Summary
Reactive lipid hydroperoxides formed by lipoxygenases and cyclooxygenases can contribute to disease through cellular oxidative damage. Several selenoproteins have lipid hydroperoxidase activity including glutathione peroxidase 4, thioredoxin reductase, and selenoprotein P (SelP). SelP is an extracellular glycoprotein that functions both in selenium distribution and has antioxidant activity. The major objective of this study was to determine if SelP, at physiological concentrations and in selenium replete media, possessed hydroperoxidase activity directed at lipid hydroperoxides generated from the metabolism of arachidonic acid by 15-lipoxygenase-1 (15-LOX-1). SelP displayed in vitro lipid hydroperoxidase activity of 15-hydroperoxyeicosatetraenoic acid (15-HpETE), attenuated 15-HpETE oxidation in cellular assays, and in a transcellular assay when 15-LOX-1 is metabolically active. These results suggest that SelP can function as an antioxidant enzyme against reactive lipid intermediates formed during inflammation, but SelP has modest activity. Nevertheless, this effect may help protect cells against the oxidative damage induced by these lipid metabolites.
Keywords: 15-hydroperoxyeicosatetraenoic acid, 15-hydroxyeicosatetraenoic acid, selenoprotein P, lipid hydroperoxidase
Introduction
A relationship between chronic inflammation and carcinogenesis has been noted in numerous malignancies, including colon cancer and hepatocellular carcinoma [1, 2]. It is believed that leukocytes and other phagocytic cells involved in the inflammatory process may lead to an induction of DNA damage in proliferating cells through the production of reactive oxygen and nitrogen species [3]. Additionally, enzymes expressed during the inflammatory process, including lipoxygenase (LOX) enzymes, have been shown to be upregulated in certain malignancies [4, 5]. Specifically, 15-LOX-1 expression is directly proportional to severity of prostate cancer, as measured by Gleason staging [5, 6]. Membrane lipids released and metabolized during inflammation, such as arachidonic acid (AA), have also been linked to various malignancies, including prostate cancer [7]. 15-LOX-1 can metabolize arachidonic acid to reactive hydroperoxy intermediates, such as 15-hydroperoxyeicosatetraenoic acid (15-HpETE), the oxidative precursor of 15-hydroxyeicosatetraenoic acid (15-HETE) [8, 9]. In addition, end products of lipid peroxidation have been implicated as being mutagenic [10], further contributing to evidence that inflammation may result in carcinogenesis through its ability to increase the oxidative tone of the cellular environment.
Cells possess several enzymes that can reduce lipid peroxides. Multiple selenoenzymes are specifically involved in the reduction of oxidized lipids. Glutathione peroxidase 4 (GPx4, also called phospholipid hydroperoxide GPx, or PHGPX) is an essential selenoenzyme that is associated with protection from lipid hydroperoxides [11]. Thioredoxin reductase (TR1) can also reduce some oxidized lipids [12], as well as indirectly modulate lipid peroxides through the reduction of peroxiredoxins [13]. The role of selenoprotein P (SelP) as a lipid hydroperoxidase is still being elucidated. SelP is one of two selenoproteins primarily found in the extracellular environment, with glutathione peroxidase 3 being the other [14, 15], and one function is in selenium distribution [16, 17]. As opposed to GPx4 and TR1, mice with the SelP gene deleted are viable but they display altered selenium distribution [17, 18]. Besides the selenium distribution function, evidence from the literature also suggests an antioxidant function of SelP. This protein has been attributed to protecting rats against diquat-induced liver toxicity through a decrease in lipid peroxidation [19]. Depletion of SelP from plasma enhances plasma protein oxidation mediated by peroxynitrite-induced oxidation and nitration [20]. In addition, SelP protects low-density lipoproteins from peroxidation [21]. It is not clear if these in vivo antioxidant results are due to direct action of SelP or if they are related to selenium redistribution and the antioxidant activity of other selenoenzymes. In a cell-free in vitro system, SelP has been shown to reduce phospholipid hydroperoxide to a greater extent than other reactive oxygen species, including hydrogen peroxide and tert-butyl hydroperoxide (t-BHP) [22].
These data suggest that an enzymatic activity of SelP may be specific for lipid-derived substrates, as opposed to other sources of reactive oxygen stress. The purpose of this study was to determine if SelP displayed lipid hydroperoxidase activity directed at 15-LOX-1-generated metabolites under selenium replete conditions with physiological levels of SelP. Herein, we report on the ability of SelP to reduce 15-HpETE, and to protect human embryonic kidney (HEK-293) cells from oxidation. Furthermore, SelP was capable of protecting a target cell population from oxidation produced by cells engineered with inducible 15-LOX-1 that were provided arachidonic acid substrate. The evidence presented suggests SelP may play a modest role in reducing lipid hydroperoxides following membrane lipid metabolism, which could serve to protect the cells from the toxic effects of chronic inflammation.
Materials and Methods
Materials
The HEK-293 cell line was purchased from American Type Culture Collection. Advanced DMEM, CD-293, and zeocin were purchased from Invitrogen. Ponasterone A was purchased from A.G. Scientific. Arachidonic acid was purchased from NuCheck Prep. E. coli thioredoxin, E. coli thioredoxin reductase, and tert-butyl hydroperoxide were purchased from Sigma-Aldrich. Purified 15-HpETE and 15-HETE, were purchased from Cayman, as was the 15-HETE enzyme immunoassay and anti-GPx4 antibody. Rat selenoprotein P and antibody were a gift from Drs. Kris Hill and Raymond Burk, Vanderbilt University. Monoclonal antibodies directed against thioredoxin reductase (B-2, sc-28321, lot# J1304); donkey polyclonal anti-mouse and anti-rabbit antibodies conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology. Diphenylpyrenylphosphin (DPPP) was purchased from Dojindo Molecular Technologies.
Cell Culture
Unless otherwise noted, the human embryonic kidney line HEK-293, as well as all subsequently engineered cells, were cultured in Advanced DMEM medium containing 2% fetal bovine serum and 2mM L-glutamine. Cells were maintained at 37°C in a humidified incubator with 5% CO2. Advanced DMEM is supplemented with 5 μg/l sodium selenite (NaSeO3) and the serum contained 37 ng/ml selenium. Therefore, even with only 2% serum, the selenite content of this media results in selenium sufficient media as determined by the plateau of glutathione peroxidase 1 activity (a measure of selenoenzyme synthesis) [23–25].
