Abstract
Colon cancer is the second leading cause of cancer-related death in industrialized countries. Many anti-cancer researches are consequently performed and individualized tumor response testing (ITRT) methods are now used to individualize patient chemotherapeutic administrations. Then, a new ITRT method, Oncogramme, was developed for colon cancer. Colon tumor fragments from different patients were dissociated and seeded in a defined culture medium. Cell preparation process as well as culture medium allowed high cell viability and a good primary culture success rate. After treatment of isolated tumoral cells by chemotherapeutics alone or in combination, cytotoxicity was determined by cell death assay allowing the Oncogramme establishment, which was validated by statistical analysis. Indeed, significant results were obtained such as different profile for each patient’s cells with various drugs, and variability between patient’s cells in the response to each drug. Procedure described here to obtain the Oncogramme is a new, fast and technically reliable ITRT method applied to colon cancer. For an individualized cancer treatment use, this test should be further validated by a phase I clinical trial.
Keywords: Colorectal cancer, Primary culture, Individualized treatment
Introduction
Colorectal cancer accounts for 10–15% of all cancers and is the second leading cause of cancer-related death in industrialized countries (Ferlay et al.2010). The treatment of colorectal cancer has undergone substantial improvements in the last 10–15 years in terms of screening, surgical management of resecable disease and adjuvant chemotherapy (Wils 2001). Nevertheless, 5-year survival for all patients has improved from only 50 to 62% during the last 25 years (Angelopoulos et al.2004; Jemal et al.2005). Lack of therapeutic activity may be greatly influenced by tumour heterogeneity with various subpopulations exhibiting different biological and pharmacological properties (Brattain et al.1981; Brattain et al.1984). Several in vitro models representative of the types of cells found in colonic cancer have been developed (Williams et al.1993). Cell lines established from human colorectal carcinomas may provide useful tools to study this disease and to develop and test new therapeutic approaches (Battu et al.1998; Vincan et al.2007). However, cell line represents just one stage of tumour progression (Paraskeva et al.1990). Primary culture from fresh tumor tissue is difficult to establish but reflect the actual antitumor agent’s responsiveness of tumours at their developmental stages.
To assist clinicians selecting the appropriate anticancer agent, individualized tumor response testing (ITRT) have been developed for all kinds of cancer (breast, ovary, lung, colon…) (Hamburger 1981; Massaro et al.1989; Möllgård et al.2003; Pieters et al.1990). ITRT in cancer treatments are ex vivo tests which appeared as indispensable assays for predicting cancer treatment. However, in contrast to other countries such as USA (paid off by Medicare) or Japan (approved by the Japanese Ministry of Health, Welfare, and Labor as “advanced clinical medicine”, in July 1999, for use at Keio University Hospital and the number of approved institutes increased to 11 in December 2005 (Kubota and Weisenthal 2006)) these promising tests are not currently used in France.
Oncomedics is then the first French company developing an ITRT, named Oncogramme, which take into account all previous studies and problems. The present paper report all the steps from fragments conservation after surgery, washing and dissociation procedure, primary cell culture to Oncogramme, which were specifically designed for colon tumor.
Patients and methods
Patients tumors
Colorectal carcinoma specimens were collected from 25 patients during surgery (2008, 2009) in collaboration with Pr M. Mathonnet from Digestive Surgery Department, and the Pathology and Anatomy Department of Limoges Regional Hospital, France. The pathologist dissected the specimen from the periphery of the tumor to avoid extracting necrosis tissue and confirmed its tumorous nature. Informed consent was obtained from all patients. Tissues were collected in DMEM/F12 (Invitrogen, Cergy Pontoise, France) complemented with a cocktail of antibiotics (B0.5X, Oncomedics, Limoges, France) which permits to keep them at least 48 h before culture processing.
