Abstract
In this study, we employed bio-derived bone scaffold and composited with the marrow mesenchymal stem cell induced into osteoblast to replicate a “biomimetic niche.” The CD34+ cells or mononuclear cells (MNC) from umbilical cord blood were cultured for 2–5 weeks in the biomimetic niche (3D system) was compared with conventional two dimensional cultures (2D system) without adding cytokine supplement. After 2 weeks in culture, the CD34+ cells from umbilical cord blood in the 3D system increased 3.3–4.8 folds when compared with the initial CD34+ cells. CD34+/CD38− cells accounted for 82–90% of CD34+ cells. After 5 weeks, CD34+/CD38− cells in the 3D system increased when compared with initial (1.3 ± 0.3 × 103 vs. 1.0 ± 0.5 × 104, p < 0.05), but were decreased in the 2D system (1.3 ± 0.3 × 103 vs. 2.5 ± 0.7 × 102, p < 0.05). The CFU progenitors were produced more in the 3D system than in the 2D system (4.6–9.3 folds vs. 1.0–1.5 folds) after 2 weeks in culture, and the colony distribution in the 3D system manifested higher percentage of BFU-E and CFU-GEMM, but in the 2D system was mainly CFU-GM. The LTC-ICs in the 3D system showed 5.2–7.2 folds increase over input at 2 weeks in culture, and maintain the immaturation of hematopoietic progenitor cells (HPCs) over 5 weeks. In conclusion, this new 3D hematopoietic progenitor cell culture system is the first to utilize natural cancellous bone as scaffold with osteoblasts as supporting cells; it is mimicry of natural bone marrow HSC niche. Our primary work has demonstrated it could maintain and expand HSC/HPC in vitro.
Keywords: Biomimetic osteoblast niche, Hematopoietic stem cells, Long term culture
Introduction
The concept of hematopoietic niche was first proposed by Schofield 30 years ago (Schofield 1978), and is composed of marrow stromal cells and extracellular matrix for supporting hematopoietic stem/progenitor cells (HSCs/HPCs). Previous studies demonstrated that endosteal osteoblasts play a critical role in creating a HSC niche, and regulate hematopoietic cells in maintenance, proliferation, and maturation (Zhang et al. 2003; Calvi et al. 2003). Earlier in vitro studies used 2D systems to culture hematopoietic cells. Hematopoietic cells in 2D systems lack support by various components in a hematopoietic niche in 3D configuration, and 2D systems have demonstrated to behave in a manner strikingly different from that in 3D culture or in vivo (Weaver et al. 1997). 3D culture systems using scaffolds made from natural materials or synthetic polymers have demonstrated superior hematopoietic supporting activity to that of traditional 2D culture systems (Bagley et al. 1999; Banu et al. 2001). However, most 3D systems afford a scaffold that provides only physical structure, and are not competent to simulate the natural pore size of cancellous, cellular population, and complicated extracellular matrix of bone marrow. In this study, we designed a 3D biomimetic osteoblast niche with bio-derived scaffold and osteoblasts induced from human marrow mesenchymal stem cells. The results of our experiments demonstrated that this system could provide excellent long-term support for the maintenance and expansion of HSCs/HPCs. Our investigation suggests that this 3D biomimetic osteoblast niche would be a new model between conventional in vitro 2D systems and experimental animals to study hematopoietic stem cell.
Materials and methods
Scaffold preparation
We use bio-derived bone as scaffold of the niche, which was kindly provided by the Division of Stem Cell and Tissue Engineering (State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu, China), and the preparation was processed into the bio-derived cancellous bone as previously reported (Xie et al. 2007). Briefly, the procedures were performed to degrease, partially deproteinize, lyophilize, sterilize by 60Co γ-ray irradiation, and finally, store at 4 °C. In our study, the bio-derived bone was cut into blocks sized about 1.0 × 0.5 × 0.5 cm, fitted into the wells of 24-well plates, and sterilized with ethylene oxide before use (Fig. 1). The bio-derived bone is characterized with respect to natural porosity, pore size, and minerals.
Fig. 1.
Bio-derived bone scaffold was placed into the 24-well plate
Preparation of bone marrow mesenchymal stem cells
Human bone marrow cells were obtained from the posterior iliac crest of healthy volunteers, after informed consent and in accordance with the ethical commission of West China Hospital of Sichuan University. The low-density mononuclear cells were isolated after centrifugation at 400g for 30 min on Ficoll-Hypaque (Sigma). Nucleated cells were plated in 25 cm2 cell culture flasks at the concentration of 5 × 105/cm2 in culture medium consisting of Dulbecco’s modified Eagle’s medium (DMEM; GIBCO) supplemented with 10% fetal bovine serum (FBS; GIBCO), 100 U/mL penicillin, 100 μg/mL streptomycin, 0.29 mg/mL l-glutamine, 100 mM HEPES buffer (R&D Systems), and incubated at 37 °C with 5% CO2. After 72 h, the nonadherent cells were discarded, and half medium change was performed twice weekly when cells grew to near confluence. Bone marrow mesenchymal stem cells (MSCs) were subsequently detached using 0.05% trypsin/0.53 mM EDTA, replated at 5 × 103 cells/cm2, and cultured similarly as first passage (P1) cells until near confluence. The MSCs were trypsinized similarly to yield second passage (P2) cells and subsequently used for scaffold seeding or monolayer cultures.
