Abstract
Omega-3 (n-3) fatty acid deficiency, and elevated inflammatory signaling and central serotonin (5-HT) turnover have separately been implicated in the pathophysiology of major depressive disorder (MDD). In the present study we investigated the interrelationship between n-3 fatty acid status, pro-inflammatory signaling activity, and central 5-HT turnover in vivo. Rats were fed diets with or without the n-3 fatty acid precursor α-linolenic acid (ALA) during perinatal development (E0-P100), and a subset of rats fed the ALA- diet were switched to the ALA+ diet post-weaning (P21-P100, repletion). In adulthood (P100), plasma interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNFα), and C-reactive protein (CRP) levels were measured. Additionally, indices of liver n-6 fatty acid biosynthesis, erythrocyte fatty acid composition, and regional brain monoamine turnover were determined. Indices of liver delta-6 desaturase activity were up-regulated in n-3-deficient rats, and were associated with greater erythrocyte membrane arachidonic acid (AA, 20:4n-6) composition. Plasma IL-6 (p=0.001), TNFα (p=0.02), and CRP (p=0.001) concentrations were significantly greater in n-3-deficient rats relative to controls. The 5-HIAA/5-HT ratio was significantly greater in frontal cortex, hypothalamus, and ventral striatum of n-3-deficient rats relative to controls. Changes in membrane n-3 and n-6 fatty acid composition, elevations in plasma IL-6 and TNFα, and increased central 5-HT turnover were all prevented by normalization of n-3 fatty acid status. Erythrocyte docosahexaenoic acid (DHA, 22:6n-3) was inversely correlated, and AA and the AA/DHA ratio were positively correlated, with plasma IL-6, TNFα, and CRP levels. Plasma IL-6 levels were positively correlated with 5-HIAA/5-HT ratios in all brain regions. These preclinical data provide evidence for a functional link between n-3 fatty acid deficiency, elevated peripheral inflammatory signaling, and increased central 5-HT turnover.
Keywords: Omega-3 fatty acids, Docosahexaenoic acid (DHA), Arachidonic acid, Erythrocyte, Inflammation, Cytokines, C-reactive protein, Serotonin, Brain, Rats
1. Introduction
Evidence from preclinical studies suggest that the long-chain omega-3 (n-3) fatty acids, eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), and their bioactive lipid metabolites have potent anti-inflammatory properties [1–6]. Clinical studies have also found an inverse correlation between blood n-3 fatty acid levels and pro-inflammatory markers, including cytokines interleukin-6 (IL-6), tumor necrosis factor-α (TNFα), and the acute phase protein C-reactive protein (CRP), in healthy subjects [7,8]. Furthermore, cross-sectional studies have found that patients with major depressive disorder (MDD) exhibit significant blood EPA and/or DHA deficits [9–13], and elevated circulating IL-6, TNFα, and CRP levels [14–16]. Together these data suggest that reducing n-3 fatty acid status up-regulates pro-inflammatory signaling cascades.
One consequence of chronic dietary n-3 fatty acid deficiency is a reciprocal increase in delta-6 destaurase-mediated n-6 fatty acid and membrane arachidonic acid (AA, 20:4n-6) composition [17,18], and pharmacological blockade of delta-6 destaurase has anti-inflammatory properties in mice [19]. Importantly, immune cell membrane AA composition is positively correlated with AA-derived prostaglandin E2 (PGE2) production [1,5], and PGE2 stimulates IL-6 biosynthesis at the level of transcription via nuclear factor (NF)-κB [20,21]. Moreover, the enzymes that regulate AA→PGE2 biosynthesis are up-regulated in n-3 fatty acid deficient rats [22]. A second consequence of chronic dietary n-3 fatty acid deficiency is elevated extracellular serotonin (5-HT) concentrations, 5-hydroxyindoleacetic acid (5-HIAA) levels, and the 5-HIAA/5-HT ratio in brain [23,24]. Importantly, peripheral administration of IL-6 also increases extracellular 5-HT concentrations, 5-HIAA levels, and the 5-HIAA/5-HT ratio in rat brain [25,26]. Together these data suggest that up-regulation of the AA→PGE2→IL-6 signaling cascade may contribute to elevated central 5-HT turnover in response to n-3 fatty acid deficiency.
