Abstract
The data derived from epidemiological and animal models confirm a beneficial effect of fish oil (rich in ω-3 polyunsaturated fatty acids) in the amelioration of tumor growth and progression, including breast cancer. The breast cancer patients often develop bone metastasis evidenced by osteolytic lesions, leading to severe pain and bone fracture. Using a mouse model of MDA-MB-231 human breast cancer cell metastasis to bone, here we show that fish oil diet enriched in DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid) prevents the formation of osteolytic lesions in bone, indicating suppression of cancer cell metastasis to bone. These results are supported by our data showing both DHA and EPA significantly attenuate the migration/invasion of MDA-MB-231 breast cancer cells in culture. The mechanism that limits breast cancer cells to selective metastasis to bone remains hitherto unexplored. Aberrant increased expression of CD44 is associated with generation of cancer stem cells, which contribute to metastasis of breast cancer cells. We demonstrate that DHA and EPA significantly inhibit the expression of CD44 protein and mRNA by a transcriptional mechanism. Furthermore, we show markedly reduced levels of CD44 mRNA and protein in the tumors of mice, which were fed fish oil diet than those in control diet. Our data provide the first evidence for a salutary effect of fish oil on breast cancer metastasis to bone. Our results identify a novel function of the fish oil active components, DHA and EPA, which target the cell-intrinsic pro-metastatic molecule CD44 to inhibit migration/invasion.
INTRODUCTION
Fish oil diet rich in n-3 polyunsaturated fatty acids have beneficial effects in many diseases including various cancers. Decreased consumption of fish oil during past decade in Japanese women correlates with high incidence of breast cancer [1]. Two main constituents of fish oil, DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid) suppress breast cancer cell growth in vitro and in animal models [2; 3; 4]. Accumulation of genetic mutations including oncogene activation and loss of tumor suppressor function along with epigenetic changes contribute invasive properties to the tumor cells to metastasize to distant organs [5]. However, development of solid tumor may not be sufficient to induce metastasis. For example, many patients with disseminated tumor cells in their circulation do not develop clinical metastasis [6]. Similarly, many mouse models of different cancers induced by oncogenically transformed cells also automatically do not establish distant metastasis [7]. It is believed that expression of specific sets of genes in the disseminated cancer cells endow them to metastasize to specific distant organs. For instance, a gene expression signature necessary for breast cancer cells to metastasize to lung has been reported [8; 9]. Mouse fed fish oil diet showed reduced tumor growth and lung metastasis of breast cancer [10; 11]. Furthermore, severity of lung metastasis was significantly reduced by the EPA- and DHA-rich diet in nude mice following surgical excision of the primary solid tumor [12]. Though other organs such as lungs, liver and brain are involved, bone remains the prevalent site for breast cancer metastasis [5; 13]. Bone metastasis of breast cancer cells is often facilitated due to the permissive nature of the fenestrated bone marrow endothelial lining, called sinusoids [14]. Colonized breast cancer cells in the bone microenvironment cause maturation and activation of osteoclasts to form osteolytic lesions leading to severe pain and bone fracture. Although, fish oil diet has been shown to affect primary breast tumor growth and metastasis to lungs, its role in bone metastasis has not been investigated. In the present study, we examined the effect of fish oil diet on breast cancer metastasis to bone and provide a mechanism by identifying a target for its action to attenuate invasiveness.
MATERIALS AND METHODS
Materials
The MDA-MB-231 cell line was purchased from American Type Culture Collection (Rockville, MD) and maintained at 37°C in Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and penicillin and streptomycin. Antibodies against CD44 and actin were purchased from Epitomics (Burlingame, CA) and Sigma (St Louis, MO), respectively. TRIZol RNA isolation kit was obtained from Sigma (St Louis, MO). The ω-3 fatty acids, DHA and EPA were purchased from Cayman Chemical Company (Ann Arbor, MI). The fish oil diet was obtained from Harlan Bioproducts (Indianapolis, IN) and prepared as described [11]. CD44-Luc reporter plasmid containing luciferase gene under the control of CD44 promoter was purchased from Addgene (Cambridge. MA; submitted by Dr. Robert A. Weinberg, Whitehead Institute, Cambridge, MA) [15].