Purification of CD34+ cells from human umbilical cord blood
Human umbilical cord blood (UCB) cells were obtained from consenting mothers at the end of full-term deliveries in West China Second Hospital. Red blood cells were sedimentated by incubating UCB samples with 0.5% (w/v) methyl cellulose dissolved in Hanks’ solution at room temperature for 30 min, mononuclear cells (MNC) were collected with Ficoll-Paque. The cells were purified further for CD34+ cells with the EasySep CD34+ positive selection Kit (StemCell Technologies) according to the manufacturer’s instructions. In all experiments, the purity of CD34+ cells was 87–95%.
Simulation of osteoblasts niche
Bio-derived bone scaffolds were soaked in DMEM for 2 days. Two hours before the MSCs seeding, a few drops of FBS were added to each scaffold to facilitate cell adherence. The MSCs at P2-P3 were suspended into 1–8 × 106/mL, and 20 μL of cell suspension at each concentration were seeded to the scaffold with caution to avoid overflow. The cells were incubated at 37 °C with 5% CO2 in a humidified atmosphere for 4 h to allow cell adherence, and then medium supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.29 mg/mL l-glutamine, 100 mM HEPES buffer was added to wells. Twelve hours after inoculation, scaffolds were placed into a new well, and the MSCs retained in scaffold were estimated from the initial seeded cell number minus the number of cell left in previous well. Retained cells divided by input cells equals the attachment frequency of bone marrow MSCs. The optimum concentration of the bone marrow MSCs was selected for subsequent research. Bone marrow MSCs on scaffolds were cultured for 3 days, then induced to osteoblast differentiation with osteogenic medium (F12 medium containing 10% FBS, 10−8 mol/L dexamethasone, 50 μg/mL ascorbic acid, 10 mmol/L β-sodium glycerophosphate). The mimic osteoblast niche with natural trabecula structure, natural extracellular matrix, and osteoblasts was constituted after 7 days MSCs osteogenic induction.
Long term culture of UCB derived hematopoietic stem/progenitor cells
2D supporting cell co-cultivation
As performed in previous reports, with slight alteration (Moore et al. 1997; Yamaguchi et al. 2001), bone marrow MSCs (5 × 104 cells/well) were irradiated (25 Gy) with 137caesium before seeding onto 24-well plate and cultured with a medium containing 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.29 mg/mL l-glutamine, 100 mM HEPES buffer, and incubated at 37 °C with 5% CO2 and grown to confluence. Purified CD34+ cells or MNCs (2 × 104 CD34+ cells/well, 1 × 105 MNCs/well) were seeded onto 24-well plate coated with irradiated BMSCs, and cultured with MyeloCult H5100 (StemCell Technologies) medium containing 1 μM freshly dissolved hydrocortisone at 37 °C with 5% CO2 without exogenous cytokine added. After 2 weeks and 5 weeks in culture, with biweekly medium change, nonadherent cells were collected with gentle washing and adherent cells were collected after incubation in Cell Dissociation Solution (CDS; Sigma) for 20 min at 37 °C. Both adherent and nonadherent cells were pooled and counted for later analysis.
3D biomimetic osteoblast niche cultivation
After 7 days of induction of bone marrow MSCs, 2 × 104 CD34+ cells or 1 × 105 MNCs from UCB were suspended in 20 μL MyeloCult H5100 medium (StemCell Technologies), and seeded into the bio-derived bone scaffold, then cultured with MyeloCult medium containing 1 μM freshly dissolved hydrocortisone at 37 °C with 5% CO2 in a humidified atmosphere without exogenous cytokine. Half medium change was performed twice weekly. After 2 weeks and 5 weeks, scaffolds were washed with PBS to harvest the nonadherent cells. Because the HSCs were characteristically adherent to the osteoblast, scaffolds were saturated by brief vortexing in CDS, and incubated for 20 min at 37 °C, and centrifuged at 300g for 10 min to harvest adherent cells. Both adherent and nonadherent cells were collected for the HSCs/HPCs assay as described below.
Flow cytometry analysis
Before and after each time point of culture, hematopoietic cells were analyzed with respect to phenotype of CD34 and CD38 to assess the percentage of stem/progenitor cells remaining in 2D or 3D culture system. After filtering with 70 μm mesh. 5 × 105 cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-human CD34 antibody and phycoerythrin (PE)-conjugated anti-human CD38 antibody at 4 °C for 30 min. Dead cells were eliminated by staining with 7AAD. A replicate sample was stained with an isotype to ensure specificity. A minimum of 2 × 104 events was analyzed by the FACSsort (Becton-Dickinson, CA, USA) using CellQuest software (BD Becton-Dickinson, CA, USA) for each sample.