To our knowledge the effects of n-3 fatty acid deficiency on inflammatory signaling and central 5-HT turnover have not been systematically investigated in vivo. Evaluation in clinical populations is complicated by difficult-to-control variables that influence n-3 fatty acid status, inflammation, and/or monoamine turnover, including medications, diet, genes, and multiple life-style variables. Therefore, a more definitive evaluation requires an animal model so that these variables can be obviated or systematically manipulated, and central effects directly investigated. In the present study, we determined the effect of chronic dietary n-3 fatty acid deficiency and repletion on peripheral indices of constitutive pro-inflammatory signaling activity, including plasma inflammatory markers (IL-6, TNFα, CRP), liver n-6 fatty acid biosynthesis and membrane AA composition, and central monoamine turnover. Our central hypothesis was that n-3 fatty acid deficiency would lead to coordinated elevations in liver n-6 fatty acid biosynthesis and membrane AA composition, pro-inflammatory signaling, and central 5-HT turnover.
2. Materials and methods
2.1. Diets
Diets were either α-linolenic acid (ALA, 18:3n-3)-fortified (ALA+, TD.04285) or ALA-free (ALA-, TD.04286)(Harlan-TEKLAD, Madison, WI). Both diets were matched for all non-fat nutrients (casein (vitamin-free) 200 g/kg, L-cystine 3 g/kg, sucrose 270 g/kg, dextrose monohydrate 99.5 g/kg, corn starch 200 g/kg, maltodextrin 60 g/kg, cellulose 50 g/kg, mineral mixture AIMN-93G-MX 35 g/kg, vitamin mixture AIN-93-VX 10 g/kg, choline bitartrate 2.5 g/kg, TBHQ (antioxidant) 0.02 g/kg). Both ALA+ and ALA- diets contained n-3 fatty acid-free hydrogenated coconut (45 g/kg) and safflower (19 g/kg) oils, and the ALA+ diet additionally contained ALA-containing flaxseed oil (6 g/kg). Analysis of diet fatty acid composition by gas chromatography confirmed that the ALA- diet did not contain ALA, but was matched with the ALA+ diet in saturated fatty acids (C8:0, C10:0, C12:0, C14:0, C16:0, C18:0), monounsaturated fatty acids (18:1n-9), and n-6 fatty acids (18:2n-6, linoleic acid, 22% of total fatty acid composition)(see Table 1 in [27]). ALA represented 4.6% of total fatty acid composition in the ALA+ diet. Neither diet contained preformed long-chain n-3 or n-6 fatty acids including DHA and AA, respectively.
2.2. Animals
Male offspring bred in-house to nulliparous female Long-Evans hooded rats were used. For perinatal ALA deficiency, dams were fed the ALA- diet for 1 month prior to mating through weaning, and male offspring were maintained on the ALA- diet post-weaning (P21) to adulthood (P100). Controls were born to dams maintained on the ALA+ diet, and received the ALA+ diet post-weaning (P21) to adulthood (P100). Repleted rats were offspring of dams maintained on the ALA- diet, and switched to ALA+ diet post-weaning (P21) to adulthood (P100). Rats were housed 2 per cage with food and water available ad libitum, and maintained under standard non-barrier vivarium conditions (i.e., not specific pathogen-free) on a 12:12 h light:dark cycle. Food intake (g/kg/d) over three separate 3-day periods and endpoint body weight (kg) were determined. Rats were sacrificed by decapitation on P100 during the light portion of the cycle in a counterbalanced manner. Trunk blood was collected into EDTA-coated tubes, plasma isolated by centrifugation, and erythrocytes washed 3× with 4°C 0.9% NaCl. The brain was dissected on ice to isolate the frontal cortex (olfactory tubercle and residual striatal tissue were removed), hypothalamus, and ventral striatum, which were flash frozen in liquid nitrogen. Liver was harvested and flash frozen in liquid nitrogen. All samples were immediately stored at −80 °C. All experimental procedures were approved by the University of Institutional Animal Care and Use Committee, and adhere to the guidelines set by the National Institutes of Health.