Generation of osteolytic metastasis in animal models
The immune-compromised (nu/nu) mice were obtained from NIH animal facilities and used according to the approved protocol by the Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio. The nude mice were fed a control lab chow diet or a diet containing 10% fish oil for 2 weeks prior to the intracardiac injection of the MDA-MB-231 cells as described previously [16]. The mice were maintained in their respective diets for 4 weeks post injection. Deeply anesthetized animals were exposed to X-ray using a Faxitron radiographic inspection unit as described [16]. The radiolucent osteolytic areas of bone metastasis were marked and quantified using a computer-assisted BIOQUANT image analysis program. For tumor generation, the animals were injected with 106 MDA-MB-231 cells in the mammary fat pad. Mice were sacrificed and the tumors were excised and frozen as described previously to isolate RNA and prepare protein lysates [11].
Migration assay
Confluent monolayers of MDA-MB-231 cells were grown in the presence and absence of DHA or EPA. A scratch was manually engineered in the monolayer using a pipette tip [17]. The cells were allowed to migrate into the scratched area for 21 hours (h) and were photomicrographed at 0h and 21h. Migration of the cells was quantified by analyzing the distances between the two edges of the scratch using BIOQUANT software [18].
Invasion assay
DHA- or EPA-treated or untreated MDA-MB-231 cells were trypsinized and 6×104 cells were seeded in trans-well migration chamber with 8 μm membranes coated with collagen (QCM cell invasion assay kit, Millipore, Billerica, MA). The migration chambers were placed on 24-well plates containing a growth medium with or without DHA or EPA and incubated at 37°C for 24 hours. The unmigrated cells on top of the membrane were removed using cotton swabs. The cells at the bottom of the membrane were stained with the reagent provided in the kit. After the cells were photographed, the stain was extracted using extraction buffer according to the manufacturer’s instructions and the absorbance was measured at 590 nm.
RNA extraction and RT-PCR analysis
Total RNAs from MDA-MB-231 cells or tumor tissues were prepared using TRIZol RNA extraction kit. Total RNA was reverse-transcribed to make cDNA, amplified by quantitative PCR using ABI Prism 7900 sequence detection system and analyzed by SDS 2.1 Software utilizing SYBR green probe method (Applied Biosystems, Foster City, CA) as described previously [18]. Human CD44 primers were used as described by Godar et al [15]. The relative mRNA levels were normalized to GAPDH expression in the same sample.
Preparation of tumor and cell lysates
Tumor and MDA-MB-231 cell lysates were prepared essentially as described previously, using radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM phenyl methyl sulfonyl fluoride, 0.05% aprotinin and 1 % Nonidet P-40) [11]. Equal amounts of protein (20 mg) in the cleared supernatants, collected by centrifugation at 4°C at 10,000xg for 30 minutes, were used for immunoblotting with indicated antibodies as described previously [19; 20; 21; 22].
Transfection and luciferase assay
CD44-Luc reporter plasmid was transfected into MDA-MB-231 breast cancer cells using the Fugene HD reagent (Roche Inc, Indianapolis, IN) and treated with DHA or EPA for 24h. The luciferase enzyme activity was determined in the cell lysates using a kit (Promega, Madison, WI) as described [11; 18; 23]. The data were normalized as a ratio of luciferase activity to total protein content [11; 18; 23].
Statistics
The significance of the data was determined by ANOVA followed by paired t test or Student-Newman-Keuls analysis using Graph Pad Prism 4 software, as described previously [18]. p value less than 0.05 was considered as significant.
