Abstract
As neuronal progenitors differentiate into neurons, they acquire a unique set of transcription factors. The transcriptional repressor REST prevents progenitors from undergoing differentiation. Notably, REST binding sites are often associated with retinal ganglion cell (RGC) genes whose expression in the retina is positively controlled by Atoh7, a factor essential for RGC formation. The key regulators that enable a retinal progenitor cell (RPC) to commit to an RGC fate have not been identified. We show here that REST suppresses RGC gene expression in RPCs. REST inactivation causes aberrant expression of RGC transcription factors in proliferating RPCs, independent of Atoh7, resulting in increased RGC formation. Strikingly, inactivating REST in Atoh7-null retinas restores transcription factor expression, which partially activates downstream RGC genes but is insufficient to prevent RGC loss. Our results demonstrate an Atoh7-independent program for initial activation of RGC genes and suggest a novel role for REST in preventing premature expression in RPCs.
Keywords: retinal ganglion cells, retinal progenitor cells, REST (NRSF), Atoh7 (Math5), Neurod1
Introduction
The vertebrate retina is an accessible, well-described sensory tissue that continuously provides valuable insights into neurogenesis, nerve structure, and nervous system circuitry (Chalupa and Williams, 2008). In particular, major advances have been made using the retina as a model for central nervous system development. In the developing retina, neurogenesis begins in the central retina as newly differentiated neurons signal to the adjacent retinal progenitor cells (RPCs) to initiate a central-to-peripheral wave of neurogenesis. During this process, multipotent RPCs must decide whether to continue to divide or to exit the cell cycle and commit to a more restricted lineage-competent state. Once committed, RPCs undergo differentiation into one of seven cell types in an evolutionarily conserved temporal order and distinct laminated pattern (Livesey and Cepko, 2001; Agathocleous and Harris, 2009). Retinal ganglion cells (RGCs) are invariably the first cell type to differentiate followed immediately by amacrine cells, horizontal cells and cone photoreceptor cells in a highly overlapping manner. Subsequently, the later cell types, bipolar cells, rod photoreceptor cells, and Müller glial cells, are produced. Much has been learned about the genetic regulatory mechanisms that are responsible for retinal development and several models have been proposed to explain virtually all aspects of this intricate process (for recent reviews, see Cayouette et al., 2006; Harada et al., 2007; Lamba et al., 2009; Mu and Klein, 2008; Agathocleous and Harris, 2009; Jadhav et al., 2009). This is especially true for RGCs, for which extensive analysis has revealed the transcriptional network circuitry critical for RGC specification and differentiation (Mu et al., 2004, 2005, 2008; Hernandez et al., 2007; Agathocleous and Harris, 2009; Souren et al., 2009). A critical gap in our knowledge, however, is the identification of the key regulators that enable an RGC-competent RPC to alter its genetic program and advance to a committed RGC fate. In this study, we provide new insight into this issue by identifying REST (also called NRSF) as one of these key regulators.
The proneural bHLH transcription factor Atoh7 (also called Math5 in the mouse and Ath5 in other vertebrates) determines the competency state for RPCs by providing a favorable intrinsic environment for advancement to an RGC fate (Figure 1A; reviewed in Mu and Klein, 2008). RGC competency occurs when a subpopulation of proliferating RPCs lose their ability to respond to Notch signaling and exit the cell cycle (Perron and Harris, 2000; Nelson et al., 2006; Le et al., 2006; Riesenberg et al., 2009). Atoh7 begins to be expressed at approximately the same time and in the same cells where the mediators of Notch signaling, Delta and Hes1/5 are upregulated (Yang et al., 2003; Willardsen et al., 2009). But Atoh7 alone is not sufficient to specify the RGC lineage. Once RPCs exit the cell cycle, Atoh7-expressing RPCs give rise to multiple retinal cell types (Figure 1A; Yang et al., 2003). Commitment to the RGC lineage is marked by downregulation of Atoh7 and onset of expression of the POU domain transcription factor Pou4f2 and the LIM-homeodomain transcription factor Isl1 (Figures 1A, 1B; Gan et al., 1999; Mu et al., 2008; Pan et al., 2008). At present, little is known about how Atoh7-expressing RPCs respond to local environmental signals to activate Pou4f2 and Isl1.
Figure 1.

