Abstract
Nitrous oxide (N2O)-induced antinociception is thought to result from nitric oxide (NO)-dependent neuronal release of endogenous opioid peptides in the central nervous system. The present study employed microdialysis to determine whether exposure to N2O stimulates proopiomelanocortin (POMC) neurons to release β-endorphin in the arcuate nucleus (ARC) of the hypothalamus and the periaqueductal gray (PAG) of the midbrain. Male Sprague Dawley rats were stereotaxically implanted with microdialysis probes in the ARC or PAG. Exposure to 70% N2O significantly increased dialysate levels of oxidation products of NO as well as β-endorphin, compared to levels in fractions collected under room air. These increases in the ARC and PAG were abolished by systemic pretreatment with L-NG-nitro arginine methyl ester (L-NAME). These findings suggest an association between increased NO activity and the stimulated release of β-endorphin during exposure of rats to N2O.
Keywords: Nitrous oxide, microdialysis, β-endorphin, nitric oxide, arcuate nucleus, periaqueductal gray, rat
1. Introduction
N2O is a widely used anesthetic gas with several important clinical applications. It is a weak general anesthetic agent and can induce anesthesia only at hyperbaric pressures (Eger, 1985). But, at normobaric pressures, N2O is generally considered an analgesic drug that supports inhalational and non-inhalational anesthetics for the range of anesthetic techniques from general anesthesia to moderate sedation (formally known as conscious sedation) in both medicine and dentistry (Jackson and Johnson, 2002; Parlow et al., 2005; Bishop, 2007).
An involvement of endogenous opioid receptors in N2O-induced analgesia is evidenced by the observation that N2O antinociception in experimental animals was antagonized by naloxone and other opioid receptor blockers (Berkowitz et al., 1976, 1977; Zuniga et al., 1987b; Quock et al., 1990, 1993). It has been directly demonstrated in vivo and in vitro that N2O exposure can stimulate the neuronal release of endogenous opioid peptides (Quock et al., 1985; Zuniga et al., 1987a). Antagonism of N2O-induced antinociception in the rat hot plate test by pretreatment with an antiserum to β-endorphin attests to a mediatory role of this opioid peptide in the antinociceptive response (Hara et al., 1994). We also previously reported that the antinociceptive effect of N2O in both rats and mice was also antagonized by inhibition of the enzyme nitric oxide synthase (NOS) and interference with NO production (McDonald et al., 1994; Ishikawa and Quock, 2003a; Li et al., 2004). Based on the ability of NOS-inhibitors to interfere with β-endorphin-stimulated neuronal release of methionine-enkephalin in the rat spinal cord (Hara et al., 1995), we suggested that NO might modulate the neuronal release of endogenous opioid peptides.
In a recent study, we reported that exposure to N2O increased levels of β-endorphin and oxidation products of NO in the artificial cerebrospinal fluid perfusate collected from urethane-anesthetized rats (Zelinski et al., 2009). These effects were antagonized by systemic pretreatment with an NOS-inhibitor suggesting that N2O may stimulate an NO-dependent neuronal release of β-endorphin. The present study was conducted to examine some possible brain sites of action of N2O. Microdialysis experiments were conducted to test the hypothesis that N2O stimulates pro-opiomelanocortin (POMC) neurons to release β-endorphin in the arcuate nucleus (ARC) of the hypothalamus and the periaqueductal gray (PAG) of the midbrain in an NO-dependent manner.
