Abstract
The present study examines the postnatal reproductive development of male rats following prenatal exposure to an atrazine metabolite mixture (AMM) consisting of the herbicide atrazine and its environmental metabolites diaminochlorotriazine, hydroxyatrazine, deethylatrazine, and deisopropylatrazine. Pregnant Long Evans rats were treated by gavage with 0.09, 0.87, or 8.73 mg AMM/kg body weight (BW), vehicle, or 100 mg ATR/kg BW positive control, on gestation days 15-19. Preputial separation was significantly delayed in 0.87 mg and 8.73 mg AMM-exposed males. AMM-exposed males demonstrated a significant treatment-related increase in incidence and severity of inflammation in the prostate on postnatal day (PND) 120. A dose-dependent increase in epididymal fat masses and prostate foci were grossly visible in AMM-exposed offspring. These results indicate that a short, late prenatal exposure to mixture of chlorotriazine metabolites can cause chronic prostatitis in male LE rats. The mode of action for these effects is presently unclear.
Keywords: Atrazine, puberty, prostate, inflammation, development, rat, metabolites, mixture
1. Introduction
Atrazine (ATR; 2-chloro-N-ethyl-N′-isopropyl-[1,3,5] triazine), a chloro-s- triazine, is one of the most widely applied herbicides in the United States for control of broadleaf weeds and grasses in crops of corn, sugar cane, and sorghum [1]. Plants, animals, and microbes each catabolize chlorotriazines and resulting metabolites are relatively persistent in the environment, with half-lives exceeding one year in soil and nearly twice as long in water [2]. Because of its environmental persistence and potential to enter drinking water supplies, ATR has been banned in the European Union [3].
Although the precise mechanism of action remains to be elucidated at certain tissue sites, studies have demonstrated that ATR adversely affects the endocrine system and reproductive tissues in the rat (reviewed in [4, 5]). In female rats, ATR has been shown to interfere with a number of endocrine processes through disruption of pituitary-ovarian function via the hypothalamus [6]. For instance, delayed pubertal development [7], prolonged estrous cycling and delayed ovulation [8-10] following ATR exposure have been attributed to suppression of the ovulatory luteinizing hormone (LH) surge by ATR. Female Long-Evans (LE) offspring of untreated dams cross-fostered to dams gavage dosed with ATR in pregnancy exhibited delayed puberty and mammary gland development associated with significantly lower proliferation-associated markers, specifically, mammary gland-specific aromatase and epidermal growth factor receptor gene expression [11].
Exposure to ATR has also been shown to affect reproductive tissues in male rats. Stoker et al. [12] observed increased myeloperoxidase activity in the lateral prostate (LP) of male offspring of Wistar rats following early lactational exposure to ATR. This inflammatory effect was attributed to inhibition of the suckling-induced prolactin (PRL) release that is involved in regulating the maturation of the dopaminergic neurons shortly before and after birth [12]. Puberty was delayed in male Wistar rats exposed to ATR peripubertally and those rats in the highest dose group exhibited decreased testicular testosterone [13]. In another study [14], male LE rats exposed in utero to 100 mg ATR/kg BW exhibited delayed attainment of puberty and increased LP weights at necropsy on PND120 and PND220, as well as visible signs of prostatic inflammation.
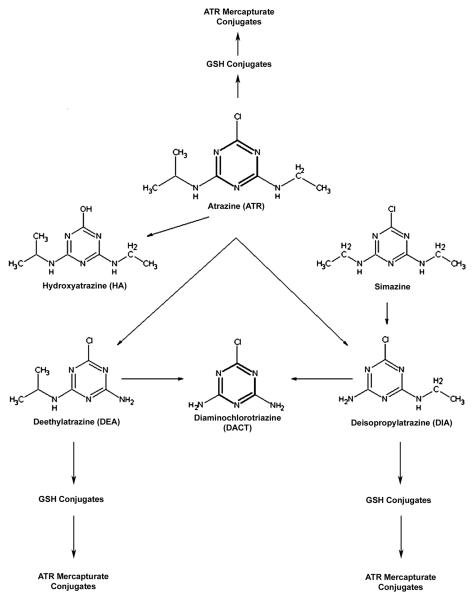
Animal catabolism of ATR and other chlorotriazines, including simazine and propazine, occurs by dealkylation, dechlorination, and conjugation (Figure 1). The parent chlorotriazines produce the same set of four metabolites that have been detected in animals, soil/sediment, and surface and ground waters; hydroxyatrazine (HA; 6-hydroxy-N-ethyl-N′-isopropyl-[1,3,5] triazine-2,4-diamine), diaminochlorotriazine (DACT; 6-chloro-[1,3,5] triazine-2,4-diamine), deisopropylatrazine (DIA; 6-chloro-N-ethyl-[1,3,5] triazine-2,4-diamine), and deethylatrazine (DEA; 6-chloro-N-isopropyl-[1,3,5] triazine-2,4-diamine). Although ATR is currently seldom detected at levels exceeding the U.S. Environmental Protection Agency maximum contaminant level (MCL) of 3 ppb (3 μg/L) in ground water [15, 16], the U.S. Geological Survey National Water-Quality Assessment Watershed Regressions for Pesticides Atrazine Model [17], recently updated with 2007 ATR agricultural use data [18], predicts “…streams with a greater than 5-percent probability of exceeding the benchmark represent about 6 percent of the Nation's stream miles (36,829 of 649,935 mi)” and that “Approximately 546 stream miles (less than 1/10th of 1 percent of the Nation's stream miles) are predicted to have more than a 50-percent probability of exceeding 3 μg/L.” Moreover, ATR metabolites commonly occur at levels higher that ATR itself [12, 19] and MCLs for the metabolites (individually or cumulatively) have not been established. In an analysis of 233 early summer pre- and post-emergence run-off event samples from 76 Midwestern stream or reservoir outflows and 70 low flow samples collected at 70 Midwestern streams, Battaglin et al. [20] reported that ATR was detected in at least 80% of the samples at a median concentration of 4.07 μg/L in pre-emergence sites and 2.69 μg/L at post-emergence sites. Furthermore, three ATR metabolites, DEA, DIA, and HA, were detected in at least 50% of the samples with concentrations of DEA (0.41 and 0.54 μg/L) and DIA (0.32 and 0.39 μg/L) being the highest in pre- and post-emergence sites, respectively, and HA (0.29 μg/L) being the highest in low flow samples [20]. And in another study, atrazine and up to three of its degradation products were identified at sites in Rock Creek National Park in Washington D.C., Chesapeake and Ohio Canal National Historic Park in Maryland, DeSoto National Wildlife Refuge in Missouri Valley, Iowa, and Seminole State Park near Sinclair, Wyoming [21].
Figure 1.
Catabolism of atrazine and simazine into primary metabolites and further breakdown products.
Studies utilizing individual ATR metabolites demonstrated delayed puberty in both male and female Wistar rats and the investigators concluded that atrazine molar equivalents (AME) of ATR chlorinated metabolites may be as potent as ATR itself and may also mediate the effects of ATR [22, 23]. Enoch et al. [24] first reported developmental effects in female rats following prenatal exposure to low doses of a mixture of ATR and its metabolites. The atrazine metabolite mixture (AMM) was formulated to contain ATR and its metabolites DACT, HA, DEA, and DIA in AME proportions and at levels reported in a survey of ground and surface water potentially containing ATR [25-27]. Late prenatal exposure of LE rats to this AMM, which was also used in the present study, delayed mammary gland development of female offspring, evident as early as weaning in all treatment groups [24]. Mammary gland developmental impairments from AMM exposure were as severe as those observed in offspring prenatally exposed to the positive control group (high dose ATR), and were present when no significant effects on day of vaginal opening, a female pubertal indicator, or decreased body weight were observed. These findings suggest that the rat mammary gland may be particularly sensitive to the effects of atrazine metabolites or that the ATR metabolites, used together, are particularly potent in mediating the effects on some, but not all reproductive tissues.