RESULTS
Fish oil prevents breast cancer cell metastasis to bone
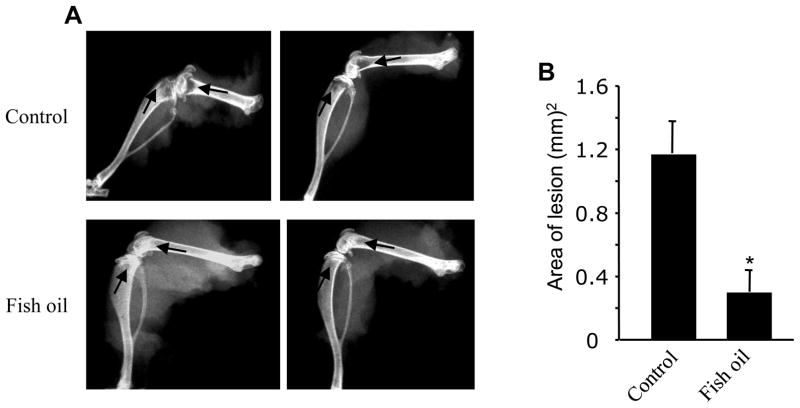
The human MDA-MB-231 breast cancer cells when inoculated in the left cardiac ventricle of immune-compromised nude mice, they prominently metastasize to bone to form osteolytic lesions [16]. We used this mouse model to examine the effect of fish oil on breast cancer metastasis to bone. A group of mice fed lab chow or a diet containing 10% fish oil for 2 weeks were injected with the MDA-MB-231 breast cancer cells in their left cardiac ventricle. Four weeks after inoculation of the breast cancer cells, the mice were sacrificed and the hind limbs were X-rayed. As expected, osteolytic bone destruction was obvious in the hind limbs of control mice fed lab chow (Fig. 1A, upper panels, indicated by arrows). Formation of osteolytic lesions was markedly prevented in the hind limbs of mice fed fish oil diet (Fig. 1A, bottom panels; indicated by arrows). The lesions in the X-rays were quantified using computer software. The histogram presented in the Fig. 1B shows that fish oil significantly attenuated the osteolytic lesions produced by the metastasis of breast cancer cells.
Figure 1.
Effect of fish oil diet on breast cancer cells bone metastasis. (A) Inhibition of osteolytic lesions in fish oil-fed mice. Two groups of animals fed lab chow and fish oil diet, respectively received MDA-MB-231 breast cancer cells in their left cardiac vein and the osteolytic lesions were visualized using X-ray analysis. Hind limb X-rays from two control lab chow- (upper panels) and two fish oil (bottom panels) diet-fed mice are shown. Arrows indicate the osteolytic lesions in the joints. (B) Quantification of the osteolytic lesions in panel A. The lesions in the control and fish oil-fed mice were marked and quantified. n = 5; *p < 0.005 vs control.
Fish oil components DHA and EPA block breast cancer cell migration/invasion
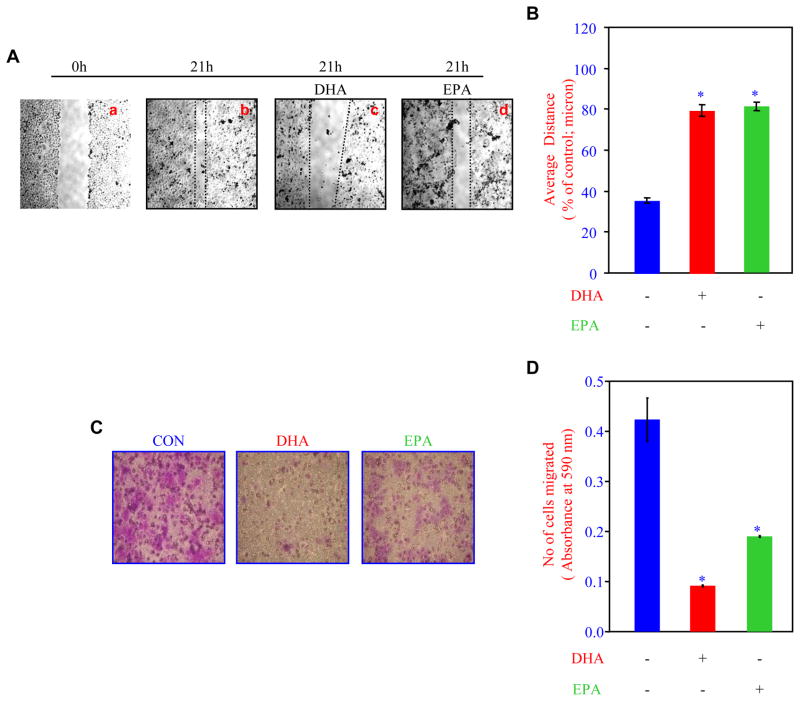
To investigate the mechanism of breast cancer cell migration, we used a scratch assay using the MDA-MB-231 breast cancer cell monolayer. At 21 hours, the cells migrated significantly (Fig. 2A, compare b with a). Treatment of the cells with DHA inhibited the migration of the cells (Fig. 2A, compare c with b). Similarly, EPA suppressed the migration (Fig. 2A, compare d with b). The distance between the cell fronts is inversely proportional to the migration of breast cancer cells. Fig. 2B shows the quantification, indicating both DHA and EPA significantly attenuated the migration of breast cancer cells.