Conditional Expression of 15-LOX-1
pVgEcR (Invitrogen) encodes the fusion transcription factor used to generate ecdysone-inducible cells. HEK-293 were transfected with pVgEcR and selected for Zeocin resistance to generate stable expression of the VgEcR gene product and are referred to as 293-EcR. 293-EcR cells that conditionally express 15-LOX-1 were used as previously described [26, 27].
Preparation and Purification of 1-Palmitoyl-2-(13-hydroperoxy-cis-9,trans-11-octdecadienoyl) Phosphatidylcholine (PLPC-OOH)
PLPC-OOH was prepared and quantified as previously described [22]. Briefly, PLPC was oxidized with soybean lipoxidase and resulting PLPC-OOH was extracted with ethyl acetate. The ethyl acetate extract was evaporated, dissolved in methanol, and PLPC-OOH was purified by HPLC. PLPC-OOH was dissolved in methanol and stored at −20°C.
Biochemical Enzyme Assay
A NADPH-coupled reaction was used to assess the ability of SelP to reduce various lipid substrates similar to assays previously published [28]. Lipid substrates tested in the assay included 10 μM 15-HETE, 10 μM 15-HpETE, 100 μM tert-butyl hydroperoxide, and 60 μM PLPC-OOH. The assay was run in a 384 well UV transparent clear bottom plate. Reaction mixtures contained 0.1M Tris-HCl, pH 7.4, 0.24 mM NADPH, 1mM EDTA, 0.025% Triton-X-100/0.3% sodium deoxycholate, ~0.1 Units E. coli thioredoxin reductase, 3.2 μg rat SelP, and appropriate lipid substrate aliquots. After a 10 min incubation at 25°C, the reaction was initiated by the addition of 6.66 μM E. coli thioredoxin to the sample wells. In control experiments, reactions mixtures without rat SelP or E. coli thioredoxin were used to evaluate the spontaneous reaction rates. The oxidation of NADPH was measured by monitoring the absorbance at 340 nm (A340) for ~500 sec.
Enrichment of Selenoprotein P
Increased transcription and translation of SelP has previously been observed in 293-EcR cells treated with the ecydsone analog ponasterone A (PonA) [29]. 293-EcR cells were maintained in serum-free CD-293 cell culture medium supplemented with 2mM L-glutamine, 1 μM sodium selenite and 10 μM PonA. After 3 days, supernatant was collected from the cells following centrifugation at 250 × g for 5 minutes. The supernatant was concentrated ~20 fold using a Centricon Plus-70 centrifugal filter (Millipore) with a 30-kDa cutoff membrane. This concentrated media retains SelP, and was used in our experiments to evaluate antioxidant properties of SelP. As a control, supernatant was collected and concentrated from vehicle (EtOH) treated 293-EcR cells.
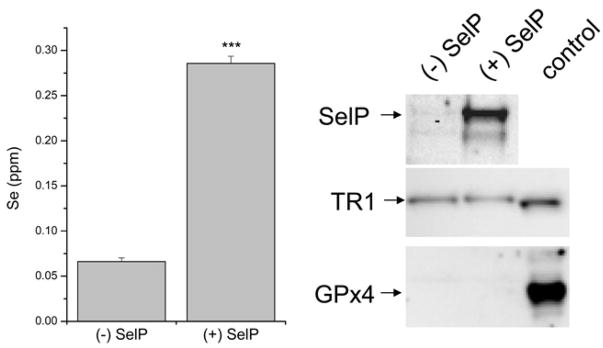
Inductively coupled plasma (ICP) spectrometry (Perkin Elmer Optima 3100 XL) was used to determine the Se content in the concentrated cell culture media. The instrument was calibrated using SPEX CertiPrep Laboratory Performance Check Standard 1 (Metuchen, NJ). The results were collected in ppm using WinLab32 for ICP software (v. 3.4.0.0253) and then converted to Se concentration. The difference in selenium content between the PonA and control supernatants was used to calculate SelP concentration based on the assumption that there are ten selenium atoms per molecule of SelP. Protein expression of SelP, GPx4, and TR1 was verified by immunoblotting. The supernatant collected from PonA-treated cells was referred to as (+) SelP, while that collected from EtOH-treated cells was referred to as (−) SelP.
Measurement of Lipid Hydroperoxides
Lipid hydroperoxides were measured using diphenylpyrenylphosphin (DPPP), a molecular probe that becomes fluorescent upon oxidation by lipid hydroperoxides [30]. HEK-293 cells were plated on a 384-well tissue culture plate at a concentration of 18,000 cells/well. Cells were labeled with 100 μM DPPP or DMSO control and were incubated overnight. Cells were supplemented with (+) SelP supernatant at a concentration of ~ 47 nM (a 1:6 dilution from the concentrated stock media) SelP. An equivalent amount of (−) SelP concentrated supernatant was also tested, as was 100 nM sodium selenite, and selenium sufficient blank control medium containing 5 μg/l selenium selenite. Immediately following the addition of these supplements, cells were treated with 0–100 μM 15-HpETE, 30 μM 15-HETE or EtOH control. Because some variability in results were observed between batches of the hydroperoxy lipids purchased from Cayman, all reported results were tested from the same batch number of 15-HpETE (13250-6) or 15-HETE (156030-19). Fluorescent intensities following excitation at 351 nm were measured at the emission wavelength of 380 nm with a Perkin-Elmer Victor3 V plate reader.
Enzyme Immunoassay
EcR-15-LOX cells were seeded on a 24-well plate at a concentration of 1 ×105 cells/well. After 24 hours culture medium was changed to serum-free CD293 supplemented with 2 mM L-glutamine and 0.1% ovalbumin. Cells were treated with 10 μM PonA for 24 hours, followed by a 2 hour treatment with 60 μM arachidonic acid. Vehicle treatment with EtOH served as controls for both treatment conditions. Culture medium was collected and 15(S)-HETE levels were measured by enzyme immunoassay according to the manufacturer’s instructions.
