Abstract
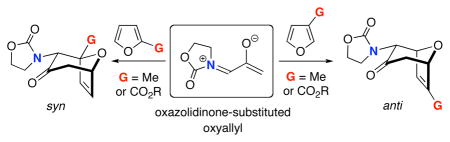
The (4+3) cycloadditions of oxazolidinone-substituted oxyallyls and unsymmetrically-substituted furans lead to syn regioselectivity when the furan has a 2-Me or 2-COOR substituent, while anti regioselectivity is obtained with a 3-Me or 3-COOR group. DFT calculations are performed to explain the selectivities. The reactivities and regioselectivities are consistent with the ambiphilic reactivity of amino-oxyallyls with furans.
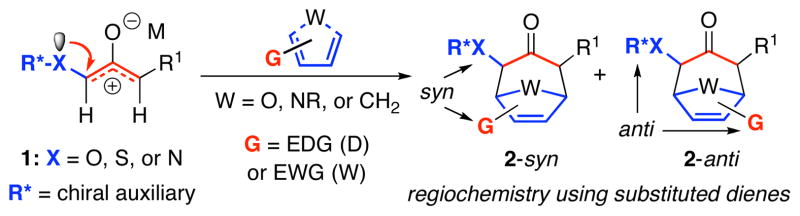
The (4+3) cycloaddition of oxyallyls with dienes (Scheme 1) is a useful route to seven-membered carbocycles.1 Theoretical studies have examined the mechanisms of these cycloadditions,2 which may be either stepwise or concerted depending on the groups X and M in 1. The use of a heteroatom-stabilized oxyallyl, in particular an oxygen-, nitrogen-, or sulfur-derivative, in conjunction with an unsymmetrical diene has often been reported to provide high levels of regioselectivity (2-syn vs 2-anti).1,3 However, there have to date been few systematic studies of how the regioselectivity for a given class of oxyallyl is influenced by the substituents on the diene.
Scheme 1.
Regioselectivities in (4+3) Cycloadditions of Oxyallyls with Substituted Dienes.
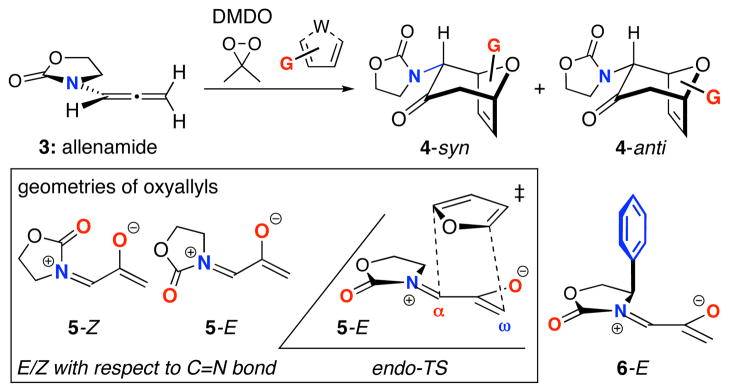
We have previously reported a method for (4+3) cycloadditions that commences with the oxazolidinone-containing allenamide 3 (Scheme 2).4 Oxidation of 3 by dimethyldioxirane (DMDO) in the presence of a furan furnishes selectively the endo5 cycloadduct 4, and can be performed successfully with either electron-rich (Me) or electron-poor (COOR) groups on the furan. The cycloaddition is believed to involve the oxyallyl 5, and is promoted by a Lewis acid (ZnCl2). We recently studied the electronic structures of the oxyallyls by density functional theory calculations on 6.6 They are zwitterions (unlike the parent oxyallyl, which is a diradical7), and there is substantial electron delocalization from the nitrogen onto the allyl group, consistent with an iminium enolate structure. The (4+3) cycloadditions of 5 and 6 with furan are calculated to be concerted processes. Only the EC=N isomer of the oxyallyl (5-E, 6-E) is involved, because the ZC=N isomer (5-Z) is destabilized by electrostatic repulsion between the oxygen atoms, even when coordinated to ZnCl2.
Scheme 2.
(4+3) Cycloadditions of Allenamide-Derived Oxyallyls.
The methodology in Scheme 2 has previously been applied to a variety of asymmetric cycloadditions.4,8 Here we present an experimental and theoretical study of the regioselectivity of the achiral oxyallyl 5 towards unsymmetrical furans.