Schematic illustration of RGC production from RPCs. (A) REST is active in proliferating RPCs and is degraded upon cell cycle exit/differentiation. Atoh7 begins to be expressed at the G2/M phase before RPCs exit the cell cycle to commence differentiation. Neighboring RPCs respond to the local environment to express different proneural bHLH genes. The onset of Pou4f2 and Isl1 expression marks the initial commitment to RGC differentiation. Note that Atoh7 is required for expression of Pou4f2 and Isl1 and for RGC formation. Atoh7-expressing cells give rise to multiple cell types, including RGC, amacrine cells (AC), horizontal cells (HC), and photoreceptor cells (PhR). (B) REST gene targets include Pou4f2 but not Isl1. BC: bipolar cells, MGC: Müller glial cells.
REST, a zinc-finger transcription factor, offers a possible route in solving the problem of RPC commitment to an RGC fate. REST was initially identified as a master repressor of neuronal gene expression in non-neuronal cell types (Chong et al., 1995; Schoenherr and Anderson, 1995; Bruce et al., 2004, 2009). REST binds to a conserved 21-bp motif termed repressor element 1 (RE1), which is found within the transcriptional regulatory regions of hundreds of neuronal genes (Mortazavi et al., 2006; Johnson et al., 2007; Otto et al., 2007). REST mediates active repression via recruitment of histone deacetylases by its corepressors mSin3 and CoREST (Ballas et al., 2005; Lunyak and Rosenfeld, 2005). In neuronal progenitor cells, REST is expressed at levels that are sufficiently high enough to maintain neuronal genes in a chromatin-inactive state poised for activation (Ballas et al., 2005). Upon neuronal differentiation, REST is degraded through ubiquitin-mediated proteolysis (Guardavaccaro et al., 2008). A wealth of information exits in the literature on the molecular mechanisms by which REST functions as a transcriptional repressor. Most investigations addressing the biological role of REST use mammalian tissue culture cell systems (Ballas et al., 2005; Su et al., 2004; Watanabe et al., 2004). Although tissue culture cells are more amenable to mechanistic analysis, they may not accurately reflect the complexity of the in vivo environment where neurogenesis occurs. REST-null mice have been generated but exhibit embryonic lethality (Chen et al., 1998), limiting their use in studying REST’s role in neurogenesis.
In a previous study, where we identified genes whose expression was downstream of Atoh7, we discovered that many Atoh7-dependent genes harbor RE1 elements within 100 kb of their coding sequences (Mu et al., 2005). Most notable among these genes was Pou4f2, which contains two RE1 sites. In a separate study, Johnson et al. (2007) using genome-wide mapping of in vivo protein-DNA interactions, identified REST-RE1 occupancy sites at 1946 loci in the human genome, many of which were close to genes known to be associated with neuronal differentiation. Intriguingly, several of these RE1 sites were the same as those we identified as Atoh7-dependent genes, including Pou4f2. On the basis of the results of Mu et al. (2005) and Johnson et al. (2007), we reasoned that release of REST-mediated repression might play an important role in activating RGC genes. If so, REST could influence RPCs in their decision whether to commit to an RGC fate.
To determine the role of REST in the developing retina of mice, we deleted a floxed allele of REST using a Six3-Cre transgene (Furuta et al., 2000). We found that REST played a critical role in suppressing RGC gene expression in proliferating RPCs. Most strikingly, deletion of REST partially restored the RGC gene expression program that is normally lost in Atoh7-null retinas. In addition, we present evidence to show that another proneural bHLH competency factor, Neurod1, is upregulated in REST-deleted retinas and plays an unexpected role in RGC development that is independent on the presence of Atoh7.
