Abstract
Myocilin, a protein associated with the development of glaucoma, is expressed in most eye tissues with highest expression observed in trabecular meshwork cells. In culture, primary human trabecular meshwork cells incubated in 10% fetal bovine serum have reduced myocilin expression compared to in vivo, but incubation in human aqueous humor, their normal in vivo nutrient source, restores myocilin expression to near in vivo levels. To investigate the mechanism by which human aqueous humor stimulates myocilin accumulation in conditioned media from normal human trabecular meshwork cells, three independent trabecular meshwork cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing various supplements: fetal bovine serum (10%), human serum (0.2%), porcine aqueous humor (50%), bovine serum albumin (0.1%), dexamethasone (10−7 M), human aqueous humor (50%) or heat-inactivated human aqueous humor (50%). Conditioned media from cultured primary trabecular meshwork cells following incubation in human aqueous humor showed significant accumulation of myocilin in a time- (15 minutes) and dose-dependent manner (half maximal effective concentration ~ 30%) while intracellular myocilin levels decreased. Minimal myocilin accumulation was observed in conditioned media isolated from trabecular meshwork cells cultured in DMEM containing fetal bovine or human serum, bovine serum albumin, porcine aqueous humor, dexamethasone or DMEM alone. Heat inactivation of human aqueous humor nearly eliminated human aqueous humor-stimulated myocilin secretion. Inhibitors of new protein synthesis, gene transcription, the endoplasmic reticulum/Golgi system and endocytic/exocytic secretory pathways failed to inhibit human aqueous humor-stimulated myocilin secretion. Using immunolabeling and transmission electron microscopy, myocilin was found associated with 70–90 nm vesicle-like structures within the cytoplasm of human aqueous humor treated trabecular meshwork cells. These studies suggest that myocilin secretion from trabecular meshwork cells occurs in a Golgi-independent manner following human aqueous humor treatment. Heat-labile factors in human aqueous humor are responsible for the time- and dose-dependent release of myocilin from vesicle-like structures within the cytoplasm of trabecular meshwork cells.
Keywords: myocilin, trabecular meshwork, aqueous humor, Golgi-independent
INTRODUCTION
Glaucoma is a multifactorial eye disease that is the leading cause of preventable blindness in the world (Quigley, 1996). Increased intraocular pressure (IOP) is a risk factor for the development of glaucoma and is due in part to changes in the cells and the extracellular matrices that make up the trabecular meshwork (Tamm, Ethier, 2009). Myocilin, the first protein genetically associated with the development of glaucoma (Adam et al., 1997; Sheffield et al., 1993; Stone et al., 1997), is a constituent of human aqueous humor (hAq) and is expressed in many ocular tissues, with highest expression observed in cells of the trabecular meshwork (Adam et al., 1997; Huang et al., 2000; Kubota et al., 1997; Ortego et al., 1997; Ricard et al., 2001; Swiderski et al., 2000; Takahashi et al., 2000; Tamm, 2002). Investigations into myocilin’s association with IOP regulation and the development of glaucoma have been productive, however, the functional role of myocilin has yet to be elucidated.
While a direct link between altered levels of myocilin and the regulation of IOP are lacking, a number of studies suggest that myocilin expression may mediate, or is the result of, increased IOP (Chen et al., 2008; Clark et al., 2001; Hart et al., 2007; Howell et al., 2010 ; Lutjen-Drecoll et al., 1998; MacKay et al., 2008a; Mackay et al., 2008b; Mackey, 2008; Naskar, Thanos, 2006; Polansky, Nguyen, 1998). Evidence for myocilin as a causative agent for increasing IOP is suggested by studies in the human anterior segment culture system where perfusion of either bacterial- or eukaryotic-derived recombinant myocilin increased IOP (Fautsch et al., 2000; Fautsch et al., 2006). Elevated levels of myocilin have been identified in the trabecular meshwork and aqueous humor of individuals suffering from primary open-angle glaucoma (POAG) (Howell et al., 2010; Konz et al., 2009; Lutjen-Drecoll et al., 1998). Similar observations have been made in rat and canine species that suffer from glaucoma (Mackay et al., 2008b; Mackay, 2008; Naskar and Thanos, 2006). Myocilin is also elevated in steroid induced ocular hypertension in both human anterior eye culture and in primates (Clark et al., 2001). Furthermore, individuals with reduced myocilin levels due to mutations in the myocilin gene do not have glaucoma (Lam et al., 2000). Taken together, there appears to be a positive correlation between the elevation of IOP and increased myocilin expression. However, transgenic and myocilin knock-out animals have not conclusively yielded a role for myocilin in the normal regulation of IOP (Gould et al., 2004; Gould et al., 2006; Khare et al., 2008; Kim et al., 2001; Senatorov et al., 2006; Shepard et al., 2007; Zhou et al., 2008; Zillig et al., 2005).
Myocilin production in trabecular meshwork cells is modulated by several molecules and environmental conditions. Primary cultures of normal human trabecular meshwork (NTM) cells treated with steroids, transforming growth factor beta-1 (TGF-β1) or exposed to mechanical or oxidative stress can stimulate transcription and protein synthesis of myocilin (Clark et al., 2001; Polansky et al., 1997; Tamm et al., 1999). This stimulation of myocilin expression occurs over a period of days rather than hours suggesting myocilin may not be the primary target but rather a secondary response to these stimuli (Shepard et al., 2001).
Culture conditions also have a significant effect on myocilin expression in NTM cells. Changes in the topology of the substrate can increase myocilin expression (Russell et al., 2008). Conversely, NTM cells cultured in media containing fetal bovine serum (FBS), the standard supplement for maintaining NTM cells in culture, exhibit decreased myocilin accumulation in the conditioned media (Fautsch et al., 2005; Liton et al., 2006). We previously reported that NTM cells initially cultured in FBS and subsequently maintained in hAq, the normal nutrient provider for NTM cells in vivo, increased myocilin levels in conditioned media (Fautsch et al., 2005). The mechanism leading to increased myocilin levels in hAq-containing conditioned media are unknown. To better understand this phenomenon, we examined the possible mechanisms mediating hAq-stimulated myocilin accumulation in conditioned media of human NTM cells following incubation with hAq.
MATERIALS AND METHODS
Normal Human Aqueous Humor
Normal human donor eyes were obtained within 12 hours of death from the Minnesota Lions Eye Bank. The donor eyes were managed in accordance with the provisions of the Declaration of Helsinki for research involving human tissue. HAq was collected as described previously (Fautsch et al., 2005). A Bradford protein assay (Bio-Rad, Hercules, CA) was performed to determine the protein concentration of the hAq. Only hAq obtained from donors with no documented eye disease, no documented treatment with topical or systemic steroids and total protein concentrations in the normal hAq range (200 – 500 ng/ml) were used in this study (Cole, 1974; Tripathi et al., 1989). HAq samples were pooled prior to use in cell culture experiments. For the stimulation of NTM cells, hAq was diluted to 50% final concentration in Dulbecco’s Modified Essential Medium (DMEM) (GIBCO, Invitrogen, Carlsbad, CA) containing 100 mU/ml penicillin and 100 ng/ml Streptomycin (GIBCO). To denature protein, DMEM containing hAq (50% final concentration) was heated to 95ºC for 10 minutes.