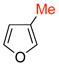
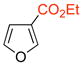
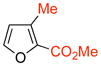
The cycloadditions of 5 with monosubstituted furans were examined first. Methyl was chosen as a representative electron-donating group, and COOMe or COOEt for the electron-withdrawing group. Cycloadditions involving the more electron-rich 2-methoxyfuran failed, due to competing oxidation and decomposition.8 The measured regioselectivities for cycloadditions of 5 with 7–12 are shown in Table 1.
Table 1.
Cycloadditions of Oxyallyl 5 with Furans 7–12.a
| furanb | additive | 4 [syn:anti ratioc] | yield (%)d |
|---|---|---|---|
|
|
– | 4a [83:17] | 90 |
| ZnCl2 | 4a [86:14] | 97 | |
|
|
– | 4b [≥95:5] | 41 |
| NaClO4e | 4b [≥95:5] | 67 | |
 9 9
|
– | 4c [13:87] | 95 |
| ZnCl2 | 4c [22:78] | 96 | |
 10 10
|
– | 4d [9:91] | 36 |
| ZnCl2 | 4d [9:91] | 53 | |
 11 11
|
ZnCl2 | 4e [≥95:5] | 65 |
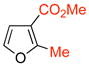 12 12
|
ZnCl2 | 4f [≥95:5] | 56 |
4.0 equiv DMDO in acetone/CH2Cl2 was added over 18 h via syringe pump to a solution of the allenamide (conc. 0.05 M) and furan in CH2Cl2 at −78 °C. Where applicable, 2.0 equiv of Lewis acid were used.
3.0 equiv of the furan were used, except for 7 and 9, where 6.0 equiv was used.
Isomer ratios were determined by 1H and/or 13C NMR.
Isolated yield.
NaClO4 gave higher yields than ZnCl2.
The cycloadditions were conducted either under thermal conditions or in the presence of a Lewis acid (ZnCl2 or NaClO4). Inclusion of the Lewis acid generally increased the yield, but did not alter the regioselectivity. Surprisingly, the regioselectivities for both the 2- and the 3-substituted furans were found to be independent of the electronic character of the substituent. Both 2-methylfuran (7) and methyl 2-furoate (8) gave predominantly the syn cycloadducts, with syn:anti ratios of 86:14 and ≥95:5, respectively. The 3-methylfuran (9) and ethyl 3-furoate (10) both gave predominantly the anti cycloadducts, with syn:anti ratios of 22:78 and 9:91, respectively.
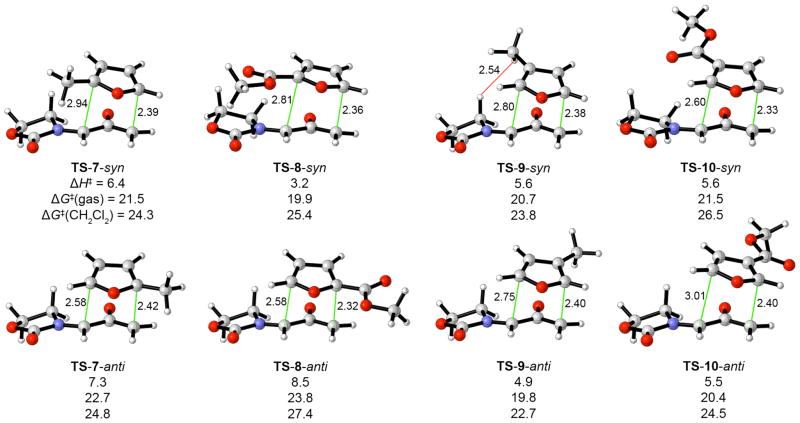
The origins of these regioselectivities were investigated with density functional theory calculations at the B3LYP/6–31G(d) level9 in Gaussian 03.10 Activation energies in CH2Cl2 were simulated by computing free energies of solvation with the Conductorlike Polarizable Continuum Model (CPCM).11 Transition states for the syn and anti cycloadditions of 5-E with furans 7–10 were located (using COOMe as a model for COOEt in 10). The TS geometries and activation energies12 are shown in Figure 1. The cycloadditions are concerted but asynchronous processes. Stepwise transition states were also located, but were at least 2.6 kcal/mol higher in energy.
Figure 1.
Transition states for the (4+3) cycloadditions of 5-E with substituted furans. Distances in Å, ΔH‡ in kcal/mol at 0 K, ΔG‡ in kcal/mol at 298.15 K and 1 mol/L.