Materials and methods
Gene targeting and animal breeding
A gene targeting vector was made that contained a floxed REST allele in which exon 2 of REST was flanked by two loxC2 sites and could be deleted by Cre-mediated recombination. To construct this vector, we used genomic DNA from G4 ES cells to PCR-amplify 1.07-, 2.21-, and 5.4-kb fragments from the REST locus (see Figure 3A) and subsequently cloned them into a knockout vector. The resulting constructs were linearized and electroporated into G4 ES cells (George et al., 2007), after which G418-resistant ES cells were selected to identify homologous recombination events. A 5 probe from outside the homologous recombination region was used to detect 11-kb wild-type and 7.6-kb targeted fragments produced by EcoRV/Nhe1 digestion of ES cell DNA (Figures 3A, 3B). Two targeted ES cell lines were identified, expanded and injected into B6(GC)-Tyrc-2J/J blastocysts, and the injected blastocysts were transferred into the uteri of pseudopregnant C57/BL/6J female mice. Chimeric males resulting from the injected blastocysts were bred to B6(GC)-Tyrc-2J/J females (Jackson Laboratory) to generate the targeted floxed REST allele. The targeted allele was further bred to a Rosa26-FlPeR line to remove the FRT-flanked Neo cassette (Farley et al., 2000). The resulting line was assigned as RESTfx, which was distinguished from the wild-type allele by PCR genotyping using primers re08 (5–CATGCGAGTACTGCCATACCCAAC-3), re09 (5–GTGATGGGGCAGTCTTCTGGAGG–3), and re11 (5–GGGCACACCTTTAATCCTAGCTTC–3) (Figures 3C–E). Atoh7 (Math5) knockout mice were genotyped as described in Wang et al. (2001). Neurod1 knockout mice were genotyped as described in Pennesi et al. (2003). Embryos were designated as E0.5 at noon on the day in which vaginal plugs were observed.
Figure 3.

REST binds to an RE1 site downstream of Pou4f2 gene. (A) Two RE1 sites are found within the Pou4f2 locus. Site A is located 1.7-kb upstream from the transcriptional start site, and site B is located 1.4-kb downstream from the transcriptional stop site. Sites A and B are highly similar to the canonical RE1 (cRE1) site. (B) Chip analysis of E14.5 wild-type retinas. qPCR was performed to detect antibody-bound DNA fragments encompassing sites B and a non-specific region 10-kb downstream of Pou4f2 (ns). REST-bound levels were normalized to input DNA. DNA sequences of qPCR primers were RE1 site B, RE1b1 (5–GTTAGCTGTTGTAGCGCTCCCTG–3), RE1b2 (5–CTGTCCCCATCCTAGGTTTCAGG–3), non-specific downstream region, ns1 (5–CCACTTATCCACTGAGTCATCTC–3), ns2 (5–GTACCCTACGAGATAGCACCATC–3).
All animal procedures in this study followed the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center.
Histology, in situ RNA hybridization, and immunohistochemical analysis
Embryos or eyes dissected from embryos and adults were fixed, cryo- or paraffin-embedded, and sectioned into 7- or 12-um slices for histology, in situ hybridization, or immunohistochemical analysis. For histological analysis, the sections were stained with hematoxylin and eosin. In situ RNA hybridization on frozen or paraffin-embedded sections was performed as described previously (Smith et al., 2000) with the following modifications. After de-waxing and rehydration, sections were fixed for 30 min at room temperature in 4% paraformaldehyde in PBS and treated for 10 min with 8 ug/ml proteinase K in 50 mM Tris-HCl, 5 mM EDTA (pH 7.0). Prior to hybridization, the sections were washed twice in 2 SSC for 15 min, and incubated in 0.1 M Tris, 0.1 M glycine for 30 min. The hybridization solution (100 ul/slide) contained 50% deionized formamide, 5x SSC (pH adjusted with citric acid to pH 6.0), 10% dextran sulfate, 1mg/ml yeast tRNA and from 10–20 ng/ul of the riboprobes, and was performed overnight at 65°C–68°C under coverslips. Next, the sections were washed for 1–2 hr in 0.5x SSC, 20% formamide at 65°C. Subsequently, they were treated with 10 ug/ml RNaseA for 30 min at 37°C in NTE, then washed for 4 hr in 0.5x SSC, 20% formamide at 65°C and for 30 min in 2x SSC, and blocked for 1 hr at room temperature in 1% blocking reagent (Roche) in MABT. A 1:400 to 1:1000 dilution of anti-digoxigenin-AP conjugated antibody (Roche) was preincubated for at least 1 hr in 1% blocking reagent and 10% normal sheep serum in MABT at 4°C. The sections were incubated with the antibody overnight at 4°C, washed for 6 hr in PBST, and for 30 min, in NTMT, and stained using centrifuged BM purple AP substrate (Roche) in 0.1% Tween 20 for 12–36 hr at 4°C or room temperature. They were washed in NTMT, dehydrated, and then mounted in Aquamount (Polysciences). Images were collected using an Olympus X70 microscope with an Olympus UPlanApo 10/0.40 objective lens and Olympus DP71 camera with Olympus DP-Controller software.