Cell Culture
NTM cells were derived from three independent human donor eyes as described previously (Stamer et al., 1995): NTM3 – 20-year-old female; NTM7 – 6-month-old male; and NTM8 – 46-year-old female. Donors had no apparent ocular pathologies. Human ciliary epithelial (HCE) cells were kindly provided by Dr. Miguel Coca-Prados (Yale University, New Haven, CT). NTM and HCE cells were passaged and maintained in DMEM containing 10% FBS (GIBCO). Cells were cultured to confluency and maintained for one week prior to 24 hours of serum starvation. NTM cells (passage 4 to 8) were treated as follows:
Incubation with media supplements. Serum starved NTM and HCE cells were treated with DMEM supplemented with 10% FBS, 0.2% human serum, 50% porcine aqueous humor, 0.1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO), or 100 nM dexamethasone (Sigma-Aldrich) for up to 48 hours. Protein concentrations of 0.2% human serum and 0.1% BSA were similar to the normal total protein concentration of human aqueous humor (200 – 500 ng/ml) (Cole, 1974; Tripathi et al., 1989).
Incubation with inhibitors of gene expression. Serum starved NTM cells were pre-treated for either 1 or 16 hours with 5 μg/ml actinomycin D (Sigma-Aldrich) (Lomri, Baron, 1992), 10 μg/ml cycloheximide (Sigma-Aldrich) (Lomri, Baron, 1992), 5 μg/ml brefeldin A (Sigma-Aldrich) (Liu et al., 1992), 10 μg/ml nocodazole (Sigma-Aldrich) (Badman et al., 1998) or 10 μM phenylarsine oxide (Sigma-Aldrich) (Covian-Nares et al., 2008) prior to addition of 50% hAq.
Growth factor-supplemented media. Serum-starved confluent NTM cells were incubated with individual growth factors and myocilin levels quantified following treatment. Cells were treated for 72 hours with 10, 100 or 1000 ng/ml of the following growth factors: recombinant human basic fibroblast growth factor (bFGF; GIBCO), transforming growth factor beta-2 (TGF-β2) (R&D Systems, Minneapolis, MN), epidermal growth factor (EGF) (GIBCO), platelet-derived endothelial growth factor (PD-ECGF) (R&D Systems), endothelin-1 (ET-1) (Sigma-Aldrich), or osteopontin (R&D Systems). Control treatments included vehicle solutions used for inhibitors and protein factors.
Myocilin Protein Quantification
Conditioned media and NTM cells were collected from treated NTM cells. Conditioned media was centrifuged at 13,000 RPM for 10 minutes to remove cellular debris. NTM cells were lysed in cell lysis buffer [50 mM Tris pH 8.0, 0.5% sodium dodecyl sulfate, 0.5% Triton, 137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4-7H2O, 1mM KH2PO4, protease inhibitors (Roche, Indianapolis, IN)] at 4oC for 30 minutes with occasional vortexing followed by sonication. Cell lysates were centrifuged at 13,000 RPM to remove cellular debris and total protein was quantified by Bradford assay. Twenty μl of conditioned media or 15 μg of total protein were separated by gel electrophoresis using precast 4-15% polyacrylamide gradient gels (Bio-Rad) and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). Membranes were blocked in 1% non-fat milk diluted in wash buffer (20 mM Tris pH 7.5, 150 mM NaCl, 0.05% Tween) for 1 hour at room temperature and probed with a polyclonal, anti-myocilin antibody generated against myocilin amino acids 108 to 131 (Fautsch et al., 2000). Horse-radish peroxidase conjugated anti-rabbit IgG (GE Healthcare, Picataway, NJ) and enhanced chemiluminesence (GE Healthcare) were used to detect myocilin immunoreactivity by film (Kodak, Rochester, NY). Digital images were obtained using an Epson Perfection 2400 scanner (Long Beach, CA) and relative myocilin levels were analyzed using Image J software (http://rsbweb.nih.gov/ij/).
Transmission Electron Microscopy
Confluent NTM cells were maintained in 10% FBS for one week and subsequently were placed in serum-free medium containing antibiotics for 24 hours prior to treatment with either 10% FBS or 50% hAq for 4 hours. Following treatment, cells were collected by trypsinization, neutralized in 10% FBS, washed, centrifuged at 300 x g and the NTM pellets were fixed in 1% paraformaldehyde/0.5% glutaraldehyde overnight at 4ºC. The pellets were suspended in 2% SeaPlaque agarose (Cambrex Bio Science Rockland, Inc., Rockland, ME) and re-immersed in 1% paraformaldehyde/0.5% glutaraldehyde overnight at 4ºC. The agared pellets were cut into 1 mm cubes and processed using a cold LRWhite (LRW) processing protocol as per manufacturer’s instructions (Ted Pella, Inc., Redding, CA). The cell blocks were dehydrated in 30% ethanol for 5 minutes at 4ºC; 50% ethanol for 5 minutes at 4ºC; 60% ethanol for 15 minutes at 4ºC; 70% ethanol for 5 minutes at 4ºC; 70% ethanol for 1 hour at −20ºC followed by 80%, 95% and 100% ethanol incubations for 1 hour each at −20ºC. The specimens were transferred to a 1:1 dilution of LRW/100% ethanol solution overnight at −20ºC. The next day, specimens were placed in fresh LRW resin for 4 hours at −20ºC followed by fresh LRW for 15 minutes at room temperature and fresh LRW for one hour at 4ºC. Specimens were equilibrated to room temperature, embedded in fresh LRW resin and allowed to polymerize at 50ºC for 3 days. Thin sections (90 nm) were cut, mounted on nickel grids and incubated with polyclonal anti-myocilin antibody overnight at room temperature (Fautsch et al., 2000). Sections were rinsed in PBS-Tween 20 and incubated at room temperature for 1 hour with goat anti-rabbit secondary antibody conjugated with 10 nm gold particles (Electron Microscopy Sciences, Hatfield, PA) at a 1:100 dilution. Grids were rinsed, dried and stained with uranyl acetate. Negative control grids were incubated with goat serum in place of the primary antibody. Positive control included a thin section of purified recombinant myocilin protein (Fautsch et al., 2006) pellet processed and embedded using the same method as described above. Specimens were examined on a JEOL 1400 electron microscope (JEOL Ltd., Tokyo, Japan).
Statistics
Myocilin is a normal component of hAq. Because of this, it was necessary to calculate extracellular myocilin levels by dividing experimental myocilin levels (determined by Image J) for each time point by the myocilin levels in unconditioned media (represents myocilin levels in media prior to addition to cells). Values were expressed as percent or fold change compared to unconditioned media. Significance of normalized data was compared by ANOVA and Student’s t-test with differences considered significant if p<0.05 (JMP 8.0, Statistical Analysis System (SAS®), Cary, NC). Each figure represents n=3 where n is the average of 2–3 experiments conducted in each of the three independent NTM cell lines. Data is represented as the mean and the standard error of the mean.