Three of the substituted furans are predicted to react more easily than furan itself, which has an activation energy of ΔH‡ = 6.4 kcal/mol (ΔG‡ 20.8 kcal/mol) (Supporting Information). The oxyallyl is therefore ambiphilic towards furans, although the Me substituent effect is quite small and the ester substituent effect is relatively large. The energies of the HOMO and LUMO of 5-E are calculated to lie between those of furan.13 The presence of both an electron-rich O− atom and electron-withdrawing iminium group on the central carbon confers both nucleophilic and electrophilic character to the unsubstituted terminus (Cω). There is, however, only a small degree of charge transfer in any of the transition states. The oxyallyl is calculated to have a charge of −0.14e in the transition state for reaction with furan; this value increases to −0.17e for the methylfurans, and drops to −0.05e in the furoate ester TSs.14 The ambiphilicity of 5 distinguishes it from other synthetically-useful oxyallyl derivatives such as 13 and 141–3 (Scheme 3), which are cationic, electrophilic species.
Scheme 3.
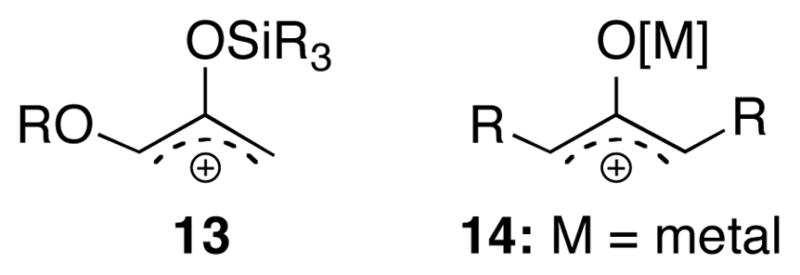
Electrophilic Oxyallyl Cations.
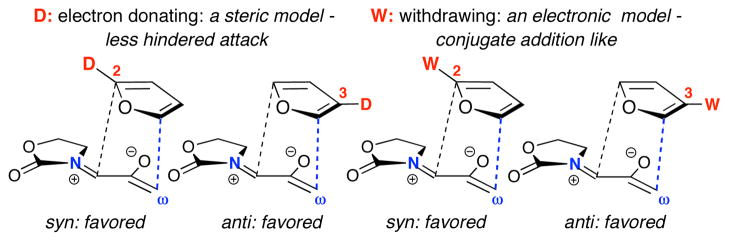
The calculated regioselectivities (ΔΔH‡ and ΔΔG‡) in Figure 1 predict the correct major product for each furan. In spite of the ambiphilic character displayed in the reactivity patterns, the transition states do all show more bonding at the enolate terminus than the iminium terminus. The ester group has a regiochemical preference consistent with electronic effects, while the regioselectivities observed for methylfurans likely result from steric effects: for 2-methylfuran, the oxyallyl attacks the less substituted (5-) terminus, while for 3-methylfuran, the favored anti TS avoids steric hindrance involving the oxazolidinone (see TS–9–syn in Figure 1). The regioselectivities are also the same as would be expected for a diradical mechanism; however, all diradical TSs that we have located15 lie at least 2.0 kcal/mol higher than the closed-shell TSs.
From our investigation of cycloadditions of 5 with the 2,3-disubstituted furans 11 and 12 (Table 1), both 11 and 12 yielded predominantly the syn cycloadducts, with selectivities of ≥95:5. The syn-directing influence of the 2-substituent, arising from steric and (for COOMe) electronic components, is stronger than the anti-directing effect of the 3-substituent. The selectivities are summarized in Scheme 4.
Scheme 4.
Summary of Preferred Regioisomers.
We have also analyzed the regioselectivities, by computing the distortion of the reactants and interactions that occur in the transition states. In Table 2 are listed the distortion energies (ΔE‡dist) and the energies of interaction between the distorted reactants at the TS (ΔE‡int); these two quantities add up to the activation energy ΔE‡.16 Both of the 2-substituted furans incur a smaller distortion penalty in their syn TS. This is supplemented, in the 2-COOMe case, by a stronger interaction energy in the syn TS. The analysis for the 3-substituted furans is less clear-cut. For 3-methylfuran, both regioisomers have similar distortion energies, and the balance is tipped in favor of the anti isomer by the interaction energy. For 3-COOMe, the distortion energies strongly favor the anti isomer, but the overall selectivity is small, because the early anti TS has only a weak interaction between the reactants. The anti preference becomes more pronounced when entropic effects and solvation are taken into consideration (ΔΔG‡ = 2.0 kcal/mol in CH2Cl2).
Table 2.
