Magnetic nanoparticles are widely used for imaging applications, diagnostic measurements, and cell separation.[1-7] Each of these applications requires unique material design, especially in the particle surface, to ensure stability in physiological conditions, biocompatibility, ideal pharmacokinetics, and cellular distribution. In general, in vitro applications have less stringent surface requirements, and it is possible to have more permissive coatings. Conversely, only dextran-coated materials are used for in vivo systemic human applications.[5, 7]
Monocrystalline magnetic nanoparticles are synthesized either via a sol-gel process in which iron salts are precipitated out of aqueous media in the presence of polymers such as dextrans,[2] or via thermal decomposition of metal-complexes, that produces ferrofluids suspended in nonpolar solvents.[8-12] The latter procedure typically results in high purity magnetic cores, better controlled particle sizes, and higher magnetic moments.[13] Unfortunately, transferring such nanocrystals (NCs) into aqueous solutions so as to meet the requirements for biological applications (e.g. stability under high salt concentrations, long circulation times, stability of coating), has been a considerable challenge. Many previous attempts to produce water-soluble magnetic NCs through surface ligand exchange, using small molecules such as dimercaptosuccinic acid, have only yielded stable conjugates for short periods.[7, 14, 15]
Herein, we report on a synthetic protocol for transferring highly magnetic NCs into an aqueous phase, thus producing highly stable NCs under varying pH and ionic strength, as well as under temperature stress (an assay commonly used to test polymer affinity to metal oxide cores). We hypothesized that carboxymethyl polyvinyl alcohol (CMPVA) polymers would provide multi-dentate groups that facilitate strong chelation of the polymer with the iron oxide surface. After exchanging the hydrophobic ligands on the NCs with a polycarboxylate coating (Scheme 1), the conjugates (CMPVA-NCs) were then amenable to further bioconjugation, and also showed excellent stability at high temperatures and under a wide range of physiological conditions for up to several months. We also tested the use of the CMPVA-NCs in cellular assays. When rendered target-specific, the CMPVA-NCs efficiently labeled cell surface markers. More importantly, the particles exhibited extremely low nonspecific binding, which could minimize false-positives in cellular assays.[16-18]
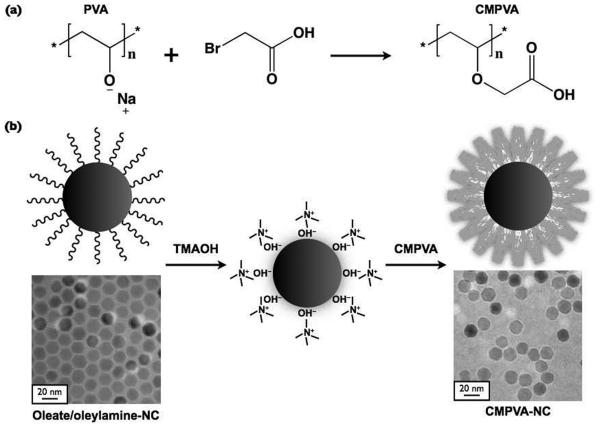
Scheme 1.
A schematic representation illustrating (a) the synthesis of carboxymethyl polyvinyl alcohol (CMPVA), and (b) the ligand exchange process using tetramethylammonium hydroxide (TMAOH), which yields water-soluble CMPVA-NCs. The transmission electron microscopy (TEM) images show the 16 nm particles that were coated with oleate/oleylamine surfactants (bottom left) and with CMPVA (bottom right, 510 μmol carboxylate per gram of polymer).
