Abstract
The risk of Alzheimer’s disease (AD) is higher in women than in men, a sex difference that likely results from the effects of sex steroid hormones. To investigate this relationship, we first compared progression of β-amyloid (Aβ) pathology in male and female triple transgenic (3xTg-AD) mice. We found that female 3xTg-AD mice exhibit significantly greater Aβ burden and larger behavioral deficits than age-matched males. Next, we evaluated how the organizational effects of sex steroid hormones during postnatal development may affect adult vulnerability to Aβ pathology. We observed that male 3xTg-AD mice demasculinized during early development exhibit significantly increased Aβ accumulation in adulthood. In contrast, female mice defeminized during early development exhibit a more male-like pattern of Aβ pathology in adulthood. Taken together, these results demonstrate significant sex differences in pathology in 3xTg-AD mice and suggest that these differences may be mediated by organizational actions of sex steroid hormones during development.
Keywords: Alzheimer’s disease, β-amyloid, estrogen, testosterone, neonatal, sex differences
1. Introduction
Women have a higher risk for the development of Alzheimer’s disease (AD) than men. Epidemiological and observational studies have demonstrated a higher prevalence (Bachman et al., 1992; Jorm et al., 1987; Rocca et al., 1986) and incidence (Andersen et al., 1999; Fratiglioni et al., 2001; Jorm and Jolley, 1998; Ruitenberg et al., 2001) of AD in women. Further, AD appears to affect men and women differently, with women showing greater vulnerability to the disease. For example, at early stages of neurofibrillary tangle development, women exhibit greater senile plaque deposition than men (Corder et al., 2004). In addition, AD pathology is more strongly associated with clinical dementia in women than in men (Barnes et al., 2005).
Sex differences in AD may reflect differences between men and women in either of the two classic actions of sex steroid hormones: organizational effects during critical periods of development that induce permanent brain dimorphisms, or activational effects during adulthood that regulate adult brain function. Such activational effects of estrogens and testosterone are differentially diminished during aging in men and women. It is well established that estrogens have many beneficial effects in the brain, reviewed in (Wise, 2002) including numerous protective actions relevant to the prevention of AD, such as promotion of neuron viability and reduction of β-amyloid (Aβ) accumulation, reviewed in (Pike et al., 2009). The loss of neuroprotective estrogen actions as a consequence of estrogen depletion at menopause may increase the vulnerability of the female brain to AD and other disorders. Consistent with this theory, estrogen-based hormone therapy in postmenopausal women is associated with reduced risk of AD in some (Henderson et al., 1994; Kawas et al., 1997; Paganini-Hill and Henderson, 1996; Tang et al., 1996; Zandi et al., 2002) but not all studies (Espeland et al., 2004; Rapp et al., 2003). Similarly, ovariectomy-induced hormone depletion in wild-type rodents (Petanceska et al., 2000) and some transgenic mouse models of AD (Carroll et al., 2007; Levin-Allerhand et al., 2002; Zheng et al., 2002) can increase levels of the AD-related protein β-amyloid (Aβ), an effect attenuated by estradiol treatment. In men, age-related testosterone loss is linked with elevated levels of Aβ (Gillett et al., 2003; Rosario et al., 2009) and increased AD risk (Hogervorst et al., 2001; Moffat et al., 2004; Rosario et al., 2004, 2009). In transgenic mouse models of AD, Aβ accumulation is accelerated under low testosterone conditions and reduced in the presence of high testosterone or dihydrotestosterone (McAllister et al., 2010; Rosario et al., 2006). Taken together, these studies suggest that adult exposure to estrogens and androgens may regulate the development of AD in women and men, respectively.
Another factor that may contribute to sex differences in AD risk is the organizational effects of sex steroid hormones during early development. Developmental patterns of estrogen and testosterone exposure induce numerous structural and functional differences between male and female brains (Cosgrove et al., 2007). Importantly, men and women also exhibit different vulnerabilities to several neurological disorders that occur prior to age-related hormone depletion, including post-traumatic stress disorder, schizophrenia, multiple sclerosis, autism, attention deficit disorder, and Tourette’s syndrome (Cahill, 2006; Vagnerova et al., 2008). Thus, differences between male and female brains established during development may contribute to sex differences in vulnerability to disease, perhaps including AD.
The higher risk of AD in women may involve sex differences in the activational effects and/or organizational effects of estrogens and testosterone. To investigate these issues, we first compared development of Aβ pathology in adult male and female triple transgenic (3xTg-AD) mice. Next, we assessed how the adult pattern of Aβ pathology is affected by disruption of the normal organizational effects of sex steroid hormones during critical developmental periods. Specifically, we defeminized neonatal female 3xTg-AD mice using transient testosterone treatment (Meek et al., 2006; Isgor and Sengelaub, 2003; Akhmadeev and Kalimullina, 2005) and demasculinized neonatal male 3xTg-AD mice by temporarily blocking testosterone action using the androgen receptor antagonist flutamide (Miyata et al., 2003; Houtsmuller et al., 1994; Meek et al., 2006; Kudwa et al., 2005). Alterations in adult pathology following these neonatal manipulations provide novel insight into the organizational role of sex steroid hormones on AD-related pathology.
2. Results
Experiment 1: Comparison of pathology development in male and female 3xTg-AD mice
To assess potential sex differences in AD-related pathology, Aβ accumulation and behavioral performance were compared in male and female 3xTg-AD mice across three age groups: 2–4 mo, 6–8 mo, and 12–14 mo. The average age of males and females at each time point were not statistically different within age groups (average age in months ± standard error): female 2–4 mo = 3.1 ± 0.0, male 2–4 mo = 3.2 ± 0.1; (p = 0.78); female 6–8 mo = 6.7 ± 0.3, male 6–8 mo = 7.0 ± 0.4 (p = 0.37); female 12–14 mo = 13.0 ± 0.4, male 12–14 mo = 13.7 ± 0.3 (p = 0.06).
Female 3xTg-AD mice display higher levels of Aβ immunoreactivity than males
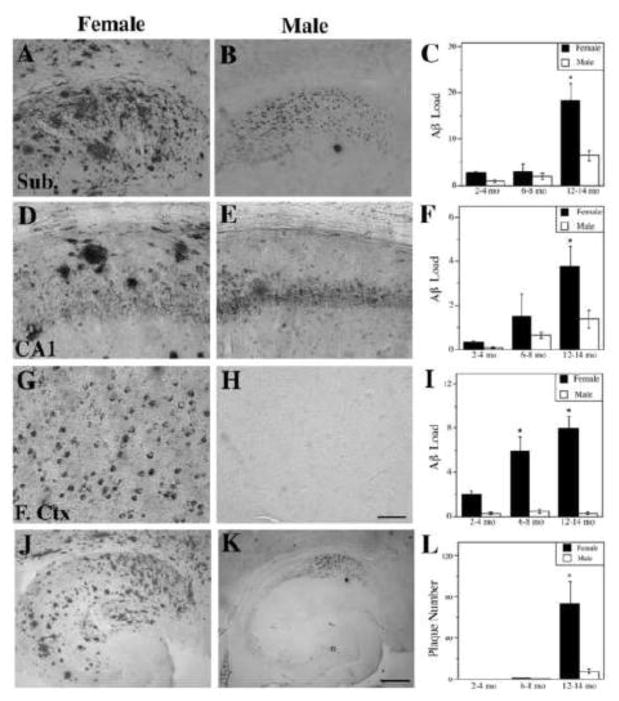
To assess the development of Aβ pathology in male and female 3xTg-AD mice, we quantified Aβ immunoreactive load in three brain regions: CA1 portion of hippocampus, subiculum, and frontal cortex. Both male and female 3xTg-AD mice exhibited an age-related increase in Aβ load in the subiculum ([F (7,46) = 24.8, p = 0.0013], [F (7,46) = 14.4, p = 0.005], respectively) and hippocampus ([F (7,46) = 60.0, p = 0.0001], [F (7,46) = 7.1, p = 0.026], respectively). In the frontal cortex, females in the frontal cortex as well [F (7,46) = 17.3, p = 0.003] but males showed no increase in this region [F (7,46) = 22.7, p = 0.21]. However, we observed that female 3xTg-AD mice had higher Aβ loads compared to males (Figure 1), a sex difference that was significant at the 12–14 mo age group in both subiculum (Figure 1A–C) and hippocampus CA1 (Figure 1D–F). The sex difference in Aβ load was most striking in the frontal cortex where it was significantly higher in females in both the 6–8 mo and 12–14 mo ages (Figure 1G–I).