2. Results
2.1. Microdialysis experiments in the ARC
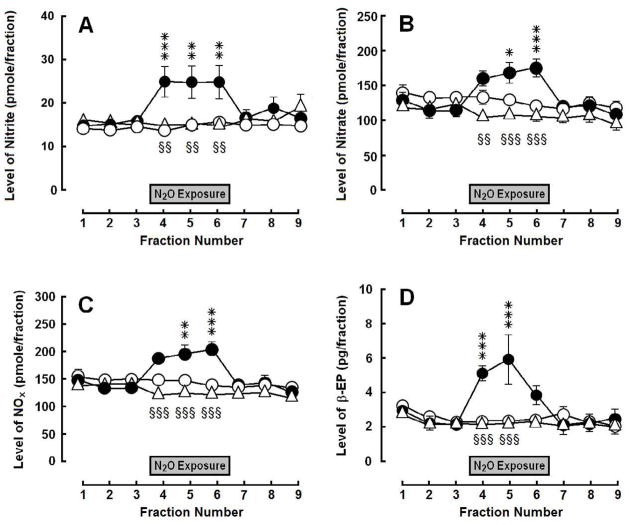
Exposure to 70% N2O increased dialysate levels of total oxidation products of NO (NOX−)—defined as nitrite (NO2−) plus nitrate (NO3−)—and β-endorphin in the ARC (Fig. 1). These N2O-induced increases were reversed by i.p. pretreatment with 50 mg/kg L-NAME 30 min prior to N2O exposure [NOx: group, F(2,176) = 2.52, p > 0.05; time, F(8,176) = 9.49, p < 0.0001; interaction, F(16,176) = 10.07, p < 0.0001; nitrite: group, F(2,176) = 2.74, p = 0.087; time, F(8,176) = 3.34, p < 0.005; interaction, F(16,176) = 4.70, p < 0.0001; and nitrate: group, F(2,176) = 2.18, p > 0.05; time, F(8,176) = 8.53, p < 0.0001; interaction, F(16,176) = 7.66, p < 0.0001; β-endorphin: group, F(2,168) = 3.96, p < 0.05; time, F(8,168) = 5.96, p < 0.0001; interaction, F(16,168) = 6.54, p < 0.0001].
Fig. 1.
Influence of N2O treatment on (A) nitrite, (B) nitrate, (C) total NOx and (D) β-endorphin (β-EP) levels in dialysate collected from the ARC: control (open circles), vehicle + N2O (closed circles) and L-NAME + N2O (open triangles). Each symbol represents the mean ± SEM (pmol/fraction) of 8–9 rats per group. Each fraction represents a 20-min collection sample. The gray box indicates the 60-min exposure to 70% N2O. Significance of difference: *, p < 0.05, **, p < 0.01, ***, p < 0.001, vehicle + N2O group compared to control group; and §§, p < 0.01, §§§, p < 0.001, L-NAME + N2O group compared to vehicle + N2O group (Bonferroni’s multiple comparison test).
2.2. Microdialysis experiments in the PAG
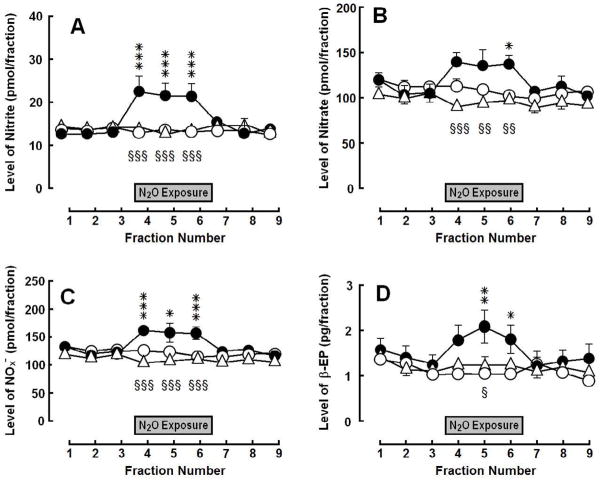
Exposure to 70% N2O increased dialysate levels of NOx and β-endorphin in samples collected from the PAG (Fig. 2). Systemic pretreatment with L-NAME abolished the N2O-induced increases [NOx: group, F(2,168) = 3.79, p = 0.0393; time, F(8,168) = 4.50, p < 0.0001; interaction, F(16,168) = 5.11, p < 0.0001; nitrite: group, F(2,168) = 10.49, p = 0.0007; time, F(8, 168) = 3.43, p = 0.0011; interaction, F(16,168) = 4.17, p < 0.0001; and nitrate: group, F(2, 168) = 2.83, p = 0.0816; time, F(8,168) = 3.19, p = 0.0021; interaction, F(16,168) = 3.15, p = 0.0001; β-endorphin: group, F(2,168) = 1.63, p = 0.219; time, F(8,168) = 5.61, p < 0.0001; interaction, F(16,168) = 4.07, p < 0.0001].