We first prepared manganese-doped ferrite (MnFe2O4) NCs using the non-hydrolytic route at high temperature, to produce monodispersed particles with strong magnetization.[8, 13, 17] The metal dopant (Mn2+) was incorporated into the ferrite framework to increase the overall magnetization of the resulting materials.[19] Thermal decomposition of the iron and manganese complexes in the presence of hydrophobic surfactants and diol reducing agents resulted in the formation of spherical particles. Since magnetization increases proportionally with the size of the NCs,[11, 17] core materials with larger particle diameters are desirable, for example, to enhance the detection sensitivity in nuclear magnetic resonance (NMR)-based or magnetometer-based assays.[18, 20] By modifying the seed-mediated growth procedure reported by Sun et al. to incrementally grow the magnetic core size from 8 nm to 16 nm (Fig. S1, Supporting Information), we produced highly monodispersed NCs soluble in organic solvents.[8] Transmission electron microscopy (TEM) analysis consequently demonstrated the crystalline structure and narrow size distribution of the 16 nm MnFe2O4 NCs. These particles, which were surrounded by an organic surfactant layer, were soluble in nonpolar solvents and readily close-packed into a hexagonal lattice upon drying. In the Fourier transform infrared (FTIR) spectra, oleic acid ligands were found to produce a carboxylate peak at around 1600 cm−1 and the oleylamine ligands produced an amine peak at approximately 1550 cm−1 (Fig. S2).[21] Measurements done using vibrating sample magnetometer showed that the NCs were superparamagnetic at 300 K (Fig. S2). Inductively coupled plasma atomic emission spectroscopy (ICP-AES) measurements then indicated that the MnFe2O4 core materials were composed of approximately 80% iron and 20% manganese metals.
A number of synthetic and natural polymers have been used to coat the surface of non-hydrolytically synthesized nanoparticles.[22-26] Here, we decided to focus on polyols, in particular polyvinyl alcohol (PVA), since it is synthetic, inexpensive, hydrophilic, biodegradable, and has long been used in hydrogels for tissue engineering and for topical pharmaceutical formulations.[27-29] Initially, our attempts using unmodified PVA as a coating were unsuccessful with the NCs precipitating during ultrafiltration. We thus hypothesized that carboxylate groups would be necessary on the polymer to provide stronger association with the metal oxide surface.[7, 30] Carboxymethylation is a common procedure in polysaccharide chemistry for derivatizing alcohol groups with carboxymethyl. Using this method, we modified the repeating hydroxyl units of PVA into carboxylate groups so as to facilitate their association with iron oxide through multiple anchoring groups. We were also able to produce hydrophilic conjugatable handles using a similar carboxymethylation procedure (Scheme 1).[31] PVA was first reacted with bromoacetic acid under basic condition to slowly convert the hydroxyl groups into carboxylate groups. FTIR analysis showed the appearance of an absorption band at around 1600 cm−1 that was associated with the carbonyl stretching from the carboxymethyl groups (Fig. S2).[32, 33] By simply modifying the molar ratio of bromoacetic acid to polyvinyl alcohol, the degree of carboxylation on the polymer could be adjusted. We then quantified the amount of carboxylate groups present on the polymer by titrating to neutrality using sodium hydroxide. We produced three samples for the surface coating optimization: 150, 380, and 510 μmol carboxymethyl groups per gram of CMPVA.
In order to transfer water-insoluble MnFe2O4 NCs into the aqueous phase, chloroform solution containing the colloidal metal oxide was mixed with a dilute aqueous solution of tetramethylammonium hydroxide (TMAOH). The quaternary ammonium solution was used as a phase transfer catalyst, which slowly displaced the oleylamine and oleic acid surfactants, and surrounded the particle surface.[34, 35] Once the colloids were transferred into water, the electrostatic double layer of ions stabilized the NCs and prevented agglomeration. A hydrodynamic diameter of 24 nm, measured by dynamic light scattering, demonstrated that the amphiphilic surfactant could temporarily stabilize the colloids in water. When CMPVA polymers were added to the aqueous solution of TMAOH-coated NCs, the resulting water-soluble colloids (CMPVA-NCs) became highly stable in salt solution. The TEM image in Scheme 1 shows that CMPVA-NCs remained well-dispersed in aqueous solution without noticeable aggregation (510 μmol carboxymethyl per gram of polymer). Loss of steric interaction caused by the removal of hydrophobic surfactant was also observed for the polymer-coated particles. The FTIR spectrum of CMPVA-NCs showed similar peaks to those of CMPVA. The oleylamine-induced amine band, however, was absent at 1550 cm−1, indicating ligand exchange with CMPVA (Fig. S2). Thus, whereas the TMAOH-coated NCs immediately precipitated upon the addition of PBS (10 mM, 0.15 M NaCl) due to disruption of the ionic double layer, the CMPVA-NCs remained stable in buffer solution, even at high electrolyte concentrations (Fig. 1).