Figure 1.
Female 3xTg-AD mice exhibit higher levels of Aβ than males. Representative images show Aβ immunoreactivity from 12–14 mo female (A, D, G) and male (B, E, H) 3xTg-AD mice in the subiculum (A,B), hippocampus CA1 (D,E), and frontal cortex (G,H). Scale bar = 100μm. Quantification of Aβ immunoreactive load shows significant differences between females (solid bars) and males (open bars) in the subiculum (C) [F (7,46) = 23.72, p < 0.0001], hippocampus CA1 (F) [F (7,46) = 5.88, p = 0.0005], and frontal cortex (I) [F (7,46) = 9.76, p <0.0001]. Representative low magnification images of Aβ immunoreactivity in hippocampus and subiculum of 12–14 mo female (J) and male (K) 3xTg-AD mice show significantly higher numbers of Aβ plaques in females (L) [F (8,43) = 9.25, p < 0.0001]. Scale bar = 250μm. Data show mean values ± SEM. * p < 0.01 relative to males of same age group.
As another measure of Aβ pathology, we compared the development of extracellular Aβ deposition into plaque-like structures in males and females. The number of extracellular Aβ plaques was totaled from the subiculum and hippocampus CA1. At 12–14 mo of age, when extracellular Aβ plaques became obviously apparent, female 3xTg-AD mice exhibited a significantly higher number of plaques compared to age-matched males (Figure 1J–L).
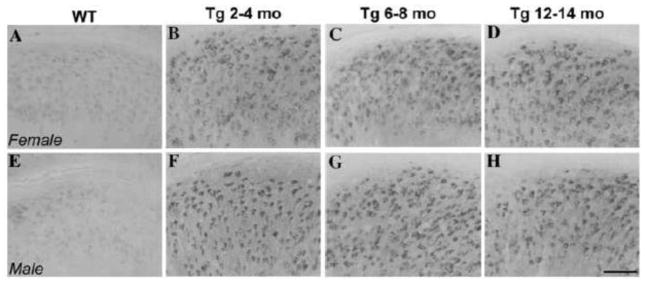
To confirm that our sex difference in Aβ pathology reflects amyloidogenic species of Aβ rather than non-amyloidogenic carboxyl-terminal fragments (CTFs) of the amyloid precursor protein, we examined CTF-immunoreactivity. In comparison to non-transgenic C57Bl6/129S mice (Figure 2A–E), high levels of CTF-immunoreactivity were observed in subiculum of both female (Figure 2B–D) and male (Figure 2F–H) 3xTg-AD mice. Levels of CTF- immunoreactivity were not significantly different between the sexes across ages [F (4,17) = 2.1, p = 0.38].
Figure 2.
No difference in levels of amyloid precursor protein (APP) C-terminal fragments (CTFs) were observed between male and female 3xTg-AD mice. Representative images show CTF immunoreactivity (CT20 antibody) in the subiculum of male and female non-transgenic C57Bl6/129S and 3xTg-AD mice. Non-transgenic mice (A–E) show low levels of CTF-immunoreactivity while both male (F–H) and female (B–D) mice show similar, high levels of CTF- immunoreactivity. Scale bar = 100μm.
Females 3xTg-AD mice display poorer hippocampal-dependent behavior than males
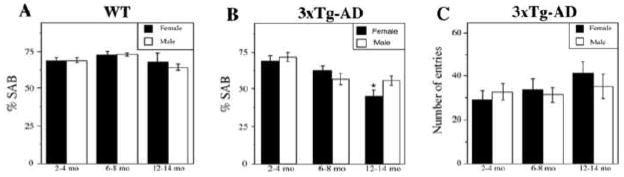
To assess behavioral changes in male and female 3xTg-AD mice, we measured performance on spontaneous alternation behavior (SAB), a hippocampal-dependent task involving working memory and visual attention. We observed an age-related decline in SAB performance in both females [F (3,20) = 0.87, p = 0.002] and males [F (3, 7) = 0.67, p = 0.031]. SAB performance in males declined most strongly at the 6–8 mo time point, whereas in females the decrease was decreased more evenly over time. Notably, we observed poorer SAB performance in females compared to males, which was statistically significant in the 12–14 mo age group (Figure 3B). To control for possible group differences in activity level, we measured the number of arm entries and found they were not significantly different across groups [F (3,19) = 0.79, p = 0.56] (Figure 3C). To evaluate the potential role of normal aging in the observed decreases in SAB performance, we assessed SAB in non-transgenic C57Bl6/129S mice in 2–4 mo, 6–8 mo, and 12–14 mo age groups. We observed no significant differences in SAB performance [F (2,15) = 1.6, p = 0.19] (Figure 3A) and arm entries [F (2,15) = 0.59, p = 0.62] in non-transgenic mice across age groups or gender.
Figure 3.
Female 3xTg-AD mice perform more poorly than males on spontaneous alternation behavior (SAB), a hippocampal-dependent working memory task. (A) SAB performance did not decline with age or significantly differ by sex in non-transgenic C57Bl6/129S (WT) mice. (B) We observed an age-related decline in SAB performance in both females (solid bars) [F (3,20) = 0.87, p = 0.002] and males (open bars) [F (3, 7) = 0.67, p = 0.031]. This effect is significantly different between females and males in the 12–14 mo age group [F (3,19) = 6.58, p = 0.0001]. * p < 0.05 from males of same age group. (C) As a control for activity level, the number of arm entries was measured; it was not significantly different across groups [F (3,19) = 0.79, p = 0.56]. Data show mean values ± SEM.
Experiment 2: Effects of neonatal hormone manipulations on adult pathology
To assess the effects of neonatal hormone exposure on the development of Aβ pathology in adult male and female 3xTg-AD mice, male pups were demasculinized by treatment with the androgen receptor antagonist flutamide during postnatal days 1–21 and female pups were defeminized by treatment with testosterone propionate (TP) during postnatal days 1–7. Hormone-treated animals as well as vehicle-treated and gonadectomized male and female control groups were sacrificed at 7 mo of age.