Colony-formation assays
To assess the colony-forming ability of hematopoietic cells harvests from 2D or 3D culture system, traditional methylcellulose assays were performed. Before and after culture, equal numbers of cells isolated from 2D and 3D system were plated in semisolid culture medium, which contained 1% methylcellulose in IMDM, 30% FBS, 1% bovine serum albumin (BSA), 2 mM l-glutamine, 1 × 10−4 M 2-mercaptoethanol, 50 ng/mL SCF, 20 ng/mL rh GM-CSF, 20 ng/mL rh IL-3, 20 ng/mL rh IL-6, 20 ng/mL rh G-CSF, 3U/mL Epo (MethoCult GF H4435, StemCell Technologies) following the manufacturer’s instruction for colony-forming unit assay. Methylcellulose-based media were aliquotted in 35-mm petri dishes and incubated at 37 °C in an atmosphere of 5% CO2 in a humidified incubator. All cultures were done in double. After 14 days of culture, burst forming unit-erythroid (BFU-E), colony forming unit-granulocyte/macrophage (CFU-GM), and colony forming unit-granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM) were scored under inverted microscope as described previously (Nissen-Druey et al. 2005).
Long term culture initiating cell assay (LTC-IC assay)
Limiting dilution assay (LDA) was used to determine the frequency of LTC-IC as described by Traycoff et al. (1995) with few modifications. In brief, irradiated (80 Gy) M2-10B4 stromal cells (StemCell Technologies) were seeded onto 96 well plate with MyeloCult medium (StemCell Technologies) containing 1 μM of freshly dissolved hydrocortisone. Four concentrations of fresh CD34+ cells and MNCs from UCB or harvested haematopoietic cells were seeded at limiting dilution on the preformed M2-10B4 cell monolayer in 20 replicates of each concentration in 100 μL of MyeloCult H5100 medium supplemented with 1 μM freshly dissolved hydrocortisone. The plates were incubated at 37 °C in an atmosphere of 5% CO2 in a humidified incubator for 5 weeks. At weekly intervals half the culture volume was removed from above the adherent cell layer and replaced with fresh culture medium. After 5 weeks, the medium was removed from each well, followed by the addition of 150 μL of a semisolid culture medium (MethoCult GF H4435, StemCell Technologies). The cells were mixed into a semisolid medium by gentle vortexing, and the plates were incubated as described above. After 14 days, the plates were scored under an inverted microscope for the presence of BFU-E, CFU-GM, or CFU-GEMM in each well, and also from the numbers of negative wells for each concentration. The data were analyzed with L-CalcTM Version 1 (StemCell Technologies) to calculate the frequency of LTC-IC in a given cell population.
Scanning electron microscopy (SEM)
The analysis with SEM was performed after 2 weeks in culture. The specimens were fixed for 30 min with 2.5% glutaraldehyde in 0.15 M PBS (PH = 7.4) and then washed in 0.15 M PBS twice. The samples were dehydrated in increasing concentrations of ethanol (from 50 to 100%). Before observation through the scanning electron microscope (JSM 5900LV), they were subsequently critical point dried with liquid CO2 and coated with gold.
Statistical analysis
The results were expressed as the mean ± SD of data obtained from three different experiments performed in duplicate. Statistical significance was determined using the Student’s t-test. The significance level was set at p < 0.05.
Results
Culture in the biomimetic niche (3D)
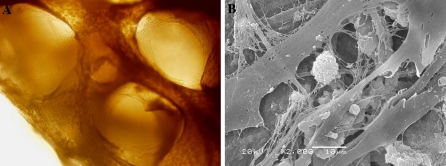
We seeded different concentrations (1–8 × 106/mL) of MSCs on the bio-derived bone scaffold to determine the optimal seeding concentration. The optimal cell attachment frequency was selected in later cultivation. It was observed that cell attachment frequency decreased with the seeding cell concentration: the higher concentration was accompanied with more cells leaking into the well. Although lower concentration had higher attachment frequency, it would take a longer time to form the cell nets between trabeculum. The favorable concentration of 4 × 106 MSCs/mL was selected in later research based on the optimal cell attachment frequency, growth rate and growth cavity. After 7 days of induction, we performed morphological and biochemical analyses of the MSCs induced osteoblasts on the bio-derived bone scaffold. The cells were defined as osteoblast-like cells by the determination of osteocalcin and alkaline phosphatase activity. Under inverted microscope, the osteoblast-like cells grew along with bone trabeculae, filling the intertrabecular cavity of the nonliving cancellous bone (Fig. 2a). After inoculation of CD34+ cells for 2 weeks, the blocks were observed through SEM. Hematopoietic cells were intimately attached to the osteoblast-like cells (Fig. 2b). The osteoblast-like cells possess numerous cellular processes, which branched between hematopoietic cells. Thin fibers were present between the cellular processes, and were organized into nets filling the intercellular vacuity.
