Abstract
It is well established that many cell functions are controlled by the PI-3K signaling pathway and the signaling lipid, phosphatidylinositol-3,4,5-triphosphate (PIP3). This is particularly true for mast cells which play a key regulatory role in allergy and inflammation through activation via high-affinity IgE receptors (FcεRI ) leading to activation of signaling cascades and subsequent release of histamine and other pro-inflammatory mediators. A pivotal component of this cascade is the activation of PI-3K and a rise in intracellular levels of PIP3. In this study, we developed a novel chimeric toxin that selectively binds to mast cells and which functions as a PIP3 phosphatase. Specifically, the chimeric toxin was composed of the FcεRI binding region of IgE and the active subunit of the cytolethal distending toxin, CdtB, which we have recently demonstrated to function as a PIP3 phosphatase. We demonstrate that the chimeric toxin retains PIP3 phosphatase activity and selectively binds to mast cells. Moreover, the toxin is capable of altering intracellular levels of PIP3, block antigen-induced Akt phosphorylation and degranulation. These studies provide further evidence for the pivotal role of PIP3 in regulating mast cell activation and for this signaling lipid serving as a novel target for therapeutic intervention of mast cell- mediated disease. Moreover, these studies provide evidence for the utilization of CdtB as a novel therapeutic agent for targeting the PI-3K signaling pathway.
1. INTRODUCTION1
Mast cells play a key regulatory role in allergy and inflammation through the activation of high-affinity IgE receptors (FcεRI ) and subsequent release of histamine and other pro-inflammatory mediators (Kinet, 2007; Rivera and Olivera, 2008). Mast cell activation is dependent upon the binding of multivalent antigen to prebound IgE molecules leading to cross-linking of FcεRI. As a consequence of these events, a complex signaling cascade is activated leading to degranulation, synthesis of pro-inflammatory lipid mediators and cytokines. The high-affinity FcεRI is composed of an α-chain which is responsible for binding the Fc portion of IgE, a β-chain and twoγ-chains. Upon antigen binding and FcεRI cross-linking, immunoreceptor tyrosine-based activation motifs (ITAMS) on the β- and γ-subunits become phosphorylated and then serve as docking sites for secondary signaling proteins such as the tyrosine kinases Lyn and Syk. These molecular interactions increase tyrosine phosphorylation events and increase enzymatic activity of signaling proteins at or near the receptor complex. Additionally, signaling pathways leading to mast cell activation are dependent upon protein-lipid interactions. In particular, the generation of the signaling lipid, phosphatidylinositol-3,4,5-triphosphate (PIP3), has been shown to be critical for mediating mast cell activation (reviewed by Nadler et al., 2001).
Phosphoinositides (PIs) are derivatives of phosphatidylinositol and while they represent minor components of membrane lipids, PIs regulate a wide range of biological processes including: cell proliferation, cell survival, differentiation, cytoskeleton organization and membrane trafficking (reviewed in Kraub and Haucke, 2007;Sasaki et al., 2007). PIs regulate these cellular processes by serving as site-specific membrane signals that mediate membrane recruitment and regulation of effector/signaling proteins (Lemmon, 2008). PIP3 is synthesized from PI(4,5)P2 following the activation of PI-3K and has received much attention for its critical role as a second messenger. In particular, FcεRI-mediated mast cell activation requires the recruitment and activation of PI-3K and concomitant synthesis of PIP3 (Rivera and Olivera, 2008;Abramson and Pecht, 2007;Nadler et al., 2001;Tkacyzk and Gilfillan, 2001). The generation of PIP3 is critical to recruiting pleckstrin homology domain containing proteins such as BTK, Akt and PDK1 to the plasma membrane where they become activated and coupled to upstream signals and, in turn, transduce those signals to downstream events ultimately leading to activation of the secretory and synthetic response. Thus, FcεRI-mediated mast cell degranulation, synthesis of lipid derived pro-inflammatory mediators and cytokine production is ultimately controlled by PIP3 thereby making this lipid messenger a potent target for pharmacologic intervention in treating mast cell-mediated allergic disorders.
Several investigators have demonstrated that a reduction in PI-3K activity resulting from mutations or the introduction of inhibitors results in decreased PIP3 synthesis with a concomitant inhibition of cell function (reviewed in Kim et al., 2008). Of particular relevance, we have recently demonstrated that the active subunit of the cytolethal distending toxin (Cdt), CdtB, exhibits potent PIP3 phosphatase activity making it a useful agent to modulate PIP3 signaling pathways and to advance our understanding of the role of PIP3 in regulating mast cell activation (Shenker et al., 2007). We now report on the effect of a novel chimeric toxin composed of CdtB to specifically deplete cells of PIP3 and the FcεRI binding region of IgE to confer mast cell specificity in order to inhibit the FcεRI/PI-3K/PIP3 signaling pathway. Our results clearly indicate that the chimeric toxin not only specifically binds to mast cells, but is capable of inhibiting antigen-IgE mediated mast cell degranulation.
2. MATERIALS and METHODS
2.1 Cell culture
RBL-2H3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, glutamine (2 mM), penicillin (100 units/ml) and streptomycin (100 μg/ml) (Venkatesha et al., 2004). Murine bone marrow derived mast cells were (BMMC) derived from bone marrow cells obtained from the femurs of C57/BL6mice (Zaidi et al., 2006). Cells were cultured for 4-6 weeks in Iscove's medium supplemented with 10% fetal bovine serum, glutamine (2 mM) penicillin (100 units/ml), streptomycin (100 μg/ml), and rmIL-3 (10 ng/ml). The homogeneity of the mast cells was confirmed by acid toluidine blue staining; the cell population used for these studies was >95% BMMC.