2.3. Fatty acid composition
The gas chromatography procedure used to determine erythrocyte and liver fatty acid composition has been described in detail previously [24]. Briefly, total fatty acid composition was determined with a Shimadzu GC-2014 (Shimadzu Scientific Instruments Inc., Columbia MD). Analysis of fatty acid methyl esters was based on area under the curve calculated with Shimadzu Class VP 4.3 software. Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Composition data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All analyses were performed by a technician blinded to group identity. We focused our analysis on fatty acids previously implicated in the regulation of inflammatory signaling, including n-3 fatty acids, DHA (22:6n-3) and EPA (20:5n-3), n-6 fatty acid AA (20:4n-6), and the AA/DHA and AA/EPA ratios. We also determined liver linoleic acid (LA, 18:2n-6) composition, the γ-linolenic acid (18:3n-6)/LA ratio (an index of delta-6 desaturase activity), and the AA/LA ratio as an index of desaturase/elongase-mediated LA→AA biosynthesis.
2.4. Plasma cytokine and CRP levels
Plasma cytokine (pg/ml) and CRP (ng/ml) concentrations were determined with a multiplexing suspension array and flow-cytometry based analyzer Luminex™ 100 IS (MiraiBio, South San Francisco, CA) using a LINCOplex Cytokine/Chemokine Luminex® Bead immunoassay Kit according to manufacturer’s protocol (LINCO Research, St. Charles, MO). All analyses were performed by a technician blinded to group identity.
2.5. HPLC-ECD
Monoamine concentrations (ng/mg protein) in the frontal cortex, hypothalamus, and ventral striatum were determined by high performance liquid chromatography with electrochemical detection (HPLC-ECD), as previously described [24]. Briefly, an antioxidant solution (0.4 N perchlorate, 1.34 mM ethylenediaminetetraacetic acid (EDTA) and 0.53 mM sodium metabisulfite) was added to the samples followed by homogenization using an ultrasonic tissue homogenizer (Biologics, Gainesville, VA). A fraction of the tissue homogenate was dissolved in 2% sodium dodecyl sulfate (SDS) (w/v) for protein determination (Pierce BCA Protein Reagent Kit, Rockford, IL). The remaining suspension was spun at 14,000 × g for 20 min in a refrigerated centrifuge. The supernatant was reserved for HPLC. Samples were separated on a Microsorb MV C-18 column (5 μm, 4.6×250 mm, Varian, Walnut Creek, CA) and simultaneously examined for 5-HT and 5-HIAA. Compounds were detected using a 12-channel coulometric array detector (CoulArray 5200, ESA, Chelmsford, MA) attached to a Waters 2695 Solvent Delivery System (Waters, Milford, MA). We focused our analysis on indices of serotonin turnover, 5-hydroxyindoleacetic acid (5-HIAA)/serotonin (5-HT), dopamine turnover, dopamine 3,4-dihydroxyphenylacetic acid (DOPAC)/dopamine (DA), and norepinephrine turnover, 3-methoxy-4-hydroxyphenylethylenglycol (MHPG)/norepinephrine (NE).
2.6. Statistical analysis
Group (control, deficient, repleted) differences in fatty acid composition, plasma inflammatory markers, and monoamine ratios were evaluated with a one-way ANOVA, and pairwise comparisons made with unpaired t-tests (2-tail, α=0.05). Non-parametric (Spearman) correlation analyses were performed to determine relationships between erythrocyte fatty acid composition, inflammatory markers, and monoamine ratios (2-tail, α=0.05). Analyses were performed with GB-STAT (V.10, Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Food intake and body weight
There were no significant group differences in food intake, F(2,44)=0.6, p=0.58 (CON: 60.8±2.6; DEF: 58±2.5; REP: 60.0±2.1 g/kg/d), or endpoint (P100) body weight, F(2,29)=1.1, p=0.34 (CON: 461±12; DEF: 431±14; REP: 441±14 kg).