RESULTS
Time-Course and Dose-Response of Aqueous Humor-Stimulated Myocilin Secretion
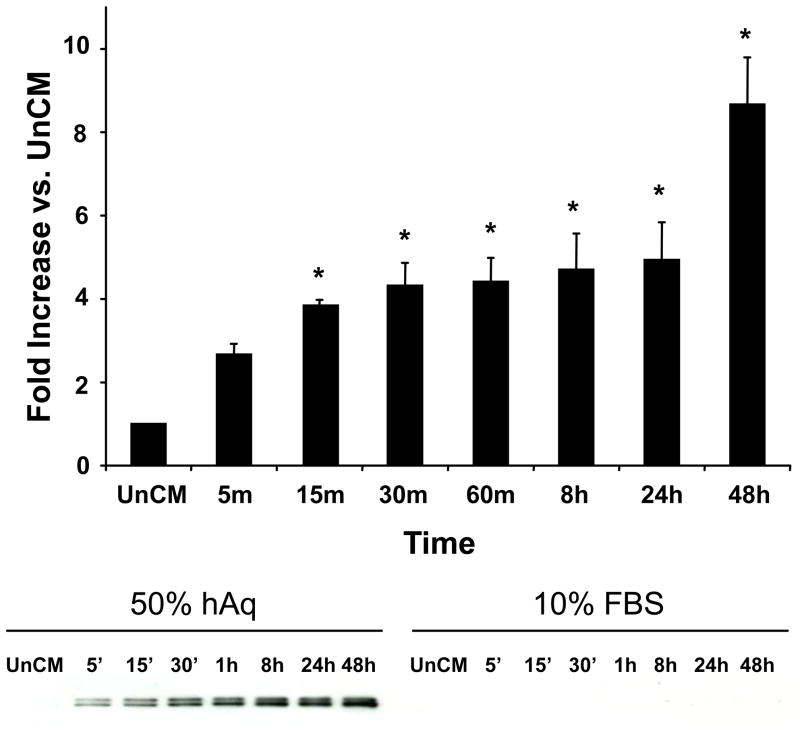
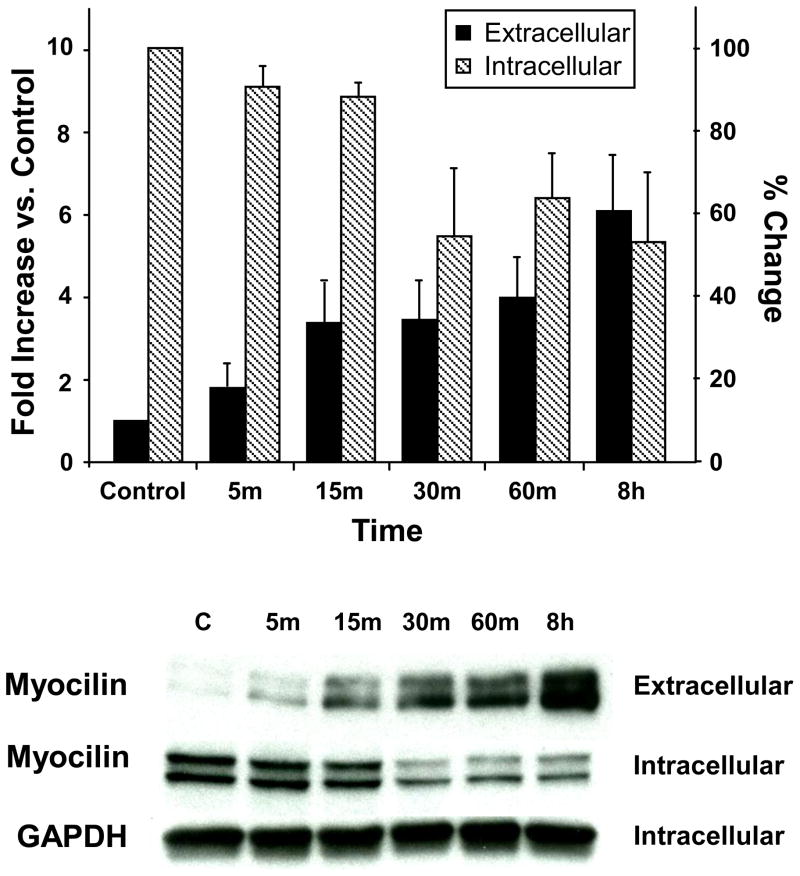
To determine the time-course required for hAq to mediate the secretion of myocilin, NTM cells were incubated with 10% FBS or 50% hAq for varying lengths of time (5 minutes to 48 hours). Myocilin levels in conditioned media from trabecular meshwork cells were elevated nearly 4-fold within 15 minutes following incubation with 50% hAq and were maintained at these levels over a 24 hour period (Figure 1). Between 24 and 48 hours, a second increase in myocilin levels was noted in conditioned media from NTM cells treated with 50% hAq. Intracellular myocilin levels were reduced over a similar time-frame, consistent with a rapid release of myocilin from the cells (Figure 2).
Figure 1. Human aqueous humor stimulation of myocilin secretion from trabecular meshwork cells.
Human NTM cells (mean ± SEM, n=3) were treated with 50% hAq or 10% FBS for up to 48 hours and myocilin accumulation in conditioned media was quantified by Western blot (representative image shown under bar graph). Myocilin in 50% hAq conditioned media was normalized to myocilin levels in unconditioned media (UnCM) containing 50% hAq. Asterisk (*) represents p< 0.008 (conditioned compared to UnCM).
Figure 2. Intracellular and extracellular myocilin levels following human aqueous humor.
Myocilin levels were compared in conditioned media and total cellular protein from NTM cells treated with 50% hAq. Fold increase (conditioned media versus unconditioned media) and percent change (intracellular myocilin vs. control) are represented as mean ± SEM (n=3) (representative image shown under bar graph). Control for extracellular myocilin calculation was 50% hAq-unconditioned media. Cell lysate isolated from serum-starved cells served as intracellular control.