2.2 Assay of degranulation
RBL-2H3 and BMMC were incubated overnight in the presence of 1 μg/ml anti-dinitrophenyl (DNP) mouse IgE (Sigma Chemical; St. Louis, MO). Cells (5 × 104) were seeded into 96-well plates in a total volume of 50μl of HEPES-buffered saline containing 0.1% BSA and exposed to DNP-BSA (Bioresearch Technologies; Novato, CA) for 30 min. For total β-hexosaminidase release, control cells were lysed in 50 μl of 0.1% Triton X-100. Aliquots of supernatants or cell lysates were incubated with 1 mM p-nitrophenyl-N-acetyl-β-D-glucosamine for 1.5 h at 37 °C. The reaction was stopped by adding 250 μl of a 0.1 M Na2CO3/0.1 M NaHCO3 buffer and absorbance was measured at 405 nm (Ali et al., 1993).
2.3 Construction and expression of chimeric toxin
Construction of the plasmid containing wildtype cdtB gene (pGEMCdtB) was previously described (Shenker et al., 2005). In order to construct the plasmid containing the cdtB gene fused to the coding sequence for the Fc binding region of IgE, designated pIVEXCdtBFcε, we inserted NcoI and NotI restriction sites by PCR using pGEMCdtB as a template with the following primers:
CAT GCC ATG GCA AAC TTG AGT GAT TTC AAA GTA GCA
ATA GTT TAG CGG CCG CGA TCA CGA ACA AAA CTA ACA GGA
A new plasmid (pIVEXCdtB) containing cdtB was generated from the PCR product which was ligated into pIVEX2.3d (Roche Applied Science; Indianapolis, IN). The Fcε fragment was generated by PCR using cDNA obtained from LPS activated murine B-cells as described (Belostotsky and Lorberboum-Galski, 2003); the following primers were used to incorporate NotI and XmaI restriction sites into the PCR product:
ATA AGA ATG CGG CCG CCA GCA ATG GAT GTC TGA AAG CA
CCC CCC GGG TGG GGT CTT GGT GAT GGA ACG CA
The PCR product contains 411 residues encoding 135 amino acids corresponding to residues 137-271 of the mouse IgE heavy chain (Genbank accession J00476); this sequence corresponds to the C terminus of domain 2 and domain 3. The PCR product was ligated into pIVEXCdtB to yield a new plasmid designated, pIVEXCdtBFcε. The chimeric protein was expressed using an in vitro coupled translation/transcription system (RTS 500 ProteoMaster; Roche Applied Science) that we routinely employ and the protein purified by nickel affinity chromatography as we incorporate a His tag into the construct (Shenker et al., 2004;Shenker et al., 2005).
2.4 Phosphatase assay
Phosphatase activity was assessed by monitoring the dephosphorylation of PIP3 as described by Maehama et al., 2000 and Shenker et al., 2007. Briefly, the reaction mixture (20 μl) consisted of 100 mM Tris-HCl (pH 8.0), 10 mM dithiothreitol, 0.5 mM diC16-phosphatidylserine (Avanti Polar Lipids; Alabaster, AL), 25 μM PIP3 (diC16; Echelon Biosciences; Salt Lake City, UT) and the indicated amount of toxin. Lipid solutions were deposited in 1.5 ml tubes, organic solvent removed, the buffer added and a lipid suspension formed by sonication. Phosphatase assays were carried out at 37°C for 30 min; the reactions were terminated by the addition of 15 μl of 100 mM N-ethylmaleimide. Inorganic phosphate levels were then measured using a malachite green assay. Malachite green solution [Biomol Green; (Biomol; Plymouth Meeting, PA)] was added to 100 μl of the enzyme reaction mixture and color was developed for 20 min at RT. Absorbance at 650 nm was measured and phosphate release quantified by comparison to inorganic phosphate standards.
2.5 Measurement of cellular PIP3 content
BMMC (5 × 105/ml) were incubated in the presence of medium or toxin for the time indicated. Replicate cultures (1 × 107 cells) were pooled and harvested. The cell pellet was treated with cold 0.5 M TCA for 5 min, centrifuged and the pellet washed twice with 5% TCA containing 1 mM EDTA. Neutral lipids were extracted twice with methanol:chloroform (2:1) at RT. Acidic lipids were extracted with 2.25 ml methanol:chloroform:12M HCl (80:40:1) for 15 min at RT; the samples were centrifuged for 5 min and the supernatant recovered. The supernatant was then treated with 0.75 ml chloroform and 1.35 ml 0.1 M HCl and centrifuged to separate organic and aqueous phases; the organic phase was collected and dried. The dried lipids were resuspended in 50 mM Hepes buffer (pH 7.4) containing 150 mM NaCl and 1.5% sodium cholate, and left overnight at 4°C. PIP3 levels were then determined using a commercially available competitive ELISA according to the manufacturers directions (PIP3 Mass ELISA Kit and PIP2 Mass ELISA Kit; Echelon Biochemicals).
2.6 Immunoassays
Samples were separated on 12% SDS-PAGE gels and then transferred to nitrocellulose. The membrane was blocked with BLOTTO and then incubated with anti-CdtB mAb (CdtB194), anti-Akt (Cell Signaling Technology; Danvers, MA) or anti-pAkt antibody (pAkt 473; Cell Signaling Technology) for 18 hr at 4°C (Shenker et al., 1999). Membranes were washed, incubated with goat anti-mouse immunoglobulin (Southern Biotech; Birmingham, AL) conjugated to horseradish peroxidase. The Western blots were developed using chemiluminescence [SuperSignal West Pico; (Thermo Scientific; Rockford, IL)] and analyzed by digital densitometry (Kodak Scientific Imaging Systems; Rochester, NY).
In order to determine if CdtBFcε influenced mast cell sensitization with IgE, BMMC were incubated with IgE as described above. Cells were then treated with CdtBFcε (20 μg/ml) for 2 hr min. The cells were then stained with anti-IgE conjugated to FITC (Southern Biotech) and analyzed by flow cytometry.