3.2. Erythrocyte fatty acid composition
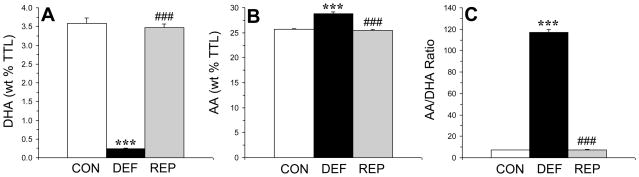
There was a significant main effect of treatment (diet) on DHA composition, F(2,29)=411, p≤0.0001 (Fig. 1A). DHA composition was significantly lower in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.52). The main effect of treatment was significant for arachidonic acid (AA, 20:4n-6) composition, F(2,29)=76.1, p≤0.0001 (Fig. 1B). AA composition was significantly greater in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.44). The main effect of treatment was significant for the AA/DHA ratio, F(2,29)=1587, p≤0.0001 (Fig. 1C). The AA/DHA ratio was significantly greater in n-3-deficient rats compared with both control (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.72). The main effect of treatment was also significant for the AA/EPA ratio, F(2,29)=1850, p≤0.0001 (data not shown). The AA/EPA ratio was significantly greater in n-3-deficient rats compared with both control (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.67).
Figure 1.
Erythrocyte compositions (wt % total fatty acid composition) of docosahexaenoic acid (DHA, 22:6n-3)(A) and arachidonic acid (AA, 20:4n-6)(B) and the AA/DHA ratio (C) in control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group means ± S.E.M. ***p≤0.0001 vs. controls, ###p≤0.0001 vs. DEF rats.
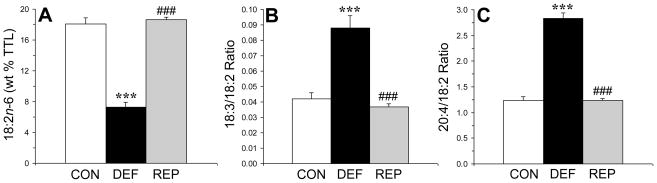
3.3. Liver n-6 fatty acid biosynthesis
There was a significant main effect of treatment on liver linoleic acid (LA, 18:2n-6) composition, F(2,29)=110, p≤0.0001 (Fig 2A). Liver LA composition was significantly lower in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.55). There was a significant main effect of treatment on the liver γ-linolenic acid (GLA)/LA ratio, F(2,29)=27.3, p≤0.0001 (Fig 2B). The GLA/LA ratio was significantly greater in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.40). There was a significant main effect of treatment on the AA/LA ratio, F(2,29)=119, p≤0.0001 (Fig 2C). The AA/LA ratio was significantly greater in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.45). Similarly, the erythrocyte AA/LA ratio was significantly greater in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001)(data not shown).
Figure 2.
Linoleic acid (18:2n-6) composition (wt % total fatty acid composition)(A), the γ-linolenic acid (GLA, 18:3n-6)/LA ratio (an index of delta-6 desaturase activity)(B), and the arachidonic acid (AA, 20:4n-6)/LA ratio (an index of LA→AA biosynthesis)(C), in the liver of control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group means ± S.E.M. ***p≤0.0001 vs. controls, ###p≤0.0001 vs. DEF rats.
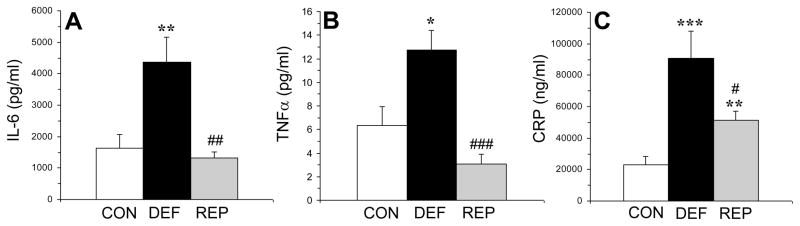
3.4. Plasma inflammatory markers
There was a significant main effect for plasma IL-6 concentrations (pg/ml), F(2,29)=8.6, p=0.001 (Fig. 3A). IL-6 was significantly higher in n-3-deficient rats compared with both controls (p=0.001) and repleted rats (p=0.004), and did not differ between control and repleted rats (p=0.57). There was a significant main effect for plasma TNFα concentrations (pg/ml), F(2,29)=11.9, p=0.0003 (Fig. 3B). TNFα levels were significantly higher in n-3-deficient rats compared with both controls (p=0.02) and repleted rats (p≤0.0001), and did not differ between controls and repleted rats (p=0.12). There was a significant main effect for plasma CRP concentrations (ng/ml), F(2,29)=11.4, p=0.0005 (Fig. 3C). CRP levels were significantly higher in n-3-deficient rats compared with both controls (p=0.001) and repleted rats (p=0.03), and was significantly greater in repleted rats relative to controls (p=0.003).