Distortion and Interaction Energies of Transition States for Cycloadditions of 5-E with Furans.a
| furan | regioisomer | ΔE‡ | ΔEdist‡ | ΔEint‡ |
|---|---|---|---|---|
| syn | 5.4 | 11.3 | −5.9 | |
| anti | 6.3 | 14.6 | −8.3 | |
| syn | 1.9 | 13.3 | −11.3 | |
| anti | 7.8 | 15.6 | −7.9 | |
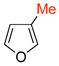 |
syn | 4.6 | 12.1 | −7.4 |
| anti | 3.9 | 12.3 | −8.4 | |
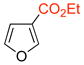 |
syn | 4.4b | 15.7 | −11.3 |
| anti | 4.7 | 10.4 | −5.7 |
Electronic energies in kcal/mol.
ΔE‡ predicts syn, but ΔH‡ and ΔG‡ favor anti.
(4+3) cycloadditions of donor-substituted oxyallyl cations have often been calculated to occur by stepwise pathways, with the oxyallyl cation exhibiting clearly electrophilic behavior.2 Amino-substituted oxyallyls such as 5 are a distinct class of oxyallyl with ambiphilic properties. Even for the achiral 5, an instantaneous facial selectivity is present at the TS, which leads to the high anti selectivity observed with 3-methylfuran. This steric feature, and their relatively electron-rich character, provide oxazolidinone-substituted oxyallyls with well-defined and unique regiochemical properties, leading to a coherent and predictive model.
Supplementary Material
Acknowledgments
We thank the NIH and Australian Research Council for generous financial support (GM-36700 to KNH, GM-66055 to RPH, and DP0985623 to EHK) and the NCSA, UCLA ATS, and NCF NI (Australia) for computer resources. EHK thanks the ARC Centre of Excellence for Free Radical Chemistry and Biotechnology for generous financial support. We also thank Dr. Vic Young and Dr. Ben Kucera of The University of Minnesota for X-ray crystallography.
Footnotes
Supporting Information Available: Experimental procedures, NMR spectra and characterizations for all new compounds, B3LYP geometries and energies are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Reviews: Harmata M. Acc Chem Res. 2001;34:595–605. doi: 10.1021/ar000064e.Harmata M, Rashatasakhon P. Tetrahedron. 2003;59:2371–2395.Hartung IV, Hoffmann HMR. Angew Chem Int Ed. 2004;43:1934–1949. doi: 10.1002/anie.200300622.The designation (m+n) is used here in accordance with Woodward–Hoffmann/IUPAC conventions for describing cycloadditions based on the number of atoms, as opposed to the bracketed [m+n] description that indicates numbers of electrons.
- 2.(a) Walters MA, Arcand HR. J Org Chem. 1996;61:1478–1486. [Google Scholar]; (b) Cramer CJ, Barrows SE. J Org Chem. 1998;63:5523–5532. [Google Scholar]; (c) Cramer CJ, Barrows SE. J Phys Org Chem. 2000;13:176–186. [Google Scholar]; (d) Cramer CJ, Harmata M, Rashatasakhon P. J Org Chem. 2001;66:5641–5644. doi: 10.1021/jo015695g. [DOI] [PubMed] [Google Scholar]; (e) Harmata M, Schreiner PR. Org Lett. 2001;3:3663–3665. doi: 10.1021/ol016611t. [DOI] [PubMed] [Google Scholar]; (f) Sáez JA, Arnó M, Domingo LR. Org Lett. 2003;5:4117–4120. doi: 10.1021/ol035652h. [DOI] [PubMed] [Google Scholar]; (g) Arnó M, Picher MT, Domingo LR, Andrés J. Chem Eur J. 2004;10:4742–4749. doi: 10.1002/chem.200400277. [DOI] [PubMed] [Google Scholar]; (h) Sáez JA, Arnó M, Domingo LR. Tetrahedron. 2005;61:7538–7545. [Google Scholar]; (i) Krenske EH, Houk KN, Harmata M. Org Lett. 2010;12:444–447. doi: 10.1021/ol902591k. [DOI] [PubMed] [Google Scholar]
- 3.For (4+3) cycloadditions of donor-substituted oxyallyls, including regioselective examples, see: Föhlisch B, Krimmer D, Gehrlach E, Kaeshammer D. Chem Ber. 1988;121:1585–1594.Murray DH, Albizati KF. Tetrahedron Lett. 1990;31:4109–4112.Walters MA, Arcand HR, Lawrie DJ. Tetrahedron Lett. 1995;36:23–26.Lee JC, Jin S, Cha JK. J Org Chem. 1998;63:2804–2805.Harmata M, Rashatasakhon P. Synlett. 2000:1419–1422.Beck H, Stark CBW, Hoffmann HMR. Org Lett. 2000;2:883–886. doi: 10.1021/ol991386p.Myers AG, Barbay JK. Org Lett. 2001;3:425–428. doi: 10.1021/ol006931x.Harmata M, Ghosh SK, Hong X, Wacharasindhu S, Kirchhoefer P. J Am Chem Soc. 2003;125:2058–2059. doi: 10.1021/ja029058z.Harmata M. Adv Synth Catal. 2006;348:2297–2306.MaGee DI, Godineau E, Thornton PD, Walters MA, Sponholtz DJ. Eur J Org Chem. 2006:3667–3680.Chung WK, Lam SK, Lo B, Liu LL, Wong WT, Chiu P. J Am Chem Soc. 2009;131:4556–4557. doi: 10.1021/ja807566t.