3. RESULTS
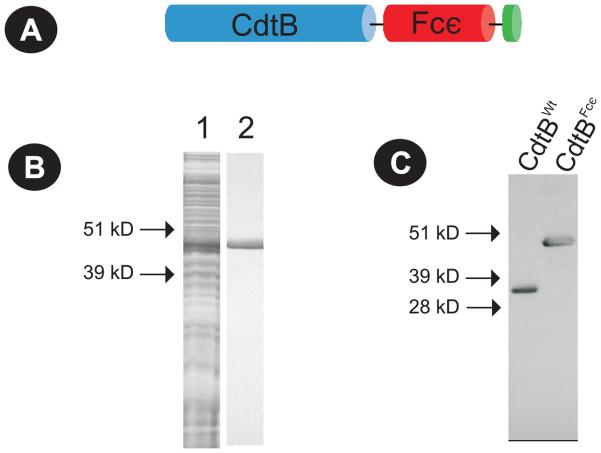
The plasmid expressing the CdtB-IgE Fc chimeric protein (pIVEXCdtBFcε) was constructed in pIVEX (Roche) as described in Materials and Methods section; the insert contains 1207 bp that encodes a fusion toxin protein consisting of 135 aa encoding the FcεRI binding region from the CH2/CH3 region of IgE and the entire sequence for CdtB (32 kDa; Fig. 1A). Expression of the chimeric protein was achieved using an in vitro coupled translation/transcription system and the protein purified by nickel affinity chromatography as we incorporated a His tag into the construct (Shenker et al., 2004;Shenker et al., 2005). Figures 1B and 1C show SDS-PAGE and Western blot analysis of samples containing both crude and purified CdtBFcε that was expressed from pIVEXCdtBFcε using the in vitro coupled translation/transcription system. The chimeric toxin (CdtBFcε) exhibits a mw of approximately 49 kDa compared to 32 kDa for CdtBWT.
Figure 1.
Expression of CdtBFcε chimeric toxin. Panel A is a schematic representation of the protein encoded by pIVEXCdtBFcε showing the CdtB subunit on the N-terminus of the chimera (blue), the Fcε binding region downstream (red) and the histidine tag (green) on the C terminus. CdtBFcε was expressed from pIVEXCdtBFcε using in vitro coupled translation/transcription system and purified as described in Materials and Methods; panel B shows a Coomassie stained SDS-PAGE gel of the unpurified expressed protein (lane 1) and the purified chimeric toxin (lane 2). Panel C shows a Western blot analysis of the expressed chimeric toxin which exhibits a mw of approximately 47 kDa; this compares to 32 kDa for CdtBwt.
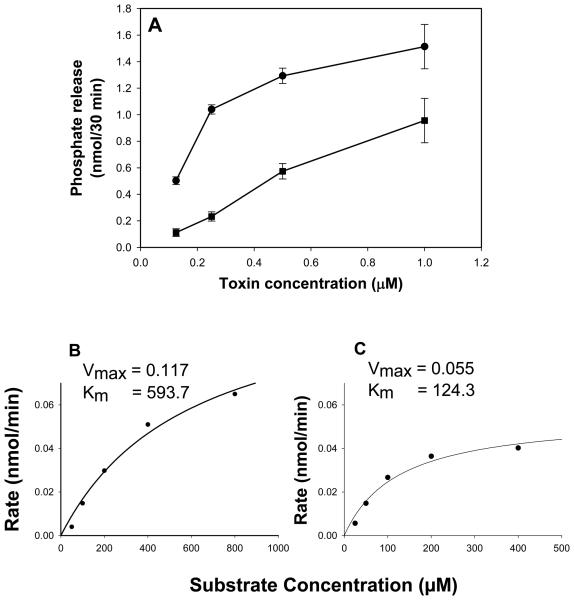
CdtBFcε was assessed for its ability to dephosphorylate PIP3 using malachite green to measure inorganic phosphate release. As shown in Fig 2A, CdtBFcε exhibits dose-dependent phosphate release which ranged from 0.5 ± 0.03 to 1.5 ± 0.17 nmol/30 min in the presence of 0.125 and 1.0 μM CdtBFcε, respectively. For comparative purposes, CdtBWT was also assessed. CdtBWT also exhibits dose-dependent phosphate release which was lower than the chimeric toxin; phosphate release ranged from 0.1 ± 0.02 to 0.96 ± 0.07 nmol/30 min in the presence of 0.125 and 1.0 μM CdtBFcε. We have previously demonstrated that CdtBWT exhibits Michaelis-Menten kinetics (Shenker et al., 2007). Therefore, to further assess the phosphatidylinositol phosphatase activity of CdtBFcε we compared the Michaelis-Menten kinetics to that of CdtBWT and determined Km and Vmax values with respect to cleavage of PIP3 (Figs. 2B and 2C). By this analysis, both CdtBFcε and CdtBWT demonstrated Km values of 593.7 and 124.3 μM, respectively. Vmax values were 0.117 nmol/min (CdtBFcε) and 0.055 nmol/min (CdtBWT) for 0.5 μM toxin. Thus, the chimeric toxin exhibits approximately twice the PIP3 phosphatase activity along with reduced substrate affinity compared to that observed with wildtype CdtB .
Figure 2.
Analysis of CdtBFcε for PIP3 phosphatase activity. Varying amounts of CdtBWT (squares) and CdtBFcε (circles) were assessed for their ability to hydrolyze PIP3 in vitro as described in Materials and Methods (panel A). The amount of phosphate release was measured using a malachite green binding assay. Data are plotted as phosphate release (nmol/30 min; mean ± S.D) versus protein concentration. CdtBFcε exhibited dose-dependent phosphatase activity; results are the mean ± S.D. of three experiments. The rate of CdtBFcε (0.5 μM; panel B) and CdtBWT (0.5 μM; panel C) mediated phosphate release in the presence of varying concentrations of PIP3 was assessed. Data were analyzed using Michaelis-Menten kinetics; Km values for CdtBwt and CdtBFcε were 124.3 and 593.7 μM, respectively and Vmax were 0.055 nmol/min (CdtBwt) and 0.117 nmol/min (CdtBFcε).