Fig. 2.
The hematopoietic cells grow in the bio-derived bone scaffold and MSCs on the plate. a MSCs were grown in the porous network of bone trabecula observed through the inverted microscope. b SEM view of ball like hematopoietic cells embeded in the mimicry osteoblast niche
Phenotypic characteristic of the cultured hematopoietic cells
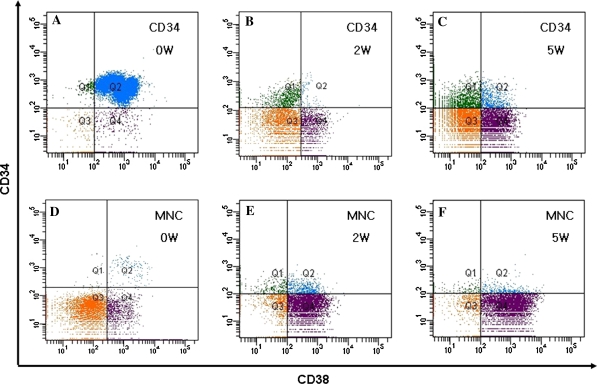
CD34 positive is used as the most common immunophenotype for human hematopoietic stem/progenitor cell, and CD34+/CD38− cells are believed to indicate a more primitive cell subpopulation. The average volume of UCB used in our studies was 121 ± 17.07 mL/unit (including anticoagulant), the average recovery of MNCs was 31 ± 8.12 × 107 cells/unit, and the fraction of CD34+ cells, CD34+/CD38− was 1.04 ± 0.57%, 0.18 ± 0.11%, respectively. The percentage of purified CD34+ cell through magnetic beads was 87–95%. 2 × 104 CD34+ cells or 1 × 105 MNCs were cultured in the 3D system. After 2 weeks and 5 weeks culture, the cells were harvested, counted and analyzed by flow cytometry. The CD34+ cells were increased 3.3–4.8 folds compared with the input (p < 0.05) at 2 weeks in culture, while the CD34+/CD38− cells accounted for most of the increase, and at 5 weeks in culture, the CD34+ cells were maintained, although there was no statistical difference when compared with the input, but the CD34+/CD38− cells showed an augmentation when compared with the initial cell number (1.3 ± 0.3 × 103 vs. 1.0 ± 0.5 × 104, p < 0.05), which represent an increase of the CD34+/CD38− subpopulation (Table 1). Similar results were obtained in the MNC group. The CD34+ cells and CD34+/CD38− cells were decreased at 2 weeks and 5 weeks in the co-culture 2D system when compared with input (Table 2). Figure 3 shows representative flow cytometry dot plot data of purified CD34+ cells or MNCs from umbilical cord blood in the 3D system at 2 weeks and 5 weeks culture.
Table 1.
Yield of CD34+ cells and CD34+/CD38− cells cultured in the biomimetic osteoblast niche
| Inplanting cells | Initial input | 2-weeks culture | 5-weeks culture |
|---|---|---|---|
| CD34+ Cells | |||
| CD34+ | 2 × 104 | 7.5 ± 1.2 × 104* | 1.7 ± 0.5 × 104 |
| CD34+/CD38− | 1.3 ± 0.3 × 103 | 5.7 ± 0.7 × 104* | 1.0 ± 0.5 × 104* |
| MNC | |||
| CD34+ | 1.3 ± 0.6 × 103 | 6.8 ± 2.3 × 103* | 2.1 ± 0.4 × 103* |
| CD34+/CD38− | 1.5 ± 0.1 × 102 | 5.3 ± 2.1 × 103* | 1.1 ± 0.3 × 103* |
Note: 2 × 104 UCB CD34+ cells or 1 × 105 MNCs were seeded in the biomimetic osteoblast niche. After 2–5 weeks in culture, CD34+ cells and CD34+/CD38− cells were evaluated by FCM. (*) Significant difference between each time point and the input (p < 0.05)
Table 2.
Yield of CD34+ cells and CD34+/CD38− cells cultured in the co-culture 2D system
| Inplanting cells | Initial input | 2-weeks culture | 5-weeks culture |
|---|---|---|---|
| CD34+ Cells | |||
| CD34+ | 2 × 104 | 1.6 ± 0.3 × 104* | 4.2 ± 1.5 × 103* |
| CD34+/CD38− | 1.3 ± 0.3 × 103 | 6.6 ± 1.8 × 102* | 2.5 ± 0.7 × 102* |
| MNC | |||
| CD34+ | 1.3 ± 0.6 × 103 | 1.2 ± 0.4 × 103 | 1.8 ± 0.3 × 102* |
| CD34+/CD38− | 1.5 ± 0.1 × 102 | 0.6 ± 0.3 × 102* | 0.3 ± 0.2 × 102* |
Note: 2 × 104 UCB CD34+ cells or 1 × 105 MNCs were seeded in 2D co-culture. After 2–5 weeks in culture, CD34+ cells and CD34+/CD38− cells were evaluated by FCM. (*) Significant difference between each time point and the input (p < 0.05)
Fig. 3.