- 4.For a review on allenamide chemistry, see: Wei L-L, Xiong H, Hsung RP. Acc Chem Res. 2003;36:773–782. doi: 10.1021/ar030029i.For chemistry of nitrogen-stabilized oxyallyls, see: Xiong H, Hsung RP, Berry CR, Rameshkumar C. J Am Chem Soc. 2001;123:7174–7175. doi: 10.1021/ja0108638.Xiong H, Huang J, Ghosh SK, Hsung RP. J Am Chem Soc. 2003;125:12694–12695. doi: 10.1021/ja030416n.Rameshkumar C, Hsung RP. Angew Chem Int Ed. 2004;43:615–618. doi: 10.1002/anie.200352632.Huang J, Hsung RP. J Am Chem Soc. 2005;127:50–51. doi: 10.1021/ja044760b.Antoline JE, Hsung RP, Huang J, Song Z, Li G. Org Lett. 2007;9:1275–1278. doi: 10.1021/ol070103n.
- 5.We use the term “endo” to denote the relationship between the diene unit and the oxyallyl oxygen in 4-syn/anti and the TSs leading to them. Hoffmann has used the term “compact” to describe this geometry, see: Hoffmann HMR. Angew Chem Int Ed. 1973;12:819–835.
- 6.Krenske EH, Houk KN, Lohse AG, Antoline JE, Hsung RP. Chem Sci. 2010;1:387–392. doi: 10.1039/C0SC00280A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Coolidge MB, Yamashita K, Morokuma K, Borden WT. J Am Chem Soc. 1990;112:1751–1754. [Google Scholar]; (b) Ichino T, Villano SM, Gianola AJ, Goebbert DJ, Velarde L, Sanov A, Blanksby SJ, Zhou X, Hrovat DA, Borden WT, Lineberger WC. Angew Chem Int Ed. 2009;48:8509–8511. doi: 10.1002/anie.200904417. [DOI] [PubMed] [Google Scholar]
- 8.Antoline JE, Hsung RP. Synlett. 2008:739–744. [Google Scholar]
- 9.(a) Becke AD. J Chem Phys. 1993;98:5648–5652. [Google Scholar]; (b) Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. J Phys Chem. 1994;98:11623–11627. [Google Scholar]; (c) Lee C, Yang W, Parr RG. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 10.Frisch MJ, et al. Gaussian 03, Revision C.02. Gaussian, Inc; Wallingford, CT: 2004. A complete citation is available in the Supporting Information. [Google Scholar]
- 11.(a) Barone V, Cossi M. J Phys Chem A. 1998;102:1995–2001. [Google Scholar]; (b) Barone V, Cossi M, Tomasi J. J Comput Chem. 1998;19:404–417. [Google Scholar]
- 12.Activation energies were calculated with respect to the oxazolidinone-substituted cyclopropanone isomer of 5-E, which is 5.2 kcal/mol more stable than 5-E.
- 13.Orbitals were calculated at the HF/6–31G//B3LYP/6–31G(d) level.
- 14.Mulliken charges at the B3LYP/6–31G(d) level.
- 15.Singlet diradicals were modeled with the guess=mix keyword in Gaussian.
- 16.(a) Ess DH, Houk KN. J Am Chem Soc. 2008;130:10187–10198. doi: 10.1021/ja800009z. [DOI] [PubMed] [Google Scholar]; (b) Hayden AE, Houk KN. J Am Chem Soc. 2009;131:4084–4089. doi: 10.1021/ja809142x. [DOI] [PubMed] [Google Scholar]; (c) Schoenebeck F, Houk KN. J Am Chem Soc. 2010;132:2496–2497. doi: 10.1021/ja9077528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.