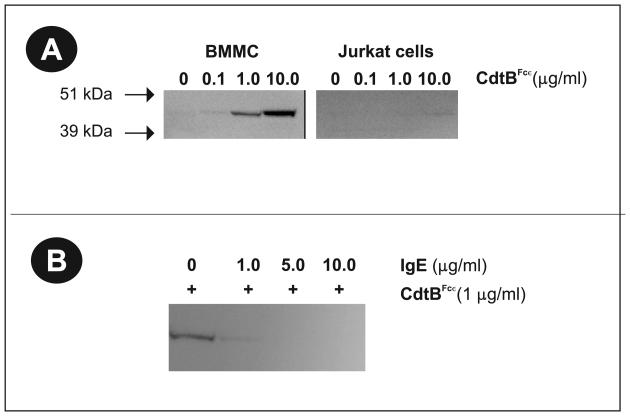
We next determined whether CdtBFcε was capable of selectively interacting with mast cells by binding to FcεRI. BMMC and Jurkat cells were exposed to varying amounts (0-10 μg/ml) of CdtBFcε for 60 min. The cells were then extensively washed, solubilized and analyzed by Western blot with anti-CdtB mAb (Fig. 3A). CdtBFcε was detected in extracts prepared from both BMMC and RBL cells (data not shown). The specificity of this interaction is demonstrated by the fact that Jurkat cells, which do not contain FcεRI, failed to bind the chimeric toxin. CdtBWT failed to associate with cells (data not shown); this observation is consistent with our earlier studies indicating that CdtB requires the binding units, CdtA and CdtC, in order for it to bind to lymphocytes (Shenker et al., 2005). These studies were extended to demonstrate specificity of CdtBFcε binding to the FcεRI. As shown in Fig. 3B, pre-exposure of BMMC to varying amounts of IgE (1-10 μg/ml) blocked CdtBFcε binding.
Figure 3.
Selective binding of CdtBFcε to mast cells. BMMC and Jurkat cells were exposed to varying amounts of CdtBFcε for 60 min at 4° C (panel A). The cells were washed, solubilized and analyzed by SDS-PAGE and Western Blot using anti-CdtB mAb. CdtBFcε was observed in BMMC samples, but not with Jurkat cells. Similar results were observed with RBL cells (data not shown). It should be noted that CdtBwt does not bind to either Jurkat cells or BMMC (data not shown). Panel B is a Western blot demonstrating that the binding of CdtBFcε to BMMC can be blocked by pre-exposing cells (30 min) to varying amounts of IgE prior to the addition of chimeric toxin.
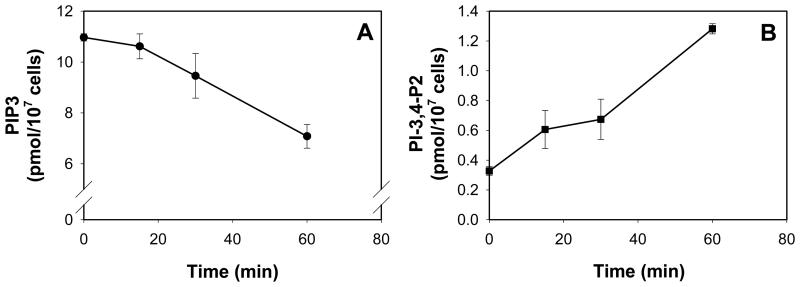
We next assessed whether the chimeric toxin was also capable of entering cells and exhibit PIP3 phosphatase activity by assessing the intracellular levels of both PIP3 and the degradative product, PI-3,4-P2. RBL cells were treated with CdtBFcε (10 μg/ml) for varying periods of time and the levels of PIP3 determined (Fig. 4A). Toxin-treated cells exhibited a time-dependent reduction in PIP3; baseline levels from 10.9 ± 0.14 pmol/107 cells to 9.6 ± 0.88 and 7.0 ± 0.47 pmol/107 cells at 30 and 60 min, respectively. Reductions in PIP3 levels were also dependent upon the dose of chimeric toxin employed (data not shown). A concomitant increase in the levels of PI-3,4-P2 (Fig. 4B) was observed from a baseline of 0.33 ± 0.03 pmol/107 cells to 0.67 ± 0.13 and 1.3 ± 0.03 at 30 and 60 min, respectively. Similar results were observed with BMMC (data not shown). It should be noted that in the absence of CdtA and CdtC, CdtBwt was not able to alter phosphatidylinositol levels.
Figure 4.
Analysis of CdtBFcε-induced changes in PIP3 and PI-3,4-P2 levels in RBL cells. RBL cells were incubated with CdtBFcε (10 μg/ml) for 15-60 min. Lipids were then extracted; PIP3 (panel A) and PI-3,4,-P2 (panel B) levels were determined by ELISA. Results are the mean ± S.D. of three experiments. The chimeric toxin induced a time dependent reduction in PIP3 and increase in PI-3,4-P2.