Representative FACS analysis is shown for purified CD34+ cells or MNCs from UCB in 3D culture system. Q1 quadrant hint CD34+/CD38− cell fragment, Q1 and Q2 quadrants hint CD34+ cells at onset (a, d). CD34+/CD38− cells account for the highest amount of CD34+ cells after 2 weeks in culture of CD34+ cells (b). CD 34+ and CD34+/CD38− cells were increased in MNC after 2 weeks in culture (e). The CD34+/CD38− cells were increased after 5 weeks in culture of CD34+ cells (c). Both CD 34+ and CD34+/CD38− cells in MNC were increased after 5 week in culture (f)
Colony forming unit (CFU) assay of cultured cells
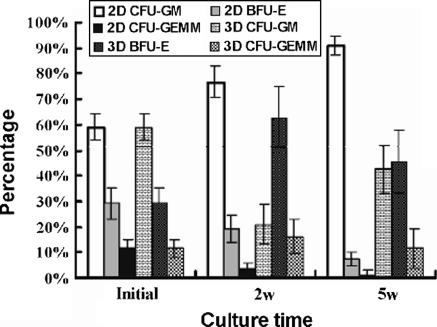
To measure the maintenance of colony-forming activity of the HSCs/HPCs, suitable aliquots of cultured cells were assayed for CFU-G, BFU-E, and CFU-GEMM. Without exogenous cytokine, the CFUs at 2 weeks culture in the 3D system showed a 4.6–9.3 folds increase in comparison with the input (p < 0.05), nevertheless, the CFUs were increased only 1.0–1.5 folds in the 2D system. At 5 weeks culture, the CFUs in the 3D system was 3.6–7.9 folds higher than in the 2D system, and there was a decrease of 18–75% of input in the 2D system. This trend was also observed in the MNCs cultivation (Fig. 4). The distribution of CFUs was analyzed through the colony morphology under inverted microscope. To verify and analyze the cell population of each colony we picked some colonies in situ and stained with Wright-Giemsa. The morphology showed that increased colonies in the 3D system were mainly composed of BFU-E, while in the 2D system were mostly myeloid lineage colonies (Fig. 5), furthermore, it revealed more megakaryocytes, eosinophile granulocytes, and basophile granulocytes in colonies from the 3D system.
Fig. 4.
Maintenance and expansion of CFU of UCB CD34+ cells and MNC in the 2D or 3D culture system. 2 × 104 UCB CD34+ cells or 1 × 105 MNC per well were cultured in the 2D or 3D culture system for 2–5 weeks. Cultured cells were recovered for methylcellulose CFU assay. To normalize the multiple experiments, the content of CFUs at each time point were expressed by the fold of initial CFU of each sample, each bar in the graphs represents mean ± SD from three separate experiments performed in duplicate. (^) Significant difference between each time point and the initial (p < 0.05). (*) significant difference between 2D and 3D culture system (p < 0.05)
Fig. 5.
Colony distribution of the hematopoietic cells at each time point. 2 × 104 UCB CD34+ cells per well were cultured in 2D or 3D system for 2–5 weeks. Cultured cells were recovered for methylcellulose CFU assay. The lineage of the colony was determined by their appearance under inverted light microscope as described in materials and methods. The percentage of each lineage was presented by mean ± SD from three separate experiments performed in duplicate. BFU-E: erythroid burst forming unit; CFU-G: granulocyte colony forming unit; CFU-GEMM: colony-forming units-granulocyte, erythroid, macrophage, megakaryocyte
Maintenance of multipotential progenitor
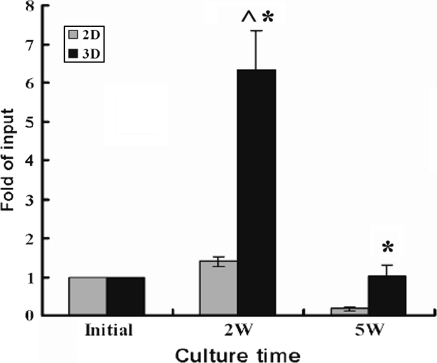
Long term culture initiating cell assay is one method to assess the most primitive hematopoietic cells ex vivo. The CD34+ cells in our study were cultured for 2–5 weeks in 2D and 3D systems, and then co-cultured for 5 weeks with M2-10B cells in long term culture medium. Cells were ultimately cultured 2 weeks in methylcellulose medium, represented multipotential progenitor cells, which is also termed extended long term culture initiating cells (Hao et al. 1996). LDA was used to determine the frequency of LTC-ICs in the CD34+ cells at initial and harvested cells after culture. Absolute LTC-ICs was calculated according to the LTC-ICs frequency and total harvested cell number. Figure 6 shows that LTC-ICs were increased 5.2–7.2 fold after 2 weeks culture in the 3D system, and 1.26–1.45 fold in the 2D system (p < 0.05). After 5 weeks in culture, the LTC-ICs were maintained in the 3D system, while were significantly decreased in the 2D system compared with the initial cell number, and the LTC-ICs were decreased 5.5–13.7 fold in the 2D system compared to the 3D system.