Immunoblotting
Following collection of culture medium for enzyme immunoassay evaluation, EcR-15-LOX cells were resuspended in lysis buffer, sonicated at 4°C, centrifuged at 14,000 rpm for 10 minutes, and supernatant was collected. For EcR-15-LOX samples, 5 μg of the supernatant protein was separated by SDS-PAGE. For enriched supernatant samples collected from 293-EcR cells, 15 μl of the sample was separated by SDS-PAGE. Following separation, proteins were transferred to a nitrocellulose membrane and membranes were probed for 15-LOX-1 (Cayman Chemical, Ann Arbor, MI), SelP (gift from Kris Hill & Raymond Burk), GPx4 (Cayman Chemical Company), or TR1 (Santa Cruz Biotechnology). A peroxidase conjugated secondary antibody was used to detect chemiluminescence indicative of protein expression. Positive controls for GPx4 and TR1 expression included 30 μg mouse testicular lysate and 10 μg HEK-293 cell lysate, respectively.
Transcellular Assay
EcR-15-LOX cells were plated in a 384-well tissue culture plate at 6,000 cells/well. Cells were treated with 10 μM PonA and incubated overnight. HEK-293 cells were grown in 25cm2 flasks in serum-free CD-293 cell culture medium supplemented with 2mM L-glutamine and 0.1% ovalbumin. These cells were labeled with 100 μM DPPP or DMSO control. After 24 hours, DPPP-labeled HEK-293 cells, or unlabeled controls, were added into the wells with the EcR-15-LOX cells at a concentration of 18,000 cells/well. Cells were allowed to recover for 1 hour prior to the addition of (+) SelP at ~ 47 nM SelP or (−) SelP control supernatant. Immediately following the addition of the concentrated supernatant, cells were treated with 60 μM arachidonic acid. Vehicle treatment with EtOH served as controls for both ponasterone A and arachidonic acid treatments. Thirty minutes after arachidonic acid addition, fluorescent intensities were measured with a Perkin-Elmer Victor3 V plate reader as described above.
Statistical Analysis
GraphPad Instat, version 3.06, was used to evaluate the statistical significance of the results. Statistical significance was determined by one-way ANOVA with Bonferroni multiple comparison post hoc tests, and differences were considered significant for p<0.05.
Results
The ability of SelP to reduce PLPC-OOH through a NADPH-coupled biochemical assay has been described previously [22, 28]. Similar methods were followed to test the ability of SelP to reduce 15-HpETE and 15-HETE. Because 15-HpETE was previously shown to inhibit that activity of mammalian TR1 [27], E. coli, rather than mammalian, thioredoxin reductase and thioredoxin were used in this assay. In experiments with the 15-LOX-1 metabolites, we were unable to demonstrate TR-activity inhibition at 30 μM (data not shown). NADPH oxidation was used as an indirect measure of the hydroperoxidase activity of SelP. Nonenzymatic NADPH oxidation rates were observed when thioredoxin was not added to the reaction mixtures and did not show substrate selectivity. NADPH oxidation when SelP was not added to the reaction mixtures reflects activity by the E. coli thioredoxin system and we did observe substrate preferences for t-BHP and 15-HpETE. However, with complete reaction mixtures, the PLPC-OOH was the best substrate as measured by the most NADPH oxidation (Figure 1). 15-HpETE was the next best substrate, with ~70% of the activity observed with the PLPC-OOH substrate. However, ~50% of that activity may be contributed by the thioredoxin system coupled in this reaction. Essentially no selective SelP activity was observed for 15-HETE and t-BHP.
Figure 1.
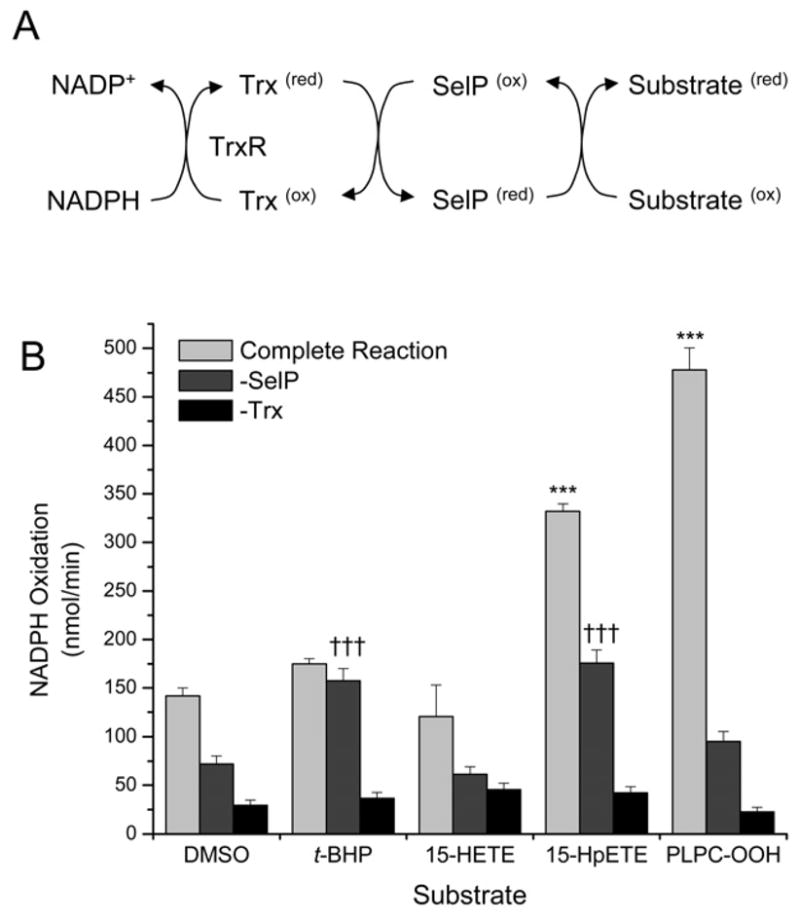
Lipid hydroperoxidase activity as measured in a NADPH-coupled reaction. NADPH oxidation was measured in each reaction mixture base, described in the Methods, with t-BHP, 15-HETE, 15-HpETE, or PLPC-OOH as potential substrates as well as the vehicle control (DMSO). Each substrate was tested with a complete reaction mixture, or mixtures lacking either SelP or Trx. The SelP activity in the complete reaction mixtures with the 15-HpETE and PLPC-OOH substrates were significantly different from the other conditions (***, p<0.001). The NADPH oxidation was increased with t-BHP and 15-HpETE substrates in the mixtures without SelP (-SelP) above the conditions (†††, p<0.001) indicating that the E. coli thioredoxin system utilized had background activity on these substrates independent of SelP.