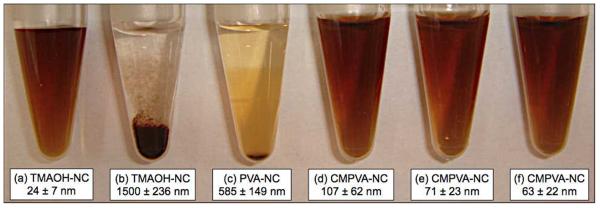
Figure 1.
Colloidal stability and dynamic light scattering measurements of surface-modified particles in aqueous solution (0.5 mg metal/mL). (a) TMAOH-coated NCs were highly stable in water, but immediately precipitated in PBS (b). (c) The absence of carboxyl groups on pure polyvinyl alcohol failed to stabilize the NCs in buffer solution. NCs were rendered water-soluble and stable in PBS after coating them with CMPVA containing (d) 150 μmol carboxylate per gram of polymer, (e) 380 μmol carboxylate per gram of polymer, and (f) 510 μmol carboxylate per gram of polymer.
To quantify the amounts of polymer bound to the particles, thermogravimetric analysis was performed. The weight loss curve shows that the oleate/oleylamine-coated core materials contained approximately 15% hydrophobic surfactant layer, whereas CMPVA-NCs contained approximately 65% CMPVA in weight percent (Fig. S2). Similar to cross-linked dextran-coated nanoparticles, the thick CMPVA coating is necessary to provide high stability to the colloids, to lower nonspecific binding, and presumably to extend the blood circulation half-life. This robust coating also enabled surface modification and attachment of biomolecules without affecting the stability of the colloids or degrading the magnetic core.
We next characterized the transverse relaxivity (r2) of CMPVA-NCs. The r2 is defined as the capacity of magnetic NCs to shorten the transverse relaxation time (T2) of water molecules in NMR. NCs with higher r2 can produce larger T2-changes, and thereby improve the detection sensitivities in NMR-based measurements. The r2 value of CMPVA-NCs was 380 mM−1 s−1 (37°C; 0.47T), considerably higher than that for dextran-coated, iron oxide nanoparticles (70 mM−1 s−1; Fig. S3), which can be attributed to the higher magnetization of MnFe2O4 NCs.[13, 17]
Carboxylate content, size and polymer amount all influenced the size and stability of the CMPVA-coated NCs. Since each strand of the CMPVA polymer consisted of multiple carboxylate anchoring groups, it was necessary to optimize the protocol to avoid inter-particle clustering. Fig. 1 summarizes the results and demonstrates the colloidal stability of the NCs with varying concentration of polymer coating. We determined that a 40-fold excess weight ratio of CMPVA polymer to metal was optimal to produce stable conjugates with the smallest hydrodynamic diameter. An excess of polymer typically led to larger particle sizes, whereas insufficient amounts of polymer failed to fully stabilize the colloids in solution. The carboxylate content of the polymer was also crucial for controlling the stability and hydrodynamic diameter of the resulting colloids. The MnFe2O4 NCs, coated with unmodified PVA, were found to precipitate after purification with membrane ultrafiltration due to the weak binding of the hydroxyl groups to the metal oxide surface. CMPVA polymers with a minimum carboxylate content of 380 μmol per gram of polymer, were necessary to fully stabilize the NCs in buffer solution. Increasing the carboxylate content of the polymer coating led to a reduction in hydrodynamic diameter and narrower colloid size distribution. For example, CMPVA-NCs with lower amount of carboxylates (150 μmol per gram of polymer) had larger diameters (107 nm) and broader size distribution (standard deviation σ = 62 nm), and were only stable in buffer solution for less than 24 h. When the carboxylate content was increased to 510 μmol per gram of polymer, the diameter of the resulting colloid decreased to 63 nm with a tighter size distribution (σ = 22 nm). Surface modification using CMPVA, synthesized from larger polyvinyl alcohol (MW 25000), yielded large-sized colloids (~150 nm) that were difficult to purify using centrifugal filtration. However, their stability was similar to that of lower molecular weight CMPVA polymers. Similar large-sized colloids (>130 nm) could also be synthesized when the ratio of CMPVA to metal was increased by two-fold.