Figure 3.
Plasma concentrations of interleukin-6 (IL-6, pg/mL)(A), tumor necrosis factor-alpha (TNFα, pg/ml)(B), and C-reactive protein (CRP, ng/ml)(C) in control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group mean ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.0001 vs. controls, #p≤0.05, ##p≤0.001, ###p≤0.0001 vs. DEF rats.
3.5. Correlations between erythrocyte fatty acids and plasma inflammatory markers
Among all rats (n=30), erythrocyte DHA composition was inversely correlated with plasma IL-6 (r = −0.50, p=0.007), TNFα (r = −0.54, p=0.003), and CRP (r = −0.23, p=0.03) concentrations. In contrast, erythrocyte AA composition was positively correlated with plasma IL−6 (r = +0.54, p=0.003) and TNFα (r = +0.40, p=0.03), and a positive trend was observed for CRP (r = +0.29, p=0.17). The erythrocyte AA/DHA ratio was positively correlated with plasma IL-6 (r = +0.47, p=0.01), TNFα (r = +0.45, p=0.01), and CRP (r = +0.45, p=0.03) concentrations. The erythrocyte AA/EPA ratio was positively correlated with plasma IL-6 (r = +0.50, p=0.008), TNFα (r = +0.45, p=0.01), and CRP (r = +0.42, p=0.04) concentrations.
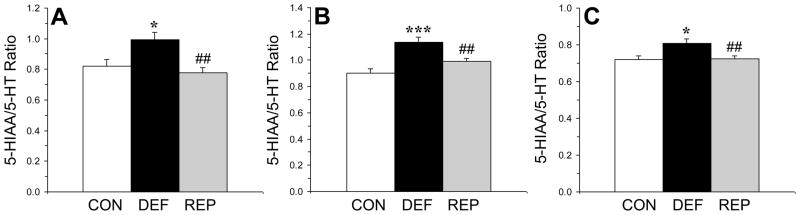
3.6. Regional brain monoamine turnover
There was a significant main effect for the 5-HIAA/5-HT ratio in the frontal cortex, F(2,29)=7.2, p=0.003 (Fig. 4A). In the frontal cortex, the 5-HIAA/5-HT ratio was significantly greater in n-3-deficient rats compared with both controls (p=0.02) and repleted rats (p=0.002), and did not differ between controls and repleted rats (p=0.45). There was a significant main effect for the 5-HIAA/5-HT ratio in the hypothalamus, F(2,29)=13.7, p≤0.0001 (Fig. 4B). In the hypothalamus, the 5-HIAA/5-HT ratio was significantly greater in n-3-deficient rats compared with both controls (p=0.0003) and repleted rats (p=0.005), and was greater in repleted rats compared with controls (p=0.02). There was a significant main effect for the 5-HIAA/5-HT ratio in the ventral striatum, F(2,29)=5.8, p=0.008 (Fig. 4C). In the ventral striatum, the 5-HIAA/5-HT ratio was significantly greater in n-3-deficient rats compared with both controls (p=0.01) and repleted rats (p=0.008), and did not differ between controls and repleted rats (p=0.91). The main effect of treatment was significant for DOPAC/DA in the frontal cortex, F(2,29)=4.7, p=0.01, but not ventral striatum, F(2,29)=0.9, p=0.43, or hypothalamus F(2,29)=2.6, p=0.09. In the frontal cortex, the DOPAC/DA ratio was significantly greater in n-3-deficient rats compared with repleted rats (p=0.01), but not controls (p=0.1), and did not differ between controls and repleted rats (p=0.17). The main effect of treatment was not significant for the MHPG/NE ratio in the ventral striatum, F(2,29)=2.9, p=0.07, frontal cortex, F(2,29)=0.7, p=0.48, or hypothalamus F(2,29)=0.6, p=0.57.
Figure 4.