Fig. 2.
Influence of N2O treatment on (A) nitrite, (B) nitrate, (C) total NOx and (D) β-endorphin (β-EP) levels in dialysate collected from the PAG: control (open circles), vehicle + N2O (closed circles) and L-NAME + N2O (open triangles). Each symbol represents the mean ± SEM (pmol/fraction) of 8–9 rats per group. Each fraction represents a 20-min collection sample. The gray box indicates the period of the 60-min exposure to 70% N2O. Significance of difference: *, p < 0.05, **, p < 0.01, ***, p < 0.001, vehicle + N2O group compared to control group; and §, p < 0.05, §§, p < 0.01, §§§, p < 0.001, L-NAME + N2O group compared to vehicle + N2O group (Bonferroni’s multiple comparison test).
3. Discussion
A major concentration of POMC neurons in the brain lies in the ARC of the basal hypothalamus (Bloom et al., 1978). POMC neurons in the ARC project axons into the PAG (Pilcher et al., 1988; Yoshida and Taniguchi, 1988) as high levels of β-endorphin are found in the PAG (Palkovits and Eskay, 1987). Electrical stimulation of the ARC was reported to increase release of β-endorphin (Bach and Yaksh, 1995; Bach 1997) as did exposure to stress or ethanol (Marinelli et al., 2004). In addition, there is evidence that this ARC-PAG pathway is of pharmacological importance in the relief of pain. l-Tetrahydropalmatine-induced antinociception in rabbits was antagonized after lesioning of the ARC or following intra-PAG pretreatment with naloxone (Hu and Jin, 2000). Microinjection of galanin into the ARC of rats elicited an antinociceptive effect that was antagonized by naloxone or β-funaltrexamine microinjected into the PAG (Sun et al., 2007).
Previous research had already drawn our attention to the possible role of β-endorphin and the ARC-PAG pathway in in N2O-induced antinociception. Zuniga et al. (1987a) elegantly demonstrated that exposure to N2O increased the release of β-endorphin from superfused basal hypothalamic cells attached to cytodex microcarrier beads. Intracerebroventricular pretreatment of rats with a rabbit antiserum to β-endorphin resulted in reduction of N2O-induced antinociception in the hot plate test (Hara et al., 1994).
Kainic acid lesions of the ventral and caudal PAG caused significant antagonism of the antinociceptive response of rats to N2O (Zuniga et al., 1987b). Intra-PAG microinjection of the μ-selective opioid antagonist D-Phe-Cys-Tyr-D-Trp-Om-Thr-Pen-Thr-NH2 produced a dose-related reduction in the intensity of N2O-induced antinociception in the rat hot plate test (Hodges et al., 1994). Finally, pretreatment with the irreversible opioid receptor antagonist β-chlornaltrexamine in the PAG caused a dose-related antagonism of N2O-induced antinociception in the mouse abdominal constriction test (Emmanouil et al., 2008).
The present study demonstrates that exposure to N2O increases extracellular levels of β-endorphin from both the ARC and PAG. There is a simultaneous increase in dialysate levels of both nitrite and nitrate in samples collected from both brain regions. A relationship between the increase in oxidation products of NO and the release of β-endorphin is suggested by abolition of both responses following inhibition of NO production. This is consistent with the conclusion of an earlier study, in which NO was implicated in the mechanism by which i.c.v. β-endorphin-induced neuronal release of methionine-enkephalin in intrathecally-perfused rats (Hara et al., 1995). The amount of methionine-enkephalin released into the spinal cord was reduced by 50% when the NOS-inhibitor, L-NG-nitro arginine (L-NOARG), was added to the artificial cerebrospinal fluid perfusate; this reduction was reversed by adding L-arginine to the already present L-NOARG in the perfusate.