Since we did observe SelP-mediated activity, we next worked to determine whether the activity of SelP observed in the biochemical assay could be translated to a cell-based system. SelP was derived from the supernatant of ponasterone A treated 293-EcR cells. Transcription and translation of SelP has previously been shown to increase in these cells when the VgEcR gene expression system is activated by ponasterone A [29]. Microarray results do not support changes in the expression of other selenoproteins under these conditions (data not shown); therefore, SelP is the only selenoprotein expected to be enriched in the concentrated supernatant collected from these cells.
Selenium content of concentrated supernatant was determined by ICP spectrometry. Increased selenium content was observed in the (+) SelP supernatant versus (−) SelP (0.285 ppm vs. 0.066 ppm). The SelP content in these media were confirmed by immunoblotting, which showed considerable SelP expression in (+) SelP supernatant, but minimal SelP (generally <5% of the induced media) in the (−) SelP supernatant. Equal levels of TR1 were observed between the (+) SelP and (−) SelP samples and GPx4 expression was not observed in either sample (Figure 2). The (−) SelP concentrated media selenium content was comparable to the background level of selenium measured in the CD-293 media when it was not concentrated, suggesting that the TR1 did not contribute significant selenium to the concentrated media (data not shown). Using the difference in selenium content between the two supernatants, as well as the assumption of ten selenium atoms per molecule of SelP [31], the (+) SelP supernatant was calculated to have a concentration ~280nM or about 5–6 fold the normal SelP concentration of selenium replete plasma [32]. While two immunoreactive bands of SelP were identified (Figure 2), densitometric analysis of these bands revealed that >93% of the SelP was found in the major band, and therefore, we did not adjust the calculation due to the minor band. The minor band is likely SelP terminated at a Sec codon (UGA) and therefore does not contributed 10 Se atoms and the SelP concentration in the concentrated media could be ~300 nM including truncated forms.
Figure 2.
Assessment of selenium and SelP content in concentrated media. Left: ICP analysis of selenium content in media samples; (+) SelP represents media from cells induced to express SelP by ponasterone A while (−) SelP represents media from cells treated with EtOH (vehicle control). The selenium difference between these concentrated media is highly significant (***, p<0.001). Right: Immunoblot analysis for SelP, TR1 and GPx4 in these media samples. The control for TR1 is 10 μg of HEK293 cell lysate, and the control for GPx4 is 30 μg of mouse testicular lysate.
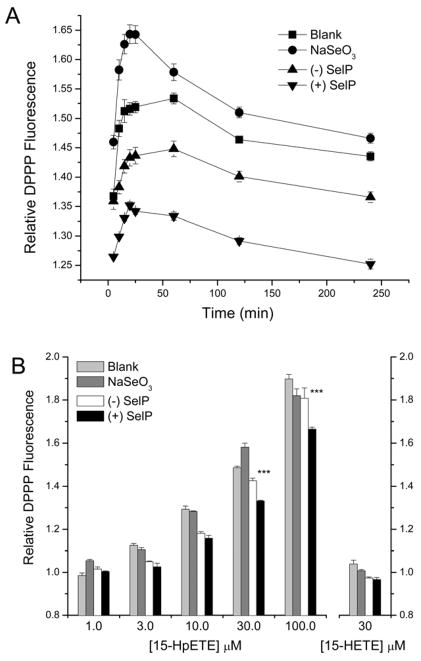
A fluorescent-based assay (DPPP) was used to detect lipid hydroperoxide levels following exposure of HEK-293 cells to the reactive lipid metabolite 15-HpETE. With cells grown in selenium sufficient medium, the cellular oxidation was evaluated from 0-240 min. Under all treatment conditions, cellular oxidation reaches its maximum after approximately 25–30 minutes of 30 μM 15-HpETE exposure. SelP enriched media consistently demonstrated a reduction of cellular oxidation over this time course (Figure 3A). Addition of 15-HpETE resulted in a dose-dependent increase in DPPP fluorescence and the cells with SelP enriched media demonstrated significant protection from oxidation at between 10 to 100 μM 15-HpETE (Figure 3B). The addition of SelP reduced relative DPPP fluorescence compared to both standard (blank) medium and (−) SelP controls (~12% and ~7% reduction, respectively). Since some studies, with viability as an outcome, have shown that both SelP and 100 nM sodium selenite can improve viability following oxidative stress [33, 34], the effect of selenium supplementation with 100 nM sodium selenite was also tested in this assay. However in this case, the addition of sodium selenite exerted an oxidative effect, as evidenced by an increase in relative DPPP fluorescence as compared to standard (identified as blank) control medium following 15-HpETE addition. In addition, no increase in DPPP fluorescence was observed following treatment with 30 μM of the less oxidative 15-HETE lipid metabolite (Figure 3B).
Figure 3.
SelP protects HEK-293 cells from oxidation by the pharmacological addition of 15-HpETE. A) Time course oxidative changes measured by DPPP fluorescence following 30 μM 15-HpETE addition in cells with standard media (blank, square), 100 nM sodium selenite (NaSeO3, circle), concentrated control media ((−) SelP, up triangle), and SelP enriched media ((+) SelP, down triangle). The (+) SelP condition is statistically different at all time points (p<0.001). B) Dose response of 15-HpETE oxidative changes measured by DPPP fluorescence (right), as well as 15-HETE at 30 μM (left). The (+) SelP condition is significantly different from the other conditions at 10 to100 μM 15-HpETE (***, p<0.001).