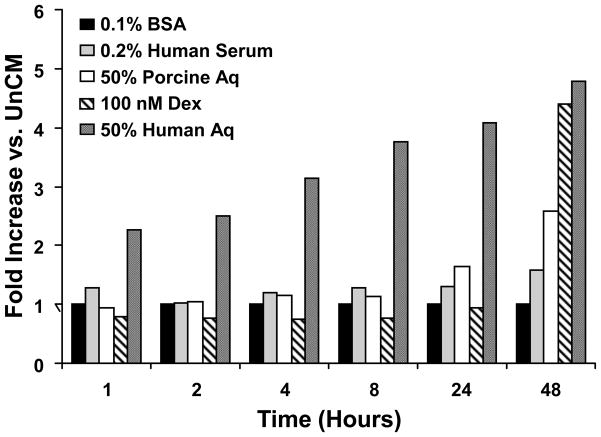
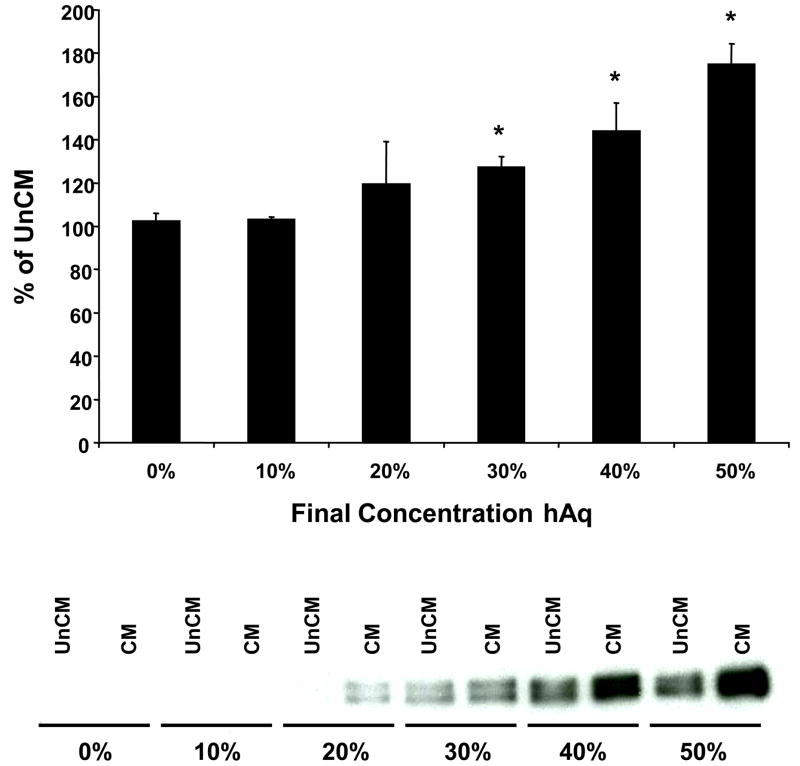
In comparison to 50% hAq, NTM cells incubated in 10% FBS showed negligible myocilin secretion over a 48 hour period. Unlike NTM cells incubated in 50% hAq, intracellular myocilin levels did not decrease with increasing incubation time in 10% FBS (data not shown). NTM cells incubated with 0.2% human serum or 0.1% bovine serum albumin also failed to yield comparable myocilin secretion levels when compared to 50% hAq (Figure 3). Only 50% porcine aqueous humor and 100 nM dexamethasone showed greater than a 2-fold increase in myocilin accumulation between 24–48 hours. The effect of hAq on myocilin accumulation in conditioned media isolated from trabecular meshwork cells was dose dependent, exhibiting a half-maximal effective concentration (EC50) of approximately 30% (Figure 4).
Figure 3. Myocilin secretion in response to various media supplements.
Bar graph depicts myocilin levels in NTM conditioned media following treatment up to 48 hours with various supplements.
Figure 4. Dose-related secretion of myocilin in response to hAq treatment.
Human NTM cells were treated with varying concentrations of hAq for 2 hours and myocilin accumulation in the conditioned media was quantified. Values are expressed as percent increase over hAq unconditioned media (mean ± SEM, n=3). Representative Western blot of myocilin expression following hAq dose-response is shown below bar graph. UnCM=unconditioned media, CM=conditioned media. Asterisk (*) represents p< 0.05 (conditioned compared to unconditioned media).
Identification of HAq Components Regulating Myocilin Accumulation in Conditioned Media
HAq contains numerous ions and proteins essential for the maintenance of anterior segment tissues. Analysis of the osmolarity of DMEM at a concentration of 50% hAq (294 osmol/L) or 10% FBS (338 osmol/L) showed minimal differences. DMEM alone, which contains ions, electrolytes and amino acids but no protein component, also did not induce myocilin accumulation in NTM conditioned media (data not shown), suggesting that chemical components of hAq were not essential for the rapid release of myocilin.
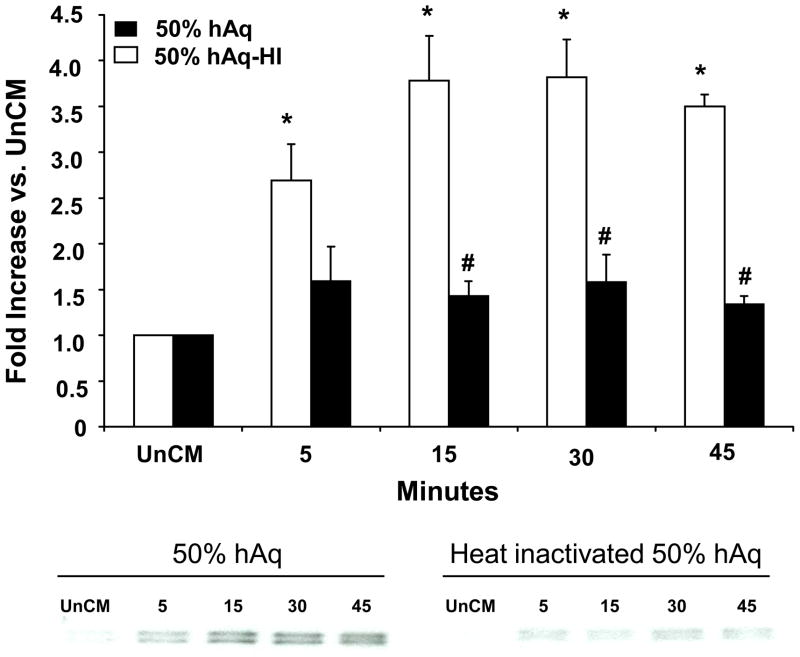
To determine whether hAq contained a heat-labile factor responsible for the rapid release of myocilin, hAq was heat-inactivated and used to treat NTM cells (Figure 5). Heat-inactivation significantly decreased hAq-stimulated myocilin secretion at 15, 30 and 45 minutes, suggesting a heat-sensitive protein factor(s) in hAq is involved in the rapid release of myocilin from NTM cells.
Figure 5. HAq-stimulated myocilin secretion is mediated by a heat sensitive factor(s).
NTM cells were treated with hAq or hAq that was heat inactivated (HI). Fold increase is represented as mean ± SEM (n=3). Representative Western blot of myocilin expression following 50% hAq and 50% hAq-HI is shown below bar graph. Asterisk (*) represents p< 0.002 (conditioned compared to unconditioned media, UnCM); number sign (#) represents p< 0.0007 (50% hAq compared to heat-inactivated 50% hAq).
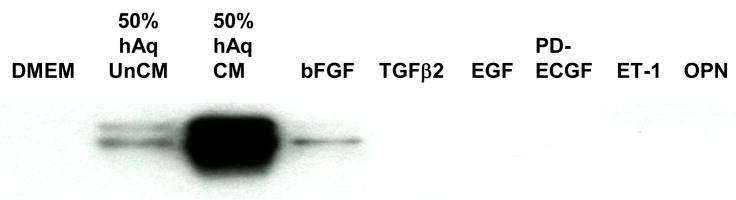
In an attempt to identify the factor(s) involved in the rapid secretion of myocilin, NTM cells were incubated with proteins that have been previously identified in hAq, associated with glaucoma, or involved in the regulation of IOP. Addition of individual proteins TGFβ2, EGF, PD-ECGF, ET-1 or osteopontin at pharmacological doses (10, 100 or 1000 ng/ml) failed to induce myocilin accumulation in NTM conditioned media (Figure 6). Conversely, bFGF when added at 1000 ng/ml, but not 10 or 100 ng/ml, exhibited a small increase in myocilin secretion when compared to DMEM treated cells. However, relative to 50% hAq stimulation, this increase was negligible.