Whilst it was originally assumed that similar approaches for coating hydrophobic, superparamagnetic NCs could be extended to other types of carboxylated polymers, attempts to coat these materials have been surprisingly unsuccessful. Slow aggregation of particles was observed after addition of poly(acrylic acid), carboxymethyl dextran, or poly(methacrylic acid) solutions, at various concentrations, to the aqueous TMAOH-coated NC solution. In addition, further purification of the modified NCs to remove excess polymer only resulted in water-dispersible aggregates that were unsuitable for labeling cells. In contrast, CMPVA-NCs could be repeatedly concentrated by ultrafiltration, and redissolved in buffer solution without risk of particle precipitation caused by the removal of the stabilizing polymer coating.
A heat stress test was used to further assess the strength of polymer-coating on magnetic nanomaterials.[36] Following the incubation of CMPVA-NCs at elevated temperature (90°C for 30 min), the amount of dissociated polymer was quantitated. We observed <2% dissociation of the polymer from CMPVA-NCs. Even with a 5-month-old sample, there was negligible dissociation (Fig. S3). In contrast, non-crosslinked dextran-coated nanoparticles showed much higher dissociation (~30%) under identical conditions. The strong binding of CMPVA polymers to the ferrite surface will be highly beneficial for surface modifications and experiments that require substantial heating and/or harsh chemical conditions, e.g. using redox reagents or cell permeabilizers.[37]
In order to test the biocompatibility of CMPVA-NCs, cytotoxicity measurements were done using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole) assay on HeLa cells and on NIH/3T3 fibroblasts (Fig. S4). Magnetic resonance imaging on animals (mice) typically requires 0.2 mg/mL of metal concentration in the blood. For in vitro experiments, however, less than 0.1 mg/mL of metal concentration is sufficient for efficient labeling. Even at the highest tested concentration of 0.4 mg/mL of metal (Fe + Mn), CMPVA-NCs did not induce noticeable cytotoxicity. Taking into account the thick stable coating of the NCs and their low cytotoxicity, these colloids thus are well suited to labeling surface receptors on live cells.
The CMPVA-NCs were further modified with fluorophores (VT680) and neutravidin molecules to label mammalian cells that were pretargeted with biotinylated antibodies. Sulfhydryl-modified neutravidin was conjugated to the amine-modified NCs using the water-soluble, heterobifunctional crosslinker sulfo-SMCC, to yield stable particle-protein complexes. Each bioconjugate (~8 × 10−18 g metal) contained approximately 39 fluorophores and 41 neutravidin molecules. Neutravidin proteins are nearly neutral in charge and have low nonspecific interactions, and they did not compromise the stability or properties of the CMPVA-NCs.