To confirm the defeminizing effect of TP treatment in females, female mice were assessed on their reproductive behavior and serum E2 levels. TP-treated females exhibited significantly lower lordosis quotients than vehicle-treated females (Table 1), demonstrating that they were effectively defeminized. Control OVX mice showed similarly low lordosis quotients. However, OVX mice are largely depleted of endogenous E2 whereas the TP-treated females show intermediate levels of serum E2 that statistically are not significantly lower than vehicle-treated females [F (2,16) = 6.9, p = 0.11] (Table 1).
Table 1.
Defeminizing effect of testosterone treatment in female 3xTg-AD mice
| Condition | Lordosis quotient | E2 (pg/ml) |
|---|---|---|
| Female + vehicle | 59.6 ±17.4 | 55.5 ± 14.2 |
| OVX | 10.0 ± 4.1* | 9.9 ± 2.2* |
| Female + testosterone | 8.6 ± 5.7* | 27.4 ± 5.2 |
p < 0.05 relative to Female + vehicle
To confirm efficacy of flutamide treatment in demasculinizing males, seminal vesicle weights were measured and mice were assessed on reproductive capacity by measuring both mounting behavior and bedding preference. Similar to control androgen depleted ORX mice, flutamide-treated males attempted to mount a female significantly fewer times and spent less time with female bedding compared to vehicle-treated males. However, unlike ORX males, flutamide-treated males did not have significantly lower seminal vesicle weight [F (2,14)= 10.7, p = 0.16] (Table 2).
Table 2.
Demasculinizing effect of flutamide treatment in male 3xTg-AD mice
| Condition | Number of mounts | % Time with female bedding | Seminal vesicle weight (mg) |
|---|---|---|---|
| Male + vehicle | 10.8 ± 1.5 | 63.4 ± 4.8 | 102.5 ± 10.7 |
| ORX | 0.0 ± 0.0* | 30.5 ± 1.7* | 12.8 ± 1.7* |
| Male + Flutamide | 3.1 ± 1.0* | 42.4 ± 4.5* | 85.4 ± 7.9 |
p <0.05 relative to male + vehicle
Neonatal hormone manipulations alter Aβ accumulation in adulthood
Several months following the neonatal hormone treatments, the TP-treated female mice and flutamide-treated male mice were compared with each other and vehicle-treated control mice for levels of Aβ accumulation. At age 7 mo, Aβ immunoreactivity was quantified in the subiculum, CA1, and frontal cortex (Figure 4). Flutamide treatment of male 3xTg-AD mice was associated with significant increases in Aβ load in hippocampus CA1 and subiculum but did not alter Aβ levels in frontal cortex. The effects of TP treatment in female 3xTg-AD mice on Aβ levels varied by brain region, showing a significant reduction in frontal cortex, no effect in subiculum, and a significant increase in hippocampus CA1.
Figure 4.
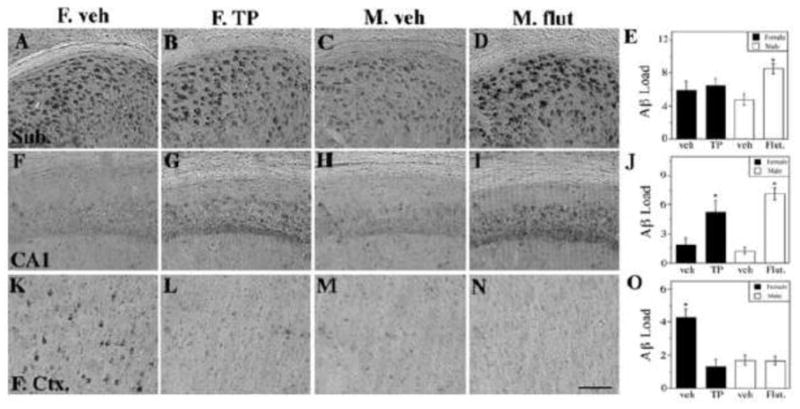
Neonatal flutamide treatment demasculinizes male 3xTg-AD adult brains and TP treatment defeminizes female brains in terms of Aβ pathology in a region-specific manner. Representative photomicrographs display Aβ immunoreactivity visualized in the subiculum (AD), CA1 of hippocampus (F–I), and frontal cortex (K-N). Scale bar = 100μm. Significant treatment differences were observed in quantification of Aβ immunohistochemistry load in the subiculum (E) [F (4,32) = 2.82, p = 0.05], the CA1 (J) [F (4,32) = 17.11, p < 0.0001] and the frontal cortex (O) [F (4,32) = 13.01, p < 0.0001]. Data are represented as mean values ± SEM. * p < 0.05 from opposite sex of same age group.
Neonatal hormone manipulations affect behavioral performance in adulthood
We also evaluated the effects of neonatal hormone manipulations on SAB performance. Compared to vehicle-treated male 3xTg-AD mice, flutamide-treated male 3xTg-AD performed significantly poorer on the SAB task (Figure 5A). In contrast, TP-treated female 3xTg-AD did not perform significantly different from vehicle control females. To confirm that SAB performance differences did not reflect changes in activity level, the numbers of arm entries across groups was measured and was not found to be statistical insignificant [F (3,19) = 1.23, p = 0.30] (Figure 5B).
Figure 5.
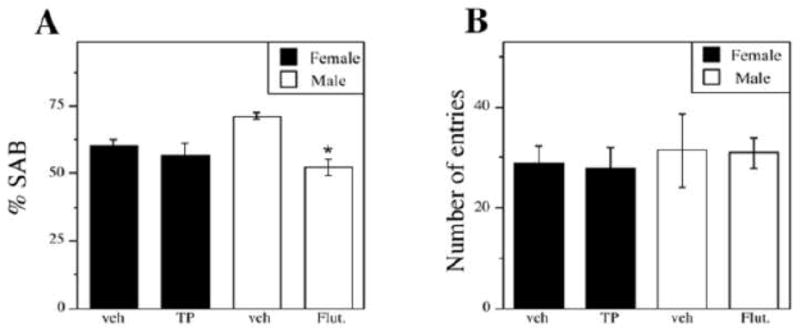
Neonatal flutamide treatment, but not TP treatment alters hippocampal-dependent behavior on the Y-maze. Neonatally-hormone treated mice exhibited a significant overall treatment effect on Y-maze performance (A) [F (3,30) = 6.37, p = 0.003]. The number of arm entries was not significantly different across groups (B). Data are represented as mean values ± SEM. * p < 0.05 from opposite sex of same age group.
3. Discussion
In this study, we investigated sex differences in the progression of Aβ pathology in male and female 3xTg-AD mice and how this relationship is affected by alterations in sex steroid hormones during a critical neonatal period of neural development and sexual differentiation. Consistent with our previous findings (Carroll et al., 2007; Rosario et al., 2006), we observed that Aβ accumulation and deficits in hippocampal-dependent behavior increase with age in adult male and female 3xTg-AD mice. These observations are consistent with the original characterization of the 3xTg-AD mouse model (Oddo et al., 2003), which reported progressive accumulation of intraneuronal Aβ in CA1 hippocampus, subiculum, entorhinal cortex, and amygdala followed by deposition of extracellular Aβ by age 12 mo. This progressive accumulation of Aβ has been associated with corresponding cognitive impairments (Oddo et al., 2003), which is also consistent with our present observations. However, behavioral deficits likely reflect not only Aβ accumulation, but also several other pathological changes characteristic of 3xTg-AD mice, including hyperphosphorylation of tau, glial activation, impaired long-term potentiation (Oddo et al., 2003).