Figure 6. Growth factor stimulation of myocilin secretion from NTM cells.
Western blot of conditioned media collected from NTM cells treated for 72 hours with 1000 ng/ml (super physiologic concentrations) of recombinant human basic fibroblast growth factor (bFGF), transforming growth factor beta-2 (TGF-β2), epidermal growth factor (EGF), platelet-derived endothelial growth factor (PD-ECGF), endothelin-1(ET-1), vascular endothelial growth factor (VEGF) or osteopontin (OPN). UnCM = unconditioned media, CM = conditioned media.
In the anterior segment of the eye, HCE cells are thought to be the source of many of the proteins found in hAq (Coca-Prados, Escribano, 2007). Incubation of NTM cells with 72-hour conditioned media obtained from HCE cells incubated in 10% FBS, 0.2% human serum (HS), 50% porcine aqueous humor (pAq) or 0.1% BSA all failed to stimulate the release of myocilin from NTM cells (Figure 7). Conditioned media obtained from HCE cells incubated in hAq was not tested due to the fact that factors present in hAq would mask potential factor(s) produced from HCE cells essential for myocilin secretion from NTM cells.
Figure 7. HCE cell conditioned media and myocilin secretion from NTM cells.
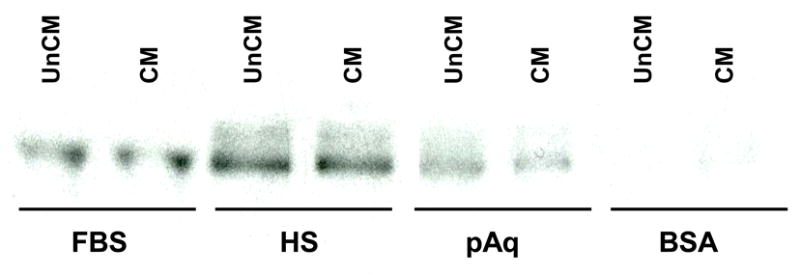
Conditioned media collected from HCE cells maintained for 72 hours in FBS (10%), human serum (HS, 0.2%), porcine aqueous humor (pAq, 50%) or BSA (0.1%) was incubated with NTM cells for 24 hours. Myocilin accumulation in HCE conditioned media following incubation with NTM cells was determined by Western blot. UnCM=unconditioned media, CM=conditioned media.
Analysis of Cellular Mechanisms Involved in HAq Mediated Rapid Secretion of Myocilin
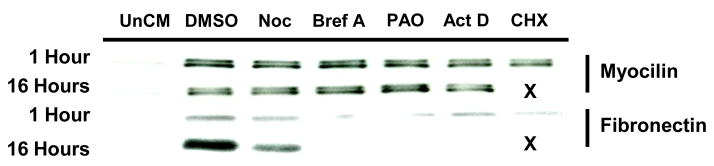
To examine possible mechanisms involved in myocilin accumulation in NTM conditioned media following incubation with hAq, NTM cells were treated with pharmacological inhibitors of gene transcription (actinomycin D; act D) or translation (cycloheximide; CHX). Pretreatment of NTM cells with act D or CHX prior to hAq incubation had no effect on myocilin accumulation in conditioned media (Figure 8). This suggests that myocilin accumulation in NTM conditioned media following hAq incubation is not due to new synthesis of myocilin mRNA or protein synthesis.
Figure 8. Inhibition of new protein synthesis or secretion of proteins by the traditional pathway fails to inhibit hAq-stimulated myocilin secretion.
NTM cells were treated with the inhibitor or vehicle control for either 1 or 16 hours prior to stimulation with 50% hAq for 2 hours. Visualization of fibronectin in the same conditioned media served as the control for the dose and duration of the inhibitor used as fibronectin is secreted through the traditional pathway. Lanes denoted with an “X” were not assayed as the treatment was cytotoxic. Unconditioned media (UnCM), nocodazole (Noc), brefeldin A (Bref A), phenylarsine oxide (PAO), actinomycin D (act D) and cycloheximide (CHX).
The traditional secretory pathway involves newly translated proteins that are processed within the rough endoplasmic reticulum with subsequent transport to the Golgi apparatus via a complex tubular system (Nickel, Rabouille, 2009). Common pharmacological agents used as inhibitors of normal tubule function and therefore Golgi-based protein secretion are nocodazole (Noc) and brefeldin A (Bref A). To determine if the endoplasmic reticulum/Golgi apparatus secretory pathway was responsible for hAq mediated myocilin secretion, NTM cells were treated with Noc or Bref A prior to hAq treatment (Figure 8). Noc and Bref A were found to have no effect on hAq-stimulated myocilin accumulation in conditioned media. Conversely, fibronectin, a golgi-dependent secretory protein, was inhibited by both Noc and Bref A, confirming the concentrations of Noc and Bref A were sufficient to inhibit Golgi-dependent secretion. These results suggest that myocilin accumulation in conditioned media after hAq treatment is not through the traditional secretory pathway, but involves a Golgi-independent mechanism.
Many proteins can be secreted from cells via Golgi-independent mechanisms, particularly using endocytic/exocytic pathways. To explore a Golgi-independent secretory pathway, NTM cells were treated with phenylarsine oxide (PAO), a trivalent arsenic compound that inhibits protein internalization in a variety of mammalian cell lines. Through this inhibition, PAO would inhibit redistribution of cytoplasmic proteins and resultant exocytic pathways that may mediate the secretion of myocilin from potential intracellular stores. NTM cells treated with PAO prior to incubation in hAq failed to inhibit myocilin accumulation in conditioned media (Figure 8).
Localization of Myocilin Stores in NTM Monolayer Cells
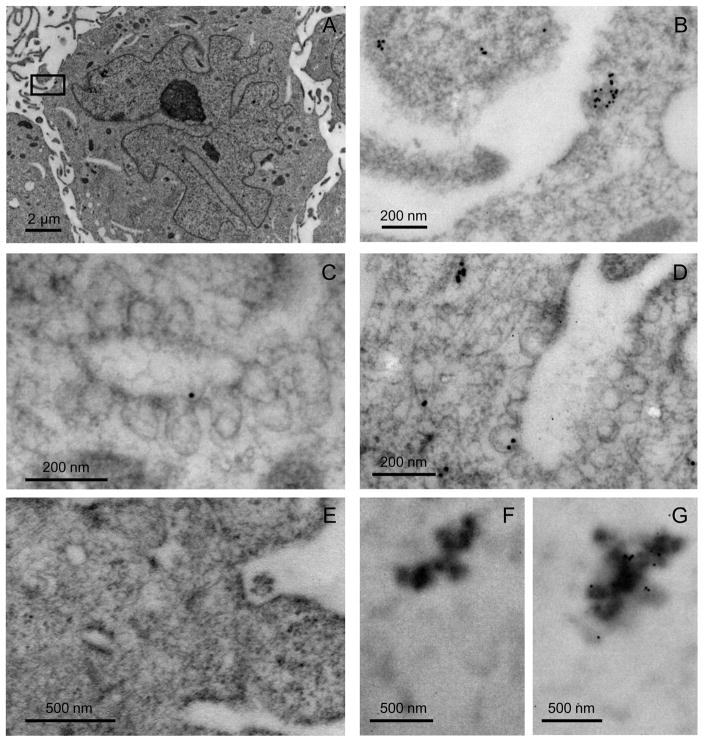
Since inhibitors of transcription, translation, the endoplasmic reticulum/Golgi apparatus and endocytic/exocytic secretory pathways did not influence myocilin accumulation in hAq treated NTM conditioned media, we postulated that NTM cells in monolayer culture must store myocilin in locations available for release upon the appropriate stimuli. Using a polyclonal anti-myocilin antibody and transmission electron microscopy, we found myocilin diffusely distributed within the cytoplasm with some rough endoplasmic reticulum, mitochondria and cytoplasmic filament association in NTM cells incubated in 50% hAq. In 50% hAq treated cells, myocilin was also found associated with the external portion of 70–90 nm vesicle-like structures (Figure 9).