To demonstrate the cell labeling efficiency of CMPVA-NCs, we chose an extracellular cancer marker, HER2/neu, as a model target. Two cancer cell lines, SK-BR-3 and MDA-MB-231 that have high and low HER2/neu expression respectively, were used for comparison. After labeling the extracellular receptors with biotinylated anti-HER2/neu monoclonal antibodies (Herceptin) and removing the free antibodies, the cells were incubated with neutravidin-modified CMPVA-NCs at room temperature. To minimize the endocytic particle uptake, the incubation time was kept short (20 min).[17, 38] As a control, cells were also incubated with only neutravidin-modified CMPVA-NCs. Fig. 2a shows confocal microscopy images of the labeled cells. Clear NIR fluorescence (red) originating from the NCs could be visualized on the surface of pretargeted SK-BR-3 cells. Experiments with MDA-MB-231 showed negligible fluorescence due to their low expression level of HER2/neu receptors. Note that no fluorescence (except for weak punctate spots) was observed on the control samples, which indicated extremely low nonspecific binding of CMPVA-NCs. This obvious difference between the control and positively labeled samples is invaluable, particularly for diagnostic magnetic resonance (DMR) assays, where changes in T2 measurements rely on the difference between positive samples and controls.[16]
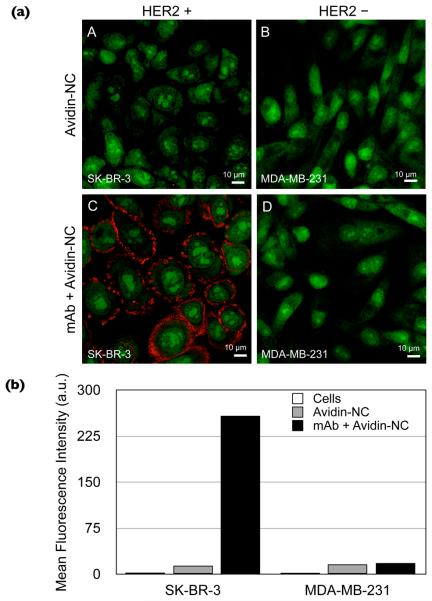
Figure 2.
Labeling of HER2/neu surface receptors with fluorescent CMPVA-NCs. (a) Confocal microscopy was used to image SK-BR-3 and MDA-MB-231 breast cancer cells that were stained with TO-PRO 1 iodide (green fluorescence). The neutravidin-conjugated NCs had low nonspecific binding and did not bind to cells that were not tagged with biotinylated anti-HER2/neu antibodies (A, B). When cells were pretargeted with anti-HER2/neu antibodies, the neutravidin-conjugated NCs, labeled with the near-infrared dye VT680 (red fluorescence), bound to SK-BR-3 cells (C), whereas the labeling was negligible on MDA-MB-231 cells (D). (b) Quantitative analysis of receptor labeling using flow cytometry. The mean fluorescence intensity of SK-BR-3 cells, tagged with biotinylated Herceptin and labeled with the NCs, was much higher than that of MDA-MB-231 cells. The control samples that had been incubated only with neutravidin-NCs had a slightly higher signal compared to the unlabeled cells.
Flow cytometry was performed to further quantify the cell targeting efficiency (Fig. 2b). The relative signal intensity on the SK-BR-3 cells, labeled with biotinylated anti-HER2/neu antibodies and incubated with neutravidin-modified NCs, was substantially higher (15-fold) than that of MDA-MB-231 cells. Cells labeled with only the neutravidin-modified NCs had a mean fluorescence intensity that was only slightly higher than that of the unlabeled cells. We also verified that SK-BR-3 cells incubated with either amine-modified NCs or with neutravidin-conjugated NCs had similar signal intensities. This result was important as it showed that protein conjugation with the NCs did not have a deleterious effect on nonspecific binding to cells.