Serotonin turnover (5-HIAA/5-HT) in the frontal cortex (A), hypothalamus (B), and ventral striatum (C) of control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group mean ± S.E.M. *p≤0.05, ***p≤0.001 vs. controls, ##p≤0.01 vs. DEF rats.
3.7. Correlations between peripheral markers and brain 5-HT turnover
Among all rats (n=30), plasma IL-6 concentrations were positively correlated with the 5-HIAA/5-HT ratio in the frontal cortex (r = +0.37, p=0.04), hypothalamus (r = +0.38, p=0.04), and ventral striatum (r = +0.48, p=0.01). Plasma TNFα concentrations were not significantly correlated with the 5-HIAA/5-HT ratio in the frontal cortex (r = +0.27, p=0.17), hypothalamus (r = +0.05, p=0.78), or ventral striatum (r = +0.15, p=0.43). Plasma CRP concentrations were not significantly correlated with the 5-HIAA/5-HT ratio in the frontal cortex (r = +0.11, p=0.63), hypothalamus (r = +0.19, p=0.38), or ventral striatum (r = +0.18, p=0.42). The 5-HIAA/5-HT ratio in the frontal cortex was inversely correlated with erythrocyte DHA composition (r = −0.56, p=0.004), and positively correlated with erythrocyte AA composition (r = +0.57, p=0.002), and the AA/DHA (r = +0.66, p=0.0002) and AA/EPA (r = +0.62, p=0.0006) ratios.
4. Discussion
The principal finding of the present study is that chronic dietary n-3 fatty acid deficiency significantly increased constitutive pro-inflammatory cytokine (IL-6, TNFα) and CRP production in rats. Chronic n-3 fatty acid deficiency also up-regulated liver n-6 fatty acid biosynthesis, and increased membrane AA composition and regional brain 5-HT turnover. Changes in erythrocyte DHA and AA composition, elevations in IL-6 and TNFα, and increases in central 5-HT turnover were all prevented by normalization of n-3 fatty acid status. Erythrocyte DHA composition was inversely correlated, and erythrocyte AA and the AA/DHA and AA/EPA ratios were positively correlated, with plasma IL-6, TNFα, and CRP levels. Moreover, plasma IL-6, but not TNFα or CRP, levels were positively correlated with 5-HT turnover all brain regions investigated. These preclinical data provide evidence that n-3 fatty acid deficiency, and associated elevations in AA biosynthesis, up-regulate constitutive pro-inflammatory signaling activity in vivo, and that these peripheral measures are positively associated with central 5-HT turnover. To our knowledge, this is the first demonstration of a link between n-3 fatty acid status, peripheral pro-inflammatory signaling, and central 5-HT turnover.
The present data are consistent with the prior finding that n-3 fatty acid deficiency is associated with greater elevations in plasma IL-6 production in response to peripheral lipopolysaccharide administration in mice [6], and additionally demonstrate that constitutive pro-inflammatory cytokine production is also elevated in response to n-3 fatty acid deficiency. It is notable that the basal plasma IL-6 levels observed in control and repleted rats in the present study are greater than those observed in control mice in the latter study [6]. This may be due in part to differences in ambient immune activators in the housing colony, diet, and/or species. Additional studies are warranted to determine whether the present findings can be replicated in rodents maintained under barrier (i.e., specific pathogen-free) housing conditions.
Consistent with a prior study, the activity of delta6-desaturase, the rate-limiting enzyme mediating both n-6 and n-3 fatty acid biosynthesis, was up-regulated in liver in response to n-3 fatty acid deficiency [17]. Elevations in n-6 fatty acid biosynthesis were associated with elevations in erythrocyte membrane AA composition which were positively correlated with plasma pro-inflammatory markers including IL-6. Additionally, we found that the IL-6-induced acute phase protein CRP was also elevated in response to n-3 fatty acid deficiency. Elevations in liver delta6-desaturase activity, membrane AA composition, and increased IL-6 production were all prevented by normalization of n-3 fatty acid status. These findings are consistent with prior studies finding that elevations in membrane AA composition and the AA/DHA and AA/EPA ratios increase pro-inflammatory signaling activity [1,6,22], and that blockade of delta-6 destaurase-mediated n-6 fatty acid biosynthesis has anti-inflammatory properties [19].