Primary cell culture
After three washing bath of 30 min (one with WBM and two with WBB5X, Oncomedics), specimens were dissected into 1 × 1 mm pieces using Mac Ilwain Tissue Chopper, immersed in an enzymatic dissociation buffer (DBColon, Oncomedics) and then incubated at 37 °C for 2 h. After stopping enzymatic activity, cells were centrifuged at 150g for 10 min. After measuring the cell viability by dye exclusion using trypan blue, the cell pellet was resuspended in defined culture medium (MDB1XColon, Oncomedics), and seeded in culture support according to appropriate density.
After 5 days culture (37 °C, 5% CO2, Binder humidity CO2 Incubator, Tuttlingen, Germany), cells were centrifuged, counted and the viability measured by dye exclusion method. Then cells were resuspended into new culture flasks in fresh defined culture medium.
Oncogramme establishment
After 7 days in culture, aliquots of cells were seeded in 8-well Labtek plates (less than 5 × 104 cells/well, Nunc, Fisher Scientific Bioblock, Illkrich, France) and exposed during 72 h to MDB1XColon (control group) or to current chemotherapeutics used during colon cancer treatment in MDB1XColon: 5-fluorouracil (5-FU) (Sigma, Saint-Quentin Fallavier, France), irinotecan (Sigma, Saint-Quentin Fallavier, France) and oxaliplatin (Sigma, Saint-Quentin Fallavier, France) (Collectif 1992; Falcone et al.2007; Tournigand et al.2004). Each drug concentration was chosen by first determining their IC50 on colon cell lines and after testing concentrations close to IC50 on colon primary cells (data not shown). Concentrations chosen were 25, 100 and 150 μg/mL for 5-FU, irinotecan and oxaliplatin, respectively. These chemotherapeutic agents were applied alone or combined in order to mimic standard protocol for in vivo treatments of colon cancer: FOLFOX (5FU and oxaliplatin), FOLFIRI (5FU and irinotecan) and FOLFIRINOX (5FU, oxaliplatin and irinotecan) (Collectif 1992; Falcone et al.2007; Tournigand et al.2004). Cell viability was determined according to Live/Dead Viability/Cytotoxicity kit for mammalian cells (Molecular probes, Leiden, Netherlands). Viable cells showed esterase activity that permit green fluorescence of calcein and dead cells were indicated by the red fluorescence of ethidium homodimer that penetrate into nucleus of dead cells that lack of membrane integrity. After incubation of cells in ethydium homodimer and calcein AM as recommended by purchaser, cells were washed in phosphate buffer saline (PBS) and fixed in 4% paraformaldehyde (Sigma) in PBS for 5 min at 22 °C (Binder Incubator). Total cells were detected with a 20 min counterstaining with 0.5 μg/mL DAPI (Sigma) in water at 22 °C. Cells were finally fixed with glycerol gelatin (Sigma) and examined by fluorescent microscope (Leica DMRX, Rueil-Malmaison, France). After pictures superposition and counting, the percentage of dead cells was determined in each treated and control wells. Then, the cell death ratio was calculated for each drug in reference to the values obtained with control cells (fixed to 1).
Immunochemistry
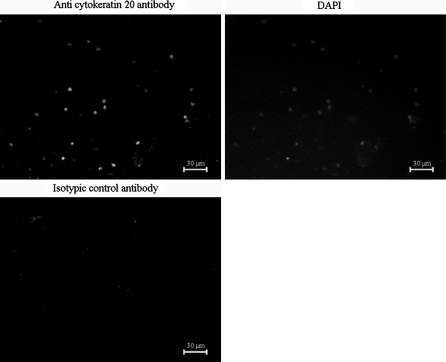
After 10 days in culture, aliquot of cells was seeded in 8-well Labtek plates (less than 5 × 104 cells/well, Nunc). Cells were fixed in paraformaldehyde (Sigma) at 4% in PBS for 10 min at 22 °C and permeabilized using Triton X100 (Sigma). After saturation with 10% goat serum (Sigma), cells were incubated for 60 min with a monoclonal mouse anti-cytokeratin 20 antibody (clone Ks20.8, Dako, Trappes, France) at 2.4 mg/L or with isotypic control (irrelevant mouse immunoglobulin G, Merck Chemical Ltd, Nottingham, United Kingdom). They were then revealed by a 30-min incubation with Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin antibodies (1/2000, Molecular Probes). Total cells were detected with a 20 min counterstaining with 0.5 μg/mL DAPI (Sigma) in water at 22 °C, fixed with glycerol gelatin (Sigma) and examined by fluorescence microscopy (Leica DMRX).