Figure 9. Intracellular distribution of myocilin in NTM cells.
Cells were treated for 4 hours in 50% hAq and immunolabeled for myocilin. A) Morphology of NTM cells following immunolabeling procedure (x5000). B) Inset box from panel A (x40,000). C–D) Myocilin association with 70-90 nm vesicle-like structures. E) Tissue negative control. F) Negative control for recombinant myocilin. G) Immunolabeling of myocilin recombinant protein
DISCUSSION
Myocilin is associated with glaucoma and may be the possible cause or effect of increased IOP (Fautsch et al., 2000; Fautsch et al., 2006; Howell et al., 2010 ; MacKay et al., 2008a; Naskar, Thanos, 2006). To date, the stimulatory factors/influences associated with increased myocilin secretion (steroids, TGF-β1, cellular stressors) require days to elicit their affects, indicating these agents do not induce the secretion of myocilin directly, but most likely influence secretion indirectly through an intermediary signaling mechanism (Polansky et al., 1997; Shepard et al., 2001; Tamm et al., 1999). This was confirmed in our study where myocilin accumulation was increased 2-fold from 24 to 48 hours in conditioned media from NTM cells treated with hAq. Unique to our study is the observation that myocilin accumulation occurs as a primary event following incubation in aqueous humor. This time- (15 minutes) and dose-dependent (EC50 ~ 30%) increase in myocilin is independent of transcription and de novo protein synthesis, occurs in a Golgi-independent manner, is associated with vesicle-like structures and is caused by a heat sensitive factor(s) in aqueous humor.
While chemical factors in hAq may contribute to myocilin secretion, our study suggests that variations in the media and its constituents do not result in an increase in myocilin secretion from NTM cells. This is evidenced by the observation that DMEM, which contains similar concentrations of ions found in serum, does not by itself stimulate myocilin secretion. Second, the osmolarity of 50% hAq is not significantly different than that of 10% FBS. Third, ascorbic acid, an important antioxidant component in hAq, failed to stimulate myocilin secretion in previous studies from our lab (Fautsch et al., 2005). Furthermore, heat denaturation of the hAq protein component failed to stimulate myocilin secretion. These observations indicate that the factor(s) involved in the accumulation of myocilin in NTM conditioned media following hAq treatment is not due to the chemical composition of hAq but rather is part of the protein composition.
Human serum (0.2%) at final protein concentration comparable to that of 50% hAq failed to stimulate appreciable myocilin accumulation in the conditioned media of NTM cells. Therefore, we suggest that the proteins involved in mediating myocilin secretion from NTM cells are present in hAq in quantities that differ from those found in serum. This would also suggest that these proteins do not solely originate from serum, but are actively produced and secreted from tissues within the anterior segment. To test this, we analyzed conditioned media from HCE cells. HCE cells produce various plasma and anterior segment proteins in addition to many growth factors, cytokines, and neural peptides (Coca-Prados, Escribano, 2007). However, HCE conditioned media failed to stimulate the secretion of myocilin from NTM cells. This suggests that the regulatory factors necessary to stimulate myocilin secretion from NTM cells are not produced from HCE cells in vitro. Whether these factors are produced from HCE cells in the presence of hAq, similar to in vivo conditions, was not possible to test since hAq already contains the necessary factor(s) involved in promoting myocilin secretion from NTM cells.
The factor(s) involved in stimulating myocilin secretion from NTM cells appear to be species specific since addition of porcine aqueous humor failed to induce myocilin release from NTM cells. While most growth factors, cytokines and hormones cross react with different species, examples where this does not occur exist (Layton et al., 1994; Smith et al., 1986; Souza et al., 1995). Human cells respond to primate growth hormone, but not to rat or bovine growth hormone. The difference between the activation of the growth hormone pathway by primate growth hormone, but not rat or bovine, is due to a single amino acid difference between the human growth hormone receptor and the growth hormone receptor of other species (Souza et al., 1995). This suggests that protein/receptor interactions involved in mediating myocilin secretion may be the result of species-specific stimuli not recapitulated in cell culture.
Since the secretion of myocilin from hAq-treated NTM cells occurs rapidly via a Golgi-independent mechanism in the absence of gene transcription and new protein synthesis, we postulated that in FBS-treated NTM cells, myocilin would be stored in the cytoplasm near the plasma membrane. Conversely, we would predict that incubation with hAq would release myocilin from NTM cells and decrease intracellular myocilin levels. Incubation of NTM cells in 50% hAq decreased intracellular stores of myocilin compared to NTM cells incubated in 10% FBS. This result is consistent with the cells releasing myocilin into the media following incubation in 50% hAq. However, no obvious difference in intracellular myocilin levels following FBS- or hAq-treatment was identified by transmission electron microscopy. In 10% FBS treated NTM cells, myocilin appeared as a diffuse protein with intermittent association with rough endoplasmic reticulum, mitochondria and filamentous structures. No apparent reservoirs of myocilin were identified near the plasma membrane. In 50% hAq treated NTM cells, vesicle-like structures with size between 70–90 nm were found posterior to the plasma membrane. Myocilin was associated with the external portion of these vesicles, indicating myocilin may be secreted via a vesicle-mediated, Golgi-independent pathway.
Previous reports have suggested an exosome-mediated, secretory system of extracellular delivery of myocilin. Exosomes range in size from 40–100 nm and are generally found inside multivesicular bodies (Hardy et al., 2005; Hoffman et al., 2009; Perkumas et al., 2007). Upon attachment to the plasma membrane, multivesicular bodies release exosomes into the extracellular milieu. Hoffman et al (2009) showed porcine aqueous humor was able to stimulate myocilin-associated exosome release following a 72-hour incubation (Hoffman et al., 2009). In our studies, porcine aqueous humor was able to stimulate release of myocilin from NTM cells following 48 hour incubation. However, porcine aqueous humor was unable to stimulate a rapid release of myocilin from NTM cells within 24 hours. Furthermore, we did not identify multivesicular-like structures within our NTM cells by transmission electron microscopy. However, given the size similarity between exosomes and the vesicle-like structures identified in our study, it is reasonable to suggest that the vesicle-like structures we observed in NTM cells may be exosomes. Future studies aimed at identifying factors associated with myocilin on these vesicles may help determine if myocilin has an intracellular function or is transported to the plasma membrane for secretion to elicit extracellular effects in the trabecular meshwork or downstream within Schlemm’s canal.