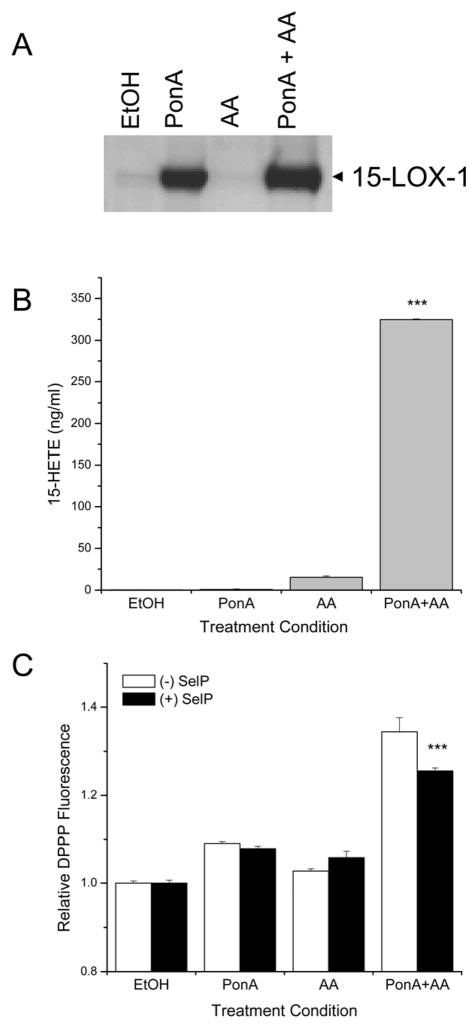
To determine if SelP could protect cells from oxidation following 15-LOX-1 catalysis of arachidonate, overexpression of 15-LOX-1 was achieved using an ecdysone-inducible gene expression system (Figure 4A). While possible that upregulation of SelP expression by this ecdysone-inducible system could confound results of this experiment, this was likely a minor effect as the culture media of these cells remained non-concentrated. This hypothesis is supported by the results of the pharmacological DPPP assay, which showed that an ~20-fold concentration of (+) SelP supernatant was required in order to see modest reductions in lipid hydroperoxides levels. Following addition of arachidonic acid, the enzymatic activity of 15-LOX-1 was confirmed through detection of 15-HETE by enzyme immunoassay. Production of the metabolite increased significantly following treatment of cells with the combination of ponasterone A and arachidonic acid, but was observed at only a minimal level under control conditions (Figure 4B). DPPP was used to detect lipid hydroperoxides following arachidonic acid metabolism by this system. A transcellular assay in which DPPP-labeled HEK-293 cells were added onto EcR-15-LOX cells allowed for the measurement of lipid hydroperoxides in cells distant from those that were responsible for metabolizing arachidonic acid. Requiring lipid metabolites to move through the extracellular environment prior to acting on DPPP-labeled cells, the ability of the predominantly extracellular SelP to reduce the reactivity of these metabolites was able to be evaluated. DPPP fluorescence of the HEK-293 cells increased following treatment of EcR-15-LOX cells with the combination of ponasterone A and arachidonic acid as compared to control conditions (Figure 4C). The addition of ~ 47 nM SelP attenuated this increase in fluorescence as compared to (−) SelP control. While the antioxidant effect was modest, the change in cellular oxidation, based on fitting the 15-HpETE dose response in figure 3, indicates ~2-fold reduction of lipid hydroperoxides in the presence of the (+) SelP media compared to the (−) SelP media.
Figure 4.
SelP can protect target cells from the oxidation by effector cells following 15-LOX-1 catalysis of arachidonate. A) Inducible expression of 15-LOX-1 in 293-EcR cells with integrated 15-LOX-1. Four conditions are evaluated; vehicle control (EtOH), ponasterone A (PonA), arachidonic acid (AA), and the combination ponasterone A and arachidonate (PonA+AA). B) Production of the 15-LOX-1 metabolite 15-HETE under the four conditions just described. C) Protection from oxidation, as measured by DPPP fluorescence, with the addition of SelP enriched media, (+) SelP, compared to the control concentrated media, (−) SelP (***, p< 0.001).
Discussion
The health effects of selenium have been studied in multiple disease states, including cancer, cardiovascular disease, and inflammatory conditions [35–40]. Benefits of supplemental selenium intake are believed to be due to antioxidant activity of selenoenzymes [41–43]; proteins capable of redox reactions through selenium atoms incorporated as the amino acid selenocysteine [44–46]. Many of the selenium-based health claims have been muted by recent clinical data. Specifically, the anti-cancer benefit from selenium appears to exist only for individuals with low serum selenium levels [47]. Earlier dietary supplementation studies demonstrated a decrease of cancer incidence that was most pronounced in individuals with lower serum selenium levels [36]. Additionally, serum selenium levels have been shown to be inversely correlated to the incidence of certain cancers [37, 38, 48]. Recent results of the Selenium and Vitamin E Trial (SELECT) did not support the utility of supplemental selenomethionine in prostate cancer prevention in selenium sufficient individuals [49]. Still, the antioxidant activity of selenoenzymes are likely important in human health.
While protection against oxidative injury by the glutathione peroxidases have been extensively characterized [50, 51], the antioxidant activity of SelP is less well characterized. Biochemical data has supported a role for SelP as a phospholipid hydroperoxidase [22, 28]. However, the reducing capacity of SelP in this assay was measured to be two orders of magnitude lower than activity observed by GPx4 [52], suggesting that the contribution of SelP as an antioxidant protein might be minimal as compared to other selenoproteins. The modest activity we observed suggest that the lipid hydroperoxidase activity of SelP may not even be enzymatic in vivo. The Sec amino acids are nucleophilic, and therefore, the modest lipid hydroperoxide protection documented could be non-enzymatic reactivity of these amino acids. Our use of sodium selenite was intended to control for non-specific Se-based antioxidant activity but the reduction of the selenite was oxidative in the timeframe of our experiments (Figure 3).