The actual mechanism by which N2O stimulates release of the opioid peptide is not known. However, previous studies have demonstrated that exposure to N2O causes an increase of NOS enzyme activity, which is correlated to the antinociceptive effect (Ishikawa and Quock, 2003b; Henry et al., 2005). These results suggest that N2O might cause a direct activation of NOS but this remains to be proven.
Other research has demonstrated that intracerebroventricular administration of the NO precursor L-arginine or the NO-donor 3-morpholinosydnoimine (SIN-1) evoked an antinociceptive effect in the mouse abdominal constriction test that was antagonized by a rabbit antiserum against rat dynorphin1-13, suggesting that increased production or presence of NO may stimulate release of dynorphin to cause antinociception (Chung et al., 2006). A species or test specificity in the opioid peptide that mediates N2O-induced antinociception is likely. While β-endorphin and μ opioid receptors are implicated in the rat hot plate test (Hara et al., 1994; Hodges et al., 1994), there is strong evidence that dynorphin and κ opioid receptors mediate N2O-induced antinociception in the mouse abdominal constriction test (Quock et al., 1990, Quock and Mueller, 1991; Branda et al., 2000; Cahill et al., 2000).
There is also evidence in the literature that N2O appears to stimulate release of the hypophysiotropic hormone/neurotransmitter corticotrophin-releasing factor (CRF) (Sawamura et al., 2003). N2O exposure causes a concentration-related increase in the c-Fos expression of CRF-positive neurons in the paraventricular nucleus of the hypothalamus; this effect was antagonized by intracerebroventricular pretreatment with α-helical CRF9-41, a CRF receptor antagonist. Alpha-helical CRF9-41 pretreatment also abolished the antinociceptive effect of N2O in the rat tail flick test (Sawamura et al., 2003). It has been hypothesized that N2O exposure stimulates CRF release, which, in turn, stimulates endogenous opioid peptide release in the PAG (Sanders et al., 2008).
It is concluded that N2O stimulates NO-dependent release of β-endorphin from POMC neurons in both the ARC and PAG. The relative contribution of β-endorphin released from each site to the antinociceptive response remains to be seen.
4. Experimental procedures
4.1. Animals
Male Sprague Dawley rats, 250–300 g body weight, were obtained from Harlan Laboratory (Indianapolis, IN). Rats were housed two per cage with food and water available ad libitum in the Wegner Hall Vivarium at Washington State University, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The facility was maintained on a 12-h light/dark cycle (lights on 0700–1900) under standard conditions (22 ± 1°C room temperature, 33% humidity). Rats were kept in the holding room for at least four days following arrival in the facility.
This research was approved by the Institutional Animal Care and Use Committee (IACUC) of Washington State University with post-approval review and was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy Press, 1996) as adopted and promulgated by the U.S. National Institutes of Health. All measures to minimize pain or discomfort were taken by the investigators.
4.2. Intracerebral cannulation procedures
Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (15 mg/kg). Then each rat was individually mounted in a stereotaxic system with electronic digital measuring scales and digital readouts that permit extremely accurate insertion and placement of microdialysis cannulae to within 1.0 μm of the target site (Cartesian Research, Inc., Sandy, OR). 23-Gauge CG-I-8 guide cannulae (Eicom, Kyoto, Japan) were implanted into the ARC (stereotaxic coordinates: −3.0 mm AP, 0.2 mm L and −8.0 mm DV) or the PAG (stereotaxic coordinates: −6.3 mm AP, 0.2 mm L and −4.0 mm DV) for microdialysis experiments (Paxinos and Watson, 2006). Each guide cannula was plugged with a CD-I-8 dummy cannula (Eicom) and cap nut. After surgery, rats were allowed a minimum of five days recovery time before insertion of the dialysis probe.