Immunohistochemical analysis was performed as described previously (Mao et al., 2008a, 2008b). Briefly, frozen or paraffin-embedded sections were placed in a microwave oven at 600 W in 10 mM sodium citrate for 18 min to expose the antigen epitopes, and then blocked in 10% normal serum and 0.1% Tween 20 for 1 hr at room temperature. Incubation with primary antibodies was performed at 4°C over 1 or 2 nights. Secondary antibodies were applied to sections for 2 hr at room temperature. The primary antibodies used were: goat anti-Pou4f2/Brn3b (1:200, Santa Cruz Biotechnology), rabbit anti-calbindin (1:500, Swant), sheep anti-Chx 10 (1:1000, Covance), rabbit anti-Eomes (1:200 Chemicon), mouse monoclonal ant-GFAP (1:300, Sigma), rabbit anti-GSK3 (1:200, Cell Signalling), mouse anti-Isl1 (1:250 DSHB, University of Iowa), Goat anti-Neurod1 (Santa Cruz Biotechnology), mouse anti-NFL (1:200 InVitrogen), rabbit anti-B-opsin and anti-R-opsin (1:500, Chemicon), mouse monoclonal anti-Pax6 (1:200, DSHB, University of Iowa), rabbit anti-Sox9 (1:200, Chemicon), and chicken anti-TUJ-1 (1:200, Chemicon). Secondary antibodies were conjugates of Alexa Fluor 488 and Alexa Fluor 555 (Invitrogen). DAPI (4, 6-diamidino-2-phenylindole) was used as a nuclear counterstain. Finally, slices were washed and mounted in fluoromount G (EMS).
Retinal flat-mount analysis
To detect RGC number, eyes were removed from postnatal mice and fixed with 4% paraformaldehyde for 30 min. The cornea, ciliary band, and lens were removed using a pair of iris scissors. The remaining retinal tissue and attached pigmented epithelium were fixed for 1 hr and then washed four times in PBS saline and 0.1% Triton X-100 at room temperature. The retinas were then incubated in blocking solution (PBST plus 5% fetal bovine serum) for 1 hr, and incubated with anti-Pou4f2/Brn3b (1:100) antibodies for 48 hr at 4C. Retinas were washed four times with PBST and stained with Alexa488-conjugated donkey anti-goat secondary antibody (Invitrogen). After the retinas were washed thoroughly with PBS, the pigmented epithelium was removed from the retinas and four or five symmetrical cuts were made halfway from the peripheral rim to the central optic disk. Retinas were then flat-mounted onto glass slides and analyzed using an Olympus FluoView1000 confocal microscope. To avoid the skewed distribution of Pou4f2-expressing RGCs along the nasal-temporal axis, Pou4f2-positive RGCs were counted in three randomly chosen areas of the dorsal temporal regions of flat-mounted retinas. Values from three littermate pairs of P20 mice were used for statistical analysis using a simple t test (STATISTICA 6). For estimating RGC number in developing retinas, retinal sections representing the same place in the retina from littermates of different genotypes were stained with anti-Pou4f2 or anti-Isl1 antibodies and the number of Pou4f2/Isl1 positive cells was determined by counting.
TUNEL assays and BrdU labeling
TUNEL assays on retinas were conducted using an in situ cell death detection kit (Roche Applied Science) following the manufacturer’s instructions. For pulse labeling with BrdU to detect S-phase RPCs, 100 ug of BrdU (Upstate Biotechnology) per gram of body weight was intraperitoneally injected into pregnant females 30 min before euthanization. The sections were processed using the microwave retrieval technique described above in the section on immunohistochemical analysis.
Chromatin immunoprecipitation analysis (ChIP)
Retinas were isolated from E14.5 wild-type embryos of C57BL/6J:129sv mixed background and were cross-linked with 1% formaldehyde for 10min. ChIP assays were performed as previously described (Tsai et al., 2008) with minimal modifications. Briefly, the fragmented, precleared chromatin lysate was incubated overnight with specific antibodies: anti-REST (Upstate/Millipore) and normal rabbit IgG (Upstate/Millipore). Quantitative (q)PCR was conducted in a 7500 FAST ABI instrument.