This study is the first, to our knowledge, to attempt to directly link SelP and the reduction of lipid hydroperoxides derived from 15-LOX-1 catalysis. This study extends work that demonstrated that selenium supplementation of endothelial cells produce significantly higher 15-HETE to 15-HpETE ratios, while selenium deficiency increased oxidation of arachidonic acid to 15-HpETE [53]. The SelP lipid hydroperoxidase activity likely extends to other lipoxygenase metabolites like 5-HpETE and 12-HpETE. The first assessment of SelP lipid hydroperoxidase activity utilized PLPC-OOH (and used herein as a control); a product of soybean lipoxygenase activity on the phospholipid substrate. Therefore, we do not expect region-specificity in the lipoxygenase reaction to be a major determinant in the SelP-mediated hydroperoxidase activity. Since the region-specific LOX enzymes have distinct roles during inflammation, e.g. 5-LOX metabolites are generally proinflammatory while 12/15-LOX metabolites are considered anti-inflammatory [54], the reductive activity of SelP may not have uniform activity during inflammation. However, the SelP activity appears to be distinct from, and considerably more modest when compared to the GPx4 modulation of lipoxygenase pathways involved in cell death [55]. Additional evidence supporting a lipid hydroperoxidase function of SelP in a cell-based system includes a study where lipid hydroperoxides are increased in myofibroblasts when SelP expression is knocked down [56]. Loss of SelP also led to apoptosis and decreased cell viability through activation of c-Jun N-terminal kinases in this model. In endothelial cells and astrocytes, SelP has been shown to protect against t-BHP-induced cytotoxicity when cells were maintained in selenium deficient medium [33, 34]. SelP protected against cell death to the same extent as selenium supplementation with 100 nM sodium selenite and this effect was attributed to increased expression and activity of cytosolic glutathione peroxidase. Both SelP and sodium selenite increased this antioxidant protein, and the use of a glutathione peroxidase specific inhibitor, counteracted SelP-mediated cytoprotection. While these results show antioxidant protection of cells by SelP during selenium deficiency, the results may simply reflect selenium distribution or non-enzymatic reactivity of the lipid reactive species with Sec moieties contained within SelP. There is little published data that delineates whether this function carries over to conditions where selenium is available at physiological sufficient levels.
Here it is shown that when HEK-293 cells were maintained in selenium sufficient medium, ~ 47 nM SelP reduced lipid hydroperoxide-dependent oxidation following exposure of the cells to 15-HpETE. This SelP concentration is considerably higher than that required to protect endothelial cells (0.6 nM) or astrocytes (2 nM) from the oxidative damage of t-BHP [33, 34]. The normal physiological concentration of SelP in selenium replete human serum is estimated at 50 nM [32]. A decrease to less than 5% of selenium-replete values has been observed in animals with severe selenium deficiency [57, 58]. This suggests that the discrepancy in SelP concentration required to exert antioxidant effects may be related to whether cells are maintained under selenium sufficient or deficient conditions.
The results presented here also show that reduction of lipid hydroperoxides in HEK-293 cells was achieved when SelP and 15-HpETE were added concurrently. In addition, short term treatment with sodium selenite lead to increased oxidative tone in the cells, as reflected by an increase in lipid hydroperoxides following simultaneous addition of sodium selenite and 15-HpETE. Protection of endothelial cells and astrocytes against t-BHP-induced cytotoxicity required pre-incubation with SelP or sodium selenite and no protection was observed in endothelial cells if SelP and t-BHP were added simultaneously [33, 34]. This delayed effect would account for the time required to synthesize cytosolic glutathione peroxidase, the enzyme ultimately responsible for SelP-mediated protection in this model. The reduction of lipid hydroperoxides that was observed following short term treatment with SelP likely represents direct enzymatic activity of the protein, rather than a genomic effect requiring the transcription and translation of secondary genes such as glutathione peroxidase.
The cellular protection from oxidation by lipid hydroperoxides afforded by SelP observed in this study, while significant, was modest (only a 7–12% reduction as compared to control conditions). This level of antioxidant activity by SelP could be a consequence of the extracellular localization of this protein. Intracellular reduced glutathione protects endothelial cells against 15-HpETE-induced cell injury and stimulates the conversion of 15-HpETE to 15-HETE [59]. Specifically, phospholipid hydroperoxide glutathione peroxidase has been shown to reduce the hydroperoxy ester lipids formed by 15-LOX-1 metabolism [60] and is capable of inhibiting the activity of lipoxygenase enzymes [61]. If intracellular selenoproteins including glutathione peroxidases are the primary source of antioxidant defense against the products of lipid metabolism, it is possible that the reactivity of these metabolites is minimized prior to reaching the extracellular environment, therefore reducing the need for SelP to act as a detoxifying protein. As GPx4 expression was not observed in the concentrated supernatants, this protein is not believed to be involved in the antioxidant protection conferred by the SelP enriched culture medium. Additionally, because the enzymatic activity of mammalian TR1 is inhibited by 15-HpETE [27]and equal levels of TR1 expression were observed between the (+) SelP and (−) SelP supernatants, it unlikely that this protein is involved in the activity attributed to (+) SelP medium.
In conclusion, SelP has been shown to reduce lipid hydroperoxide-dependent oxidation in HEK-293 cells after exposure to 15-HpETE. This was observed following pharmacological treatment with the metabolite, as well as endogenous production through ecdysone-inducible expression of 15-LOX-1. These results provide evidence that the lipid hydroperoxidase activity of SelP initially observed in biochemical assays may also occur in a cell-based model of 15-LOX-1 catalyzed arachidonic acid metabolism. By reducing lipid hydroperoxides following cell membrane metabolism, SelP may serve to decrease oxidative tone of tissues under inflammatory conditions. This could provide protection against the toxic effects of lipid peroxidation, leading to a decrease in DNA damage and mutations and potentially contributing to any anti-carcinogenic effects of selenium supplementation. The modest antioxidant effects observed in our cellular and transcellular experiments suggest that the selenium distribution function of SelP is more consistent with its primary function compared to lipid hydroperoxidase activity.
Acknowledgments
This project was supported by a USPHS Grants CA115616. We thank Drs. Hill and Burk (Vanderbilt University) for the rat SelP protein and antisera. We also thank Dr. Cassidy (University of Utah) for helping us with the coupled SelP activity assay. We also acknowledge the use of core facilities supported by P30 CA042014 awarded to the Huntsman Cancer Institute.