4.3. Microdialysis procedures
On the day prior to the microdialysis experiment, the rats were lightly anesthetized with 2.5% isoflurane, U.S.P. (Butler Animal Health Supply, Dublin, OH), and C-I-8-02HP microdialysis probes (Eicom) with 2-mm-long AN69 polyacrylonitrile membranes (100 kDa cutoff; 0.32 mm outer diameter) were inserted into the guide cannulae. Probes were continuously perfused with artificial cerebrospinal fluid (aCSF) containing 147 mM NaCl, 4 mM KCl, 1 mM MgCl2·6H2O, 2.3 mM CaCl2·2H2O (pH 7.3–7.5). Probes were attached to TSU-20 dual-channel liquid swivels (Eicom) with fluoroethylene polypropylene tubing in cylindrical cages to permit free movement of the animal. After rats recovered from anesthesia, the aCSF flow rate was set at 1.0 μl/min. After a 120-min washout, 20-μl dialysis samples were collected every 20 min before, during and following a 60-min exposure to N2O/O2 or breathing air (all medical gases from A-L Compressed Gases, Inc., Spokane, WA) for a total of nine samples. After collection, samples for β-endorphin were immediately stored on dry ice and later frozen at −80°C until analyzed.
4.4. Exposure to N2O
N2O and O2 were mixed and delivered using a dental-sedation system (Porter, Hatfield, PA) at a total flow rate of 10 L/min. Rats were individually exposed in a Plexiglas® exposure chamber (20 cm W × 35 cm L × 15 cm H), through which circulated compressed breathing air during the 120-min washout and 60-min pre-exposure periods followed by 70% N2O/30% O2 during the 60-min exposure period, and compressed breathing air again during the 60-min post-exposure period. The concentrations of N2O and O2 delivered into the box were monitored using a POET II® anesthetic monitoring system (Criticare, Milwaukee, WI). Control rats received compressed breathing air throughout the 300-min session. Exhausted gases were routed by polyethylene tubing to a nearby fume hood.
4.5. Quantification of oxidation products of NO (NOx−)
An index of NO was determined by measuring the formation of nitrite and nitrate or collectively NOX−. Because NO in oxygen-containing solutions is chemically unstable and undergoes rapid oxidation to nitrite, it was necessary to measure both nitrite and nitrate to accurately determine the level of total NO. The perfused dialysates (20 μl) were collected every 20 min in the sample loop of an EAS-20 automated sample injector (Eicom) connected to an ENO-20 automated NO detector-HPLC system (Eicom). Nitrite and nitrate in the dialysate were separated by a reverse-phase separation column packed with polystyrene polymer (NO-PAK, 4.6 × 50 mm, Eicom), and nitrate was reduced to nitrite in a reduction column packed with copper-plated cadmium filings (NO-RED, Eicom). Nitrite was mixed with a Griess reagent to form a purple azo dye in a reaction coil. The separation and reaction columns and the reaction coil were placed in a column oven which was set at 35°C. The absorbance of the color of the product dye at 540 nm was measured using a flow-through spectrophotometer. The mobile phase consisted of 10% methanol containing 0.15 M NaCl-NH4Cl and 0.5 g/l of 4Na-EDTA. The Griess reagent, consisting of 1.25% HCl containing 5 g/l sulfanilamide with 0.25 g/l N-naphthylethylenediamine, was delivered at a rate of 0.1 ml/min. The contamination of nitrite and nitrate in aCSF and the reliability of the reduction column were examined in each experiment.