Importantly, we also observed sex differences with females demonstrating higher levels of both Aβ accumulation and a behavioral deficit in comparison to age-matched males at the 12–14 mo time point. Interestingly, the most pronounced sex difference in Aβ accumulation was observed in the frontal cortex, where females displayed significantly higher Aβ levels by age 6–8 mo. These observed sex differences in 3xTg-AD mice are consistent with findings from other studies. In the Tg2576 (Callahan et al., 2001; Lee et al., 2002), APP/PS1 (Wang et al., 2003), APPswe/PSEN1E9 (Halford and Russell, 2009), and (Sturchler-Pierrat and Staufenbiel, 2000) transgenic mouse models of AD, females are also reported to show higher levels of Aβ deposition than males. Similarly, in an APP/PS1 mouse model, females demonstrate a more severe conditioned taste aversion deficit accompanied by a faster onset of plaque deposition (Pistell et al., 2008). These results mirror those reported in another APP model showing significant elevation of C99 and Aβ in female mice compared to males and a decrease in BACE-activity in males (Schafer et al., 2007) as well as reports from an APP model demonstrating worse impairment in water maze retention and circular platform performance in females, even at a young age (3mo) (King et al., 1999). Even in a KO mouse model of glycogen synthase kinase 5 (GRK5KO), female GRK5KO mice had a 2.5-fold increase in hippocampal swollen axonal clusters compared to males and showed lower hippocampal levels of several synaptic proteins such as synaptophysin (Li et al., 2009). Furthermore, our sex difference results are in parallel with a recent study demonstrating higher Aβ burden in female 3xTg-AD mice (Hirata-Fukae et al., 2008). Interestingly, Hirata-Fukae and colleagues reported the absence of a sex difference in tau hyperphosphorylation, another AD-related pathology expressed in 3xTg-AD mice. We did not evaluate levels of tau hyperphosphorylation in this study since tau pathology is only beginning to develop in 3xTg-AD mice at age 7 months, the key time point utilized in this study. Across several transgenic mouse lines, these data demonstrate a common pattern of greater Aβ pathology in female mice exposed to the same genetic factors, suggesting an inherent sex difference in vulnerability to a critical component of AD pathogenesis.
The increased Aβ pathology in AD transgenic mice is consistent with reports in humans of greater neuropathological AD changes in women compared to age-matched men (Corder et al., 2004; Swaab et al., 2001). Such sex differences are generally thought to reflect activational, neuroprotective effects of sex steroid hormones in the adult, which are diminished more abruptly and completely in women as a consequence of menopause. In fact, our prior observations in 3xTg-AD mice demonstrate a significant role of activational effects of sex steroid hormones in adult male (Rosario et al., 2006) and female (Carroll et al., 2007; Carroll and Pike, 2008; Carroll et al., 2010) mice as gonadectomy-induced loss of hormones worsens pathology in a manner that is prevented by sex steroid hormone replacement. An alternative theory is the emerging suggestion that the rise in gonadotrophin hormones such as luteinizing hormone (LH) in aged men and women may contribute to neurodegneration and AD progression (reviewed in Barron et al., 2006; Casadesus et al., 2005; Meethal et al., 2005). However, this theory remains controversial and some evidence from our lab has demonstrated that certain gonadotrophins do not play a role in AD-like pathogenesis (Rosario and Pike, unpublished observations). Finally, another alternative theory supported by our current data, suggests that adult activational effects of either sex steroid hormones or gonadrotrophin hormones alone are unlikely to explain the observed sex differences. Unlike women, female rodents do not experience menopause and the associated depletion of estrogens and progesterone. However, between 11–16 mo of age female mice do experience a transition period characterized by an altered pattern of estrus cycles but normal E2 levels. This transition period is followed by a state of persistent diestrus characterized by low serum E2 levels (Felicio et al., 1984, Nelson et al., 1981). Middle-aged mice can show alterations in E2-mediated activational effects that are linked to reproductive senescence (Alkayed et al., 2000; Jezierski and Sohrabji, 2001; Nordell et al., 2003), however these changes do not typically manifest until 14–16 mo of age, which is later than the female mice used in this study. Furthermore, our data demonstrate significant sex differences in the frontal cortex as early as 6–8 mo of age. Therefore, it is reasonable to hypothesize that although adult activational effects of sex steroid hormones are significant regulators of AD-like pathology in 3xTg-AD mice, other hormone effects also have significant roles.
One contributing factor for the observed sex differences in AD-like pathology in 3xTg-AD mice may be the organizational effects of sex steroid hormones in the developing brain. The brain is particularly sensitive to hormones during critical developmental periods (Krohmer and Baum, 1989; Slob et al., 1980; Weisz and Ward, 1980) when even small hormone alterations can permanently alter brain structure and function (Gore, 2008; McCarthy, 2010). The brain is also acutely sensitive to hormone effects in utero (reviewed in vom Saal, 1989). One limitation of this study is that we were unable to assess the influences of sex hormones in utero, however future studies using pregnant dams will elucidate the earliest influences of sex hormones on adult brain changes. Elevated testosterone and estrogen levels in neonates affect structural organization of several brain regions, particularly hypothalamic and hippocampal structures (Gore, 2008; McCarthy et al., 2008; Morris et al., 2004). Experimental manipulations that affect the normal developmental hormone levels results in neural changes that persist in the adult brain (Anderson et al., 2005; Bakker and Baum, 2008; Becu-Villalobos et al., 1997; Breedlove, 1997; Dominguez-Salazar et al., 2002; Houtsmuller et al., 1994; Isgor and Sengelaub, 1998; McCormick et al., 1998; McCormick and Mahoney, 1999; Miyata et al., 2003; Seale et al., 2005; Yang et al., 2004). Our data show that disruption of androgen signaling in neonatal male 3xTg-AD mice by administration of the androgen receptor antagonist flutamide resulted in elevated Aβ levels in CA1 hippocampus and subiculum. This increased Aβ burden is a more female-like pattern of Aβ accumulation and suggests that neonatal demasculinization of male 3xTg-AD mice altered their adult vulnerability to AD-like pathology. In parallel, demasculinized male 3xTg-AD mice exhibited significantly poorer hippocampal-dependent behavior that was similar to age-matched female 3xTg-AD mice. Female 3xTg-AD mice pups that were defeminized by neonatal testosterone exposure showed significantly lower Aβ levels in frontal cortex, which is a more male-like pattern. Our result that TP administration to female 3xTg-AD mice did not improve hippocampal-dependent behavior is seemingly inconsistent with Raber and colleagues who demonstrate that androgen administration to ApoE4 −/− female mice improved cognitive deficits (Raber et al., 2002). However, it is not reasonable to directly compare these models as the 3xTg-AD is a model of direct AD-like neuropathology while the ApoE4 KO mouse is a model for an AD risk factor. Further both studies utilized different hormone paradigms (organizational vs. activational). Regardless, our findings provide novel evidence that developmental sexual differentiation of the brain significantly affects subsequent progression of Aβ pathology.