Figure 2.
Effects of DHA and EPA on breast cancer cells migration and invasion. (A) DHA and EPA inhibit breast cancer cell migration. MDA-MB-231 cell monolayers were scratched and incubated with 50 ng/ml DHA or EPA for 21 hours [11]. After the incubation, the cell monolayers were photographed. (B) Quantification of the data in panel A. The distance between the cell edges were determined by BIOQUANT. n = 6; *p < 0.001 vs control. (C) DHA and EPA attenuate invasion of breast cancer cells. MDA-MB-231 breast cancer cells used in the presence and absence of DHA or EPA were allowed to pass through the collagen-coated membrane and stained. The photographs of the stained cells are shown. (D) Quantification of the data in panel C. The stains in the migrated cells were eluted and the absorbance measured at 590 nm. n = 3; *p < 0.01.
The metastasis of the tumor cells requires the local intravasation, which involves passing through the extracellular matrix. Therefore, to examine the effect of DHA and EPA, we performed an invasion assay using a collagen-coated membrane in the culture wells. After incubation of the cells with DHA or EPA, the bottom part of the membrane was stained to determine the cell migration. As shown in the Fig. 2C, both DHA and EPA inhibited the migration of the breast cancer cells through the collagen membrane. Note that DHA was more effective than EPA in this assay. To quantitatively assess the results, the stains were eluted and absorbance measured, which is directly proportional to the number of migrated cells. Fig. 2D shows that both DHA and EPA significantly inhibited the invasion of human MDA-MB-231 breast cancer cells.
DHA and EPA suppress expression of CD44
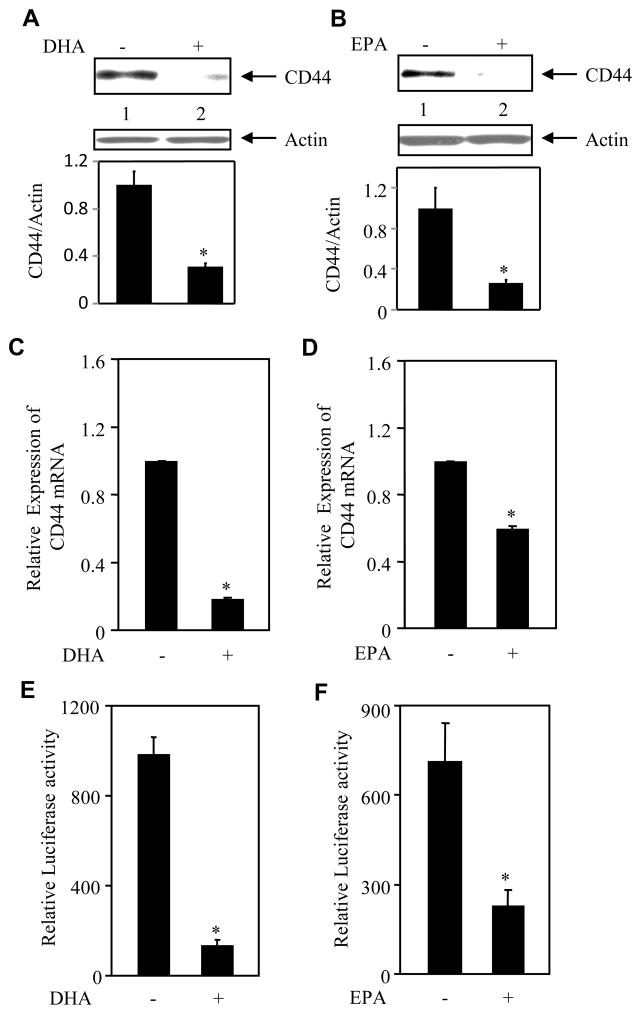
Recent reports demonstrate a positive role of CD44 in the progression of metastasis [24; 25]. Many cancer cells including breast cancer cells hyperexpress the cell surface adhesion protein CD44 [26; 27]. We therefore examined the effect of the ω-3 fatty acids on the expression of CD44 in the MDA-MB-231 breast cancer cells. Incubation of MDA-MB-231 cells with DHA significantly inhibited the levels of CD44 protein (Fig. 3A). Similarly, EPA attenuated the CD44 protein levels (Fig. 3B). To elucidate the mechanism, we performed real time qRT-PCR analyses using total RNAs prepared from DHA- or EPA-treated MDA-MB-231 cells. Both DHA and EPA significantly suppressed the expression of CD44 mRNA (Figs. 3C and 3D). To explore the possibility that these two ω-3 fatty