4.6. Quantification of β-endorphin
β-Endorphin was quantified using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (EK-022-33, Phoenix Pharmaceutical, Belmont, CA). The microwells were pre-coated with a secondary antibody and non-specific binding sites were blocked. Fifty μl of either an unknown dialysate sample or a known concentration of β-endorphin standard (prepared from serial dilutions across a range of 0–100 ng/ml) were added to the well, together with 25 μl biotin-labeled β-endorphin. A 2-hr incubation allowed the unlabeled β-endorphin (either unknown or standard) to compete with the biotin-labeled peptide for binding to the immobilized antipeptide IgG. The addition of 100 μl streptavidin-horseradish peroxidase (SA-HRP) for 60 min, then bound SA-HRP to the immobilized IgG-biotin complex. Following a thorough rinse, 100 μl of the SA-HRP substrate tetramethylbenzidine dihydrochloride (TMB) was added to each well, forming a blue color. An absorbance plate-reader (Synergy™ HT, BioTek Instruments, Inc., Winooski, VT) was used to read the results at 450 nm. A standard curve (10 points between 0 and 100 ng/ml) was fit to the data using nonlinear regression (Graphpad Prism, Graphpad Software, San Diego CA). The β-endorphin content of the dialysate samples were computed against the standard curve. The intra-assay coefficient of variance was < 5%; the inter-assay coefficient of variance was < 14%.
4.8. Drugs
L-NG-nitro arginine methyl ester (L-NAME) was purchased from Research Biochemicals International (Natick, MA). The drug was freshly prepared in 0.9% physiological saline solution and administered i.p. in a dose of 50 mg/kg 30 min prior to the start of N2O exposure. Control animals received an i.p. injection of vehicle only. The volume of i.p. injection in both cases was 0.1 ml/100 g body weight.
4.9. Statistical analysis of data
The mean dialysate levels of total NOx, nitrite, nitrate and β-endorphin in different treatment groups of rats were compared using repeated measures two-way analysis of variance (ANOVA). When a significant F value was found, post-hoc analysis was performed by Bonferroni’s multiple comparison test.
Research Highlights.
Nitrous oxide increases levels of oxidation products of NO in dialysate collected from the ARC and PAG.
Nitrous oxide increases levels of β-endorphin in dialysate collected from the ARC and PAG.
Pretreatment with a NOS-inhibitor antagonizes the nitrous oxide-induced increases in both oxidation products of NO and β-endorphin.
Acknowledgments
This research was supported by NIH Grant GM-77153, State of Washington Initiative Measure No. 171 and the Allen I. White Distinguished Professorship from Washington State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach FW. Beta-endorphin in the brain. A role in nociception. Acta Anaesthesiol Scand. 1997;41:133–140. doi: 10.1111/j.1399-6576.1997.tb04627.x. [DOI] [PubMed] [Google Scholar]
- Bach FW, Yaksh TL. Release into ventriculo-cisternal perfusate of beta-endorphin- and Met-enkephalin-immunoreactivity: effects of electrical stimulation in the arcuate nucleus and periaqueductal gray of the rat. Brain Res. 1995;690:167–176. doi: 10.1016/0006-8993(95)00600-u. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Finck AD, Ngai SH. Nitrous oxide analgesia: reversal by naloxone and development of tolerance. J Pharmacol Exp Ther. 1977;203:539–547. [PubMed] [Google Scholar]
- Berkowitz BA, Ngai SH, Finck AD. Nitrous oxide ‘analgesia’: resemblance to opiate action. Science. 1976;194:967–968. doi: 10.1126/science.982058. [DOI] [PubMed] [Google Scholar]