Interestingly, organizational actions of sex steroid hormones did not uniformly affect development of pathology. Demasculinization of male 3xTg-AD mice did not alter Aβ levels in frontal cortex. Similarly, defeminization of female 3xTg-AD mice did not affect Aβ levels in subiculum and actually elevated them in hippocampus CA1. These seemingly inconsistent observations are likely related in part to the various, and as yet incompletely defined, mechanisms by which neonatal hormones induce their effects. Sex steroid hormones regulate apoptosis, cell proliferation, and developmental cell migration in the neonatal brain in a manner that is at least partially mediated by aromatase action (Gore, 2008; McCarthy et al., 2008; Morris et al., 2004), which converts testosterone to E2. However, aromatase expression can vary across brain regions (Negri-Cesi et al., 1996), with cortical neurons showing lower expression than hypothalamic neurons, for example. Thus, manipulations of sex steroid hormones during the critical neonatal period would be expected to differentially affect brain regions. Also, it is likely that the observed sex differences in pathology of 3xTg-AD mice reflect influences beyond the organizational and activational effects of sex steroid hormones. For example, recent evidence shows that sexual differentiation of neonatal rodent brain is significantly affected by Müllerian inhibiting substance independently of sex steroid hormones (Wang et al., 2009). We suggest that the organizational effects of sex steroids and related developmental factors including Müllerian inhibiting substance combine to significantly affect neural development in a manner that makes the female brain more vulnerable to AD pathogenesis than the male brain.
The concept that male and female brains exhibit an inherent difference in vulnerability to AD is consistent with the literature on sexual dimorphisms in brain regions related to AD. For example, imaging studies have shown higher hippocampal volumes in females compared to males (Goldstein et al., 2001) and sex differences in hippocampal cholinergic function (Madeira and Lieberman, 1995). In the amygdala, volumes have been shown to be greater in males than in females (Goldstein et al., 2001) and a sexually dimorphic function has been demonstrated in the consolidation of emotional memories (Cahill et al., 2001; Canli et al., 2002; Cooke and Woolley, 2005; Hamann, 2005). In the prefrontal cortex, there are many reports of sex differences in memory performance (Duff and Hampson, 2001; Speck et al., 2000). In addition to sex differences in neural structure and function, men and women are differentially vulnerable to several neurological disorders that develop prior to age-related depletion of sex steroid hormones (Cahill, 2006; Vagnerova et al., 2008). One example is schizophrenia (Arato et al., 2004; Mendrek, 2007), which is characterized by an increased risk with developmental feminization (Lutchmaya et al., 2004) and protective effects in adulthood by estrogens (Bergemann et al., 2005; Mendrek, 2007) and possibly testosterone (Akhondzadeh et al., 2006). Therefore, the organizational and activational effects of sex steroid hormones contribute not only to sexual dimorphisms in brain structure and function, but also to its vulnerability to specific diseases, perhaps including AD.
In summary, our data confirm and extend reports of sex differences in AD transgenic mouse models in which females exhibit more severe pathology. Further, our results implicate perinatal effects of sex steroid hormones as modulators of adult vulnerability to pathology, suggesting a significant role by the established organizational actions of estrogens and testosterone on neural development. The mechanism by which developmental hormone exposure influences AD-related Aβ pathology in adults is not known, but is consistent with the possibility that the feminized brain is inherently more vulnerable to AD pathogenesis. In this case, further investigation of neural sex differences and the organizational actions of sex steroid hormones may contribute to our understanding of AD and its prevention and treatment.
4. Experimental Procedures
Experimental design
Male and female 3xTg-AD mice harboring three human transgenes, APP(Swe), PS1(M146V) and tau(P301L) (Oddo et al., 2003), were maintained at the USC Gerontology vivarium on 12h light on/off cycle and given ad libitum access to food and water. Mice were handled in accordance with the NIH Health and Wellness of Animal Subjects procedures and an institutionally approved IACUC protocol.
Experiment 1
To assess normal, age-related sex differences in Aβ pathology in 3xTg-AD mice, male and female 3xTg-AD mice were divided into 6 groups according to sex and age (N=7 per group). Groups of both male and females were sacrificed at 2–4 mo, 6–8 mo, and 12–14 mo of age.
Experiment 2
To assess the effects of neonatal hormone exposure on the development of Aβ pathology, both male and female 3xTg-AD pups were identified by sex at birth by measuring anogenital distance (pups with a distance from the anterior edge of the anus to the base of the genital tubercle >1 mm were classified as male) (Hurd et al., 2008) and marked by toe clips. Males were divided into two groups (N=7 per group): demasculinized males and control males. Males were demasculinized by treatment with the androgen-receptor antagonist flutamide, a treatment that induces irreversible demasculization that persists in adulthood in both reproductive behavior and function of the hypothalamo-pituitary-adrenal axis (Anahara et al., 2004; Dominguez-Salazar et al., 2002; Husmann and McPhaul, 1991; Isgor and Sengelaub, 1998; Seale et al., 2005). Flutamide (Sigma, St. Louis, MO) was injected daily in neonates from postnatal day 1 through day 20 at a dose of 50 mg/kg/day (ip) while control males were injected daily with vehicle (sesame oil, ip) (Sigma, St. Louis, MO). Such demasculinization using flutamide treatment was based on previous protocols demonstrating irreversible abnormalities in adulthood in both reproductive behavior and in the brain. For example, neonatal flutamide exposure in male rodents leads to decreased seminal vesicle weight in adulthood (Miyata et al., 2003), undescended testicles (Husmann and McPhaul, 1991), lower female partner preference (Houtsmuller et al., 1994; Meek et al., 2006), lower preference for female-soiled bedding (Kudwa et al., 2005) and lower sexual behavior (longer latency and frequency of mounts) (Houtsmuller et al., 1994). For comparison, a group (N=5) of 3 mo old C57Bl6/129S mice were depleted of endogenous testosterone (T) by orchidectomy (ORX) at age 3 mo.
In parallel, on the day of birth, females were divided into defeminized and control groups (N=7 per group). Females were defeminized by treatment with testosterone propionate (TP) (Steraloids, Newport, RI) at a dose of 100 μg/day (ip) as neonates from postnatal day 1 through day 7. This defeminization protocol was based on previous studies demonstrating irreversible abnormalities in both behavior and in the brain. For example, testosterone propionate exposure in neonatal female rodents leads to abnormal vaginal cornification in adulthood (Hutter and Gibson, 1998; Iguchi and Takasugi, 1981), increased body weight (Swanson and van der, 1963; Tarttelin et al., 1975), decreased lordosis (Meek et al., 2006), altered neuronal organization of the amygdala (Akhmadeev and Kalimullina, 2005), altered hippocampal pyramidal cell morphology and dendritic morphology (Isgor and Sengelaub, 2003), altered spatial learning (Isgor and Sengelaub, 2003), and altered development of the anteroventral periventricular preoptic area (Lansing and Lonstein, 2006). Control females were injected (ip) daily with sesame oil vehicle. For comparison, we also included a group (N=5) of female C57Bl6/129S mice that were depleted of endogenous estrogens by ovariectomy (OVX) at age 3 mo. After postnatal hormone or vehicle treatment, both male and female 3xTg-AD mice were then weaned at 3 weeks of age and housed with littermates of the same-sex and treatment condition until sacrifice at age 7 mo. We chose to assess mice at 7 mo because at this age the mice are mature adults with significant AD-like pathology but have not yet entered middle age.
