, et al. Fluorescence in situ hybridization for detecting transitional cell carcinoma: implications for clinical practice. BJU Int. 2005;96(9):1280–5. doi: 10.1111/j.1464-410X.2005.05826.x. [DOI] [PubMed]
- 18.Sarosdy MF, Schellhammer P, Bokinsky G, Kahn P, Chao R, Yore L, et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J Urol. 2002;168(5):1950–4. doi: 10.1016/S0022-5347(05)64270-X. [DOI] [PubMed]
- 19.Placer J, Espinet B, Salido M, Sole F, Gelabert-Mas A. Clinical utility of a multiprobe FISH assay in voided urine specimens for the detection of bladder cancer and its recurrences, compared with urinary cytology. Eur Urol. 2002;42(6):547–52. doi: 10.1016/s0302-2838(02)00448-7. [DOI] [PubMed]
- 20.Dalquen P, Kleiber B, Grilli B, Herzog M, Bubendorf L, Oberholzer M. DNA image cytometry and fluorescence in situ hybridization for noninvasive detection of urothelial tumors in voided urine. Cancer. 2002;96(6):374–9. doi: 10.1002/cncr.10881. [DOI] [PubMed]
- 21.Constantinou M, Binka-Kowalska A, Borkowska E, Zajac E, Jalmuzna P, Matych J, et al. Application of multiplex FISH, CGH and MSSCP techniques for cytogenetic and molecular analysis of transitional cell carcinoma (TCC) cells in voided urine specimens. J Appl Genet. 2006;47(3):273–5. doi: 10.1007/BF03194636. [DOI] [PubMed]
- 22.Sarosdy MF, Kahn PR, Ziffer MD, Love WR, Barkin J, Abara EO, et al. Use of a multitarget fluorescence in situ hybridization assay to diagnose bladder cancer in patients with hematuria. J Urol. 2006;176(1):44–7. doi: 10.1016/S0022-5347(06)00576-3. [DOI] [PubMed]
- 23.Skacel M, Fahmy M, Brainard JA, Pettay JD, Biscotti CV, Liou LS, et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol. 2003;169(6):2101–5. doi: 10.1097/01.ju.0000066842.45464.cc. [DOI] [PubMed]
- 24.Marin-Aguilera M, Mengual L, Ribal MJ, Burset M, Arce Y, Ars E, et al. Utility of a multiprobe fluorescence in situ hybridization assay in the detection of superficial urothelial bladder cancer. Cancer Genet Cytogenet. 2007;173(2):131–5. doi: 10.1016/j.cancergencyto.2006.10.011. [DOI] [PubMed]
- 25.Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol. 2008;26(6):646–51. doi: 10.1016/j.urolonc.2007.06.002. [DOI] [PubMed]
- 26.Riesz P, Lotz G, Paska C, Szendroi A, Majoros A, Nemeth Z, et al. Detection of bladder cancer from the urine using fluorescence in situ hybridization technique. Pathol Oncol Res. 2007;13(3):187–94. doi: 10.1007/BF02893498. [DOI] [PubMed]
- 27.Bollmann M, Heller H, Bankfalvi A, Griefingholt H, Bollmann R. Quantitative molecular urinary cytology by fluorescence in situ hybridization: a tool for tailoring surveillance of patients with superficial bladder cancer? BJU Int. 2005;95(9):1219–25. doi: 10.1111/j.1464-410X.2005.05509.x. [DOI] [PubMed]
- 28.Bubendorf L, Grilli B, Sauter G, Mihatsch MJ, Gasser TC, Dalquen P. Multiprobe FISH for enhanced detection of bladder cancer in voided urine specimens and bladder washings. Am J Clin Pathol. 2001;116(1):79–86. doi: 10.1309/K5P2-4Y8B-7L5A-FAA9. [DOI] [PubMed]
- 29.Skacel M, Pettay JD, Tsiftsakis EK, Procop GW, Biscotti CV, Tubbs RR. Validation of a multicolor interphase fluorescence in situ hybridization assay for detection of transitional cell carcinoma on fresh and archival thin-layer, liquid-based cytology slides. Anal Quant Cytol Histol. 2001;23(6):381–7. [PubMed]
- 30.Yoder BJ, Skacel M, Hedgepeth R, Babineau D, Ulchaker JC, Liou LS, et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol. 2007;127(2):295–301. doi: 10.1309/ADJL7E810U1H42BJ. [DOI] [PubMed]
- 31.Savic S, Zlobec I, Thalmann GN, Engeler D, Schmauss M, Lehmann K, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guerin therapy. Int J Cancer. 2009;124(12):2899–904. doi: 10.1002/ijc.24258. [DOI] [PubMed]
- 32.Kipp BR, Karnes RJ, Brankley SM, Harwood AR, Pankratz VS, Sebo TJ, et al. Monitoring intravesical therapy for superficial bladder cancer using fluorescence in situ hybridization. J Urol. 2005;173(2):401–4. doi: 10.1097/01.ju.0000149825.83180.a4. [DOI] [PubMed]
- 33.Kipp BR, Fritcher EG, del Rosario KM, Stevens CL, Sebo TJ, Halling KC. A systematic approach to identifying urothelial cells likely to be polysomic by fluorescence in situ hybridization. Anal Quant Cytol Histol. 2005;27(6):317–22. [PubMed]
- 34.Zellweger T, Benz G, Cathomas G, Mihatsch MJ, Sulser T, Gasser TC, et al. Multi-target fluorescence in situ hybridization in bladder washings for prediction of recurrent bladder cancer. Int J Cancer. 2006;119(7):1660–5. doi: 10.1002/ijc.21704. [DOI] [PubMed]
- 35.Kipp BR, Sebo TJ, Griffin MD, Ihrke JM, Halling KC. Analysis of polyomavirus-infected renal transplant recipients’ urine specimens: correlation of routine urine cytology, fluorescence in situ hybridization, and digital image analysis. Am J Clin Pathol. 2005;124(6):854–61. [PubMed]
- 36.von Willebrand E, Savikko J, Merenmies J, Jalanko H. Human polyoma virus in kidney transplants: SV40 T-antigen demonstration in the urine. Transplant Proc. 2005;37(2):945–6. doi: 10.1016/j.transproceed.2004.12.073. [DOI] [PubMed]
- 37.Cimbaluk D, Pitelka L, Kluskens L, Gattuso P. Update on human polyomavirus BK nephropathy. Diagn Cytopathol. 2009;37(10):773–9. doi: 10.1002/dc.21147. [DOI] [PubMed]
- 38.Wojcik EM, Bassler TJ, Jr, Orozco R. DNA ploidy in seminal vesicle cells. A potential diagnostic pitfall in urine cytology. Anal Quant Cytol Histol. 1999;21(1):29–34. [PubMed]