acids may utilize transcriptional mechanism to downregulate CD44 expression, we used a reporter plasmid, which contains luciferase gene under the control of CD44 promoter. MDA-MB-231 cells transfected with this reporter construct were incubated with DHA or EPA. Both fatty acids markedly inhibited the transcription of CD44 (Figs. 3E and 3F). These results indicate that DHA and EPA, two main components of dietary fish oil, suppressed the prometastatic CD44 expression via a transcriptional mechanism.
Figure 3.
Effects of DHA and EPA on CD44 expression. (A and B) DHA and EPA inhibit CD44 protein expression. Lysates of MDA-MB-231 breast cancer cells incubated with DHA (panel A) or EPA (panel B) were immunoblotted with CD44 and actin antibodies, respectively. Bottom panels show quantification of the protein bands. (panel A) n = 3; *p = 0.03; (panel B) n = 4; *p = 0.04. (C and D) DHA and EPA block CD44 mRNA expression. RNAs prepared from DHA- or EPA-treated MDA-MB-231 cells were used for real time RT-PCR to determine CD44 mRNA expressions using specific primers. Mean ± SE of triplicate measurements is shown. *p < 0.01. (E and F) DHA and EPA inhibit transcription of CD44. MDA-MB-231 breast cancer cells transiently transfected with CD44-Luc were incubated with DHA or EPA. Luciferase activity was determined in the cell lysates and the data are presented as described in the methods as mean ± SE of triplicate measurements. n = 3; *p < 0.01.
Fish oil diet prevents the expression of CD44 in the tumor xenografts
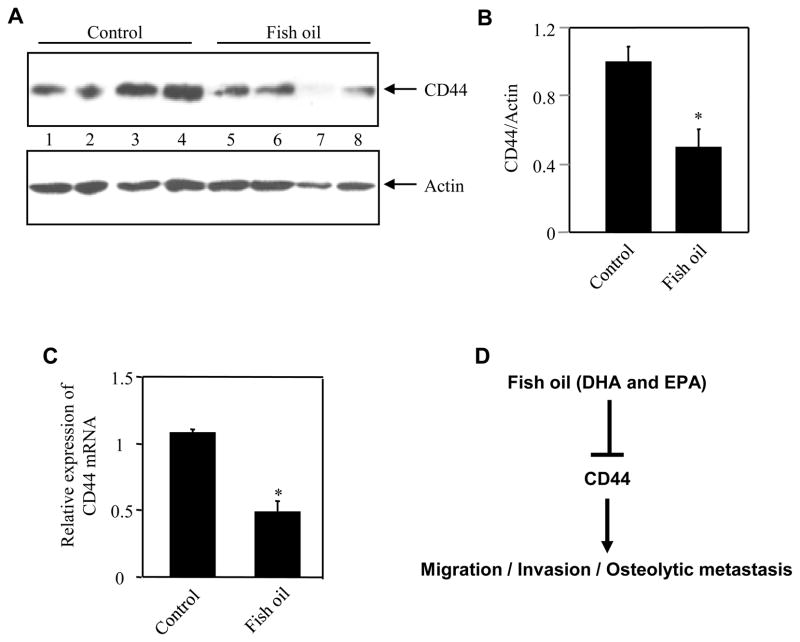
Increased expression of CD44 has been reported in both in situ and in invasive breast carcinoma [26; 27]. We tested the expression of CD44 in the xenograft tumors from MDA-MB-231 in mice, which were fed fish oil diet. Significant levels of CD44 protein were detected in the tumors of control mice (Fig. 4A, lanes 1 – 4). However, tumors in the fish oil diet fed group displayed markedly low levels of CD44 than that in the control mice (Fig. 4A, compare lanes 5 – 8 with lanes 1 – 4). Quantification of the data showed a significant inhibition of CD44 protein expression in the tumors of mice that received fish oil diet (Fig. 4B). Furthermore, analysis of total RNA by real time qRT-PCR revealed significantly reduced levels of CD44 mRNA in the breast tumors in fish oil fed group as compared to that in the control animals (Fig. 4C). These results suggest that fish oil attenuates the expression of CD44 in vivo in the breast tumors of mice.