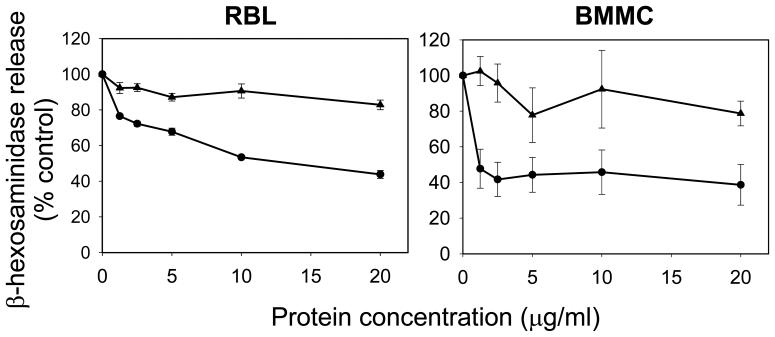
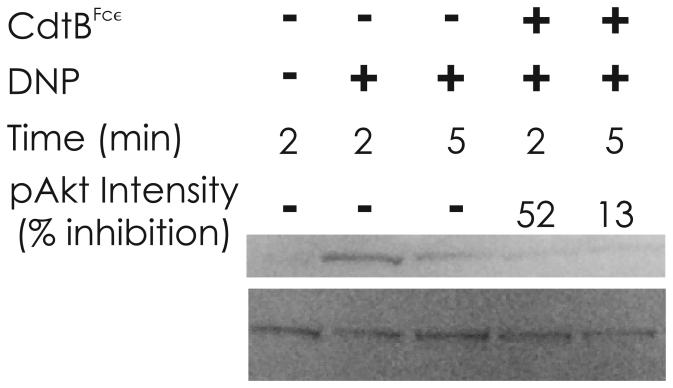
The next series of experiments focused on whether CdtBFcε was capable of inhibiting antigen-IgE-FcεRI mediated activation of mast cells. For these studies we employed both RBL cells and murine BMMC and assessed the ability of the toxin to inhibit antigen (DNP)-induced degranulation in anti-DNP IgE sensitized cells. As shown in Fig 5, CdtBFcε-treated RBL cells exhibit a dose-dependent inhibition of DNP-induced degranulation measured as a function of β-hexosaminidase release. Degranulation was reduced from 100% in control cells (exposed to antigen in the absence of of toxin) to 76.5%, 67% and 43.% in the presence of 1.25, 5.0 and 20.0 μg/ml CdtBFcε, respectively. In contrast, degranulation was minimally impaired in cells similarly treated with CdtBWT; β-hexosaminidase release was 81.8% ± 2.7 in cells treated with 20 μg/ml CdtBWt. In a parallel series of experiments, the chimeric toxin was also assessed for its ability to inhibit BMMC degranulation. CdtBFcε reduced β-hexosaminidase release to 47.7% ± 10.9 in the presence of 1.25 μg/ml CdtBFcε and to 38.7% ± 11.4 in the presence of 20 μg/ml chimeric toxin. CdtBWT had a minimal effect on degranulation as exposure to 20 μg/ml only reduced β-hexosaminidase release to 79% . In addition to degranulation, targets downstream of PIP3 were also assessed. Figure 6 demonstrates the effect of CdtBFcε on IgE-FcεRI-mediated activation of mast cells as a function of phosphorylation of Akt (pAkt), a PIP3-dependent event. Antigen activation of mast cells resulted in increased pAkt within 2 and 5 min in sensitized BMMC. By contrast, BMMC treated with CdtBFcε exhibited reduced pAkt at both 2 and 5 min.
Figure 5.
Effect of CdtBFcε on mast cell degranulation. RBL cells (left panel) and BMMC (right panel) were sensitized to anti-DNP IgE. Varying concentrations of CdtBFcε (circles) or CdtBwt (triangles) were added to cells 60 min prior to exposure to antigen (DNP-BSA). Degranulation was measured 30 min after the addition of antigen as a function of the release of β-hexosaminidase. Data are expressed as percent control (sensitized cells treated with antigen in the absence of toxin) and are the mean ± S.D of three experiments. CdtBFcε inhibited degranulation in both RBL and BMMC. All data points are statistically significant differences (p<0.05) between CdtBFcε and CdtBwt except for the 10 μg/ml values for BMMC (p=0.138).
Figure 6.
Western blot analysis of pAkt during BMMC activation. BMMC were sensitized with IgE as described in Materials and Methods. Cells were then treated with medium (control) or CdtBFcε (10 μg/ml) for 60 min and challenged with DNP-BSA for the time indicated. Replicate samples were pooled, the cells solubilized, fractionated by SDS-PAGE and analyzed for Akt and pAkt by Western blot. DNP-BSA induced phosphorylation of Akt within 2-5 min. Akt phosphorylation was reduced in the presence of CdtBFcε. The pAkt intensity is indicated and expressed as inhibition of the relative control for both 2 and 5 min. Results are representative of three experiments.
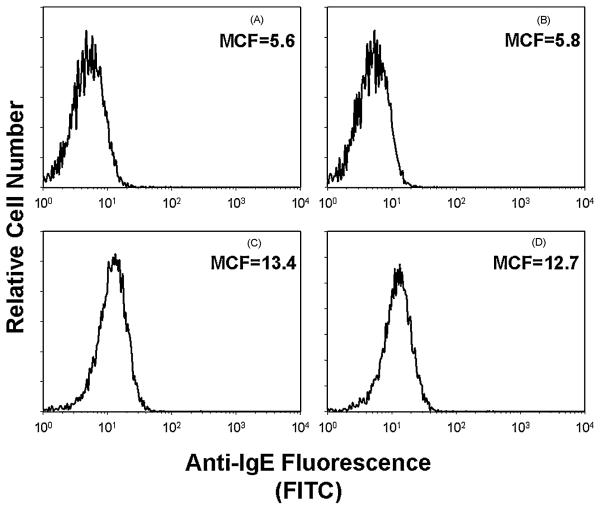
Finally, we wanted to eliminate the possibility that CdtBFcε impairment of mast cell function was the result of reduced IgE binding to BMMC as opposed to direct effects of reduced PIP3 levels. The chimeric toxin was assessed for its ability to reduce IgE sensitization of BMMC. As shown in Figure 7, BMMC were incubated in medium alone or containing CdtBFcε (controls) ; additionally, cells were sensitized to IgE (panels C and D) as described in Materials and Methods and then incubated in medium (panel C) or with CdtBFcε (panel D) for 2 hr. Surface IgE binding was assessed by immunofluorescence following staining of cells with anti-IgE conjugated to FITC and fluorescence measured by flow cytometry. CdtBFcε did not reduce IgE binding to mast cells as evident by the level of immunofluorescence; mean channel fluorescence (MCF) was 12.7 in control cells sensitized with IgE and 13.4 in cells pre-exposed to CdtBFcε. Background MCF for control cells that were not treated with chimeric toxin or IgE was5.6. It should be noted that the immunofluorescence analysis assesses surface IgE as the anti-IgE antibody did not recognize CdtBFcε (panel B; MCF=5.8).
Figure 7.