What is the protein(s) in hAq responsible for myocilin release from NTM cells? While we did not identify the protein(s) in this study, we did analyze several molecules found in hAq, some that have a reported association with glaucoma (bFGF, TGF-β2, EGF, PD-ECGF, ET-1 or osteopontin) (Chauhan, 2008; Duncan, Collison, 2003; Gartaganis et al., 2001; Min et al., 2006; Yu et al., 2007). Only bFGF at 1000 ng/ml induced myocilin secretion, however, these levels were significantly lower than what was secreted from 50% hAq-treated NTM cells. While hAq contains numerous growth factors, cytokines, catalytic enzymes and structural proteins (Roy Chowdhury et al., 2010), identifying the proteins responsible for myocilin secretion will be a challenge. Hoffman et al (2009) previously reported that the protein(s) involved in increasing extracellular myocilin from aqueous humor treated human TM cells had molecular weights ranging from 3–100 kDa (Hoffman et al., 2009). We also attempted to attribute a size to the factor(s) involved in stimulating myocilin release from NTM cells, but failed to yield a conclusive result due to increase in myocilin secretion over a large molecular weight range. The variability and the large size range suggests more than one factor may be responsible for this effect. While one could fractionate hAq based on size and/or charge, obtaining enough hAq to perform such studies will limit this approach. Activation of second messenger signaling pathways may prove to be more beneficial for the identification of the protein(s) responsible for stimulating myocilin secretion from NTM cells.
Acknowledgments
Supported in part by National Institutes of Health research grants EY 15736, EY 07065; Mayo Foundation, Rochester, MN; and Research to Prevent Blindness, New York, NY (MPF is a recipient of a Lew R. Wasserman Merit Award and the Department of Ophthalmology, Mayo Clinic is the recipient of an unrestricted grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zachary T. Resch, Email: resch.zachary@mayo.edu.
Cheryl R. Hann, Email: hann.cheryl@mayo.edu.
Kimberly A. Cook, Email: cook.kimberly@mayo.edu.
Michael P. Fautsch, Email: fautsch.michael@mayo.edu.
References
- Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bechetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet. 1997;6:2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- Badman MK, Pryce RA, Charge SB, Morris JF, Clark A. Fibrillar islet amyloid polypeptide (amylin) is internalised by macrophages but resists proteolytic degradation. Cell Tissue Res. 1998;291:285–294. doi: 10.1007/s004410050998. [DOI] [PubMed] [Google Scholar]
- Chauhan BC. Endothelin and its potential role in glaucoma. Can J Ophthalmol. 2008;43:356–360. doi: 10.3129/i08-060. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jiang D, Yu L, Katz B, Zhang K, Wan B, Sun X. CYP1B1 and MYOC mutations in 116 Chinese patients with primary congenital glaucoma. Arch Ophthalmol. 2008;126:1443–1447. doi: 10.1001/archopht.126.10.1443. [DOI] [PubMed] [Google Scholar]
- Clark AF, Steely HT, Dickerson JE, Jr, English-Wright S, Stropki K, McCartney MD, Jacobson N, Shepard AR, Clark JI, Matsushima H, Peskind ER, Leverenz JB, Wilkinson CW, Swiderski RE, Fingert JH, Sheffield VC, Stone EM. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2001;42:1769–1780. [PubMed] [Google Scholar]
- Coca-Prados M, Escribano J. New perspectives in aqueous humor secretion and in glaucoma: the ciliary body as a multifunctional neuroendocrine gland. Prog Retin Eye Res. 2007;26:239–262. doi: 10.1016/j.preteyeres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Cole DF. Comparative aspects of the intraocular fluids. In: Davson H, Graham LT Jr, editors. The Eye. Academic Press; New York: 1974. pp. 71–121. [Google Scholar]
- Covian-Nares JF, Smith RM, Vogel SS. Two independent forms of endocytosis maintain embryonic cell surface homeostasis during early development. Dev Biol. 2008;316:135–148. doi: 10.1016/j.ydbio.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G, Collison DJ. Role of the non-neuronal cholinergic system in the eye: a review. Life Science. 2003;72:2013–2019. doi: 10.1016/s0024-3205(03)00064-x. [DOI] [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci. 2000;41:4163–4168. [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Vrabel AM, Howell KG, Loewen N, Teo WL, Poeschla EM, Johnson DH. Perfusion of his-tagged eukaryotic myocilin increases outflow resistance in human anterior segments in the presence of aqueous humor. Invest Ophthalmol Vis Sci. 2006;47:213–221. doi: 10.1167/iovs.05-0334. [DOI] [PubMed] [Google Scholar]
- Fautsch MP, Howell KG, Vrabel AM, Charlesworth MC, Muddiman DC, Johnson DH. Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Invest Ophthalmol Vis Sci. 2005;46:2848–2856. doi: 10.1167/iovs.05-0101. [DOI] [PubMed] [Google Scholar]
- Gartaganis SP, Georgakopoulos CD, Exarchou AM, Mela EK, Lamari F, Karamanos NK. Increased aqueous humor basic fibroblast growth factor and hyaluronan levels in relation to the exfoliation syndrome and exfoliative glaucoma. Acta Ophthalmology Scand. 2001;79:572–575. doi: 10.1034/j.1600-0420.2001.790605.x. [DOI] [PubMed] [Google Scholar]
- Gould DB, Miceli-Libby L, Savinova OV, Torrado M, Tomarev SI, Smith RS, John SW. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol Cell Biol. 2004;24:9019–9025. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Reedy M, Wilson LA, Smith RS, Johnson RL, John SW. Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma. Mol Cell Biol. 2006;26:8427–8436. doi: 10.1128/MCB.01127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy KM, Hoffman EA, Gonzalez P, McKay BS, Stamer WD. Extracellular trafficking of myocilin in human trabecular meshwork cells. J Biol Chem. 2005;280:28917–28926. doi: 10.1074/jbc.M504803200. [DOI] [PubMed] [Google Scholar]
- Hart H, Samuelson DA, Tajwar H, MacKay EO, Lewis PA, Kallberg M, Gelatt KN. Immunolocalization of myocilin protein in the anterior eye of normal and primary open-angle glaucomatous dogs. Vet Ophthalmol. 2007;10(Suppl 1):28–37. doi: 10.1111/j.1463-5224.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Perkumas KM, Highstrom LM, Stamer WD. Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2009;50:1313–1318. doi: 10.1167/iovs.08-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KG, Vrabel AM, Chowdhury UR, Stamer WD, Fautsch MP. Myocilin levels in primary open-angle glaucoma and pseudoexfoliation glaucoma human aqueous humor. Journal of Glaucoma. 2010 Feb 22;19 doi: 10.1097/IJG.0b013e3181d13020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Jaroszewski J, Ortego J, Escribano J, Coca-Prados M. Expression of the TIGR gene in the iris, ciliary body, and trabecular meshwork of the human eye. Ophthalmic Genet. 2000;21:155–169. [PubMed] [Google Scholar]