RT-PCR analysis
Total RNA was collected from two E14.5 retinas using Trizol reagent (Invitrogen). RNAs were reversed transcribed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) following the manufacture’s instructions. A twentieth of the total cDNA was used for PCR. For quantitative reverse transcriptase-PCR analysis, cDNAs were amplified using SYBR green PCR master mix (Applied Biosystems, CA). The relative expression levels were normalized to that of GAPDH and calculated using the comparative Ct method (7500 Fast Real-time PCR systems SDS software, Applied Biosystems). DNA sequences of PCR primers were as follows: REST rrt01 (5–GTGCGAACTCACACAGGAGA–3), REST rrt02 (5–AAGAGGTTTAGGCCCGTTGT–3), GAPDH forward (5–AGGTCGGTGTGAACGGATTTG-3), GAPDH reverse (5 -TGTAGACCATGTAGTTGAGGTCA-3), Stmn2 forward (5–CTGAAGTTGTTGTTCTCCTCC–3), Stmn2 reverse (5–CTCCACGAACTCTAGCTTCTC–3), GAP43 forward (5–GTGCTGCTAAAGCTACCACT–3), GAP43 reverse (5–GTACAAAGTGTCACCTCAGT–3), Persyn forward (5–GTACAAAGTGTCACCTCAGT–3), Persyn reverse (5–CAGCAGCATCTGATTGGTGA–3), Atoh7 forward (5–CAGGACAAGAAGCTGTCCAAG–3), Atoh7 reverse (5–GGTCTACCTGGAGCCTAGCA–3).
Results
REST is expressed in the embryonic and postnatal retina
Since REST was discovered as a major repressor of neuronal genes, five additional alternatively spliced isoforms, namely REST1 to REST5, have been shown to be expressed in the differentiated neurons of the rat brain (Palm et al). In mice, REST1, REST3, and REST4 isoforms are also expressed in neuroblastoma cell lines. Among these, REST4, encoding a truncated protein (Palm et al., 1998, Figure 2B), is the major isoform acts as a de-repressor to modulate REST-mediated repressing activity (Shimojo et al., 1999; Lee et al., 2000). To determine when REST and REST4 were expressed in the mouse retina, we used semi-quantitative RT-PCR with embryonic and adult retinas. Between E12.5 and E18.5, REST was the major isoform expressed. This was also the case in the E14.5 developing cortex (Figure 2A, lanes E12.5R to E18.5R and E14.5B). In contrast, REST4 was expressed weakly in the developing embryonic retina and cortex, but it was the predominant isoform in postnatal retinas (Figure 2A, lanes P10R – P60R). The embryonic retina largely consists of proliferating progenitor cells, whereas the postnatal retina is composed of differentiated neurons and Müller glial cells. Thus, the temporal expression profile of REST isoforms in retinas supports the notion that REST is expressed in neural progenitor cells and REST4 in differentiated neurons.
Figure 2.

The expression of alternative spliced REST isoforms in retinas. (A) Semi-quantitative RT-PCR for REST expression in developing and adult retinas. The primers used in the PCR for REST, rrt01 and rrt02, are indicated as arrows in B. Note that the forward primer rrt01 aligns to 3′ end of exon 2 and extends to exon 3. One-fourth of GAPDH primers were used in the same reaction. M: 1 kb ladder; E12.5R – E18.5R: wild-type embryonic retinas; P10R – P60R: postnatal retinas; E14.5B: E14.5 forebrain; E14.5R-mut: E14.5 RESTfx/fx: Six3-Cre retinas. The alternatively spiced isoform, RERST4, was found mainly in postnatal retinas. (B) A schematic illustration and partial sequences of REST exons. The location of exons 2, 3, N, and 4 are indicated in Figure 4. The sequence of exon N is identical to that in Lee et al. (2000).
REST binds to an RE1 site downstream of Pou4f2
Two conserved RE1 sites are found in close proximity to Pou4f2. Site A is 1.7-kb upstream and site B is 1.4-kb downstream (Figure 3A). To determine if REST occupies these sites in the developing retina, we performed ChIP assays with ten E14.5 retinas using an anti-REST antibody. The immunoprecipitated DNA fragments were amplified by qPCR using primers to detect site A and site B (Figure 2A). REST bound to site B with a 2.4-fold enrichment compared to non-specific occupancy by rabbit IgG (Figure 2B). Moreover, no REST or rabbit IgG binding was observed within a randomly selected sequence 10-kb downstream of Pou4f2 (Figure 3B). In contrast to site B, we detected only weak REST binding to site A (data not shown). Site B’s location was consistent with it being a cis-regulatory element within a transcriptional regulatory region downstream of Pou4f2.