The objective of the current study was to examine the potential for alterations in the reproductive development of male rat offspring following late prenatal exposure, during the period of fetal prostate development, to a relatively low concentration mixture of ATR metabolites as described in Enoch et al. [24]. In recent ATR risk assessment documents [15, 28], the ATR “Short-term” (1-30 days) exposure value was determined using the no observed adverse effect level (NOAEL), and the lowest observed adverse effect level (LOAEL) for effects on male pup reproductive tissue as the endpoints of concern. The data presented here provide information regarding the effects of exposure to a metabolite mixture administered at doses considerably lower than the current developmental ATR NOAEL of 6.25 mg/kg/day and LOAEL of 12.5 mg/kg/day [15, 28].
2. Methods
2.1 Animals
Timed pregnant Long-Evans rats (9-15 wk old, sperm positive = Day 0) were obtained from Charles River Breeding Laboratories (Raleigh, NC). Females were housed in an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility in clear plastic cages containing heat-treated pine shavings (Beta Chips, North Eastern Products Inc., Warrensburg, NY) and given food (Purina 5008 Rodent Chow, Ralston Purina Co., St. Louis, MO) and water ad libitum. Once weaned, the male offspring were housed 3-4/cage and fed Purina 5001 chow ad libitum. The animals were maintained in a room with a 14:10-hour light/dark cycle (2100 hr lights out), at 20-24° C, and 40-50% relative humidity. All animals were treated humanely and with regard for alleviation of potential suffering, as approved by the U.S. EPA National Health and Environmental Effects Research Laboratory's Institutional Animal Care and Use Committee.
2.2 Dosing Solutions
ATR and all metabolites (Syngenta Crop Protection, Inc. Greensboro, NC; 94.5-98.2% purity) were prepared as a suspension in 1.0% methylcellulose (Sigma-Aldrich Chemical Co., Inc., St. Louis, MO) in distilled water. The AMM consists of ATR (25% by weight), DACT (35%, 1.482 AME), HA (20%, 1.094 AME), DEA (15%, 1.149 AME) and DIA (5%, 1.242 AME) and was prepared as detailed in Enoch et al. [24]. Dose groups included 0 (1% methylcellulose vehicle), 0.09, 0.87, 8.73 mg AMM/kg BW/d and 100 mg ATR/kg BW/d, which were delivered in a volume of 5.0 ml vehicle/kg BW. Solutions were stirred constantly between dosing periods and were agitated between dosing of individual animals to maintain as consistent a fine suspension as possible.
2.3 Experimental design
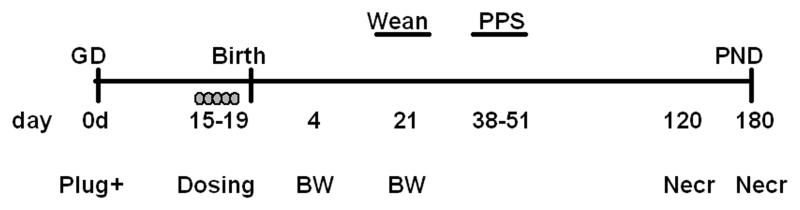
The study was conducted in two replicates (blocks) with five treatment groups per block (positive and negative controls present in each block) as depicted in Figure 2. Based on results from these blocks, a third block was conducted to include a 180 day necropsy. Pregnant Long Evans rats (n ≥ 6/treatment/block) were gavage dosed with 0, 0.09, 0.87 or 8.73 mg AMM/kg BW/d (hereafter referred to as mg AMM) or 100 mg ATR/kg BW/d (hereafter referred to as mg ATR) on GD15-19. The animals in this study were the siblings to those in Enoch et al. [24], which focused on the effects of AMM on mammary gland development. Exposure was conducted on GD15-19 because this dosing window is a critical period of development for both the mammary gland and prostate [29, 30]. Because ATR is rapidly metabolized in the adult rat, half-doses were administered b.i.d. (0700 and 1400 hr), adjusted for morning BW, in order to emulate near steady state exposure. Total single day dose of 0, 0.09, 0.87, or 8.73 mg AMM or 100 mg ATR are reported for consistency with previous studies. The 100 mg ATR dose was chosen as a positive control to compare directly with results from previous studies [14]. On PND4, pups were weighed and litters equalized to six female and four male pups. Animals were weighed on PND4, 21, 120, and 180. Typically, two male pups were randomly selected from each of at least five dams (n ≥ 10, specific n provided in legends) for pubertal assessment and necropsy. Necropsy was conducted on PND120 in blocks one and two and on PND120 and 180 in block three.
Figure 2.
Experimental design. Dams were gavaged on GD15-19 with AMM, 100 mg ATR/kg body weight, or vehicle. Body weight was recorded on PND4, 21, at PPS, PND120, and 180. Preputial separation (PPS) was observed from PND38-51. Necropsy was conducted on PND120 and PND180.
Fetal BW was obtained as described in Enoch et al. [24]. In a separate study, timed-pregnant Long-Evans dams were treated with 0 or 8.73 mg AMM/kg BW (n = 8 dams/treatment) on GD15–GD19 and euthanized on GD20. Litters were removed from the dam and the sex and weight of each fetus was recorded. One control dam died during this portion of the study.
2.4 Preputial Separation
The separation of the prepuce from the glans penis, preputial separation (PPS), is a reliable indicator of puberty in the male rat [31]. In LE rats, PPS generally occurs from 40-42 days of age. Male rats were examined daily as described in Korenbrot et al. [31], at approximately the same time of day, beginning on PND38 and continuing until PPS was achieved in all animals. Age and weight were recorded when full PPS was observed.
2.5 Necropsy and tissue collection
The necropsy from which the prostate tissues were obtained for these studies was conducted by a contracted team (Experimental Pathology Laboratory, EPL; Research Triangle Park, NC) that included a board certified veterinary pathologist. All weights and observations during necropsy were recorded. Animals were isolated in a quiet holding room for at least 12 hours prior to necropsy, which was conducted between 0800 and 1400 hr. At necropsy on PND120 and PND180, animals were weighed, euthanized by decapitation, and trunk blood was collected. Serum was separated from the blood by centrifugation at 4°C for 15 min. at 3500 rpm and stored frozen at −80°C for hormone and cytokine assays. The anterior pituitary (AP) was removed, weighed, and frozen at −80°C for pituitary prolactin and thyroid-stimulating hormone assays. Both horns of the seminal vesicle (SV) were removed as one part and the wet weight was recorded. The SV was then drained of fluid and the weight recorded as weight without fluid (SVd). Seminal fluid weight was recorded as the difference between the wet weight and SVd. The testes, as well as ventral and lateral lobes of the prostate (VP and LP, respectively) were removed and weighed. The dorsal prostate was not removed. Macroscopically visible masses presenting as light orange to dark red nodular lipomatous discolorations (presumptive inflammation +/− hemorrhage) measuring about 2-30 mm in the inguinal region and macroscopic pale prostatic foci (presumptive inflammatory cell infiltrates present on only the lateral and ventral lobes) measuring 1-2 mm were observed and recorded by the pathologist at necropsy and were collected for microscopic evaluation. Tissues for histology were fixed in neutral buffered formalin (10%).
2.6 Radioimmunoassays
Radioimmunoassays utilizing rat specific antibodies were performed as previously described [6] to measure prolactin (sPRL), thyroid-stimulating hormone (sTSH), luteinizing hormone (sLH) in serum, and prolactin (pPRL) and thyroid-stimulating hormone (pTSH) in pituitary tissue. Serum testosterone (sT) concentrations were determined using Coat-A-Count® antibody-coated tube kits from Diagnostic Products Corp. (Los Angeles, CA), as instructed by the manufacturer. Serum estradiol (sE2) and estrone (sEN) concentrations were determined using kits from Diagnostic Systems Laboratory, Inc. (Webster, TX), as instructed by the manufacturer. All assays were performed in duplicate, using 2-3 quality control samples dispersed throughout the experimental samples and inter-assay coefficients of variation for T, PRL, E2, EN, and TSH were 6.3%, 7.0%, 6.0%, 6.3%, and 4.2%, respectively..