For both Experiments 1 and 2, mice were deeply anesthetized (100 mg/kg Nembutal) on day of sacrifice, transcardially perfused with PBS, and sacrificed by decapitation. To confirm the efficacy of hormone treatments, blood was collected for serum hormone analysis in females and seminal vesicles were dissected, blotted and weighed in males. Estradiol (E2) serum levels were measured using radioimmunoassay (RIA) as previously described (Slater et al., 2001). Brains were sagitally bisected then immersion fixed for 48 h in 4% paraformaldehyde/0.1 M PBS and stored at 4°C in 0.1 M PBS/0.2% sodium azide. Fixed hemibrains were cut into blocks containing frontal cortex or subiculum plus hippocampus. Tissue blocks were sectioned exhaustively (40 μm sections) with a vibratome, the frontal cortex block in the coronal plane, and the subiculum/hippocampus block in the horizontal plane. This process yields ~45 and ~100 sections/brain, respectively which were stored sequentially.
Immunohistochemistry
Aβ accumulation and C-terminal fragments (CTF’s) were visualized by immunohistochemistry as previously described (Carroll et al., 2007; Carroll and Pike, 2008; Carroll et al., 2010) using the following antibodies: Aβ (1:300 dilution; catalog # 71-5800; Zymed, San Fransisco, CA; antibody recognizes Aβ peptides and APP fragments), and CT20 (1:16,000 dilution; catalog # 171610, Anti-0443, Anti-APP-CT20; Calbiochem, San Diego, CA; antibody recognizes amino acids 571-770 of APP). Of the 100 sections/brain in the hippocampus block, every 8th section was stained for Aβ immunoreactivity (#1, 9, 17…) and every 8th section was stained for CTF’s (#2, 10, 18…) so that 12 sections total were stained for each antibody. Similarly, of the 45 sections/brain in the frontal cortex block, every 8th section was stained for Aβ immunoreactivity so that 6 sections total were stained. Sections were stained using standard immunohistochemistry techniques (Pike, 1999) according to the ABC method (Vector Laboratories, Burlingame, CA) and developed using diaminobenzidine. Antigen unmasking consisting of a wash in 99% formic acid prior to application of primary antibodies was used to enhance Aβ immunoreactivity.
Aβ immunoreactivity quantification
Levels of Aβ immunoreactivity were quantified using two approaches. First, all stained sections were quantified for immunoreactive load or burden as previously described (Carroll et al., 2007; Carroll and Pike, 2008). Briefly, high magnification fields from immunolabeled sections were collected and digitized using a video capture system (B/W CCD camera coupled to an Olympus BX40 upright microscope) by an experimenter blinded to treatment groups. Immunoreactivity was converted to positive and negative signal with a constant, predetermined threshold using NIH Image software 1.61. The percentage of positive pixels detected is termed immunoreactive load. Quantification was completed on 5 images per section on 12 sections for hippocampus and subiculum and 6 sections for frontal cortex. In hippocampus CA1, the capture frame was centered over the pyramidal layer with the first captured field corresponding to the narrow zone that marks the division between the end of the regio inferior (CA2/3) and the start of regio superior (CA1) as defined by West et al. (1991). Progressing across CA1 toward the subiculum, the first three adjacent but non-overlapping fields were captured for load analysis. In the subiculum, we similarly captured two adjacent non-overlapping fields per section beginning at the sharp transition from the large pyramidal cells of the CA1 to the smaller cells of the presubiculum (West et al., 1991). In the frontal cortex, fields were captured from every sixth coronal section beginning rostrally with the appearance of somatosensory cortex (Franklin and Paxinos, 1997) for a total of six sections per brain. For each section, the frame was centered over layers 4–5, and five adjacent non-overlapping fields were captured beginning in cingulate cortex area 1 and progressing laterally to somatosensory cortex (Franklin and Paxinos, 1997). Second, in all animals in Experiment 1, the numbers of extracellular plaque-like, Aβ-immunorreactive deposits were quantified as previously described (Carroll et al., 2007; Carroll and Pike, 2008). Every 8th section (~12 per brain) was counted for the number of Aβ deposits in the hippocampus and subiculum. Aβ plaques were classified as diffuse or dense congregations of immunoreactivity >2x the size of a neuron.
Spontaneous Alternation Behavior (SAB)
To measure hippocampal-dependent behavior deficits, 3xTg-AD mice were tested for SAB in a Y-maze using a standard protocol within the week prior to sacrifice (Carroll et al., 2007; Carroll and Pike, 2008). The SAB task capitalizes on the natural exploratory behavior of rodents. During exploration of a Y-maze, mice will repeatedly enter maze arms in an alternating pattern. This behavior is used as a measure of working memory since the animals must remember which arms it previously entered and in which order. Thus, relatively higher alternation percentages correspond to better behavioral performance. SAB is thought to be dependent upon hippocampal function in part because impaired alternation is observed in rodents with hippocampal lesions (Conrad et al., 1996). Further, SAB deficits are associated with increasing age and pathology in mouse models of AD (King and Arendash 2002; Hsiao et al., 1996; Holcomb et al., 1998). SAB score was calculated as the proportion of alternations (an arm choice differing from the previous two choices) to the total number of alternation opportunities (total arm entries-2) (King and Arendash, 2002) during an 8 minute test period. For example, if a mouse made the following arm entries: “A,B,C,B,C,A,B,C” then % SAB would = 4/(8-2)= 66.7%. For comparison, male and female wild-type C57Bl6/129S mice were also tested at ages 2–4 mo, 6–8 mo and 12–14 mo.
Male reproductive behaviors
Male mice were tested on their sexual reproductive behavior using two approaches, mounting and stimulus bedding preference. First, mounting behavior of males when paired with a sexually receptive female was conducted as adapted from a previously described procedure (Houtsmuller et al., 1994; Meek et al., 2006). At 7 mo of age, mice were habituated to the testing room at 18:00 h at the beginning of “lights off.” At 19:00 h, mice were introduced into a female cage containing a receptive female, brought into estrus by treatment with injections of 35 μg (ip) of estradiol benzoate (Steraloids) 48 h prior and 100 μg (ip) progesterone (Steraloids) 6 h prior to behavioral testing (Nwagwu et al., 2005). Mice were evaluated on latency to mount the female and number of attempted mounts during a 60 min testing period. Second, male mice were also evaluated on their preference for female stimulus bedding as adapted from preceding work (Kudwa et al., 2005). Mice were habituated to the testing room at 18:00 h at the beginning of “lights off.” At 19:00 h, mice were introduced into a small, glass cage (8″×20″×10″) with three Petri dishes (10 cm) filled with approximately equal amounts of either male-soiled bedding, female-soiled bedding, or neutral bedding. Male- and female-soiled bedding was retrieved from cages housing sexually active mice not involved in this study. Neutral bedding consisted of unused bedding squares (Ancare Corp., Bellmore, NY). Male mice were placed in the testing chamber for 6 min and the amount of time spent with male, female and neutral bedding was recorded. Preference for female stimulus bedding was calculated as the proportion of the time spent with the female bedding to the total time spent with any bedding.
Female reproductive behavior
Females were tested on their sexual reproductive behavior by evaluating lordosis quotient as adapted from a previously described protocol (Meek et al., 2006). Mice were habituated to the testing room at 18:00 h at the beginning of “lights off.” At 19:00 h, a sexually active male mouse was introduced into each of the females’ cage. During each bout of mounting attempted by the male, the female lordosis position was recorded, with lordosis being characterized as classic arching of the back (Meek et al., 2006). Mice were observed for a maximum of 60 minutes or until 10 mounts were attempted; the percentage of full lordosis behavior per mounting attempts (lordosis quotient) was calculated.