Cell size analysis
After 10 days in culture, a 256 channel Multisizer II Coulter Counter (Beckman Coulter, Fullerton, CA) was used to determine the mean cell population diameter. Cells were diluted in Isoton® to a final volume of 15 mL. The counting conditions were: 500 μL sample volume, cumulating three successive assays. Results are displayed as the mean ± standard deviation for three different experiments.
Statistical analysis
Statistical analyses were done performing ANOVA, with Tukey and Bonferroni comparison post-tests, using GrahPad InStat software.
Results
Here we show results concerning surgical specimens obtained from 25 patients (Limoges Regional Hospital, France) with colorectal tumor.
With the method described here, primary cell cultures were out of bacterial contamination in 92% of cases (Table 1). Outside of bacterial contamination, the procedure and the defined medium allowed a primary culture success rate of 95.6% (Table 1). High cell viabilities were observed either after cell individualization or after 5 days in culture flasks with the defined culture medium MDColon (Table 1). Using this procedure and MDColon medium, isolated cells could be maintained in a long-term culture system: at least 18 months in some cases (data not shown). Tumor cells, isolated from the surgical specimens of colon cancer and grown by the present methods, were epithelial small non-adherent floating cells (Table 1; Fig. 1).
Table 1.
Characteristics of the 25 colon primary cultures obtained with the procedure developed for the Oncogramme
| 25 Surgical specimens of colon cancer | ||
|---|---|---|
| 22 Successful culture procedures | 2 Bacterial contaminations 3 days after procedure | 1 Failing culture procedure |
| Mean viability after cell dissociation: 90.11 ± 3.65% | Viability after cell dissociation: 41% | |
| Mean viability after 5 days of culture: 88.56 ± 5.15% | – | – |
| Mean cells diameter: 5.29 ± 0.24 μm | – | – |
| Oncogrammes: done | – | – |
Fig. 1.
Expression of cytokeratin 20 in colorectal cancer primary cultures. Ten days after primary cell culture procedure, immunochemistry of the specific epithelial marker of colorectal cells, cytokeratin 20 (clone Ks20.8, 2.4 mg/L, see “Methods” section), revealed that the majority of the cells are epithelial
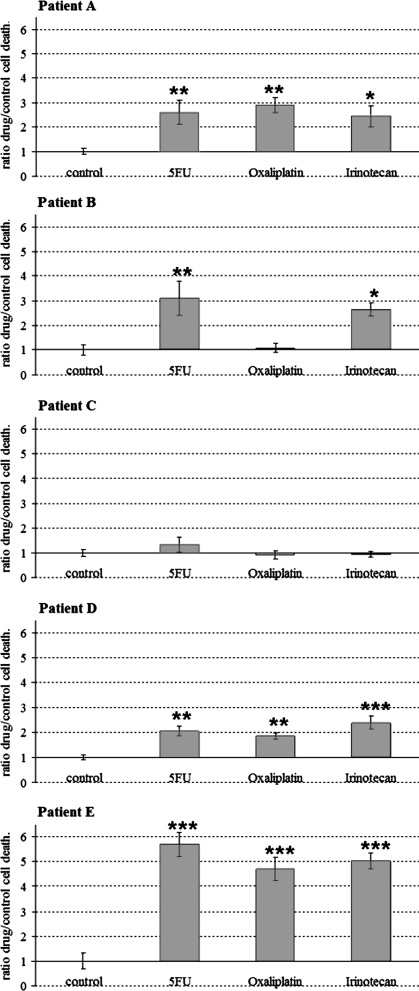
Representative Oncogrammes, obtained by this method, are shown in Fig. 2. After a 72 h drug treatment, cell death ratios were compared to control cell death. Statistical analysis revealed different representative Oncogramme profiles. Oncogramme of patients A and D showed a relatively good response in vitro to the three tested drugs. Patient B Oncogramme showed an in vitro resistance to oxaliplatin, with a cell death ratio statistically similar to the control (p > 0.05). Whereas patient C Oncogramme showed a global chemoresistance of the cells (p > 0.05), patient E Oncogramme revealed a strong sensibility of the cells to the same drugs (p < 0.001).