Figure 4.
Fish oil attenuates expression of CD44 protein and mRNA in breast tumors. (A) Tumor lysates from control lab chow-fed or fish oil diet-fed animals were immunoblotted with CD44 and actin antibodies, respectively. (B) Quantification of results in panel B. The ratio of the intensity of CD44 to actin is shown. n = 4; *p = 0.02 vs control. (C) Fish oil inhibits CD44 mRNA expression in breast tumor. Total RNAs isolated from tumors from control and fish oil fed animals were examined for expression of CD44 mRNA. Mean ± SE of 4 independent experiments is shown. *p = 0.004 vs control. (D) Schema for mechanism of fish oil-mediated attenuation of breast cancer cell migration/invasion/osteolytic metastasis.
DISCUSSION
The mortality of breast cancer patients is significantly impacted by the metastasis of the cancer cells to bone [13]. Osteolytic lesions frequently occur in the load-bearing bones with increased susceptibility to pathological facture. Furthermore, osteolysis resulting from metastasis in the pelvis, neck or in the femur is associated with treatment problem. Our results for the first time demonstrate that fish oil diet significantly prevent the formation of the osteolytic bone lesion in a model of breast cancer metastasis to bone (Fig. 1).
Osteolysis does not occur due to direct effect of breast cancer cells metastasized to bone. Rather stimulation of the osteoclast maturation and their activation cause formation of the lesions [5; 13]. PTHrP and IL-11 secreted by the disseminated breast cancer cells do not provide any advantage to these cells in the primary tumor; however these factors contribute to increase osteoclast activity [5; 28]. Furthermore, the colonized breast cancer cells produce TNF-α and IL-6, which along with PTHrP and IL-11 stimulate the bone marrow stromal cells and residing osteoblasts to synthesize more RANKL to activate osteoclasts through RANK present on their surface [5]. Thus establishment of this cell non-autonomous signaling pathway results in the formation of osteolytic lesions. Our results demonstrating the reduction in osteolysis by the fish oil diet do not distinguish its effect on metastasis and on production of these osteoclastogenic factors by the breast cancer cells. However, our in vitro data conclusively show that the active components of fish oil, both DHA and EPA, significantly prevent the migration/invasion of the metastatic human MDA-MB-231 mammary cancer cells (Fig. 2).
Stem cell migration and cancer metastasis are mediated by the overlapping sets of molecules indicating that cancer stem cells may contribute to metastasis [29]. Acquisition of phenotype to express increased levels of CD44 by the noninvasive breast cancer cells correlates with induction of epithelial mesenchymal transdifferentiation necessary for metastatic potential [30]. The aggressive MDA-MB-231 breast cancer cells possess a mesenchymal phenotype and express high levels of CD44 on their surface [31]. A recent report by Nakamura et al has demonstrated that metastasized breast cancer cells expressing CD44 are present on the bone surface, which face the bone resorbing osteoclasts [32].