- Bishop JT. Administration of nitrous oxide in labor: expanding the options for women. J Midwifery Womens Health. 2007;52:308–309. doi: 10.1016/j.jmwh.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Bloom F, Battenberg E, Rossier J, Ling N, Guillemin R. Neurons containing beta-endorphin in rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc Nat Acad Sci USA. 1978;75:1591–1595. doi: 10.1073/pnas.75.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda EM, Ramza JT, Cahill FJ, Tseng LF, Quock RM. Role of brain dynorphin in nitrous oxide antinociception in mice. Pharmacol Biochem Behav. 2000;65:217–222. doi: 10.1016/s0091-3057(99)00202-6. [DOI] [PubMed] [Google Scholar]
- Cahill FJ, Ellenberger EA, Mueller JL, Tseng LF, Quock RM. Antagonism of nitrous oxide antinociception in mice by intrathecally administered opioid peptide antisera. J Biomed Sci. 2000;7:299–303. doi: 10.1007/BF02253248. [DOI] [PubMed] [Google Scholar]
- Chung E, Burke B, Bieber AJ, Doss JC, Ohgami Y, Quock RM. Dynorphin-mediated antinociceptive effects of l-arginine and SIN-1 (an NO donor) in mice. Brain Res Bull. 2006;70:245–250. doi: 10.1016/j.brainresbull.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Eger EI., II . MAC. In: Eger OEI II, editor. Nitrous Oxide/N. 2. Elsevier; New York: 1985. pp. 125–156. [Google Scholar]
- Emmanouil DE, Dickens AS, Heckert RW, Ohgami Y, Chung E, Han S, Quock RM. Nitrous oxide-antinociception is mediated by opioid receptors and nitric oxide in the periaqueductal gray region of the brain. Eur Neuropsychopharmacol. 2008;18:194–199. doi: 10.1016/j.euroneuro.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S, Gagnon MJ, Quock RM, Shibuya T. Effect of opioid peptide antisera on nitrous oxide antinociception in rats. Pharmacol Biochem Behav. 1994;48:699–702. doi: 10.1016/0091-3057(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Hara S, Kuhns ER, Ellenberger EA, Mueller JL, Shibuya T, Endo T, Quock RM. Involvement of nitric oxide in intracerebroventricular β-endorphin-induced neuronal release of methionine-enkephalin. Brain Res. 1995;675:190–194. doi: 10.1016/0006-8993(95)00065-x. [DOI] [PubMed] [Google Scholar]
- Henry ED, Ohgami Y, Li S, Chung E, Quock RM. Correlation of inbred mouse sensitivity to nitrous oxide antinociception with brain nitric oxide synthase. Pharmacol Biochem Behav. 2005;81:764–768. doi: 10.1016/j.pbb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Hodges BL, Gagnon MJ, Gillespie TR, Breneisen JR, O’Leary DF, Hara S, Quock RM. Antagonism of nitrous oxide antinociception in the rat hot plate test by site-specific μ- and ε-opioid receptor blockade. J Pharmacol Exp Ther. 1994;269:596–600. [PubMed] [Google Scholar]
- Hu JY, Jin GZ. Arcuate nucleus of hypothalamus involved in analgesic action of l-THP. Acta Pharmacol Sin. 2000;21:439–444. [PubMed] [Google Scholar]
- Ishikawa M, Quock RM. Role of nitric oxide synthase isoforms in nitrous oxide antinociception in mice. J Pharmacol Exp Ther. 2003a;306:484–489. doi: 10.1124/jpet.103.049551. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Quock RM. N2O stimulates NOS enzyme activity in C57BL/6 but not DBA/2 mice. Brain Res. 2003b;976:262–263. doi: 10.1016/s0006-8993(03)02654-4. [DOI] [PubMed] [Google Scholar]
- Jackson DL, Johnson BS. Inhalational and enteral conscious sedation for the adult dental patient. Dent Clin North Am. 2002;46:781–802. doi: 10.1016/s0011-8532(02)00029-0. [DOI] [PubMed] [Google Scholar]
- Li S, Bieber AJ, Quock RM. Antagonism of nitrous oxide antinociception in mice by antisense oligodeoxynucleotide directed against neuronal nitric oxide synthase enzyme. Behav Brain Res. 2004;152:361–363. doi: 10.1016/j.bbr.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- McDonald CE, Gagnon MJ, Ellenberger EA, Hodges BL, Ream JK, Tousman SA, Quock RM. Inhibitors of nitric oxide synthesis antagonize nitrous oxide antinociception in mice and rats. J Pharmacol Exp Ther. 1994;269:601–608. [PubMed] [Google Scholar]