2.7 Histopathology
The fixed VP and LP were processed by routine methods and embedded in a paraffin block. Each block was then step-sectioned; one 5 μm section was retained every 20-25 sections to provide five sections of each of the lateral and ventral lobes of the prostate for staining with hematoxylin and eosin. Each slide was examined microscopically by three pathologists without knowledge of treatment and because the inflammation involved both the VP and LP, the lobes were graded as a whole. Severity of inflammation was scored as follows: 1 - minimal multifocal interstitial infiltration of inflammatory cells involving less than 10% of the gland, 2 – multifocal to coalescing moderate interstitial inflammation affecting 10-25% of the gland, with or without focally marked accumulations of perivascular collections of inflammatory cells or mild fibrosis, 3 – multifocal, marked interstitial inflammation involving 25-75% of the gland, with or without focally marked accumulations of perivascular collections of inflammatory cells, moderate fibrosis, or sloughing and necrosis of the epithelial lining with intraluminal accumulations of neutrophils (micro-abscesses), or 4- diffuse and extensive interstitial inflammation involving > 75% of the gland often with interstitial fibrosis and occasional microabscesses.
A section of the VP was stained for the presence of proliferating cell nuclear antigen (PCNA). At 200X magnification, ten random images of the VP were selected from each animal. It was determined that there was an average of 79.3 countable nuclei present in each image. Therefore, 800 nuclei per animal were used as the denominator for calculation of the labeling indices. A nucleus was considered positive if it stained moderately dark brown or darker. Mitotic figures were also included in the count for the numerator.
2.8 Statistical Analysis
Unless otherwise indicated, treated dams were utilized as the unit of measure for endpoints measured prior to and including PPS. Data were analyzed for effects by analysis of variance (ANOVA) using the general linear model (GLM) or mixed model in SAS 9.1 (SAS Institute, Inc., Cary, NC). Replicate (block) effects were assessed for each parameter and no block effect was found to exist outside of block two PRL data, which is addressed in section 3.4. Body weight was utilized as a covariate where appropriate. Means were evaluated and treatment groups were compared to the control group using Dunnett's multiple comparisons post-hoc test. Treatment groups were evaluated independently of positive controls to conserve linearity. Prostate pathology incidence and severity scores were analyzed for dose-dependent associations in a contingency table using Mantel-Haenszel chi-square analysis and Wilcoxon non-parametric analysis, respectively. All data are reported as means ±standard error and p<0.05 was used to indicate significant differences.
3. Results
3.1 Maternal weight gain
Maternal weight gain of 100 mg ATR dams (21.2 ±7.7 g) during the dosing period was significantly lower than controls (54.8 ±2.5 g; p<0.05). Unlike the ATR exposure, AMM treated dams had a mean weight gain that was 4-12% more than vehicle controls, but this difference was not significant (see also [24]).
3.2 Offspring Body Weights
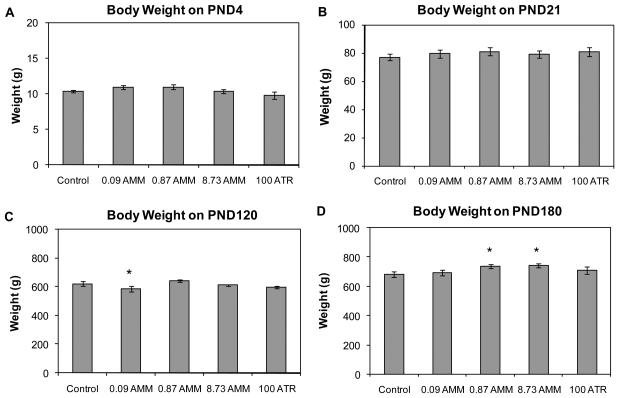
There were no significant differences in the litter size of treated dams compared to control dams and fetal (GD20) BW of males in the 8.73 mg AMM treatment group was significantly greater than that of control males (data is reported in [24]). No statistically significant differences in body weight were observed between control males and males in any treatment group during the lactational period (PND4 or PND21; Figure 3). On PND120, male offspring of the 0.09 mg AMM treatment group (586.7 ±19.0 g; p=0.05) weighed significantly less than male offspring of the control group (622.1 ±15.5 g); an effect not present in that treatment group on PND180. On PND180, male offspring in the 0.87 mg AMM treatment group (739.1 ±13.2 g; p=0.05) and in the 8.73 mg AMM treatment group (744.1 ±14.6 g; p<0.05) weighed significantly more than controls (681.8 ±21.9 g). Besides the single exception of the 0.09 mg AMM group on PND120, the male offspring were similar in weight to controls until PND180, at which time the two highest AMM dose groups were significantly elevated above control levels.
Figure 3.
Body weight of male offspring exposed to vehicle (control), AMM or positive control (100 ATR) on (A) PND4, (B) PND21, (C) PND120, and (D) PND180. Data are presented as means ± SE for respective treatment group. * Significantly different from control by Dunnett's multiple comparison (p<0.05). n > 10 dam/group for PND4 and 21; PND120 n = 10, 12, 13, 14, 8, respectively; PND180 n = 21, 18, 21, 19, 16, respectively.
3.3 Preputial Separation
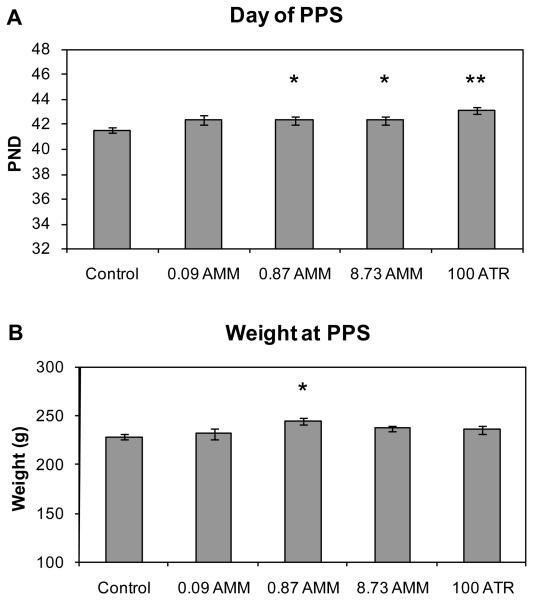
Preputial separation was significantly delayed in male offspring of the 0.87 mg AMM (42.3 ±0.3 d, p<0.05), 8.73 mg AMM (42.3 ±0.3 d, p<0.05) and 100 mg ATR (43.1 ±0.3 d, p<0.01) treatment groups compared to control males (41.5 ±0.2 d) (Figure 4). Although the mean day of PPS was the same for all AMM-exposed offspring, the delay in PPS was only nearly statistically significant in males of the 0.09 mg AMM treatment group (42.3 ±0.4 d, p=0.06) due to the slight increase in variability of response in that group. Weight at PPS was significantly greater in males of the 0.87 mg AMM treatment group (240.6 ±3.6 g, p<0.05), when compared to control males (228.8 ±3.2 g; Fig. 4B), suggesting that decrements in body weight gain in AMM-exposed males do not account for the slight delay in puberty. In addition, where PPS had not occurred by PND44 (PPS ≥ 45 days; a day randomly chosen based on the general window for PPS timing) in only 4.5% of control males, it had yet to occur by PND44 in nearly 13% or more of males in each of the AMM treatment groups and 28% of males in the ATR treatment group. All animals had achieved PPS by PND51.
Figure 4.
(A) Age in days at time of preputial separation. Data are presented as mean ± SE for the respective treatment group. Pup n = 67, 47, 64, 66, and 40, respectively. * Significantly different from control by Dunnett's multiple comparison (p<0.05). ** Significantly different from control by Dunnett's multiple comparison (p<0.01). (B) Body weight at PPS. * Significantly different from control by Dunnett's multiple comparison (p<0.05).