In this report, we describe how magnetic NCs prepared in nonpolar solvents (ferrofluids) can be efficiently transferred and stabilized via carboxymethylated, but not unmodified, PVA. CMPVA-coated NCs were highly soluble in water, showed little decomposition under heat stress, were stable for prolonged periods without precipitation, and had substantially higher r2 relaxivity compared to clinically approved products. Furthermore, the NCs were not toxic to cells, showed little nonspecific uptake, and could easily be targeted to cell surface receptors. We believe that the developed method could be used in conjunction with other types of materials,[39] and would be beneficial to a wide variety of biological targeting applications including imaging, diagnostics, and cell separation.
Supplementary Material
Acknowledgments
We thank T.-J. Yoon, M. Fernandez-Suarez, R. Mazitschek, and T. Reiner for their helpful experimental suggestions; S. Earley, V. Cortez-Retamozo, A. Newton and V. Pruzinsky for their assistance in cell culture and flow cytometry; I. Bagyadev for help in confocal microscopy; N. Sergeyev for providing cross-linked dextran-coated iron oxide nanoparticles; Y. Fisher-Jeffes for reviewing the manuscript.
Footnotes
This work was supported in part by NIH Grants 2RO1EB004626, U01-HL080731, U54-CA119349 and T32-CA79443.
Supporting Information is available online from Wiley InterScience or from the author.
References
- 1.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, Wittenberg J, Ferrucci JT. Radiology. 1988;168:297. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 2.Shen T, Weissleder R, Papisov M, Bogdanov A, Jr., Brady TJ. Magn. Reson. Med. 1993;29:599. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 3.Lewin M, Carlesso N, Tung C-H, Tang X-W, Cory D, Scadden DT, Weissleder R. Nat. Biotech. 2000;18:410. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 4.Perez JM, Josephson L, O'Loughlin T, Hogemann D, Weissleder R. Nat. Biotech. 2002;20:816. doi: 10.1038/nbt720. [DOI] [PubMed] [Google Scholar]
- 5.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. N. Engl. J. Med. 2003;348:2491. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 6.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Nat. Biotech. 2005;23:1418. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 7.Tsourkas A, Josephson L. Molecular Imaging: Principles and Practice. 2010:523. [Google Scholar]
- 8.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. J. Am. Chem. Soc. 2003;126:273. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 9.Jana NR, Chen Y, Peng X. Chem. Mater. 2004;16:3931. [Google Scholar]
- 10.Yu WW, Falkner JC, Yavuz CT, Colvin VL. Chem. Commun. 2004:2306. doi: 10.1039/b409601k. [DOI] [PubMed] [Google Scholar]
- 11.Jun Y-W, Huh Y-M, Choi J-S, Lee J-H, Song H-T, Kim S, Yoon S, Kim K-S, Shin J-S, Suh J-S, Cheon J. J. Am. Chem. Soc. 2005;127:5732. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 12.Kwon SG, Piao Y, Park J, Angappane S, Jo Y, Hwang N-M, Park J-G, Hyeon T. J. Am. Chem. Soc. 2007;129:12571. doi: 10.1021/ja074633q. [DOI] [PubMed] [Google Scholar]
- 13.Lee J-H, Huh Y-M, Jun Y-W, Seo J-W, Jang J-T, Song H-T, Kim S, Cho E-J, Yoon H-G, Suh J-S, Cheon J. Nat. Med. 2007;13:95. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 14.Fauconnier N, Pons JN, Roger J, Bee A. J. Colloid Interface Sci. 1997;194:427. doi: 10.1006/jcis.1997.5125. [DOI] [PubMed] [Google Scholar]