Abbreviations
- 15-HETE
15-hydroxyeicosatetraenoic acid
- 15-HpETE
15-hydroperoxyeicosatetraenoic
- 15-LOX-1
15-lipoxygenase-1
- PLPC-OOH
1-palmitoyl-2-(13-hydroperoxy-cis-9,trans-11-octdecadienoyl) phosphatidylcholine
- AA
arachidonic acid
- DMSO
dimethyl sulfoxide
- DPPP
diphenylpyrenylphosphin
- EtOH
ethanol
- GPx4
glutathione peroxidase 4
- PonA
ponasterone A
- Sec
selenocysteine
- SelP
selenoprotein P
- NaSeO3
sodium selenite
- t-BHP
tert-butyl hydroperoxide
- TR1
thioredoxin reductase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 2.Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–20. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737–43. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Kelavkar UP, Cohen C, Kamitani H, Eling TE, Badr KF. Concordant induction of 15-lipoxygenase-1 and mutant p53 expression in human prostate adenocarcinoma: correlation with Gleason staging. Carcinogenesis. 2000;21:1777–87. doi: 10.1093/carcin/21.10.1777. [DOI] [PubMed] [Google Scholar]
- 6.Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22:1765–73. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- 7.Hursting SD, Thornquist M, Henderson MM. Types of dietary fat and the incidence of cancer at five sites. Prev Med. 1990;19:242–53. doi: 10.1016/0091-7435(90)90025-f. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–8. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 9.Sordillo LM, Streicher KL, Mullarky IK, Gandy JC, Trigona W, Corl CM. Selenium inhibits 15-hydroperoxyoctadecadienoic acid-induced intracellular adhesion molecule expression in aortic endothelial cells. Free Radic Biol Med. 2008;44:34–43. doi: 10.1016/j.freeradbiomed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40:1213–32. [PubMed] [Google Scholar]
- 11.Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 12.Bjornstedt M, Hamberg M, Kumar S, Xue J, Holmgren A. Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J Biol Chem. 1995;270:11761–4. doi: 10.1074/jbc.270.20.11761. [DOI] [PubMed] [Google Scholar]
- 13.Mitsumoto A, Takanezawa Y, Okawa K, Iwamatsu A, Nakagawa Y. Variants of peroxiredoxins expression in response to hydroperoxide stress. Free Radic Biol Med. 2001;30:625–35. doi: 10.1016/s0891-5849(00)00503-7. [DOI] [PubMed] [Google Scholar]
- 14.Akesson B, Bellew T, Burk RF. Purification of selenoprotein P from human plasma. Biochim Biophys Acta. 1994;1204:243–9. doi: 10.1016/0167-4838(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Cohen HJ. Selenium-dependent glutathione peroxidase protein and activity: immunological investigations on cellular and plasma enzymes. Blood. 1986;68:640–5. [PubMed] [Google Scholar]
- 16.Renko K, Werner M, Renner-Muller I, Cooper TG, Yeung CH, Hollenbach B, Scharpf M, Kohrle J, Schomburg L, Schweizer U. Hepatic selenoprotein P (SePP) expression restores selenium transport and prevents infertility and motor-incoordination in Sepp-knockout mice. Biochem J. 2008;409:741–9. doi: 10.1042/BJ20071172. [DOI] [PubMed] [Google Scholar]
- 17.Hill KE, Zhou J, McMahan WJ, Motley AK, Atkins JF, Gesteland RF, Burk RF. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem. 2003;278:13640–6. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 18.Burk RF, Hill KE, Motley AK, Austin LM, Norsworthy BK. Deletion of selenoprotein P upregulates urinary selenium excretion and depresses whole-body selenium content. Biochim Biophys Acta. 2006;1760:1789–93. doi: 10.1016/j.bbagen.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burk RF, Hill KE, Awad JA, Morrow JD, Kato T, Cockell KA, Lyons PR. Pathogenesis of diquat-induced liver necrosis in selenium-deficient rats: assessment of the roles of lipid peroxidation and selenoprotein P. Hepatology. 1995;21:561–9. [PubMed] [Google Scholar]
- 20.Arteel GE, Mostert V, Oubrahim H, Briviba K, Abel J, Sies H. Protection by selenoprotein P in human plasma against peroxynitrite-mediated oxidation and nitration. Biol Chem. 1998;379:1201–5. [PubMed] [Google Scholar]
- 21.Traulsen H, Steinbrenner H, Buchczyk DP, Klotz LO, Sies H. Selenoprotein P protects low-density lipoprotein against oxidation. Free Radic Res. 2004;38:123–8. doi: 10.1080/10715760320001634852. [DOI] [PubMed] [Google Scholar]
- 22.Saito Y, Hayashi T, Tanaka A, Watanabe Y, Suzuki M, Saito E, Takahashi K. Selenoprotein P in human plasma as an extracellular phospholipid hydroperoxide glutathione peroxidase. Isolation and enzymatic characterization of human selenoprotein p. J Biol Chem. 1999;274:2866–71. doi: 10.1074/jbc.274.5.2866. [DOI] [PubMed] [Google Scholar]
- 23.Leist M, Raab B, Maurer S, Rosick U, Brigelius-Flohe R. Conventional cell culture media do not adequately supply cells with antioxidants and thus facilitate peroxide-induced genotoxicity. Free Radic Biol Med. 1996;21:297–306. doi: 10.1016/0891-5849(96)00045-7. [DOI] [PubMed] [Google Scholar]
- 24.Baker RD, Jr, Baker SS, Rao R. Selenium deficiency in tissue culture: implications for oxidative metabolism. J Pediatr Gastroenterol Nutr. 1998;27:387–92. doi: 10.1097/00005176-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Saito Y, Yoshida Y, Akazawa T, Takahashi K, Niki E. Cell death caused by selenium deficiency and protective effect of antioxidants. J Biol Chem. 2003;278:39428–34. doi: 10.1074/jbc.M305542200. [DOI] [PubMed] [Google Scholar]
- 26.Cordray P, Doyle K, Edes K, Moos PJ, Fitzpatrick FA. Oxidation of 2-Cys-peroxiredoxins by arachidonic acid peroxide metabolites of lipoxygenases and cyclooxygenase-2. J Biol Chem. 2007;282:32623–9. doi: 10.1074/jbc.M704369200. [DOI] [PubMed] [Google Scholar]
- 27.Yu MK, Moos PJ, Cassidy P, Wade M, Fitzpatrick FA. Conditional expression of 15-lipoxygenase-1 inhibits the selenoenzyme thioredoxin reductase: modulation of selenoproteins by lipoxygenase enzymes. J Biol Chem. 2004;279:28028–35. doi: 10.1074/jbc.M313939200. [DOI] [PubMed] [Google Scholar]
- 28.Takebe G, Yarimizu J, Saito Y, Hayashi T, Nakamura H, Yodoi J, Nagasawa S, Takahashi K. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J Biol Chem. 2002;277:41254–8. doi: 10.1074/jbc.M202773200. [DOI] [PubMed] [Google Scholar]