3.4 Hormone Measures
Because previous studies [6, 12] implicated altered maternal/pup PRL regulation as a possible mechanism of action for later life effects (including prostatitis) following ATR exposure, these studies evaluated that hormone, and others, for persistent effects that may be treatment related. The mean sPRL of all block two animals was elevated to more than three times the mean sPRL of block one and three animals, and was evident as a block effect. The elevated sPRL levels could indicate that these animals experienced environmental stress at some point just prior to necropsy and, therefore, sPRL data from block two was not considered in data analysis. Additionally, two control animals and one treated animal from block one exhibited sPRL levels>20 ng/ml, which was also could be attributed to unidentified environmental stress. These samples were considered outliers using statistical methods and were not included in data analysis. Once excluded, there were no further block effects detected in this study. Based on our results from PND120, only serum T, PRL, E2, and EN were measured on PND180. The results of hormone analyses for PND120 and PND180 are shown in Table 2.
Table 2.
Hormone Measures at Necropsy
| sT (ng/ml) |
sPRL (ng/ml) |
pPRL (μg/ml) |
sE2 (pg/ml) |
sEN (pg/ml) |
sTSH (ng/ml) |
pTSH (μg/ml) |
sLH (ng/ml) |
|
|---|---|---|---|---|---|---|---|---|
| PND120 | ||||||||
| Control | 1.48 ± 0.15 | 2.71 ± 0.51 | 2.91 ± 0.35 | 17.8 ± 1.0 | 39.4 ± 2.9 | 3.19 ± 0.31 | 0.179 ± 0.013 | 0.159 ± 0.009 |
| 0.09 AMM | 1.75 ± 0.18 | 2.80 ± 0.48 | 2.39 ± 0.74 | 19.0 ± 1.2 | 48.3 ± 4.1 | 2.84 ± 0.26 | 0.173 ± 0.016 | 0.195 ± 0.045 |
| 0.87 AMM | 1.89 ± 0.24 | 2.77 ± 0.34 | 3.93 ± 0.88 | 20.2 ± 1.0 | 49.8 ± 3.1 | 3.11 ± 0.48 | 0.188 ± 0.017 | 0.164 ± 0.015 |
| 8.73 AMM | 1.83 ± 0.21 | 3.11 ± 0.48 | 3.35 ± 0.63 | 18.6 ± 1.2 | 50.0 ± 4.1 | 2.80 ± 0.20 | 0.183 ± 0.014 | 0.158 ± 0.009 |
| 100 ATR | 2.37 ± 0.38* | 3.08 ± 0.74 | 1.89 ± 0.21 | 19.7 ± 2.9 | 55.3 ± 4.6* | 3.23 ± 0.57 | 0.172 ± 0.022 | 0.205 ± 0.026 |
| PND180 | ||||||||
| Control | 2.72 ± 0.65 | 4.46 ± 0.69 | 32.8 ± 2.6 | 46.4 ± 7.0 | ||||
| 0.09 AMM | 2.62 ± 0.53 | 5.14 ± 0.81 | 22.1 ± 2.1* | 27.1 ± 2.2 | ||||
| 0.87 AMM | 1.50 ± 0.32 | 5.44 ± 1.46 | 19.2 ± 2.2** | 36.5 ± 4.0 | ||||
| 8.73 AMM | 1.09 ± 0.18** | 2.78 ± 0.51 | 20.9 ± 1.9** | 43.9 ± 13.9 | ||||
| 100 ATR | 1.41 ± 0.41 | 4.50 ± 1.04 | 19.6 ± 1.8* | 24.5 ± 4.2* |
Note: sT, serum testosterone; sPRL, serum prolactin; pPRL, pituitary prolactin; sE2, serum estradiol; sEN, serum estrone; sTSH, serum thyroid-stimulating hormone; pTSH, pituitary thyroid-stimulating hormone; sLH, serum luteinizing hormone; PND, postnatal day; AMM, atrazine metabolite mixture; ATR, atrazine. n ≥ 10 for all treatment groups.
Significantly different from control by t-test comparison (p < 0.05)
Significantly different from control by Dunnett's multiple comparison (p < 0.01).
On PND120, males of the 100 mg ATR treatment group had significantly higher mean sT (2.37 ±0.38 ng/ml, p<0.01) and sEN (55.3 ±4.6 pg/ml, p<0.05), compared to controls (1.48 ±0.15 ng/ml and 39.4 ±2.9 pg/ml, respectively). Although these were the only significant differences noted on PND120, all treatment groups exhibited a non-significant trend of elevated sT, sPRL, sE2, and sEN. Conversely, there was a significant treatment-related decrease in sT and sE on PND180. The mean sT of 8.73 mg AMM males (1.09 ±0.18 ng/ml, p<0.05) was significantly lower than controls (2.72 ±0.65 ng/ml) and mean sE2 was significantly lower (p<0.05-0.01) in all treatment groups compared to controls. Despite these significant changes in hormones levels, the patterns of which were not consistent over time, nearly all mean hormone concentrations were within the normal reported ranges for the respective hormones measured. These data do not support an association between altered serum or pituitary PRL concentrations and prenatal exposure to ATR or AMM in LE rats.
3.5 Necropsy observations and analyses
Tissue weights at PND120 and PND180 are summarized in Table 1. AP weight on PND120 was significantly greater in 0.87 AMM-exposed (11.72 ± 0.29 mg, p<0.01) and 100 mg ATR-exposed (11.82 ± 0.49 mg, p<0.01) males, compared to control males (10.11 ± 0.37 mg). Neither mean testes nor mean SV weights were significantly different in exposed males of any treatment group compared to controls on PND120. The mean VP weight of 8.73 mg AMM-exposed males (410.5 ± 19.1 mg, p<0.05) was significantly less compared to the control mean (473.6 ± 19.1 mg), while statistical analysis of a treatment-associated decrease in VP weight in AMM exposed males on PND 120 resulted in p=0.06. There were no statistical differences in mean LP weight between any treatment group and controls on either PND120 or PND180. No significant differences in mean tissue weights of treated males compared to control males were observed on PND180.
Table 1.
Reproductive tissue weights
| BW (g) | AP (mg) | Testes (g) | LP (mg) | VP (mg) | SVd (mg) | |
|---|---|---|---|---|---|---|
| PND120 | ||||||
| Control | 622 ± 16 | 10.11 ± 0.37 | 3.81 ± 0.11 | 144.5 ± 5.3 | 473.6 ± 19.1 | 634.9 ± 24.6 |
| 0.09 AMM | 587 ± 19* | 11.19 ± 0.35 | 3.82 ± 0.08 | 147.5 ± 7.6 | 448.1 ± 21.3 | 580.0 ± 22.8 |
| 0.87 AMM | 646 ± 8 | 11.72 ± 0.29** | 3.85 ± 0.10 | 152.9 ± 6.1 | 440.0 ± 20.4 | 614.5 ± 21.8 |
| 8.73 AMM | 613 ± 6 | 11.07 ± 0.23 | 3.76 ± 0.09 | 154.4 ± 5.8 | 410.5 ± 19.1* | 612.1 ± 23.0 |
| 100 ATR | 600 ± 16 | 11.82 ± 0.49** | 3.93 ± 0.06 | 154.1 ± 7.7 | 467.9 ± 25.9 | 608.4 ± 32.2 |
| PND180 | ||||||
| Control | 682 ± 22 | 12.86 ± 1.02 | 3.82 ± 0.13 | 164.9 ± 12.6 | 472.0 ± 26.7 | 648.0 ± 57.8 |
| 0.09 AMM | 693 ± 19 | 12.33 ± 0.77 | 3.96 ± 0.17 | 172.2 ± 11.9 | 479.2 ± 34.2 | 763.8 ± 74.5 |
| 0.87 AMM | 739 ± 13 | 10.81 ± 0.80 | 4.17 ± 0.09 | 192.6 ± 13.5 | 524.5 ± 32.3 | 698.0 ± 72.2 |
| 8.73 AMM | 744 ± 15 | 11.95 ± 0.42 | 3.98 ± 0.10 | 180.8 ± 8.9 | 498.3 ± 35.0 | 701.0 ± 78.5 |
| 100 ATR | 709 ± 21 | 12.56 ± 0.90 | 4.05 ± 0.13 | 176.8 ± 15.0 | 450.5 ± 41.0 | 661.2 ± 82.9 |
Note: AP, anterior pituitary; LP, lateral prostate; VP, ventral prostate; SV, seminal vesicle; PND, postnatal day; AMM, atrazine metabolite mixture; ATR, atrazine. Body weight was utilized as a covariate for these data when interactions were present. n ≥ 20 for all treatment groups.