Assessment of CdtBFcε for effects on IgE binding to mast cells. BMMC were incubated in medium alone (panel A), medium containing CdtBFcε (panel B; 10 μg/ml) or cells were sensitized with IgE (panels C and D) as described in Materials and Methods. The cells were then exposed to medium only (panels A-C) or CdtBFcε (panel D; 10 μg/ml) for 2 hr. The level of IgE bound to BMMC was determined by flow cytometry following staining with anti-IgE antibody conjugated to FITC. Mean channel fluorescence (MCF) is shown and indicates that similar amounts of IgE were present on BMMC in the presence or absence of chimeric toxin. It should also be noted that the anti-IgE antibody does not recognize CdtBFcε (panel B). Results are representative of three experiments.
4. DISCUSSION
The Cdts are a family of heat-labile protein cytotoxins produced by several different bacterial species including diarrheal disease-causing enteropathogens such as Escherichia coli, Campylobacter jejuni, Shigella species, Haemophilus ducreyi and Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans (Comayras et al., 1997;Okuda et al., 1997;Okuda et al., 1995;Scott and Kaper, 1994;Pickett et al., 1994;Mayer et al., 1999;Pickett and Whitehouse, 1999). Cdts are encoded by three genes, designated cdtA, cdtB, and cdtC, which are arranged as an apparent operon encoding three polypeptides designated CdtA, CdtB and CdtC with apparent molecular masses of 28, 32 and 20 kDa, respectively, that form a heterotrimeric holotoxin (Shenker et al., 1999;Shenker et al., 2000;Shenker et al., 2001;Pickett and Whitehouse, 1999;De Rycke and Oswald, 2001;Thelastam et al., 2004). There is considerable agreement that the heterotrimeric holotoxin functions as an AB2 toxin where CdtB is the active (A) unit and the complex of CdtA and CdtC comprise the binding (B) unit (Nesic et al., 2004;Elwell et al., 2001;Lara-Tejero and Galan, 2001). In this regard, we have shown that CdtA and CdtC are required for the holotoxin to associate with lipid microdomains within lymphocyte membranes and that the CdtC subunit specifically binds to cholesterol (Boesze-Battaglia et al., 2006;Boesze-Battaglia et al., 2009). Moreover, we recently determined that the active subunit, CdtB, functions as a lipid phosphatase (Dlakic, 2001). Specifically, CdtB exhibits PIP3 phosphatase activity similar to that of the tumor suppressor phosphatases, PTEN and SHIP1 (Horn et al., 2004;Seminario et al., 2003). Mutation analysis indicates that CdtB-mediated toxicity correlates with phosphatase activity; furthermore, lymphocytes treated with Cdt exhibit reduced PIP3 levels. Moreover, lymphoid cell line sensitivity to CdtB-induced cell cycle arrest correlates with PTEN deficiencies, elevated basal levels of PIP3 and dependence on the PI-3K signaling pathway for survival and proliferation (Shenker et al., 2007).
In addition to cell proliferation and survival, it is well documented that PIP3 plays a central regulatory role in a diverse array of cell functions involving a wide range of cell types. Thus PIP3 represents a potent target for modulating cell function, in general, and mast cell-mediated inflammatory disorders in particular. We propose that the novel lipid phosphatase activity exhibited by CdtB makes this toxin a potentially useful agent for manipulating cellular responses for both pharmacologic purposes and for advancing our understanding of the regulatory role of PIP3. However, CdtB by itself has minimal capacity to associate with cells and the holotoxin lacks cell specificity as it is capable of binding to a wide range of cell types. In order to harness the pharmacologic potential of CdtB, we chose to develop a chimeric toxin in which we confer cell binding specificity by fusing the toxin's active subunit to the CH2/CH3 region of IgE thereby targeting the fusion toxin to FcεRI-bearing cells. Indeed, we have demonstrated that the chimeric toxin retains PIP3 phosphatase activity. The explanation for this elevation in phosphatase activity is not clear but most likely reflects increased stability of the complex. Additionally, the chimeric toxin selectively associates with cells expressing FcεR1, RBL and BMMC , as opposed to Jurkat cells which do not express this receptor. Evidence that the chimeric toxin binding to cells involves FcεRI is further supported by our observation that IgE can block toxin binding.
It is well established that in order for the Cdt holotoxin to induce toxicity, the active subunit, CdtB, must be internalized following binding. This requirement is consistent with its functioning as a lipid phosphatase requiring that it gains access to intracellular pools of PIP3. Our observations clearly indicate that RBL cells treated with CdtBFcε exhibit reduced intracellular levels of CdtB substrate (PIP3) and increased levels of enzymatic product (PI-3,4-P2) providing indirect evidence that the chimera is indeed able to gain access to intracellular compartments within these cells. It should be noted that the kinetics of these changes are dependent upon toxin concentration. The ultimate test, however, was whether the toxin was capable of preventing mast cell activation. To this end, we assessed the chimeric toxin for its ability to prevent antigen (DNP-BSA)-IgE-mediated mast cell degranulation. Our results clearly demonstrate that pre-exposure to CdtBFcε results in up to 60% inhibition of antigen-induced degranulation of both RBL and BMMC. Furthermore, the increase in pAkt, normally observed following antigen-induced activation was inhibited in CdtBFcε-treated BMMC; control cells treated with CdtBWT were unaffected. Moreover these affects were not the result of the chimeric toxin blocking IgE binding to FcεRI since both control and toxin treated mast cells bound similar amounts of IgE.