Fig. 2.
Five representative Oncogrammes showing the effects of 5-FU, irinotecan and oxaliplatin on patients’ cell death. After 72 h drug treatment, cell death ratios were compared to control cell death (means ± standard error of the mean (SEM); *p < 0.05; **p < 0.01; ***p < 0.001)
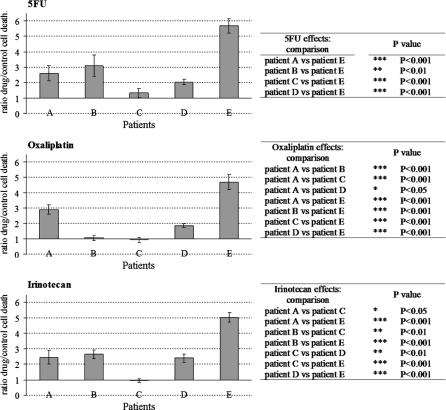
In order to represent the variability between patient cells, Fig. 3 compared cell death ratios in response to 5FU, oxaliplatin and irinotecan. Statistical analysis revealed differences between patient cell responses, which were resumed in corresponding tables. Results showed that for one drug at a determined concentration, patient cells responded differently each other. It was the case for all drugs tested here.
Fig. 3.
Cell death ratios of the five representative patients in response to 5FU, oxaliplatin and irinotecan. After 72 h drug treatment, cell death ratios provoked by each drug were compared between patients (means ± SEM)
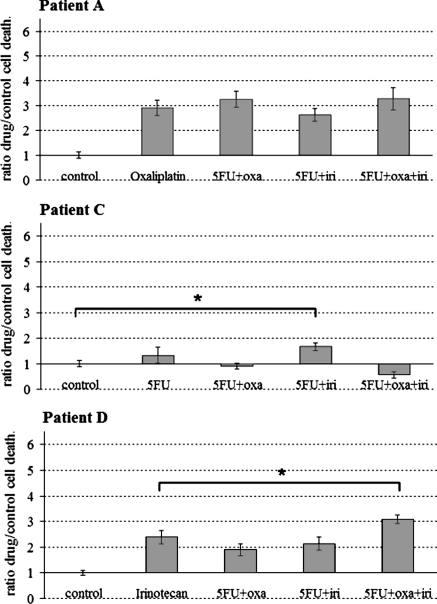
To illustrate the interest of studying the effects of chemotherapeutic associations on cells such as standard clinical protocol for in vivo treatments of colon cancer, Fig. 4 compared it to the most efficient drug observed in patients A, C and D. Although patient A Oncogramme did not show any supplementary effects of combined chemotherapeutics on cell death, patient D Oncogramme showed that the 5FU associated with oxaliplatin and irinotecan was more effective than irinotecan alone (p > 0.05). Concerning patient C for which any chemotherapeutic agent was considered as effective (Fig. 2), 5FU combined to irinotecan enhanced significantly the cell death from control (p > 0.05; Fig. 4).
Fig. 4.