Significantly different from control by Dunnett multiple comparison (p < 0.05)
Significantly different from control by Dunnett multiple comparison (p < 0.01).
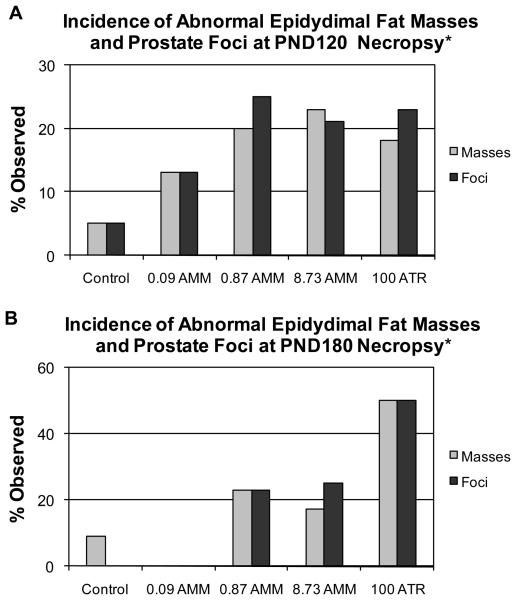
There was a significant dose-dependent increase in the percentage of males exhibiting lipomatous epididymal fat masses collected at necropsy at both PND120 and PND180 (p<0.05; Figure 5). These masses were followed up with histopathologic evaluation, the results of which are reported below. There was also a significant increase in the incidence of pale prostatic foci of discoloration (presumptive inflammatory cell infiltrates) noted in the exposed males at necropsy at both at 120 and 180 days (p<0.05; Figure 5).
Figure 5.
Percentage of animals exhibiting abnormal epididymal fat masses and pale prostatic foci at necropsy on A) PND120 (n = 10, 12, 13, 14, 8, respectively) and B) PND180 (n = 21, 18, 21, 19, 16, respectively). * Significant treatment related effect on the gross observation of masses and foci at each time point by chi-square analysis (p<0.05).
3.6 Histopathology
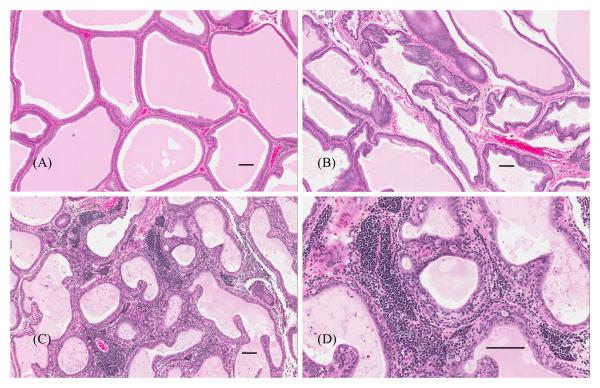
There was a significant dose-dependent increase in prostate inflammation in AMM-exposed offspring on PND120 and the severity of prostate inflammation on PND120 was significantly greater in male offspring of all AMM exposure groups compared to controls (p<0.05; Table 3). The inflammation involved both the lateral prostate and ventral prostate, and was not specific to particular regions of a lobe. Overall, prostate effects included cell infiltrates that were multifocal to regionally extensive and comprised primarily by mature lymphocytes with occasional plasma cells, macrophages, mast cells, and neutrophils. The PND120 prostate effects were typically characterized by multifocal to coalescing moderate interstitial inflammation affecting 10-25% of the gland, with or without focally marked accumulations of perivascular collections of inflammatory cells with minimal to moderate fibrosis (Figure 6). In addition to the significant increase in incidence of prostatitis in the ATR-exposed males, both the mean overall severity scores and severity scores of affected males on PND120 were greater in all AMM treatment groups than those of the ATR exposed group (Table 3). The significance of effect noted in incidence, overall severity scores, and severity among affected animals in PND 120 males was not continued on PND180 (Table 4). The effects in exposed animals appeared to dissipate over time while the control group did not vary between time points.
Table 3.
Prostate Pathology on PND 120
| Incidence of inflammation* |
Severity (affected) | |
|---|---|---|
| Control | 12/25 (48%) | 0.6 ± 0.2 (1.3 ± 0.2) |
| 0.09 AMM | 14/21 (67%) | 1.4 ± 0.3 (2.1 ± 0.2)** |
| 0.87 AMM | 19/25 (76%) | 1.4 ± 0.2 (1.8 ± 0.2)** |
| 8.73 AMM | 20/25 (80%) | 1.5 ± 0.2 (1.9 ± 0.2)** |
| 100 ATR | 17/21 (81%) | 1.2 ± 0.2 (1.5 ± 0.2) |
Note: PND, postnatal day; AMM, atrazine metabolite mixture; ATR, atrazine. Data represent incidence of alteration in the ventral and lateral prostate combined, followed by the mean severity score ± SE (mean severity score ± SE for the subset of animals with the alteration).
Significant dose-dependent increase in the incidence of inflammation by Mantel-Haenszel chi-square analysis (p<0.05).
Significantly higher severity of inflammation compared to controls by ANOVA (p<0.05).
Figure 6.
Histological sections of lateral prostate, hematoxylin and eosin (HE) stain. Scale bars are 100 μm. A) Unaffected prostate of control male on PND120 (score=0, unaffected). B) Lateral prostate section of offspring in the 0.09 mg AMM treatment group on PND120 with severity score=1; minimal multifocal interstitial infiltration of inflammatory cells involving less than 10% of the gland. C&D) Lateral prostate section of offspring in the 8.73 mg AMM treatment group on PND120 at low and high magnification, respectively, demonstrating prostatitis with predominant lymphoid infiltrate (severity score=4; diffuse and extensive interstitial inflammation involving > 75% of the gland often with interstitial fibrosis).
Table 4.
Prostate Pathology on PND 180
| Incidence of inflammation |
Severity (affected) | |
|---|---|---|
| Control | 11/20 (55%) | 0.7 ± 0.2 (1.3 ± 0.1) |
| 0.09 AMM | 4/18 (22%) | 0.4 ± 0.2 (2.0 ± 0.4) |
| 0.87 AMM | 13/21 (62%) | 0.8 ± 0.2 (1.2 ± 0.1) |
| 8.73 AMM | 11/19 (58%) | 0.9 ± 0.2 (1.6 ± 0.2) |
| 100 ATR | 6/16 (34%) | 0.7 ± 0.3 (1.8 ± 0.3) |
Note: PND, postnatal day; AMM, atrazine metabolite mixture; ATR, atrazine. Data represent incidence of alteration in the ventral and lateral prostate combined, followed by the mean severity score ± SE (mean severity score ± SE for the subset of animals with the alteration).
Although there was a high incidence and degree of inflammation in the prostate, there was no indication of progression to neoplasia at PND 120 or 180 in tissues of control or AMM or ATR-exposed males. No significant difference was observed in the percentage of proliferating epithelial cells in PND120 prostate sections. On PND180, only prostate sections of the 0.09 mg AMM treatment groups exhibited significantly more labeled epithelial cells in the prostate than controls (data not shown).