Fig. 6.
Maintenance of LTC-IC in the 2D and 3D culture system. CD34+ cells were cultured for 2–5 weeks in the 2D and 3D culture system, after five additional weeks in culture, limiting dilution methylcellulose-CFU assays were performed. To normalize the multiple experiments, the content of LTC-IC at each time point was expressed by the fold of initial LTC-IC, each bar in the graph represents mean ± SD from three separate experiments. (^) Significant difference between each time point and the initial (p < 0.05). (*) Significant difference between 2D and 3D culture system (p < 0.05). 2D: 2 dimensional culture system; 3D: 3 dimensional culture system
Discussion
A suitable culture system is indispensable to study HSCs/HPCs biological characteristics and to expand hematopoietic stem cells ex vivo. It is essential to maintain the self-renewal and differentiation potential during cultivation. Many investigators have tried culture systems with different combinations or concentrations of various cytokines. Although the content of HSCs/HPCs was increased, the augmentation was at the expense of self-renewal capability (McNiece et al. 2002). In living bone marrow, the interaction and movement of HSCs and supportive cells typically follow a chemical signal or molecular gradient in 3D. Such a gradient is important for signal transduction, but is absent in 2D system. Departing from the natural 3D microenvironments might be the reason for the inability to maintain HSCs character in ex vivo culture. In light of the structure–function relationship of bone marrow, simulating a 3D microenvironment which offers an intricate cell–cell and cell–matrix interaction of HSCs might be an avenue to retain or expand HSCs/HPCs.
3D systems have been employed to culture HSCs in recent years. Many scaffolds have been used, which included natural materials, such as collagen carriers and cellulose porous microspheres, or synthetic materials, such as porous biomatrix, polyethylene terephthalate, porous polyvinyl formal, porous gelatin microspheres, polyester nonwoven fabric porous disc carriers, and colloidal crystals (Bagley et al. 1999; Li et al. 2001; Zhang et al. 2006; Tun et al. 2002; Tomimori et al. 2000; Nichols et al. 2009). These culture systems were superior to traditional 2D systems for maintaining and expanding the HSCs/HPCs. Hematopoietic progenitor cells were increased in 3D systems without exogenous cytokines, serum, and stromal cell, which suggested that spatial structure of a culture system would influence biological behavior of hematopoietic cells (Ehring et al. 2003). Most 3D culture investigations merely provided physical scaffold for HSCs or supportive cells. There are still many differences in the scaffold pore size and scaffold matrix components when compared with natural bone marrow, even if collagen, tantalum, and heparin were coated onto the scaffold to improve the adhesiveness. We applied bio-derived bone as scaffold in 3D culture. It has good biocompatibility, natural scale, pore size, and more importantly, partially reserved calcium, phosphonium, and other extracellular matrix, which are demonstrated to promote osteogenic differentiation of MSCs in vitro (Xie et al. 2007). The MSCs attached to this natural scaffold very well (65–80% attachment) and more efficiently than the PET scaffold (30–50% attachment) (Li et al. 2001).
Osteoblasts have proven to be the crucial components of HSC niche in bone marrow. They not only anchor HSCs on the endosteum, but most importantly maintain the quiescence of HSCs through signaling of receptors-ligands and cell-adhesion molecules. We presumed that co-culture with osteoblast might retard the HSCs differentiation during ex vivo culture, which has been demonstrated reasonably in 2D cultures (Rochet et al. 2003; Taichman and Emerson 1994). Our previous studies have demonstrated the osteoblast induced from MSCs was more efficient in supporting hematopoiesis than primary osteoblast or MSCs (Huang et al. 2006). N-cadherin is the key molecular for HSCs quiescence; we have demonstrated that the osteoblast-like cells expressed high level of N-cadherin after 7–10 days induction (Hou et al. 2009). Osteoblasts and osteoblast-like cells induced from MSCs were used in our study.
Our results showed that CD34+ cells and CD34+/CD38− cells were maintained and increased after cultivation of the CD34+ cells or the MNCs from UCB, while the CD34+/CD38+ cell population was decreased in the biomimetic osteoblast niche. It demonstrated that cell to cell contact was critical for HSCs quiescence and survival. Previous studies have found that direct contact with the microenvironment recruited significant numbers of CD34+/CD38− cells into cell cycle and increased asymmetric division, which was required for maintaining HSCs self-renewal potential (Punzel et al. 2003). The augmentation of CD34+/CD38− cells in the biomimetic niche might be attributed to the asymmetric division of HSCs, of the relatively increased nutrition and supportive cell signal in the microenvironment.