Consistent with our prior study [24], perinatal n-3 fatty acid deficiency was associated with elevated 5-HIAA/5-HT ratios in all brain regions examined and were normalized in n-3 fatty acid repleted rats. Based on prior studies finding that peripheral administration of IL-6 significantly increases the 5-HIAA/5-HT ratio in rat brain [25,26], we evaluated the relationship between plasma IL-6 concentrations and regional brain 5-HT turnover. It was found that plasma IL-6, but not TNFα or CRP, concentrations were positively correlated with 5-HT turnover in the frontal cortex, hypothalamus, and ventral striatum. Furthermore, 5-HT turnover was positively correlated with erythrocyte AA composition and the AA/DHA ratio. Prior in vivo microdialysis studies suggest that central elevations in 5-HT turnover following IL-6 administration [26] and n-3 fatty acid deficiency [23] are attributable to higher extracellular 5-HT concentrations. Together, these data suggest that constitutive elevations in IL-6 production secondary to n-3 fatty acid deficiency are also associated with increases in central 5-HT release and metabolism. In view of prior evidence that n-3 fatty acid deficiency increases central pro-inflammatory signaling cascades [22], future studies are warranted to evaluate whether elevations in brain cytokine production are also associated with increased 5-HT turnover.
The present preclinical findings are consistent with prior clinical studies finding an inverse correlation between blood n-3 fatty acid levels and pro-inflammatory markers, including IL-6, TNFα, and CRP, in healthy subjects [7,8]. Moreover, patients with MDD exhibit significant blood EPA and/or DHA deficits [9–13], elevated indices of n-6 fatty acid biosynthesis [12], elevated pro-inflammatory cytokine (IL-6, TNFα) and CRP production [14–16], and elevations in central 5-HT turnover [28,29] relative to healthy controls. Furthermore, n-3 fatty acid deficiency and elevated CRP levels are also associated with increased risk for coronary heart disease (CHD), a leading cause of mortality in MDD patients [30]. Taken collectively, these data suggest that elevated pro-inflammatory cytokine production and central 5-HT turnover represent a plausible mechanism by which n-3 fatty acid deficiency may contribute to the pathophysiology of MDD and comorbid CHD (see Fig. 5).
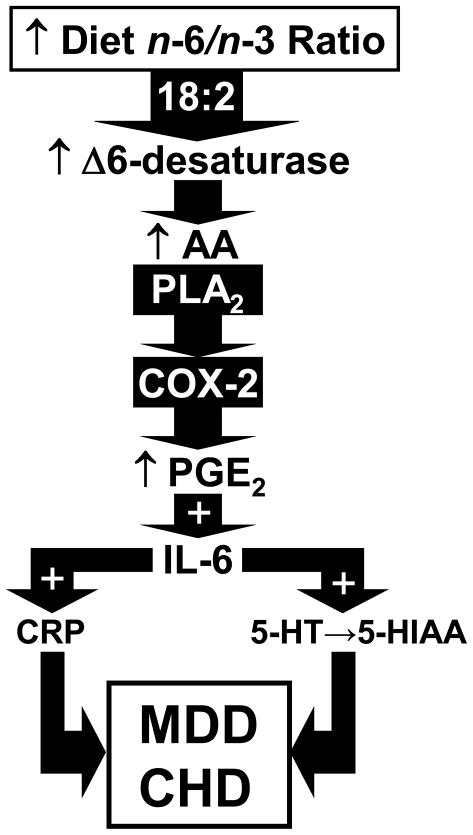
Figure 5.
Diagram illustrating proposed pro-inflammatory signaling events resulting from n-3 fatty acid deficiency. According to this model, n-3 fatty acid deficiency up-regulates liver delta6-desaturase activity, and increases n-6 fatty acid biosynthesis (18:2→18:3, 18:2→20:4) and membrane AA (20:4) composition. Increased AA availability and mobilization via phospholipase A2 (PLA2) augments AA→PGE2 production via cyclooxygenase-2 (COX-2), and increases PGE2→IL-6 production. Increased IL-6→CRP production contribute to cardiovascular-related pathology and IL-6→5-HT turnover contribute to depressed mood in MDD patients.