The observations reported in this study are the first demonstration that direct depletion of PIP3 via increased degradation results in impaired mast cell function. In this context, it is important to recognize that our extensive knowledge of the regulatory role for PIP3 in cellular processes in general, and mast cells, in particular, has been derived primarily from indirect evidence in which the synthesis of PIP3 has been altered or degradation blocked. As a key regulatory molecule, it is essential that PIP3 be maintained at low levels until its synthesis is stimulated by a variety of signals involving activation of PI-3K which utilizes PI(4,5)P2 to generate PIP3 (Nadler et al, 2001;Bachelet et al., 2006;Furumoto et al., 2006;Huber et al., 2002;Huber et al., 1998). There are essentially three classes of PI-3K (I, II, and III); class I is subdivided into two subclasses, IA and IB. It is generally accepted that class IA PI-3K isoforms are involved in FcεRI signaling (Kim et al, 2008). Inhibition of PI-3K activity via mutation or inhibitor results in defective mast cell degranulation and cytokine production as well as anaphylactic reactions in vivo (Rivera and Olivera, 2008;Rivera and Olivera, 2007;Kim et al., 2008;Marone et al., 2008). It should be noted that similar results were not noted for all isoforms suggesting a high level of redundancy of regulatory PI-3K isoforms in mast cell function. As is often the case with enzyme inhibitors, the specificity of PI-3K inhibitors is questionable; for example, wortmannin and LY294002 target a broad range of PI-3K related enzymes (Marone et al., 2008). The importance of PIP3 in mast cell responsiveness has also been demonstrated in studies that involve reduced expression of the degradative enzymes, PTEN, SHIP 1 and 2, resulting in sustained increases in intracellular PIP3; these increases represent a “gain of function” which is manifest in mast cells as a state of hyperreactivity (Seminario and Wange, 2002;Kyrstal, 2000;March and Ravichandran, 2002).
In summary, the observations reported in this paper provide further evidence that CdtB represents a highly potent lipid phosphatase. These observations are consistent with our previous demonstration that the action of the Cdt holotoxin is dependent upon CdtB-mediated depletion of intracellular levels of PIP3. It should be noted that our previous studies were based upon the toxins ability to induce cell cycle arrest and subsequent cell death. We now demonstrate that this bacterial-derived phosphatase is also capable of inhibiting a PIP3-dependent functions that do not involve proliferation. Moreover, these studies further establish the central regulatory role of this signaling lipid in regulating mast cell function and provides further evidence that PIP3 is a potent target for pharmacologic intervention to modify mast cell activity and associated pathologic processes.
ACKNOWLEDGMENTS
The authors wish to acknowledge the SDM flow cytometry core facility for their technical expertise. This work was supported by U.S.P.H.S. grant DE06014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: phosphatidylinositol-3,4,5-triphosphate, PIP3; high affinity IgE receptors, FcεRI; cytolethal distending toxin, Cdt; bone marrow derived murine mast cells, BMMC; mean channel fluorescence, MCF
References
- 1.Abramson J, Pecht I. Regulation of the mast cell response to the type 1 Fce receptor. Immunological Reviews. 2007;217:231–254. doi: 10.1111/j.1600-065X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 2.Ali H, Richardson R, Tomhave E, Didsbury J, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–24254. [PubMed] [Google Scholar]
- 3.Bachelet I, Munitz A, Levi-Schaffer F. Abrogation of allergic reactons by a bispecific antibody fragment linking IgE to CD300a. J Allergy Clin Immunol. 2006;117:1314–1320. doi: 10.1016/j.jaci.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Belostotsky R, Lorberboum-Galski H. Utilizing Fce-Bak chimeric protein for studying IgE-FceRI interactions. J. Clin. Immunol. 2003;110:89–99. doi: 10.1016/j.clim.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Boesze-Battaglia K, Besack D, McKay TL, Zekavat A, Otis L, Jordan-Sciutto K, Shenker BJ. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Cellular Microbiology. 2006;8:823–836. doi: 10.1111/j.1462-5822.2005.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boesze-Battaglia K, Brown A, Walker L, Besack D, Zekavat A, Wrenn S, Krummenacher C, Shenker BJ. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J Biol Chem. 2009;284:10650–10658. doi: 10.1074/jbc.M809094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comayras C, Tasca C, Peres SY, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infection & Immunity. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Rycke J, Oswald E. Cytolethal distending toxin (CDT): a bacterial weapon to control host cell proliferaton? FEMS Microbiol. Lett. 2001;203:141–148. doi: 10.1111/j.1574-6968.2001.tb10832.x. [DOI] [PubMed] [Google Scholar]
- 9.Dlakic M. Is CdtB a nuclease or a phosphatase? Science. 2001;291:547. doi: 10.1126/science.291.5504.547a. [DOI] [PubMed] [Google Scholar]
- 10.Elwell CA, Chao K, Patel K, Dreyfus LA. Escherichia coli CdtB Mediates Cytolethal Distending Toxin Cell cycle Arrest. Infect. Immun. 2001;69:3418–3422. doi: 10.1128/IAI.69.5.3418-3422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven M, Gilfillan A, Rivera J. Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J. Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- 12.Horn S, Endl E, Fehse B, Weck M, Mayr G, Jucker M. Restoraton of SHIP activity in a huma leukemia cell line downregulates constitutively activated phosphatidylinositol 3-kinase/Akt/BSK-3b signaling and leads to an increased transit time through the G1 phase of the cell cycle. Leukemia. 2004;18:1839–1849. doi: 10.1038/sj.leu.2403529. [DOI] [PubMed] [Google Scholar]