- Palkovits M, Eskay RL. Distribution and possible origin of β-endorphin and ACTH in discrete brainstem nuclei of rats. Neuropeptides. 1987;9:123–137. doi: 10.1016/0143-4179(87)90051-5. [DOI] [PubMed] [Google Scholar]
- Parlow JL, Milne B, Tod DA, Stewart GI, Griffiths JM, Dudgeon DJ. Self-administered nitrous oxide for the management of incident pain in terminally ill patients: a blinded case series. Palliat Med. 2005;19:3–8. doi: 10.1191/0269216305pm958oa. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Academic Press; San Diego: 2006. [Google Scholar]
- Pilcher WH, Joseph SA, McDonald JV. Immunocytochemical localization of pro-opiomelanocortin neurons in human brain areas subserving stimulation analgesia. J Neurosurg. 1988;68:621–629. doi: 10.3171/jns.1988.68.4.0621. [DOI] [PubMed] [Google Scholar]
- Quock RM, Best JA, Chen DC, Vaughn LK, Portoghese PS, Takemori AE. Mediation of nitrous oxide analgesia in mice by spinal and supraspinal κ-opioid receptors. Eur J Pharmacol. 1990;175:97–100. doi: 10.1016/0014-2999(90)90158-3. corrigendum 187–564. [DOI] [PubMed] [Google Scholar]
- Quock RM, Curtis BA, Reynolds BJ, Mueller JL. Dose-dependent antagonism and potentiation of nitrous oxide antinociception by naloxone in mice. J Pharmacol Exp Ther. 1993;267:117–122. [PubMed] [Google Scholar]
- Quock RM, Kouchich FJ, Tseng LF. Does nitrous oxide induce release of brain opioid peptides? Pharmacology. 1985;30:95–99. doi: 10.1159/000138056. [DOI] [PubMed] [Google Scholar]
- Quock RM, Mueller J. Protection by U-50,488H against β-chlornaltre-xamine antagonism of nitrous oxide antinociception in mice. Brain Res. 1991;549:162–164. doi: 10.1016/0006-8993(91)90615-3. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide. A mechanistic and toxicologic review. Anesthesiology. 2008;109:707–722. doi: 10.1097/ALN.0b013e3181870a17. [DOI] [PubMed] [Google Scholar]
- Sawamura S, Obara M, Takeda K, Maze M, Hanaoka K. Corticotropin-releasing factor mediates the antinociceptive action of nitrous oxide in rats. Anesthesiology. 2003;99:708–715. doi: 10.1097/00000542-200309000-00028. [DOI] [PubMed] [Google Scholar]
- Sun YG, Gu XL, Yu LC. The neural pathway of galanin in the hypothalamic arcuate nucleus of rats: activation of beta-endorphinergic neurons projecting to periaqueductal gray matter. J Neurosci Res. 2007;85:2400–2406. doi: 10.1002/jnr.21396. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Taniguchi Y. Projection of pro-opiomelanocortin neurons from the rat arcuate nucleus to the midbrain central gray as demonstrated by double staining with retrograde labeling and immunohistochemistry. Arch Histol Cytol. 1988;51:175–183. doi: 10.1679/aohc.51.175. [DOI] [PubMed] [Google Scholar]
- Zelinski LM, Ohgami Y, Quock RM. Exposure to nitrous oxide stimulates a nitric oxide-dependent neuronal release of β-endorphin in ventricular cisternally-perfused rats. Brain Res. 2009;1300:37–40. doi: 10.1016/j.brainres.2009.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga JR, Joseph SA, Knigge KM. The effects of nitrous oxide on the secretory activity of pro-opiomelanocortin peptides from basal hypothalamic cells attached to cytodex beads in a superfusion in vitro system. Brain Res. 1987a;420:66–72. doi: 10.1016/0006-8993(87)90240-x. [DOI] [PubMed] [Google Scholar]
- Zuniga J, Joseph S, Knigge K. Nitrous oxide analgesia: partial antagonism by naloxone and total reversal after periaqueductal gray lesions in the rat. Eur J Pharmacol. 1987b;142:51–60. doi: 10.1016/0014-2999(87)90653-4. [DOI] [PubMed] [Google Scholar]