We further examined the colony-forming function of cultured hematopoietic cells. The CFU content was shown to be increased in the 3D culture, with higher CFU production than in traditional 2D systems. Interestingly, the HSCs produced higher proportion of BFU-E and CFU-GEMM after culture in 3D than in 2D culture systems. In addition to the erythroid cells, other cell lineage colonies (megakaryocytes, eosinophile granulocytes, and basophile granulocytes) were present at significant levels in the 3D system. Mantalaris et al. cultured marrow cells in a reactor packed with porous microspheres with low concentration EPO, which produced more erythroid cells than the Dexter culture system (Mantalaris et al. 1998). Erythropoiesis of the HSCs was preserved in our studies without exogenous EPO. These observations demonstrated that the multiple differentiation potential of the HSCs was partially preserved when cultured in the “biomimetic osteoblast niche”. The LTC-IC is the most primitive hematopoietic cell which can be measured ex vivo. Our study showed that the content of LTC-ICs was increased after 2 weeks culture in the 3D system, and obviously decreased after 5 weeks culture in the 2D system, which was about 8–11% of input, while the 3D system culture generated 8.8–13.7 fold more of LTC-ICs than that of the 2D system. This indicates than the biomimetic niche is capable of maintaining and expanding primitive hematopoietic cells in long term culture.
Recent studies suggest that the expansion of HSCs/HPCs can be implemented by the augmentation of quiescent signal supporting cells by genetic technology or adherence factors with artificial extracellular matrix (Zhang et al. 2003; Oswald et al. 2006). In our study, the space of osteoblast niche was expanded through co-culture with mesenchymal stem cells induced osteoblasts, which would afford HSC quiescent signal during culture. Osteoblasts were cultured in bio-derived bone with natural geometric structure and extracellular matrix components mimicry bone marrow structure and function to a large extent in vitro. It should be mention than the biomimetic niche in our study is a mimicry of bone marrow microenvironment, which is not the natural niche for primitive HSCs/HPCs of UCB. The HSCs/HPCs of UCB survive and maintain their multilineage potential in a physiological natural environment with higher oxygen concentration without osteoblasts. A study showed that osteoblast-derived factors, such as VLA-4/VCAM-1 and SDF-1, can make bone marrow into a permissive environment for UCB-HSCs/HPCs, maintaining both survival and possible self-renewal and multilineage differentiation properties (Dao et al. 2007).
It appears that the function of bone marrow niche in vivo could be better simulated through the cancellous bone scaffold and stromal cells in vitro. It might be a new tool to bridge this gap between a Petri dish and an animal model. Through its use, it may open a new avenue for the studies on HSC/HPC behavior and the relationship between hematopoietic stem cells and environment, and assist in the maintenance and expansion of hematopoietic stem cells in vitro.
Acknowledgments
The authors would like to thank the Laboratory of Stem Cell and Tissue Engineering in State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu, Sichuan, and P.R.China for kindly providing bio-derived bone. We also would like to thank Dr. William J. Burke, a professor of Ohio University, for his kind help to make language correction to this paper.
This work was supported by a grant from National Natural Science Foundation of People’s Republic of China (No. 30870637).
References
- Bagley J, Rosenzweig M, Marks DF, Pykett MJ. Extended culture of multipotent hematopoietic progenitors without cytokine augmentation in a novel three-dimensional device. Exp Hematol. 1999;27:496–504. doi: 10.1016/S0301-472X(98)00053-8. [DOI] [PubMed] [Google Scholar]
- Banu N, Rosenzweig M, Kim H, Bagley J, Pykett M. Cytokine-augmented culture of haematopoietic progenitor cells in a novel three-dimensional cell growth matrix. Cytokine. 2001;13:349–358. doi: 10.1006/cyto.2001.0836. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Dao MA, Creer MH, Nolta JA, Verfaillie CM. Biology of umbilical cord blood progenitors in bone marrow niches. Blood. 2007;110:74–81. doi: 10.1182/blood-2006-08-034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring B, Biber K, Upton TM, Plosky D, Pykett M, Rosenzweig M. Expansion of hpcs from cord blood in a novel 3d matrix. Cytotherapy. 2003;5:490–499. doi: 10.1080/14653240310003585. [DOI] [PubMed] [Google Scholar]