- 13.Huber M, Helgason C, Damen J, Liu L, Humphries R. The src homology2-containing inositol phosphatase is the gatekeeper of mast cell degranulation. Proc. Natl. Acad. SCi. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber M, Kalesnikoff J, Reth M, Krystal G. The role of SHIP in mast cell degranulation and IgE-induced mast cell survival. Immunol. Lett. 2002;82:17–21. doi: 10.1016/s0165-2478(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Radinger M, Gilfillan A. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends in Immunology. 2008;29:493–501. doi: 10.1016/j.it.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinet J. The essential role of mast cells in orchestrating inflammation. Immunological Reviews. 2007;217:5–7. doi: 10.1111/j.1600-065X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 17.Kraub M, Haucke V. Phosphoinositides: regulators of membrane traffic and protein function. FEBS Lett. 2007;581:2105–2111. doi: 10.1016/j.febslet.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 18.Kyrstal G. Lipid phosphatases in the immune system. Seminars in Immunology. 2000;12:397–403. doi: 10.1006/smim.2000.0222. [DOI] [PubMed] [Google Scholar]
- 19.Lara-Tejero M, Galan JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 2001;69:4358–4365. doi: 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmon M. Membrane recognition by phospholipd-binding domains. Nature Reviews. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 21.Maehama T, Taylor G, Slama J, Dixon J. A sensitive assay for phosphoinositide phosphatases. Analytical Biochemistry. 2000;279:248–250. doi: 10.1006/abio.2000.4497. [DOI] [PubMed] [Google Scholar]
- 22.March M, Ravichandran K. Regulation of the immune response by SHIP. Seminars in Immunology. 2002;14:37–47. doi: 10.1006/smim.2001.0340. [DOI] [PubMed] [Google Scholar]
- 23.Marone R, Cmiljanovic V, Giese B, Wymann M. Targeting phosphoinositide 3-kinase-moving towards therapy. Biochimica et Biophysica Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Mayer M, Bueno L, Hansen E, DiRienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect. Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadler M, Matthews S, Turner H, Kinet J. Signal transduction by the high-affinity immunoglobulin E receptor FceRI: coupling form to function. Advances in Immunology. 2001;76:325–355. doi: 10.1016/s0065-2776(01)76022-1. [DOI] [PubMed] [Google Scholar]
- 26.Nesic D, Hsu Y, Stebbins CE. Assembly and ;function of a bacterial genotoxin. Nature. 2004;429:429–433. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- 27.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infection & Immunity. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella dn Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb. Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 29.Pickett CL, Cottle DL, Pesci EC, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infection & Immunity. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickett CL, Whitehouse CA. The cytolethal distending toxin family. Trends in Microbiology. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- 31.Rivera J, Olivera A. A current understanding of FceRI-dependent mast cell activation. Current Allergy and Asthma Reports. 2008;8:14–20. doi: 10.1007/s11882-008-0004-z. [DOI] [PubMed] [Google Scholar]
- 32.Rivera J, Olivera R. Src family kinases and lipid mediators in control of allergic inflammation. Immunological Reviews. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki t., Sasaki J, Sakai T, Takasuga S, Suzuki A. The physiology of phosphoinositides. Biol. Pharm. Bull. 2007;30:1599–1604. doi: 10.1248/bpb.30.1599. [DOI] [PubMed] [Google Scholar]
- 34.Scott DA, Kaper JB. Cloning and sequencing of the genes encoding Escherichia Colli cytolethal distending toxin. Infect. Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seminario M, Precht P, Wersto R, Gorospe M, Wange R. PTEN expression in PTEN-null leukeamic T cell lines leads to reduced proliferation via slowed cell cycle progression. Oncogene. 2003;22:8195–8204. doi: 10.1038/sj.onc.1206872. [DOI] [PubMed] [Google Scholar]
- 36.Seminario M, Wange R. Signaling pathways of D3-phsphoinositide-bidning kinaes in T cells and their regulation by PTEN. Seminars in Immunology. 2002;14:27–36. doi: 10.1006/smim.2001.0339. [DOI] [PubMed] [Google Scholar]
- 37.Shenker BJ, Besack D, McKay TL, Pankoski L, Zekavat A, Demuth DR. Actinobacillus actinomycetemcomitans cytolethal distending toxin (Cdt): evidence that teh holotoxin is commposed of three subunits: CdtA, CdtB, and CdtC. J. Immunol. 2004;172:410–417. doi: 10.4049/jimmunol.172.1.410. [DOI] [PubMed] [Google Scholar]
- 38.Shenker BJ, Besack D, McKay TL, Pankoski L, Zekavat A, Demuth DR. Induction of cell cycle arrest in lymphocytes by Actinobacillus actinomycetemcomitans cytolethal distending toxin rquires three subunits formaximum activity. J. Immunol. 2005;174:2228–2234. doi: 10.4049/jimmunol.174.4.2228. [DOI] [PubMed] [Google Scholar]
- 39.Shenker BJ, Dlakic M, Walker L, Besack D, Jaffe E, Labelle E, Boesze-Battaglia K. A novel mode of action for a microbial-derived immunotoxin: the cytolethal distending toxin subunit B exhibits phosphatidylinositol (3,4,5) tri-phosphate phospatase activity. J. Immunol. 2007;178:5099–5108. doi: 10.4049/jimmunol.178.8.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenker BJ, Hoffmaster RH, McKay TL, Demuth DR. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinoimycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cyclein human T-cells. J. Immunol. 2000;165:2612–2618. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- 41.Shenker BJ, Hoffmaster RH, Zekavat A, Yamguchi N, Lally ET, Demuth DR. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 2001;167:435–441. doi: 10.4049/jimmunol.167.1.435. [DOI] [PubMed] [Google Scholar]
- 42.Shenker BJ, McKay TL, Datar S, Miller M, Chowhan R, Demuth DR. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 43.Thelastam M, Frisan T. Cytolethal distending toxins. Rev. Physiol. Biochem. Pharmacol. 2004;152:111–133. doi: 10.1007/s10254-004-0030-8. [DOI] [PubMed] [Google Scholar]
- 44.Tkacyzk C, Gilfillan A. FceRI-dependent signaling pathways in human mast cells. Clinical Immunology. 2001;99:198–210. doi: 10.1006/clim.2001.4992. [DOI] [PubMed] [Google Scholar]
- 45.Venkatesha R, Ahamed J, Nuesch C, Zaidi A, Ali H. Platelet-activating Factor-induced chemokine gene expression requires NF-kb activation and Ca2+ /calcineurin signaling pathways. J Biol Chem. 2004;279:44606–44612. doi: 10.1074/jbc.M408035200. [DOI] [PubMed] [Google Scholar]
- 46.Zaidi A, Thangam E, Ali H. Distinct roles of Ca2+ mobilization and G protein usage on regulation of Toll-like receptor function in human and murine mast cells. Immunol. 2006;119:412–420. doi: 10.1111/j.1365-2567.2006.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]