CD44 represents an ensemble of transmembrane cell adhesion proteins generated by inclusion of alternatively spliced exons from the same parental gene [33]. CD44 acts as the main glycoprotein receptor for the disaccharide hyaluronan (HA), a major component of the luminal surface of the bone marrow capillary endothelium. Thus, metastatic breast cancer cells expressing high levels of CD44 may be efficiently recruited to bone marrow. Similarly, collagen I, a constituent of bone matrix, serves as another ligand for CD44, indicating efficient recruitment of breast cancer cells to the bone. Furthermore, the MMP9 present on the surface of breast cancer cells has been shown to be retained through its binding to CD44 [34]. MMP9 cleaves collagen I in the bone matrix and may contribute to induce osteolysis found in bone, resulting from breast cancer metastasis. Our results show that the active constituents of fish oil, DHA and EPA, inhibit the expression of CD44 in MDA-MB-231 breast cancer cells (Fig. 3), thus providing a mechanism for the attenuation of breast cancer cell migration/invasion, we observed in our in vitro experiments (Fig. 4D). We also show significantly low levels of CD44 mRNA and protein expression in the tumors from mice that received fish oil diet than those from control animals (Fig. 4). Furthermore, for the first time we demonstrate that fish oil significantly prevents the formation of osteolytic lesions in the bone (Fig. 1).
Bone metastasis of breast cancer cells is the single most catastrophic complication for the morbidity and mortality of breast cancer patients. Our results provide evidence for the use of fish oil supplements as an important adjuvant therapy for this devastating disease. Furthermore, our data identify the prometastatic protein CD44 as one of the targets of the fish oil.
Acknowledgments
NIH RO1 AR52425 and VA Merit review grants and Ronald Williams Orthopedic Award (Cancer therapy and Research Center, University of Texas Health Science Center at San Antonio) to NGC supported this work. GGC is a recipient of VA Senior Research Career Scientist Award and is supported by VA Research Service Merit Review grant, NIH RO1 DK50190 grant and Juvenile Diabetes Research Foundation 1-2008-185 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lands WE, Hamazaki T, Yamazaki K, Okuyama H, Sakai K, Goto Y, Hubbard VS. Changing dietary patterns. Am J Clin Nutr. 1990;51:991–3. doi: 10.1093/ajcn/51.6.991. [DOI] [PubMed] [Google Scholar]
- 2.Chajes V, Sattler W, Stranzl A, Kostner GM. Influence of n-3 fatty acids on the growth of human breast cancer cells in vitro: relationship to peroxides and vitamin-E. Breast Cancer Res Treat. 1995;34:199–212. doi: 10.1007/BF00689711. [DOI] [PubMed] [Google Scholar]
- 3.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83:217–44. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 4.Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2005;92:187–95. doi: 10.1007/s10549-005-2415-z. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 6.Klein CA. The systemic progression of human cancer: a focus on the individual disseminated cancer cell--the unit of selection. Adv Cancer Res. 2003;89:35–67. doi: 10.1016/s0065-230x(03)01002-9. [DOI] [PubMed] [Google Scholar]
- 7.Minna JD, Kurie JM, Jacks T. A big step in the study of small cell lung cancer. Cancer Cell. 2003;4:163–6. doi: 10.1016/s1535-6108(03)00221-6. [DOI] [PubMed] [Google Scholar]
- 8.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todoova K, Massague J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–70. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 9.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose DP, Connolly JM, Rayburn J, Coleman M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst. 1995;87:587–92. doi: 10.1093/jnci/87.8.587. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh-Choudhury T, Mandal CC, Woodruff K, St Clair P, Fernandes G, Choudhury GG, Ghosh-Choudhury N. Fish oil targets PTEN to regulate NFkappaB for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res Treat. 2009;118:213–28. doi: 10.1007/s10549-008-0227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose DP, Connolly JM, Coleman M. Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin Cancer Res. 1996;2:1751–6. [PubMed] [Google Scholar]