- Hao QL, Thiemann FT, Petersen D, Smogorzewska EM, Crooks GM. Extended long-term culture reveals a highly quiescent and primitive human hematopoietic progenitor population. Blood. 1996;88:3306–3313. [PubMed] [Google Scholar]
- Hou L, Liu T, Tan J, Meng W, Deng L, Yu H, Zou X, Wang Y. Long-term culture of leukemic bone marrow primary cells in biomimetic osteoblast niche. Int J Hematol. 2009;90:281–291. doi: 10.1007/s12185-009-0392-4. [DOI] [PubMed] [Google Scholar]
- Huang XB, Liu T, Meng WT, Zhi W. Osteoblasts differentiated from human marrow bone mesenchymal stem cells support hematopoietic stem/progenitor cells from umbilical cord blood. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006;14:552–556. [PubMed] [Google Scholar]
- Li Y, Ma T, Kniss DA, Yang ST, Lasky LC. Human cord cell hematopoiesis in three-dimensional nonwoven fibrous matrices: in vitro simulation of the marrow microenvironment. J Hematother Stem Cell Res. 2001;10:355–368. doi: 10.1089/152581601750288966. [DOI] [PubMed] [Google Scholar]
- Mantalaris A, Keng P, Bourne P, Chang AY, Wu JH. Engineering a human bone marrow model: a case study on ex vivo erythropoiesis. Biotechnol Prog. 1998;14:126–133. doi: 10.1021/bp970136+. [DOI] [PubMed] [Google Scholar]
- McNiece IK, Almeida-Porada G, Shpall EJ, Zanjani E. Ex vivo expanded cord blood cells provide rapid engraftment in fetal sheep but lack long-term engrafting potential. Exp Hematol. 2002;30:612–616. doi: 10.1016/S0301-472X(02)00805-6. [DOI] [PubMed] [Google Scholar]
- Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–4347. [PubMed] [Google Scholar]
- Nichols JE, Cortiella J, Lee J, Niles JA, Cuddihy M, Wang S, Bielitzki J, Cantu A, Mlcak R, Valdivia E, Yancy R, McClure ML, Kotov NA. In vitro analog of human bone marrow from 3d scaffolds with biomimetic inverted colloidal crystal geometry. Biomaterials. 2009;30:1071–1079. doi: 10.1016/j.biomaterials.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen-Druey C, Tichelli A, Meyer-Monard S. Human hematopoietic colonies in health and disease. Acta Haematol. 2005;113:5–96. doi: 10.1159/000081987. [DOI] [PubMed] [Google Scholar]
- Oswald J, Steudel C, Salchert K, Joergensen B, Thiede C, Ehninger G, Werner C, Bornhauser M. Gene-expression profiling of cd34+ hematopoietic cells expanded in a collagen i matrix. Stem Cells. 2006;24:494–500. doi: 10.1634/stemcells.2005-0276. [DOI] [PubMed] [Google Scholar]
- Punzel M, Liu D, Zhang T, Eckstein V, Miesala K, Ho AD. The symmetry of initial divisions of human hematopoietic progenitors is altered only by the cellular microenvironment. Exp Hematol. 2003;31:339–347. doi: 10.1016/S0301-472X(03)00024-9. [DOI] [PubMed] [Google Scholar]
- Rochet N, Leroy P, Far DF, Ollier L, Loubat A, Rossi B. Cal72: a human osteosarcoma cell line with unique effects on hematopoietic cells. Eur J Haematol. 2003;70:43–52. doi: 10.1034/j.1600-0609.2003.02766.x. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomimori Y, Takagi M, Yoshida T. The construction of an in vitro three-dimensional hematopoietic microenvironment for mouse bone marrow cells employing porous carriers. Cytotechnology. 2000;34:121–130. doi: 10.1023/A:1008157303025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traycoff CM, Kosak ST, Grigsby S, Srour EF. Evaluation of ex vivo expansion potential of cord blood and bone marrow hematopoietic progenitor cells using cell tracking and limiting dilution analysis. Blood. 1995;85:2059–2068. [PubMed] [Google Scholar]
- Tun T, Miyoshi H, Aung T, Takahashi S, Shimizu R, Kuroha T, Yamamoto M, Ohshima N. Effect of growth factors on ex vivo bone marrow cell expansion using three-dimensional matrix support. Artif Organs. 2002;26:333–339. doi: 10.1046/j.1525-1594.2002.06842.x. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Yang F, Deng L, Luo J, Qin T, Li X, Zhou GQ, Yang Z. The performance of a bone-derived scaffold material in the repair of critical bone defects in a rhesus monkey model. Biomaterials. 2007;28:3314–3324. doi: 10.1016/j.biomaterials.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Hirayama F, Kanai M, Sato N, Fukazawa K, Yamashita K, Sawada K, Koike T, Kuwabara M, Ikeda H, Ikebuchi K. Serum-free coculture system for ex vivo expansion of human cord blood primitive progenitors and scid mouse-reconstituting cells using human bone marrow primary stromal cells. Exp Hematol. 2001;29:174–182. doi: 10.1016/S0301-472X(00)00653-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chai C, Jiang XS, Teoh SH, Leong KW. Co-culture of umbilical cord blood cd34+ cells with human mesenchymal stem cells. Tissue Eng. 2006;12:2161–2170. doi: 10.1089/ten.2006.12.2161. [DOI] [PubMed] [Google Scholar]