- 29.Rock C, Moos PJ. Selenoprotein P regulation by the glucocorticoid receptor. Biometals. 2009;22:995–1009. doi: 10.1007/s10534-009-9251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi M, Shibata M, Niki E. Estimation of lipid peroxidation of live cells using a fluorescent probe, diphenyl-1-pyrenylphosphine. Free Radic Biol Med. 2001;31:164–74. doi: 10.1016/s0891-5849(01)00575-5. [DOI] [PubMed] [Google Scholar]
- 31.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005;25:215–35. doi: 10.1146/annurev.nutr.24.012003.132120. [DOI] [PubMed] [Google Scholar]
- 32.Mostert V. Selenoprotein P: properties, functions, and regulation. Arch Biochem Biophys. 2000;376:433–8. doi: 10.1006/abbi.2000.1735. [DOI] [PubMed] [Google Scholar]
- 33.Steinbrenner H, Alili L, Bilgic E, Sies H, Brenneisen P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med. 2006;40:1513–23. doi: 10.1016/j.freeradbiomed.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Steinbrenner H, Bilgic E, Alili L, Sies H, Brenneisen P. Selenoprotein P protects endothelial cells from oxidative damage by stimulation of glutathione peroxidase expression and activity. Free Radic Res. 2006;40:936–43. doi: 10.1080/10715760600806248. [DOI] [PubMed] [Google Scholar]
- 35.Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strauss R, Meier-Hellmann A, Insel R, Radke J, Schuttler J, Gartner R. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118–26. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]
- 36.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr, Park HK, Sanders BB, Jr, Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276:1957–63. [PubMed] [Google Scholar]
- 37.Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, Fraumeni JF, Jr, Blot WJ, Dong ZW, Taylor PR. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753–63. doi: 10.1093/jnci/92.21.1753. [DOI] [PubMed] [Google Scholar]
- 38.Nomura AM, Lee J, Stemmermann GN, Combs GF., Jr Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:883–7. [PubMed] [Google Scholar]
- 39.Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4:593–9. doi: 10.1079/phn2001143. [DOI] [PubMed] [Google Scholar]
- 40.Angstwurm MW, Gaertner R. Practicalities of selenium supplementation in critically ill patients. Curr Opin Clin Nutr Metab Care. 2006;9:233–8. doi: 10.1097/01.mco.0000222105.30795.7f. [DOI] [PubMed] [Google Scholar]
- 41.Diwadkar-Navsariwala V, Diamond AM. The link between selenium and chemoprevention: a case for selenoproteins. J Nutr. 2004;134:2899–902. doi: 10.1093/jn/134.11.2899. [DOI] [PubMed] [Google Scholar]
- 42.Irons R, Carlson BA, Hatfield DL, Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–7. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 43.Diwadkar-Navsariwala V, Prins GS, Swanson SM, Birch LA, Ray VH, Hedayat S, Lantvit DL, Diamond AM. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci U S A. 2006;103:8179–84. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tujebajeva RM, Copeland PR, Xu XM, Carlson BA, Harney JW, Driscoll DM, Hatfield DL, Berry MJ. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Rep. 2000;1:158–63. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Small-Howard A, Morozova N, Stoytcheva Z, Forry EP, Mansell JB, Harney JW, Carlson BA, Xu XM, Hatfield DL, Berry MJ. Supramolecular complexes mediate selenocysteine incorporation in vivo. Mol Cell Biol. 2006;26:2337–46. doi: 10.1128/MCB.26.6.2337-2346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard MT, Moyle MW, Aggarwal G, Carlson BA, Anderson CB. A recoding element that stimulates decoding of UGA codons by Sec tRNA[Ser]Sec. Rna. 2007;13:912–20. doi: 10.1261/rna.473907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–10. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 48.Clark LC, Hixson LJ, Combs GF, Jr, Reid ME, Turnbull BW, Sampliner RE. Plasma selenium concentration predicts the prevalence of colorectal adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 1993;2:41–6. [PubMed] [Google Scholar]
- 49.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57:1825–35. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009;1790:1478–85. doi: 10.1016/j.bbagen.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta. 1985;839:62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- 53.Weaver JA, Maddox JF, Cao YZ, Mullarky IK, Sordillo LM. Increased 15-HPETE production decreases prostacyclin synthase activity during oxidant stress in aortic endothelial cells. Free Radic Biol Med. 2001;30:299–308. doi: 10.1016/s0891-5849(00)00466-4. [DOI] [PubMed] [Google Scholar]
- 54.Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–56. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–48. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Kabuyama Y, Oshima K, Kitamura T, Homma M, Yamaki J, Munakata M, Homma Y. Involvement of selenoprotein P in the regulation of redox balance and myofibroblast viability in idiopathic pulmonary fibrosis. Genes Cells. 2007;12:1235–44. doi: 10.1111/j.1365-2443.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- 57.Nakayama A, Hill KE, Austin LM, Motley AK, Burk RF. All regions of mouse brain are dependent on selenoprotein P for maintenance of selenium. J Nutr. 2007;137:690–3. doi: 10.1093/jn/137.3.690. [DOI] [PubMed] [Google Scholar]
- 58.Yang JG, Hill KE, Burk RF. Dietary selenium intake controls rat plasma selenoprotein P concentration. J Nutr. 1989;119:1010–2. doi: 10.1093/jn/119.7.1010. [DOI] [PubMed] [Google Scholar]
- 59.Ochi H, Morita I, Murota S. Roles of glutathione and glutathione peroxidase in the protection against endothelial cell injury induced by 15-hydroperoxyeicosatetraenoic acid. Arch Biochem Biophys. 1992;294:407–11. doi: 10.1016/0003-9861(92)90704-z. [DOI] [PubMed] [Google Scholar]
- 60.Schnurr K, Belkner J, Ursini F, Schewe T, Kuhn H. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J Biol Chem. 1996;271:4653–8. doi: 10.1074/jbc.271.9.4653. [DOI] [PubMed] [Google Scholar]
- 61.Huang HS, Chen CJ, Suzuki H, Yamamoto S, Chang WC. Inhibitory effect of phospholipid hydroperoxide glutathione peroxidase on the activity of lipoxygenases and cyclooxygenases. Prostaglandins Other Lipid Mediat. 1999;58:65–75. doi: 10.1016/s0090-6980(99)00017-9. [DOI] [PubMed] [Google Scholar]