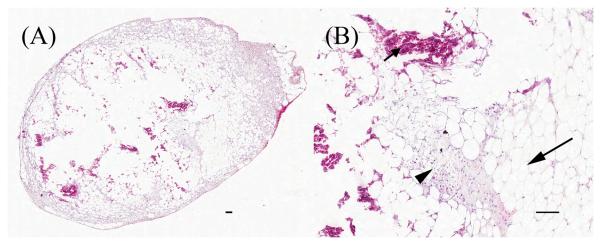
Although gross characterization of the epididymal fat masses suggested a possible differential diagnosis of lipoma, histological analysis demonstrated multifocal fat necrosis and infarction of fat lobules with associated chronic histiocytic steatitis and chronic hemorrhage of epididymal fat (Figure 7). The exact cause is not apparent but considerations would include vascular compromise, local trauma, or steatitis related to a systemic disease such as uremia, autoimmunity, or pancreatitis, though no information was available in the present study to confirm or refute this (data not shown).
Figure 7.
Histological section of abnormal epididymal fat mass, H&E stained. Scale bars are 100 μm. A) Low magnification of mass from 8.73mg AMM treatment group. B) High magnification of (A). Larger arrow indicates necrotic fat, smaller arrow indicates hemorrhage, and arrowhead indicates histiocytic steatitis.
4. Discussion
This is, to our knowledge, the first study to report the effects of exposure to a biologically relevant mixture of ATR and its environmental metabolites on reproductive development in the male rat. The effective AMM concentrations utilized in this study, administered during a critical period of male reproductive tract development, are considerably lower than the current no observed adverse effect level (NOAEL) for ATR derived from previous studies that focused on male reproductive end points. Our key findings were significant treatment-related delays in PPS and increased incidence and severity of prostatitis on PND120 following a 5-day in utero exposure to the AMM. The fact that these health effects were treatment-related suggests that the AMM may be as potent as or more so than ATR alone.
The data reported here are from the male siblings of females studied by Enoch et al. [24]. In that report, significant treatment-related increases in female offspring BW on PND4 in all AMM doses tested and on PND60 in 0.87 and 8.73 mg AMM exposed offspring were described. In the current study, there was no significant increase in male offspring BW on PND4, PND21, or PND120, but BW was significantly greater than controls in the 0.87 mg AMM and 8.73 mg AMM treatment groups on PND180. Thus, in contrast to ATR alone, this low dose AMM not only had no deleterious effect on BW, but BW was in fact elevated in both male and female offspring at the same doses, though there were variations in the rate of the response across gender.
In the present study, there was a significant, albeit marginal, delay in PPS in males of the two highest AMM treatment groups, and a nearly significant delay (p<0.06) in males of the lowest AMM treatment group. While the biological significance of a less than one day delay in PPS is not clear, these data, in accordance with the Tier 1 Screening Battery of the U.S. EPA Endocrine Disruptor Screening Program Test Guidelines [32], indicate that PPS is clearly a particularly sensitive endpoint following developmental exposure to ATR and its metabolites and that an AMM treatment effect does exist at low levels. Further, the observation of pubertal delays in the absence of any significant reductions in peripubertal BW are consistent with studies reviewed in Cooper et al. [4] where both male and female pubertal onset was delayed while BW was increased following early-life exposure to ATR or its individual metabolites. Data in the present study, Enoch et al. [24], and of those described in Cooper et al. [4] suggest that significant effects on pubertal timing following developmental exposures to low levels of ATR, its individual metabolites, or the AMM are not associated with peripubertal BW effects. To this point, the later life ramifications of delayed puberty and the mechanism(s) of action driving this response are not known.
In addition to delayed puberty, treatment-dependent alterations in the weights of other reproductive tissues have been reported in male rats following prenatal [14] and peripubertal [33] exposure to ATR and following peripubertal exposure to individual ATR metabolites [23]. However, whereas puberty was mildly delayed by biologically relevant levels of the metabolite mixture in the present study, there were no persistent effects on reproductive tract or other tissue weights, a characteristic that has been observed in at least one other study which utilized similar doses of ATR (0 to 100 mg/kg/d) and a similar dosing period (GD 14-parturition) [34]. While PPS, normally androgen dependent, was delayed in the Rosenberg study, there were no concomitant changes in intratesticular testosterone (iT) concentrations. A similar effect was also observed in the present study in which there were no significant changes in sT in males of the AMM treatment groups on PND120 and only in males in the highest AMM treatment group was sT reduced on PND180. Interestingly, in males of the 100 mg ATR treatment group, where PPS was delayed at the p<0.01 level, sT was actually elevated on PND120. However, in that treatment group, no T-regulated tissue weights were altered; only mean AP weight was significantly greater than that of controls. Trentacoste et al. [33] reported decreased SV and VP weights in Sprague-Dawley rats exposed to 100 mg ATR/kg BW peripubertally, but also reported decreased iT and sT, a correlation not observed in the present study. These differences may be due entirely to the timing of exposure, as the animals in the Trentacoste study were dosed peripubertally and not gestationally, as were those in the present study. While the absence of persistent effects on male reproductive tract tissue weights in the present study is certainly understandable considering the lack of changes in circulating hormone concentrations, there does not appear to be a relationship between AMM or ATR-induced pubertal delays and changes in male reproductive tract tissue weights or circulating hormone concentrations.
It is well known that hormonal changes can influence prostate development and a number of studies indicate that PRL in particular stimulates prostatic growth in rats [35-38]. Although there were significant treatment-related effects on the incidence of prostate inflammation and severity in the current study, there were no concomitant changes in circulating hormone concentrations that would suggest a mode of action for prostate effects. In addition to hormonal changes, timing of exposure can also impart late-life prostate effects and a number of studies have described a critical window for male reproductive tract development following exposure to various environmental contaminants [39-42]. The window of sensitivity for prostate development occurs on GD14-19, when androgen receptors are activated and the testes begin producing androgens [41, 42] and the animals utilized in the present study were exposed to the AMM on GD15-19. Atrazine has been shown to have an anti-androgenic effect through suppression of the conversion of testosterone to 5α-dihyrdrotestosterone (DHT) in the hypothalamus, anterior pituitary, and prostate [43] and that 5α-DHT prostate receptors are strongly inhibited in offspring of dams exposed to ATR and DEA during pregnancy [44]. In addition, Babić-Gojmerac et al. [45] demonstrated that DEA, a component of the AMM, inhibited 5α-reductase at the same rate as ATR in vivo and at a greater rate than ATR in vitro. Though DEA is likely less toxic due to rapid metabolism and biodegradation, the metabolic product, DACT, is also a component of the AMM and has been to shown have developmental effects similar to that of ATR [22, 23]. These observations suggest that GD15-19 is an effective window for AMM exposure to elicit prostate effects and that other hormones, in addition to those measured in the present study, be investigated for a potential role in a mode of action for prostate effects. Furthermore, we suggest that hormones which may be affecting these developmental outcomes should be evaluated during earlier stages of life, as persistent effects have been absent in both of our studies [present, 14].
An elevated incidence of lipomatous nodules was indicated in AMM/ATR-exposed rats. Similar fat nodules have been commonly observed in dogs, horses, and humans. The pale prostatic foci observed at necropsy on the outer edges of the tissue indicated focal regions of inflammation. While there is an evident AMM-related effect on the incidence and severity of inflammation in the prostate on PND120, this was not the case over time as these effects had dissipated by PND180. It is possible that the appropriate time point necessary to observe related changes pertinent to the inflammatory effects was not assessed. In a preliminary study, PND120 males in the 8.73 mg AMM treatment group, which had the most severe prostate effects, exhibited the highest concentrations of serum IL-1α and IL-6 (data not shown), two interleukins known to have roles in the resolution of inflammation [46]. Cowin et al. [41] also observed increased activation of nuclear factor-kappa B-dependent genes, including IL-1α and IL-6, in prostates of Sprague-Dawley rats exposed in utero on GD14-19 to vinclozolin. This would suggest that the prostate lesions could have been resolving or under repair and it is possible that had necropsy been conducted earlier than PND120 in the present study, the severity might have been greater, a more prominent treatment effect would have been discerned, and/or exposure-related effects (changes in hormones or tissue weight) would have been detected. Further, this study has added to the knowledge base the fact that low dose, biologically relevant exposures to ATR and its metabolites induce prostate inflammation following early life, acute exposures and this study and others [12, 14] confirm that these lesions show no signs of progression to neoplasm at the time points evaluated and in a strain of rat that is fairly resistant to tumor formation.