Statistical Analysis
Raw data were statistically evaluated using the JMP statistical software (SAS Institute Inc.; Cary, NC). Main effects were assessed by one-way ANOVA followed between group comparisons using Fisher’s Least Squared Differences test. Effects with a P value < 0.05 were considered to be statistically significant.
Acknowledgments
This research was funded by NIH grants AG23739 (CJP) and AG026572 (RD Brinton/CJP). JCC was supported by NIH grant F31NS059174. ERR was supported by NIH grant NS52143. The authors thank Dr. Elizabeth Adkins-Regan and Dr. Ruth Wood for their insightful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhmadeev AV, Kalimullina LB. [Neonatal androgenization of female rats during sex differentiation of the brain modifies neuronal organization of the amygdala complex] Ontogenez. 2005;36:64–7. [PubMed] [Google Scholar]
- Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa AA, Kashani L, Abbasi SH. Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res. 2006;84:405–10. doi: 10.1016/j.schres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–8. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Anahara R, Toyama Y, Mori C. Flutamide induces ultrastructural changes in spermatids and the ectoplasmic specialization between the Sertoli cell and spermatids in mouse testes. Reprod Toxicol. 2004;18:589–96. doi: 10.1016/j.reprotox.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JRM, Dartigues J-F, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology. 1999;53:1992–7. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Anderson LI, Leipheimer RE, Dluzen DE. Effects of neonatal and prepubertal hormonal manipulations upon estrogen neuroprotection of the nigrostriatal dopaminergic system within female and male mice. Neuroscience. 2005;130:369–82. doi: 10.1016/j.neuroscience.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Arato M, Frecska E, Beck C, An M, Kiss H. Digit length pattern in schizophrenia suggests disturbed prenatal hemispheric lateralization. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:191–4. doi: 10.1016/j.pnpbp.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, D’Agostino RB, White LR. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–9. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685–91. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- Barron AM, Verdile G, Martins RN. The role of gonadotropins in Alzheimer’s disease: potential neurodegenerative mechanisms. Endocrine. 2006;29:257–69. doi: 10.1385/ENDO:29:2:257. [DOI] [PubMed] [Google Scholar]
- Becu-Villalobos D, Gonzalez Iglesias A, Diaz-Torga G, Hockl P, Libertun C. Brain sexual differentiation and gonadotropins secretion in the rat. Cell Mol Neurobiol. 1997;17:699–715. doi: 10.1023/A:1022542221535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann N, Mundt C, Parzer P, Jannakos I, Nagl I, Salbach B, Klinga K, Runnebaum B, Resch F. Plasma concentrations of estradiol in women suffering from schizophrenia treated with conventional versus atypical antipsychotics. Schizophr Res. 2005;73:357–66. doi: 10.1016/j.schres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Neonatal androgen and estrogen treatments masculinize the size of motoneurons in the rat spinal nucleus of the bulbocavernosus. Cell Mol Neurobiol. 1997;17:687–97. doi: 10.1023/A:1022590104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–7. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A. 2002;99:10789–94. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–65. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149:2607–11. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Villamagna A, Pike CJ. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3xTransgenic-Alzheimer’s disease mice. Endocrinology. 2010;151:2713–22. doi: 10.1210/en.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G, Atwood CS, Zhu X, Hartzler AW, Webber KM, Perry G, Bowen RL, Smith MA. Evidence for the role of gonadotropin hormones in the development of Alzheimer disease. Cell Mol Life Sci. 2005;62:293–8. doi: 10.1007/s00018-004-4384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–34. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci. 2005;25:10759–67. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004;1019:24–8. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Salazar E, Portillo W, Baum MJ, Bakker J, Paredes RG. Effect of prenatal androgen receptor antagonist or aromatase inhibitor on sexual behavior, partner preference and neuronal Fos responses to estrous female odors in the rat accessory olfactory system. Physiol Behav. 2002;75:337–46. doi: 10.1016/s0031-9384(01)00674-6. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A sex difference on a novel spatial working memory task in humans. Brain Cogn. 2001;47:470–93. doi: 10.1006/brcg.2001.1326. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31:446–53. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Fratiglioni L, Small BJ, Winblad B, Bäckman L. The transition from normal functioning to dementia in the ageing population. In: Iqbal K, Sisodia S, Winblad B, editors. Alzheimer’s Disease: Advances in Etiology, Pathogenesis and Therapeutics. John Wiley & Sons, Ltd; Chichester: 2001. pp. 3–10. [Google Scholar]
- Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis. 2003;5:267–9. doi: 10.3233/jad-2003-5401. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front Neuroendocrinol. 2008;29:358–74. doi: 10.1016/j.yfrne.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford RW, Russell DW. Reduction of cholesterol synthesis in the mouse brain does not affect amyloid formation in Alzheimer’s disease, but does extend lifespan. Proc Natl Acad Sci U S A. 2009;106:3502–6. doi: 10.1073/pnas.0813349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005;11:288–93. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- Hirata-Fukae C, Li HF, Hoe HS, Gray AJ, Minami SS, Hamada K, Niikura T, Hua F, Tsukagoshi-Nagai H, Horikoshi-Sakuraba Y, Mughal M, Rebeck GW, LaFerla FM, Mattson MP, Iwata N, Saido TC, Klein WL, Duff KE, Aisen PS, Matsuoka Y. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Biudge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer’s disease. Neuro Endocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, van de Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56:535–41. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hurd PL, Bailey AA, Gongal PA, Yan RH, Greer JJ, Pagliardini S. Intrauterine position effects on anogenital distance and digit ratio in male and female mice. Arch Sex Behav. 2008;37:9–18. doi: 10.1007/s10508-007-9259-z. [DOI] [PubMed] [Google Scholar]
- Husmann DA, McPhaul MJ. Time-specific androgen blockade with flutamide inhibits testicular descent in the rat. Endocrinology. 1991;129:1409–16. doi: 10.1210/endo-129-3-1409. [DOI] [PubMed] [Google Scholar]
- Hutter HS, Gibson MJ. Effect of neonatal androgenization on positive feedback in female mice. Biol Reprod. 1988;38:636–8. doi: 10.1095/biolreprod38.3.636. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Takasugi N. Occurrence of permanent anovulation in mouse ovaries and permanent changes in the vaginal and uterine epithelia following neonatal treatment with large doses of 5 alpha-dihydrotestosterone. Endocrinol Jpn. 1981;28:207–13. doi: 10.1507/endocrj1954.28.207. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–98. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;55:179–90. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–79. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–33. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–21. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ. Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer’s disease. Behav Brain Res. 1999;103:145–62. doi: 10.1016/s0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav. 2002;75:627–42. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- Krohmer RW, Baum MJ. Effect of sex, intrauterine position and androgen manipulation on the development of brain aromatase activity in fetal ferrets. J Neuroendocrinol. 1989;1:265–71. doi: 10.1111/j.1365-2826.1989.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc Natl Acad Sci U S A. 2005;102:4608–12. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing SW, Lonstein JS. Tyrosine hydroxylase-synthesizing cells in the hypothalamus of prairie voles (Microtus ochrogaster): sex differences in the anteroventral periventricular preoptic area and effects of adult gonadectomy or neonatal gonadal hormones. J Neurobiol. 2006;66:197–204. doi: 10.1002/neu.20212. [DOI] [PubMed] [Google Scholar]
- Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci U S A. 2002;99:7705–10. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin-Allerhand JA, Lominska CE, Wang J, Smith JD. 17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice. J Alzheimers Dis. 2002;4:449–57. doi: 10.3233/jad-2002-4601. [DOI] [PubMed] [Google Scholar]
- Li L, Rasul I, Liu J, Zhao B, Tang R, Premont RT, Suo WZ. Augmented axonal defects and synaptic degenerative changes in female GRK5 deficient mice. Brain Res Bull. 2009;78:145–51. doi: 10.1016/j.brainresbull.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77:23–8. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S, Harada N, Staufenbiel M, Shen Y, Li R. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30:7326–34. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Schwarz JM, Wright CL, Dean SL. Mechanisms mediating oestradiol modulation of the developing brain. J Neuroendocrinol. 2008;20:777–83. doi: 10.1111/j.1365-2826.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. How it’s Made: Organizational Effects of Hormones on the Developing Brain. Journal of Neuroendocrinology. 2010;22:736–742. doi: 10.1111/j.1365-2826.2010.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have ‘organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mahoney E. Persistent effects of prenatal, neonatal, or adult treatment with flutamide on the hypothalamic-pituitary-adrenal stress response of adult male rats. Horm Behav. 1999;35:90–101. doi: 10.1006/hbeh.1998.1500. [DOI] [PubMed] [Google Scholar]
- Meek LR, Schulz KM, Keith CA. Effects of prenatal stress on sexual partner preference in mice. Physiol Behav. 2006;89:133–8. doi: 10.1016/j.physbeh.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Meethal SV, Smith MA, Bowen RL, Atwood CS. The gonadotropin connection in Alzheimer’s disease. Endocrine. 2005;26:317–26. doi: 10.1385/ENDO:26:3:317. [DOI] [PubMed] [Google Scholar]
- Mendrek A. Reversal of normal cerebral sexual dimorphism in schizophrenia: evidence and speculations. Med Hypotheses. 2007;69:896–902. doi: 10.1016/j.mehy.2007.01.064. [DOI] [PubMed] [Google Scholar]
- Miyata K, Yabushita S, Sano M, Miyashita K, Okuno Y, Matsuo M. Effects of perinatal exposure to flutamide on sex hormone responsiveness in F1 male rats. J Toxicol Sci. 2003;28:149–63. doi: 10.2131/jts.28.149. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–93. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–9. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Poletti A, Celotti F. Metabolism of steroids in the brain: a new insight into the role of 5alpha-reductase and aromatase in brain differentiation and functions. J Steroid Biochem Mol Biol. 1996;58:455–66. doi: 10.1016/0960-0760(96)00083-0. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod. 1981;24:784–94. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- Nordell VL, Scarborough MM, Buchanan AK, Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol Aging. 2003;24:733–743. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Nwagwu MO, Baines H, Kerr JB, Ebling FJ. Neonatal androgenization of hypogonadal (hpg) male mice does not abolish estradiol-induced FSH production and spermatogenesis. Reprod Biol Endocrinol. 2005;3:48. doi: 10.1186/1477-7827-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156:2213–7. [PubMed] [Google Scholar]
- Petanceska SS, Nagy V, Frail D, Gandy S. Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer’s amyloid beta peptides in brain. Exp Gerontol. 2000;35:1317–25. doi: 10.1016/s0531-5565(00)00157-1. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem. 1999;72:1552–63. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–58. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Zhu M, Ingram DK. Acquisition of conditioned taste aversion is impaired in the amyloid precursor protein/presenilin 1 mouse model of Alzheimer’s disease. Neuroscience. 2008;152:594–600. doi: 10.1016/j.neuroscience.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–9. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Amaducci LA, Schoenberg BS. Epidemiology of clinically diagnosed Alzheimer’s disease. Ann Neurol. 1986;19:415–24. doi: 10.1002/ana.410190502. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J Neurosci. 2006;26:13384–9. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. Jama. 2004;292:1431–2. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2009 May 8; doi: 10.1016/j.neurobiolaging.2009.04.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MMB. Incidence of dementia: does gender make a difference? Neurobiology of Aging. 2001;22:575–580. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- Schafer S, Wirths O, Multhaup G, Bayer TA. Gender dependent APP processing in a transgenic mouse model of Alzheimer’s disease. J Neural Transm. 2007;114:387–94. doi: 10.1007/s00702-006-0580-9. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J Physiol. 2005;563:265–74. doi: 10.1113/jphysiol.2004.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater CC, Zhang C, Hodis HN, Mack WJ, Boostanfar R, Shoupe D, Paulson RJ, Stanczyk FZ. Comparison of estrogen and androgen levels after oral estrogen replacement therapy. J Reprod Med. 2001;46:1052–6. [PubMed] [Google Scholar]
- Slob AK, Ooms MP, Vreeburg JT. Prenatal and early postnatal sex differences in plasma and gonadal testosterone and plasma luteinizing hormone in female and male rats. J Endocrinol. 1980;87:81–7. doi: 10.1677/joe.0.0870081. [DOI] [PubMed] [Google Scholar]
- Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the functional organization of the brain for working memory. Neuroreport. 2000;11:2581–5. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Staufenbiel M. Pathogenic mechanisms of Alzheimer’s disease analyzed in the APP23 transgenic mouse model. Ann N Y Acad Sci. 2000;920:134–9. doi: 10.1111/j.1749-6632.2000.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman MA, Ishunina TA. Structural and functional sex differences in the human hypothalamus. Horm Behav. 2001;40:93–8. doi: 10.1006/hbeh.2001.1682. [DOI] [PubMed] [Google Scholar]
- Swanson HE, van der WTBJ. Sex differences in growth of rats, and their modification by a single injection of testosterone propionate shortly after birth. J Endocrinol. 1963;26:197–207. doi: 10.1677/joe.0.0260197. [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Shryne JE, Gorski RA. Patterns of body weight change in rats following neonatal hormone manipulation: a “critical period” for androgen-induced growth increases. Acta Endocrinol (Copenh) 1975;79:177–91. doi: 10.1530/acta.0.0790177. [DOI] [PubMed] [Google Scholar]
- Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–14. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS. Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J Anim Sci. 1989;67:1824–40. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- Wang J, Tanila H, Puolivali J, Kadish I, van Groen T. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol Dis. 2003;14:318–27. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Wang PY, Protheroe A, Clarkson AN, Imhoff F, Koishi K, McLennan IS. Mullerian inhibiting substance contributes to sex-linked biases in the brain and behavior. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0902253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–16. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estrogens and neuroprotection. Trends Endocrinol Metab. 2002;13:229–30. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- Yang SL, Chen YY, Hsieh YL, Jin SH, Hsu HK, Hsu C. Perinatal androgenization prevents age-related neuron loss in the sexually dimorphic nucleus of the preoptic area in female rats. Dev Neurosci. 2004;26:54–60. doi: 10.1159/000080712. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. Jama. 2002;288:2123–9. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, Refolo LM, Petanceska S, Wang R, Duff K. Modulation of A(beta) peptides by estrogen in mouse models. J Neurochem. 2002;80:191–6. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]