- 15.Roca AG, Veintemillas-Verdaguer S, Port M, Robic C, Serna CJ, Morales MP. J. Phys. Chem. B. 2009;113:7033. doi: 10.1021/jp807820s. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Sun E, Ham D, Weissleder R. Nat. Med. 2008;14:869. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Yoon T-J, Figueiredo J-L, Swirski FK, Weissleder R. Proc. Natl. Acad. Sci. U. S. A. 2009;106:12459. doi: 10.1073/pnas.0902365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Yoon T-J, Weissleder R. Angew. Chem., Int. Ed. 2009;48:5657. doi: 10.1002/anie.200901791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang J-T, Nah H, Lee J-H, Moon SH, Kim MG, Cheon J. Angew. Chem., Int. Ed. 2009;48:1234. doi: 10.1002/anie.200805149. [DOI] [PubMed] [Google Scholar]
- 20.Gaster RS, Hall DA, Nielsen CH, Osterfeld SJ, Yu H, Mach KE, Wilson RJ, Murmann B, Liao JC, Gambhir SS, Wang SX. Nat. Med. 2009;15:1327. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerung H, Bunge SD, Boyle TJ, Brinker CJ, Han SM. Chem. Comm. 2005;14:1914. doi: 10.1039/b416066e. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RE, Chan WCW. ACS Nano. 2008;2:1341. doi: 10.1021/nn700450g. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Greytak AB, Lee J, Wong CR, Park J, Marshall LF, Jiang W, Curtin PN, Ting AY, Nocera DG, Fukumura D, Jain RK, Bawendi MG. J. Am. Chem. Soc. 2009;132:472. doi: 10.1021/ja908137d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrino T, Manna L, Kudera S, Liedl T, Koktysh D, Rogach AL, Keller S, Radler J, Natile G, Parak WJ. Nano Lett. 2004;4:703. [Google Scholar]
- 25.Yu WW, Chang E, Falkner JC, Zhang J, Al-Somali AM, Sayes CM, Johns J, Drezek R, Colvin VL. J. Am. Chem. Soc. 2007;129:2871. doi: 10.1021/ja067184n. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale F, Bruchez MP. Nat. Biotech. 2003;21:41. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 27.Peppas NA, Berner RE., Jr Biomaterials. 1980;1:158. doi: 10.1016/0142-9612(80)90039-3. [DOI] [PubMed] [Google Scholar]
- 28.Rosenblatt KM, Bunjes H. Mol. Pharmaceutics. 2008;6:105. doi: 10.1021/mp8000759. [DOI] [PubMed] [Google Scholar]
- 29.Schmedlen RH, Masters KS, West JL. Biomaterials. 2002;23:4325. doi: 10.1016/s0142-9612(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 30.Qiao R, Yang C, Gao M. J. Mater. Chem. 2009;19:6274. [Google Scholar]
- 31.McNeil M, Szalecki W, Albersheim P. Carbohydr. Res. 1984;131:139. [Google Scholar]
- 32.Chaosheng Y, Bin L. Polym. Compos. 2008;29:998. [Google Scholar]
- 33.Zhang R, Tang M, Bowyer A, Eisenthal R, Hubble J. Biomaterials. 2005;26:4677. doi: 10.1016/j.biomaterials.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 34.Euliss LE, Grancharov SG, O'Brien S, Deming TJ, Stucky GD, Murray CB, Held GA. Nano Lett. 2003;3:1489. [Google Scholar]
- 35.Salgueirino-Maceira V, Liz-Marzan LM, Farle M. Langmuir. 2004;20:6946. doi: 10.1021/la049300a. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Reynolds F, Yu L, Weissleder R, Josephson L. J. Mater. Chem. 2009;19:6387. [Google Scholar]
- 37.Xing Y, Chaudry Q, Shen C, Kong KY, Zhau HE, Chung LW, Petros JA, O'Regan RM, Yezhelyev MV, Simons JW, Wang MD, Nie S. Nat. Protocols. 2007;2:1152. doi: 10.1038/nprot.2007.107. [DOI] [PubMed] [Google Scholar]
- 38.Schulze E, Ferrucci JT, Jr., Poss K, Lapointe L, Bogdanova A, Weissleder R. Invest. Radiol. 1995;30:604. doi: 10.1097/00004424-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Salgueiriño-Maceira V, Correa-Duarte M. Adv. Mater. 2007;19:4131. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.