In summary, the present study demonstrates n-3 fatty acid deficiency up-regulates n-6 fatty acid biosynthesis and membrane AA composition, pro-inflammatory cytokine (IL-6, TNFα) and CRP production, and central 5-HT turnover in rats. Elevations in erythrocyte AA composition, plasma IL-6 concentrations, and central 5-HT turnover were all prevented by normalization of n-3 fatty acid status and were positively correlated. These preclinical data demonstrate that the n-3 fatty acid deficiency rat model recapitulates several important features associated with the pathophysiology of MDD, and support a pathogenic signaling model that warrants further evaluation in future clinical trials.
Acknowledgments
This work was supported in part by National Institutes of Health grants MH073704 and MH074858 to R.K.M., DK59630 to P.T., and DA017399 to J.W.L. The authors thank the laboratory of Dr. M. Wills-Karp for performing the cytokine assays.
Role of Funding Source. Funding for this study was provided by National Institutes of Health grants MH073704 and MH074858 to R.K.M., DK59630 to P.T., and DA017399 to J.W.L.; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest Statement. None of the authors have any actual or potential conflict of interest, including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted that could inappropriately influence, or be perceived to influence, their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997;400:589–597. [PubMed] [Google Scholar]
- 4.Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8:115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 fatty acids on interleukin-1beta-induced anxiety, stress, and inflammatory responses in rats. J Lipid Res. 2003;44:1984–1991. doi: 10.1194/jlr.M300217-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Mingam R, Moranis A, Bluthé RM, De Smedt-Peyrusse V, Kelley KW, Guesnet P, Lavialle M, Dantzer R, Layé S. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur J Neurosci. 2008;28:1877–1886. doi: 10.1111/j.1460-9568.2008.06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 8.Micallef MA, Munro IA, Garg ML. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr. 2009;63:1154–1156. doi: 10.1038/ejcn.2009.20. [DOI] [PubMed] [Google Scholar]
- 9.Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One. 2010;5:10635. doi: 10.1371/journal.pone.0010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 11.Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 12.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010 doi: 10.1016/j.jad.2010.03.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 14.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 15.Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O, Duncko R, Drevets WC, Manji HK, Charney DS, Gold PW, Neumeister A. Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biol Psychiatry. 2007;62:309–313. doi: 10.1016/j.biopsych.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N., Jr Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res. 2001;42:419–427. [PubMed] [Google Scholar]
- 19.Obukowicz MG, Welsch DJ, Salsgiver WJ, Martin-Berger CL, Chinn KS, Duffin KL, Raz A, Needleman P. Novel, selective delta6 or delta5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. J Pharmacol Exp Ther. 1998;287:157–166. [PubMed] [Google Scholar]
- 20.Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298:1445–1456. doi: 10.1152/ajpcell.00508.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 23.Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- 24.McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: Dissociation from estrogenic effects. J Psychiatry Res. 2009;43:656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Dunn AJ. Mouse interleukin-6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem Int. 1998;33:143–154. doi: 10.1016/s0197-0186(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Terreni L, De Simoni MG, Dunn AJ. Peripheral interleukin-6 administration increases extracellular concentrations of serotonin and the evoked release of serotonin in the rat striatum. Neurochem Int. 2001;38:303–308. doi: 10.1016/s0197-0186(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 27.McNamara RK, Sullivan J, Richtand NM. Omega-3 fatty acid deficiency augments the development of behavioral sensitization in adult mice: Prevention by chronic lithium treatment. J Psychiatric Res. 2008;42:458–468. doi: 10.1016/j.jpsychires.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, Socratous F, Hastings J, Guo L, Wiesner G, Kaye DM, Bayles R, Schlaich MP, Lambert GW. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch Gen Psychiatry. 2008;65:38–46. doi: 10.1001/archgenpsychiatry.2007.11. [DOI] [PubMed] [Google Scholar]
- 29.Sheline Y, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1997;17:11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 30.McNamara RK. Membrane omega-3 fatty acid deficiency as a preventable risk factor for comorbid coronary heart disease in major depressive disorder. Cardiovasc Psychiatry Neurology. 2009;9:1–13. doi: 10.1155/2009/362795. [DOI] [PMC free article] [PubMed] [Google Scholar]