The present study, using the lowest effective concentrations of AMM, suggests that late prenatal exposure to a mixture of atrazine and its metabolites significantly delays puberty (albeit modest compared to high dose ATR) and can affect adult prostate incidence and severity of inflammation in Long-Evans rats. The low doses and short duration of exposure may be the reason that typical dose-related increases in many of the responses were limited and these findings may be exacerbated by extended exposure. However, the fact that statistically significant low-dose responses were evident following prenatal exposure to metabolites of chlorotriazine herbicides is noteworthy as these low dose effects may stem from the late prenatal time of exposure, during which growth and development of male reproductive organs are particularly sensitive to environmental agents. The importance of these findings on late life health effects is not known, as no report to date has extended past those reported in Rayner et al. [14] or herein, but evidence in this study supports the notion that time points earlier than 120d may be more important than those in late life with respect to a mechanism of AMM effect on male reproductive development. The effects reported here occurred with no decrease in body weight at puberty, as has been previously reported for ATR alone [13, 14] and puberty was significantly delayed at 0.87 mg AMM which is lower than the current NOAEL for ATR or DACT (6.25 mg/kg/day total dose [28]). Additionally, effects were consistently observed in the AMM treatment groups, where the highest effective concentration (8.73 mg AMM/kg BW) contains only 1.79 mg ATR/kg BW. These studies suggest that low dose exposures to the chlorinated metabolites of ATR, as a mixture, may elicit effects that are relevant to human health, in a manner similar to high dose ATR exposure in male rats exposed just prior to birth.
ACKNOWLEDGEMENTS
We appreciate the excellent assistance provided by Angela Buckalew, Keith McElroy (RIAs), Alvin Moore, Faye Poythress, and Arthur Walden (New Year Tech), Dr.'s Abraham Nyska and Gordon Flake (NIEHS, RTP, NC, histopathology and PCNA), Judy Schmid (statistics), Mike Narotsky (mixture development advice), and Gary Klinefelter (constructive manuscript comments) and Experimental Pathology Laboratories (Durham, NC, contract necropsy assistance).
FUNDING AND DISCLAIMER Financial support for Rolondo Enoch by U.S. EPA/NCCU Cooperative Research Training Grant No. CT829460, North Carolina Central University, Durham, NC 27707 and for Jennifer Rayner by U.S. EPA, NHEERL-DESE Cooperative Training Agreement No. CT826513, University of North Carolina, Chapel Hill, NC 27599. The U.S. Environmental Protection Agency and the Division of Intramural Research of the NIH, National Institute of Environmental Health Sciences have funded the studies in this document. The contents do not necessarily reflect the views of the Agency or the Institute, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. Portions of these data were presented at the Society for the Study of Reproduction meeting in San Antonio, TX, July 2007.
Abbreviations
- AME
atrazine molar equivalents
- AMM
atrazine metabolite mixture
- ANOVA
analysis of variance
- AP
anterior pituitary
- ATR
atrazine
- BW
body weight
- DACT
diaminochlorotriazine
- DEA
deethylatrazine
- DIA
deisopropylatrazine
- DHT
5α-dihydrotestosterone
- E2
estradiol
- EN
estrone
- GD
gestation day
- HA
hydroxyatrazine
- LE
Long Evans
- LH
luteinizing hormone
- LOAEL
Lowest observable adverse effect limit
- LP
lateral prostate
- MCL
maximum contamination level
- NOAEL
no observable adverse effect limit
- PND
postnatal day
- PPS
preputial separation
- PRL
prolactin
- SEM
standard error of the mean
- SVd
dry seminal vesicle
- T
testosterone
- TSH
thyroid stimulating hormone
- VP
ventral prostate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST The authors declare they have no conflicting financial interests.
REFERENCES
- 1.U.S. EPA . Pesticides Industry Sales and Usage. 2000 and 2001 Market Estimates. EPA-733-R-04-001. U. S. Environmental Protection Agency Office of Prevention, Pesticides, and Toxic Substances; Washington, D.C.: 2004. Available at http://www.epa.gov/oppbead1/pestsales/01pestsales/market_estimates2001.pdf. Accessed 3/3/10. [Google Scholar]
- 2.Giddings JM, Anderson TA, Hall LW, Jr, Hosman AJ, Kendall RJ, Richards RP, et al. In Atrazine in North American Surface Waters: A Probabilistic Aquatic Ecological Risk Assessment. SETAC Press; Pensacola, FL.: 2005. pp. 108–112. [Google Scholar]
- 3.Sass JB, Colangelo A. European Union bans atrazine, while the United States negotiates continued use. Int J Occup Environ Health. 2006;12(3):260–7. doi: 10.1179/oeh.2006.12.3.260. [DOI] [PubMed] [Google Scholar]
- 4.Cooper RL, Laws SC, Das PC, Narotsky MG, Goldman JM, Tyrey L, Stoker TE. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):98–112. doi: 10.1002/bdrb.20110. [DOI] [PubMed] [Google Scholar]
- 5.Dooley GP, Prenni JE, Prentiss PL, Cranmer BK, Andersen ME, Tessari JD. Identification of a novel hemoglobin adduct in Sprague Dawley rats exposed to atrazine. Chem Res Toxicol. 2006;19(5):692–700. doi: 10.1021/tx060023c. [DOI] [PubMed] [Google Scholar]
- 6.Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol Sci. 2000;53(2):297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- 7.Laws SC, Ferrell JM, Stoker TE, Schmid J, Cooper RL. The effects of atrazine on female Wistar rats: an evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol Sci. 2000;58(2):366–76. doi: 10.1093/toxsci/58.2.366. [DOI] [PubMed] [Google Scholar]
- 8.Cooper RL, Stoker TE, Goldman JM, Parrish MB, Tyrey L. Effect of atrazine on ovarian function in the rat. Reprod Toxicol. 1996;10(4):257–64. doi: 10.1016/0890-6238(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 9.Eldridge JC, Wetzel LT, Stevens JT, Simpkins JW. The mammary tumor response in triazine-treated female rats: a threshold-mediated interaction with strain and species-specific reproductive senescence. Steroids. 1999;64(9):672–8. doi: 10.1016/s0039-128x(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 10.Wetzel LT, Luempert LG, 3rd, Breckenridge CB, Tisdel MO, Stevens JT, Thakur AK, Extrom PJ, Eldridge JC. Chronic effects of atrazine on estrus and mammary tumor formation in female Sprague-Dawley and Fischer 344 rats. J Toxicol Environ Health. 1994;43(2):169–82. doi: 10.1080/15287399409531913. [DOI] [PubMed] [Google Scholar]
- 11.Rayner JL, Wood C, Fenton SE. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol Appl Pharmacol. 2004;195(1):23–34. doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Stoker TE, Robinette CL, Cooper RL. Maternal exposure to atrazine during lactation suppresses suckling-induced prolactin release and results in prostatitis in the adult offspring. Toxicol Sci. 1999;52(1):68–79. doi: 10.1093/toxsci/52.1.68. [DOI] [PubMed] [Google Scholar]
- 13.Stoker TE, Guidici DL, Laws SC, Cooper RL. The effect of atrazine on puberty in male wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol Sci. 2000;58(1):50–9. doi: 10.1093/toxsci/58.1.50. [DOI] [PubMed] [Google Scholar]
- 14.Rayner JL, Enoch RR, Wolf DC, Fenton SE. Atrazine-induced reproductive tract alterations after transplacental and/or lactational exposure in male Long-Evans rats. Toxicol Appl Pharmacol. 2007;218(3):238–48. doi: 10.1016/j.taap.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 15.U.S. EPA . Atrazine IRED SAB report. U.S. Environmental Protection Agency; Washington, D.C.: 2003. Available at http://www.epa.gov/oppsrrd1/REDs/atrazine_ired.pdf. Accessed 3/3/10. [Google Scholar]
- 16.U.S. Geological Survey . Pesticides in the Nation's Streams and Ground Water, 1992–2001—A Summary. U.S. Geological Survey; Washington, D.C.: 2006. Available at http://pubs.usgs.gov/fs/2006/3028/. Accessed 3/3/10. [Google Scholar]
- 17.U.S. Geological Survey Watershed Regressions for Pesticides (WARP) Atrazine Model. 2009 Available at http://infotrek.er.usgs.gov/warp/. Accessed 3/3/10.