Three representative Oncogrammes showing the effects of chemotherapeutics associations on patients’ cell death. After 72 h drug treatment, cell death ratios were compared to the cell death ratio of the most effective agent alone, or to control cell death when any chemotherapeutic was revealed as effective (see Fig. 2) (means ± SEM; * p < 0.05). Oxa: oxaliplatin; iri: irinotecan
Discussion
The aim of this study was to demonstrate the interest of a new ITRT method called Oncogramme. Indeed, while this type of test is currently used in some other countries (Cortazar et al.1997; Kawamura et al.1997; Kurbacher et al.1998; Loizzi et al.2003), Oncogramme is the first ITRT method developed in France. The interest of this study was to validate both parts of the test, primary cell culture from surgical specimens and cell death analysis from isolated tumoral cells leading to the Oncogramme establishment.
Primary cell culture was performed according to a determined process using a defined medium (MDColon, Oncomedics) allowing high cell viability after tissue dissociation and 5 days culture. MDColon medium prevented fibroblasts survival (<10%; Fig. 1) and avoided medium variations. As we obtained a very satisfying primary culture success rate (Table 1), this dissociation procedure and culture medium could be routinely used in ITRT.
Chemotherapeutic activities were quantified using cell death essays. These analyses were performed on a low cell amount for each condition (<5 × 104), suggesting Oncogramme applications on small specimen material such as biopsies. Moreover, chemotherapeutic associations can also be tested, as for standard protocol for in vivo treatments (Fig. 4).
Significant results were obtained: different profile for each patient’s isolated cells is in favor for the interest of personalized treatments (Fig. 2).
In order to represent the variability between patient’s cells in response to each drug, we compared cell death ratios in response to 5FU, oxaliplatin and irinotecan (Fig. 3). Various profiles were obtained, showing that for one drug, the response of each patient was different and confirming that each drug concentration tested here was appropriate for ITRT.
Moreover, the Oncogramme can include the effects of chemotherapeutics associations on cell death in vitro (Fig. 4). Such Oncogrammes could permit to avoid heavy treatment for some patients, such as patient A for which any chemotherapeutic association in vitro is significantly more effective than oxaliplatin alone. It also could be used to select the appropriate chemotherapeutic cocktail for patients, such as for patient C and D.
In order to validate the Oncogramme as an ITRT, it would be essential to plan a phase I clinical trial in which Oncogramme results should be compared to clinical responses.
One important interest of the Oncogramme is the short lap time between patient surgery and results. Oncogramme can be obtained only 15 days after surgery, which is the classical latency period before any chemotherapeutics administration.
To conclude, cell culture process and Oncogramme are fast and technically reliable. The variability between patients shows that the Oncogramme should be a solid ITRT method for cancer treatment (p < 0.001, Fig. 3), which should be validated with clinical trials. Moreover, the use of such a procedure to test new drug response or to evaluate mechanisms of molecule on cell signaling can be envisaged. Contrary to cell lines, the use of primary cultures reflects patient’s reality and represents the best tool after the patient itself.
Acknowledgments
The authors thank Professor F. Labrousse and Professor F. Paraf, Department of Anatomy and Pathology, Hospital Dupuytren, Limoges, for providing specimens.