- Khare PD, Loewen N, Teo W, Barraza RA, Saenz DT, Johnson DH, Poeschla EM. Durable, safe, multi-gene lentiviral vector expression in feline trabecular meshwork. Mol Ther. 2008;16:97–106. doi: 10.1038/sj.mt.6300318. [DOI] [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konz DD, Flugel-Koch C, Ohlmann A, Tamm ER. Myocilin in the trabecular meshwork of eyes with primary open-angle glaucoma. Graefes Archive for Clinical & Experimental Ophthalmology. 2009;247:1643–1649. doi: 10.1007/s00417-009-1152-0. [DOI] [PubMed] [Google Scholar]
- Kubota R, Noda S, Wang Y, Minoshima S, Asakawa S, Kudoh J, Mashima Y, Oguchi Y, Shimizu N. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997;41:360–369. doi: 10.1006/geno.1997.4682. [DOI] [PubMed] [Google Scholar]
- Lam DS, Leung YF, Chua JK, Baum L, Fan DS, Choy KW, Pang CP. Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1386–1391. [PubMed] [Google Scholar]
- Layton MJ, Lock P, Metcalf D, Nicola NA. Cross-species receptor binding characteristics of human and mouse leukemia inhibitory factor suggest a complex binding interaction. J Biol Chem. 1994;269:17048–17055. [PubMed] [Google Scholar]
- Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- Liu ES, Ou JH, Lee AS. Brefeldin A as a regulator of grp78 gene expression in mammalian cells. J Biol Chem. 1992;267:7128–7133. [PubMed] [Google Scholar]
- Lomri A, Baron R. 1 alpha,25-dihydroxyvitamin D3 regulates the transcription of carbonic anhydrase II mRNA in avian myelomonocytes. Proc Natl Acad Sci U S A. 1992;89:4688–4692. doi: 10.1073/pnas.89.10.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, May CA, Polansky JR, Johnson DH, Bloemendal H, Nguyen TD. Localization of the stress proteins alpha B-crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 1998;39:517–525. [PubMed] [Google Scholar]
- MacKay EO, Kallberg ME, Barrie KP, Miller W, Sapienza JS, Denis H, Ollivier FJ, Plummer C, Rinkoski T, Scotty N, Gelatt KN. Myocilin protein levels in the aqueous humor of the glaucomas in selected canine breeds. Vet Ophthalmol. 2008a;11:234–241. doi: 10.1111/j.1463-5224.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- Mackay EO, Kallberg ME, Gelatt KN. Aqueous humor myocilin protein levels in normal, genetic carriers, and glaucoma Beagles. Vet Ophthalmol. 2008b;11:177–185. doi: 10.1111/j.1463-5224.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- Mackey DA. Gillies lecture: dissecting glaucoma: understanding the molecular risk factors. Clin Exp Ophthalmol. 2008;36:403–409. doi: 10.1111/j.1442-9071.2008.001798.x. [DOI] [PubMed] [Google Scholar]
- Min SH, Lee TI, Chung YS, Kim HK. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uvietic eyes. Korean J Ophthalmol. 2006;20:162–165. doi: 10.3341/kjo.2006.20.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naskar R, Thanos S. Retinal gene profiling in a hereditary rodent model of elevated intraocular pressure. Mol Vis. 2006;12:1199–1210. [PubMed] [Google Scholar]
- Nickel W, Rabouille C. Mechanisms of unconventional protein secretion. Nature Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- Ortego J, Escribano J, Coca-Prados M. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett. 1997;413:349–353. doi: 10.1016/s0014-5793(97)00934-4. [DOI] [PubMed] [Google Scholar]
- Perkumas KM, Hoffman EA, McKay BS, Allingham RR, Stamer WD. Myocilin-associated exosomes in human ocular samples. Exp Eye Res. 2007;84:209–212. doi: 10.1016/j.exer.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211:126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Nguyen TD. The TIGR gene, pathogenic mechanisms, and other recent advances in glaucoma genetics. Curr Opin Ophthalmol. 1998;9:15–23. doi: 10.1097/00055735-199804000-00004. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard CS, Agapova OA, Salvador-Silva M, Kaufman PL, Hernandez MR. Expression of myocilin/TIGR in normal and glaucomatous primate optic nerves. Exp Eye Res. 2001;73:433–447. doi: 10.1006/exer.2001.1063. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury U, Madden BJ, Charlesworth MC, Fautsch MP. Proteome analysis of human aqueous humor. Investigative Ophthalmology & Vision Science. 2010 doi: 10.1167/iovs.10-5531. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P, Gasiorowski JZ, Nealy PF, Murphy CJ. Response of human trabecular meshwork cells to topographic cues on the nanoscale level. Invest Ophthalmol Vis Sci. 2008;49:629–635. doi: 10.1167/iovs.07-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov V, Malyukova I, Fariss R, Wawrousek EF, Swaminathan S, Sharan SK, Tomarev S. Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J Neurosci. 2006;26:11903–11914. doi: 10.1523/JNEUROSCI.3020-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet. 1993;4:47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Fingert JH, Stone EM, Sheffield VC, Clark AF. Delayed secondary glucocorticoid responsiveness of MYOC in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2001;42:3173–3181. [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Millar JC, Pang IH, Steely HT, Searby CC, Sheffield VC, Stone EM, Clark AF. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16:609–617. doi: 10.1093/hmg/ddm001. [DOI] [PubMed] [Google Scholar]
- Smith RA, Kirstein M, Fiers W, Baglioni C. Species specificity of human and murine tumor necrosis factor. J Biol Chem. 1986;261:14871–14874. [PubMed] [Google Scholar]
- Souza SC, Frick GP, Wang X, Kopchick JJ, Lobo RB, Goodman HM. A single arginine residue determines species specificity of the human growth hormone receptor. Proc Natl Acad Sci. 1995;92:959–963. doi: 10.1073/pnas.92.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Swiderski RE, Ross JL, Fingert JH, Clark AF, Alward WL, Stone EM, Sheffield VC. Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Invest Ophthalmol Vis Sci. 2000;41:3420–3428. [PubMed] [Google Scholar]
- Takahashi H, Noda S, Mashima Y, Kubota R, Ohtake Y, Tanino T, Kudoh J, Minoshima S, Oguchi Y, Shimizu N. The myocilin (MYOC) gene expression in the human trabecular meshwork. Curr Eye Res. 2000;20:81–84. [PubMed] [Google Scholar]
- Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Ethier CR. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88:648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- Tripathi RC, Millard CB, Tripathi BJ. Protein composition of human aqueous humor: SDS-PAGE analysis of surgical and post-mortem samples. Exp Eye Res. 1989;48:117–130. doi: 10.1016/0014-4835(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Yu XB, Sun XH, Dahan E, Guo WY, Qian SH, Meng FR, Song YL, Simon GJ. Increased levels of transforming growth factor-beta1 and -beta2 in the aqueous humor of patients with neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2007;38:6–14. doi: 10.3928/15428877-20070101-01. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Grinchuk O, Tomarev SI. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1932–1939. doi: 10.1167/iovs.07-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig M, Wurm A, Grehn FJ, Russell P, Tamm ER. Overexpression and properties of wild-type and Tyr437His mutated myocilin in the eyes of transgenic mice. Invest Ophthalmol Vis Sci. 2005;46:223–234. doi: 10.1167/iovs.04-0988. [DOI] [PubMed] [Google Scholar]