- 13.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 14.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–56. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 15.Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, Liu A, Miu K, Watnick RS, Reinhardt F, McAllister SS, Jacks T, Weinberg RA. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraga T, Williams PJ, Mundy GR, Yoneda T. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res. 2001;61:4418–24. [PubMed] [Google Scholar]
- 17.Bange J, Prechtl D, Cheburkin Y, Specht K, Harbeck N, Schmitt M, Knyazeva T, Muller S, Gartner S, Sures I, Wang H, Imyanitov E, Haring HU, Knayzev P, Iacobelli S, Hofler H, Ullrich A. Cancer progression and tumor cell motility are associated with the FGFR4 Arg(388) allele. Cancer Res. 2002;62:840–7. [PubMed] [Google Scholar]
- 18.Mandal CC, Ghosh Choudhury G, Ghosh-Choudhury N. Phosphatidylinositol 3 kinase/Akt signal relay cooperates with smad in bone morphogenetic protein-2-induced colony stimulating factor-1 (CSF-1) expression and osteoclast differentiation. Endocrinology. 2009;150:4989–98. doi: 10.1210/en.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh Choudhury G, Abboud HE. Tyrosine phosphorylation-dependent PI 3 kinase/Akt signal transduction regulates TGFbeta-induced fibronectin expression in mesangial cells. Cell Signal. 2004;16:31–41. doi: 10.1016/s0898-6568(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277:33361–8. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh-Choudhury N, Mandal CC, Choudhury GG. Statin-induced Ras Activation Integrates the Phosphatidylinositol 3-Kinase Signal to Akt and MAPK for Bone Morphogenetic Protein-2 Expression in Osteoblast Differentiation. J Biol Chem. 2007;282:4983–93. doi: 10.1074/jbc.M606706200. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh-Choudhury N, Singha PK, Woodruff K, St Clair P, Bsoul S, Werner SL, Choudhury GG. Concerted action of Smad and CREB-binding protein regulates bone morphogenetic protein-2-stimulated osteoblastic colony-stimulating factor-1 expression. J Biol Chem. 2006;281:20160–70. doi: 10.1074/jbc.M511071200. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh-Choudhury N, Mandal CC, Ghosh-Choudhury N, Ghosh Choudhury G. Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit breast cancer cell growth. Cell Signal. 2010;22:749–58. doi: 10.1016/j.cellsig.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouhtit A, Abd Elmageed ZY, Abdraboh ME, Lioe TF, Raj MH. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am J Pathol. 2007;171:2033–9. doi: 10.2353/ajpath.2007.070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber GF, Bronson RT, Ilagan J, Cantor H, Schmits R, Mak TW. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res. 2002;62:2281–6. [PubMed] [Google Scholar]
- 26.Auvinen P, Tammi R, Tammi M, Johansson R, Kosma VM. Expression of CD 44 s, CD 44 v 3 and CD 44 v 6 in benign and malignant breast lesions: correlation and colocalization with hyaluronan. Histopathology. 2005;47:420–8. doi: 10.1111/j.1365-2559.2005.02220.x. [DOI] [PubMed] [Google Scholar]
- 27.Gotte M, Yip GW. Heparanase, hyaluronan, and CD44 in cancers: a breast carcinoma perspective. Cancer Res. 2006;66:10233–7. doi: 10.1158/0008-5472.CAN-06-1464. [DOI] [PubMed] [Google Scholar]
- 28.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–9. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 30.Uchino M, Kojima H, Wada K, Imada M, Onoda F, Satofuka H, Utsugi T, Murakami Y. Nuclear beta-catenin and CD44 upregulation characterize invasive cell populations in non-aggressive MCF-7 breast cancer cells. BMC Cancer. 2010;10:414. doi: 10.1186/1471-2407-10-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Jr, Badve S, Nakshatri H. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura H, Hiraga T, Ninomiya T, Hosoya A, Fujisaki N, Yoneda T, Ozawa H. Involvement of cell-cell and cell-matrix interactions in bone destruction induced by metastatic MDA-MB-231 human breast cancer cells in nude mice. J Bone Miner Metab. 2008;26:642–7. doi: 10.1007/s00774-008-0857-1. [DOI] [PubMed] [Google Scholar]
- 33.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 34.Hill A, McFarlane S, Johnston PG, Waugh DJ. The emerging role of CD44 in regulating skeletal micrometastasis. Cancer Lett. 2006;237:1–9. doi: 10.1016/j.canlet.2005.05.006. [DOI] [PubMed] [Google Scholar]