References
- Angelopoulos S, Kanellos I, Sapidis N, Vasiliadis K, Kanellou A, et al. Survival after curative resection for rectal cancer by the end of the 20th century. Tech Coloproctol. 2004;8(Suppl 1):s167–s169. doi: 10.1007/s10151-004-0146-5. [DOI] [PubMed] [Google Scholar]
- Battu S, Rigaud M, Beneytout JL. Resistance to apoptosis and cyclooxygenase-2 expression in a human adenocarcinoma cell line HT29 CL.19A. Anticancer Res. 1998;18:3579–3583. [PubMed] [Google Scholar]
- Brattain MG, Fine WD, Khaled FM, Thompson J, Brattain DE. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981;41:1751–1756. [PubMed] [Google Scholar]
- Brattain MG, Levine AE, Chakrabarty S, Yeoman LC, Willson JK, et al. Heterogeneity of human colon carcinoma. Cancer Metastasis Rev. 1984;3:177–191. doi: 10.1007/BF00048384. [DOI] [PubMed] [Google Scholar]
- Collectif Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced colorectal cancer meta-analysis project. J Clin Oncol. 1992;10:896–903. doi: 10.1200/JCO.1992.10.6.896. [DOI] [PubMed] [Google Scholar]
- Cortazar P, Gazdar AF, Woods E, Russell E, Steinberg SM, et al. Survival of patients with limited-stage small cell lung cancer treated with individualized chemotherapy selected by in vitro drug sensitivity testing. Clin Cancer Res. 1997;3:741–747. [PubMed] [Google Scholar]
- Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Hamburger AW. Use of in vitro tests in predictive cancer chemotherapy. J Natl Cancer Inst. 1981;66:981–988. doi: 10.1093/jnci/66.6.981. [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Ikeda K, Takiyama I, Terashima M. The usefulness of the ATP assay with serum-free culture for chemosensitivity testing of gastrointestinal cancer. Eur J Cancer. 1997;33:960–966. doi: 10.1016/S0959-8049(97)00075-0. [DOI] [PubMed] [Google Scholar]
- Kubota T, Weisenthal L. Chemotherapy sensitivity and resistance testing: to be “standard” or to be individualized, that is the question. Gastric Cancer. 2006;9:82–87. doi: 10.1007/s10120-006-0366-7. [DOI] [PubMed] [Google Scholar]
- Kurbacher CM, Cree IA, Bruckner HW, Brenne U, Kurbacher JA, et al. Use of an ex vivo ATP luminescence assay to direct chemotherapy for recurrent ovarian cancer. Anticancer Drugs. 1998;9:51–57. doi: 10.1097/00001813-199801000-00006. [DOI] [PubMed] [Google Scholar]
- Loizzi V, Chan JK, Osann K, Cappuccini F, DiSaia PJ, et al. Survival outcomes in patients with recurrent ovarian cancer who were treated with chemoresistance assay-guided chemotherapy. Am J Obstet Gynecol. 2003;189:1301–1307. doi: 10.1067/S0002-9378(03)00629-X. [DOI] [PubMed] [Google Scholar]
- Massaro EJ, Elstein KH, Zucker RM, Bair KW. Limitations of the fluorescent probe viability assay. Mol Toxicol. 1989;2:271–284. [PubMed] [Google Scholar]
- Möllgård L, Prenkert M, Smolowicz A, Paul C, Tidefelt U. In vitro chemosensitivity testing of selected myeloid cells in acute myeloid leukemia. Leuk Lymphoma. 2003;44:783–789. doi: 10.1080/1042819031000067594. [DOI] [PubMed] [Google Scholar]
- Paraskeva C, Corfield AP, Harper S, Hague A, Audcent K, et al. Colorectal carcinogenesis: sequential steps in the in vitro immortalization and transformation of human colonic epithelial cells (review) Anticancer Res. 1990;10:1189–1200. [PubMed] [Google Scholar]
- Pieters R, Loonen AH, Huismans DR, Broekema GJ, Dirven MW, et al. In vitro drug sensitivity of cells from children with leukemia using the MTT assay with improved culture conditions. Blood. 1990;76:2327–2336. [PubMed] [Google Scholar]
- Tournigand C, André T, Achille E, Lledo G, Flesh M, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- Vincan E, Brabletz T, Faux MC, Ramsay RG. A human three-dimensional cell line model allows the study of dynamic and reversible epithelial-mesenchymal and mesenchymal-epithelial transition that underpins colorectal carcinogenesis. Cells Tissues Organs. 2007;185:20–28. doi: 10.1159/000101299. [DOI] [PubMed] [Google Scholar]
- Williams AC, Hague A, Manning AM, Stappen JW, Paraskeva C. In vitro models of human colorectal cancer. Cancer Surv. 1993;16:15–29. [PubMed] [Google Scholar]
- Wils J. Adjuvant therapy for colon cancer: the European experience. Tumori. 2001;87:S85. [PubMed] [Google Scholar]