- 18.Stone W, Gilliom R. Update of Watershed Regressions for Pesticides (WARP) for Predicting Atrazine Concentration in Streams. U.S. Geological Survey; Washington, D.C.: 2009. Available at http://pubs.usgs.gov/of/2009/1122/. Accessed 3/3/10. [Google Scholar]
- 19.Shipitalo MJ, Owens LB. Atrazine, deethylatrazine, and deisopropylatrazine in surface runoff from conservation tilled watersheds. Environ Sci Technol. 2003;37(5):944–50. doi: 10.1021/es020870b. [DOI] [PubMed] [Google Scholar]
- 20.Battaglin WA, Thurman EM, Kalkhoff SJ, Porter SD. Herbicides and Transformation Products in Surface Waters of the Midwestern United States. Journal of the American Water Resources Association. 2003:743–756. [Google Scholar]
- 21.Battaglin WA, Rice KC, Focazio MJ, Salmons S, Barry RX. The occurrence of glyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa, and Wyoming, 2005-2006. Environ Monit Assess. 2009;155(1-4):281–307. doi: 10.1007/s10661-008-0435-y. [DOI] [PubMed] [Google Scholar]
- 22.Laws SC, Ferrell JM, Stoker TE, Cooper RL. Pubertal development in female Wistar rats following exposure to propazine and atrazine biotransformation by-products, diamino-S-chlorotriazine and hydroxyatrazine. Toxicol Sci. 2003;76(1):190–200. doi: 10.1093/toxsci/kfg223. [DOI] [PubMed] [Google Scholar]
- 23.Stoker TE, Guidici DL, Laws SC, Cooper RL. The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol Sci. 2002;67(2):198–206. doi: 10.1093/toxsci/67.2.198. [DOI] [PubMed] [Google Scholar]
- 24.Enoch RR, Stanko JP, Greiner SN, Youngblood GL, Rayner JL, Fenton SE. Mammary gland development as a sensitive end point after acute prenatal exposure to an atrazine metabolite mixture in female Long-Evans rats. Environ Health Perspect. 2007;115(4):541–7. doi: 10.1289/ehp.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balu K, Holden PW, Johnson LC, Cheung MW. Summary of Ciba Crop Protection groundwater monitoring study for atrazine and its degradation products in the United States. In: Coats J, Somasundaram L, editors. Pesticide Transformation Byproducts: Fate and Significance in the Environment. American Chemical Society; Washington D.C.: 1998. pp. 227–238. [Google Scholar]
- 26.U.S. Geological Survey . Water Quality Assessment Program. U.S. Geological Survey; Washington, D.C.: 1999. Available at http://water.usgs.gov/nawqa/. Accessed 3/3/10. [Google Scholar]
- 27.Wade H, Bailey C, Padmore J, Rudo K, Williams B, York A. The interagency study of the impact of pesticide use on ground water in North Carolina, Raleigh, N.C. North Carolina Pesticide Board, N.C. Department of Agriculture; Raleigh, NC: 1997. [Google Scholar]
- 28.U.S. EPA . Triazine Cumulative Risk Assessment. PC Code: 080808. DP Barcode: 317976. U.S. Environmental Protection Agency; Washington, D.C.: 2006. Available at http://www.epa.gov/oppsrrd1/REDs/triazine_cumulative_risk.pdf. Accessed 3/3/10. [Google Scholar]
- 29.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(6 Suppl):S18–24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 30.Welsh M, Saunders PT, Sharpe RM. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology. 2007;148(7):3185–95. doi: 10.1210/en.2007-0028. [DOI] [PubMed] [Google Scholar]
- 31.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- 32.U.S. EPA . Endocrine Disruptor Screening Program Test Guidelines, OPPTS 890.1500: Pubertal Development and Thyroid Function in Intact Juvenile/Peripubertal Male Rats. EPA 740-C-09-012. U.S. Environmental Protection Agency; Washington, D.C.: 2009. Available at http://www.epa.gov/oppts/pubs/frs/publications/Test_Guidelines/series890.htm. Accessed 3/3/10. [Google Scholar]
- 33.Trentacoste SV, Friedmann AS, Youker RT, Breckenridge CB, Zirkin BR. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Androl. 2001;22(1):142–8. [PubMed] [Google Scholar]
- 34.Rosenberg BG, Chen H, Folmer J, Liu J, Papadopoulos V, Zirkin BR. Gestational exposure to atrazine: effects on the postnatal development of male offspring. J Androl. 2008;29(3):304–11. doi: 10.2164/jandrol.107.003020. [DOI] [PubMed] [Google Scholar]
- 35.Grayhack JT, Bunce PL, Kearns JW, Scott WW. Influence of the pituitary on prostatic response to androgen in the rat. Bull Johns Hopkins Hosp. 1955;96(4):154–63. [PubMed] [Google Scholar]
- 36.Keenan EJ, Ramsey EE, Kemp ED. The role of prolactin in the growth of the prostate gland. Prog Clin Biol Res. 1981;75B:9–18. [PubMed] [Google Scholar]
- 37.Manandhar MS, Thomas A. Effect of prolactin on the metabolism of androgens by the rat ventral prostate gland in vitro. Invest Urol. 1976;14(1):20–2. [PubMed] [Google Scholar]
- 38.Negro-Vilar A, Saad WA, McCann SM. Evidence for a role of prolactin in prostate and seminal vesicle growth in immature male rats. Endocrinology. 1977;100(3):729–37. doi: 10.1210/endo-100-3-729. [DOI] [PubMed] [Google Scholar]
- 39.Barlow NJ, McIntyre BS, Foster PM. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicol Pathol. 2004;32(1):79–90. doi: 10.1080/01926230490265894. [DOI] [PubMed] [Google Scholar]
- 40.Carruthers CM, Foster PM. Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res B Dev Reprod Toxicol. 2005;74(3):277–85. doi: 10.1002/bdrb.20050. [DOI] [PubMed] [Google Scholar]
- 41.Cowin PA, Foster PM, Pedersen J, Hedwards S, McPherson SJ, Risbridger GP. Early-onset endocrine disruptor-induced prostatitis in the rat. Environ Health Perspect. 2008;116(7):923–9. doi: 10.1289/ehp.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf CJ, LeBlanc GA, Ostby JS, Gray LE., Jr Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicol Sci. 2000;55(1):152–61. doi: 10.1093/toxsci/55.1.152. [DOI] [PubMed] [Google Scholar]
- 43.Kniewald J, Mildner P, Kniewald Z. Effects of s-triazine herbicides on hormone-receptor complex formation, 5 alpha-reductase and 3 alpha-hydroxysteroid dehydrogenase activity at the anterior pituitary level. J Steroid Biochem. 1979;11(1C):833–8. doi: 10.1016/0022-4731(79)90018-9. [DOI] [PubMed] [Google Scholar]
- 44.Kniewald J, Peruzović M, Gojmerac T, Milković K, Kniewald Z. Indirect influence of s-triazines on rat gonadotropic mechanism at early postnatal period. J Steroid Biochem. 1987;27(4-6):1095–100. doi: 10.1016/0022-4731(87)90195-6. [DOI] [PubMed] [Google Scholar]
- 45.Babic-Gojmerac T, Kniewald Z, Kniewald J. Testosterone metabolism in neuroendocrine organs in male rats under atrazine and deethylatrazine influence. J Steroid Biochem. 1989;33(1):141–6. doi: 10.1016/0022-4731(89)90369-5. [DOI] [PubMed] [Google Scholar]
- 46.Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol. 2003;58(1):1–26. doi: 10.1016/s0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]