Aberrant RGC gene expression and increase RGC number in REST-deleted retinas
To determine REST’s function during retinogenesis, we generated mice with a floxed allele of REST (Figure 3A) and bred them with a Six3-Cre transgenic line to delete exon 2 of REST in the developing retina beginning at E10 (Figures 4B–3E; Furuta et al., 2000). We first examined histological sections from control and RESTfx/fx;Six3-Cre retinas twenty days after birth (P20) and found a significant increase in the thickness of the inner plexiform and ganglion cell layers (Figures 5A, 4B, arrow). In addition, many cell clumps protruded toward the retinal pigment epithelia (Figure 5B, arrowheads), resulting in a mis-patterned retina. Aside from these histological abnormalities, we did not detect significant differences in the formation of non-RGC retinal cell types (Figure S1, Table S1.). To quantify the number of RGCs in REST mutant retinas, we immunostained flat-mounted retinas from P20 mice with an anti-Pou4f2 antibody. We randomly selected three areas within the dorsal temporal regions of the retinas from three pairs of P20 littermates and observed 18.2% more RGCs in RESTfx/fx:Six3-Cre retinas than in heterozygous controls (Figure 5C, 5D). These data suggested that removing REST from RPCs during early retinal development substantially increases RGC production.
Figure 4.

Generation of REST conditional mutant mice. (A) Genomic structure for REST and the targeting vector, and predicted structure of the targeted floxed REST allele. Exon 2 encoding the N-terminal repressor domain and part of the zinc-finger DNA-binding domain, is flanked by loxC2 recombination sites. The black bars underneath indicate the DNA fragments amplified from genomic DNA to make making the targeting vector. Arrows indicate the PCR primers re08, re09, and re11 that were used for PCR genotyping of wild-type, floxed and deleted REST alleles. (B) Southern blot analysis using a 5 probe to distinguish wild-type and REST-targeted alleles from genomic DNA of targeted ES cells digested with EcoRV/Nhe1. Arrow indicates a targeted ES cell clone. (C) Representative PCR genotyping using re08 and re09 for wild-type and floxed REST alleles, and representative PCR genotyping of the Six3-Cre transgene using c01 and c02. Arrows indicate the RESTfx/fx;Six3-Cre allele. (D) The RESTdel/+ allele was generated by breeding mice containing the RESTfx allele with a CMV-Cre mouse line to remove exon 2 from the germline. PCR genotyping with re08 and re11 was used to distinguish the wild-type and deleted REST alleles of DNA from E10.5 embryos from interbred RESTdel/+ mice. Arrows indicate dying E10.5 RESTdel/del embryos with phenotypes identical to that described previously (Chen et al., 1998).
Figure 5.

Increased RGC formation in retinas of REST mutant mice. (A, B) Histological sections of P20 eyes from Restfx/+ (A) and RESTfx/fx;Six3-Cre (B) littermates. Arrow points to the ganglion cell layer. Arrowheads point to the cell clumps protruding toward the retinal pigmented epithelia. (C, D) Immunostaining of Pou4f2-positive RGCs on flat-mounted RESTfx/+ (C) and RESTfx/fx;Six3-Cre (D) retinas. (E) A Box-Whisker plot of a t-test (n=3, p<0.05) reveals a significant difference in RGC numbers between control and REST mutant retinas. GCL: ganglion cell layer; INL: inner nuclear layer; IPL: inner plexiform layer; ONL: outer nuclear layer.
The increase in numbers of RGCs could arise from two possible mechanisms. First, REST is expressed in neural stem cells and neuronal progenitors (Ballas et al., 2005; Sun et al., 2008; Westbrook et al., 2008). In the absence of REST, proliferating RPCs might exit the cell cycle prematurely, as has been shown for neural stem cells (Sun et al., 2008; Westbrook et al., 2008). Because RGCs are the first retinal cells to differentiate, this would cause more RPCs to adopt an RGC fate. However, using BrdU labeling as an indicator of S-phase, we detected a 40.2% increase of mitotic RPCs in retinal sections of RESTfx/fx: Six3-Cre mice versus RESTfx/+ littermate controls (Brd/total cell in NBL: 41.464.15% vs. 29.576.13%; n=3, P<0.01). A second possibility is that removing REST in mitotically active RPCs might result in aberrant activation of RGC genes. If this were the case, we would expect to see greater numbers of RPCs giving rise to RGCs. To determine whether RGC genes were expressed in proliferating RPCs, we looked for Isl1 and Pou4f2 expression in the neuroblast layer of E15.5 retinas. We observed many more Isl1- and Pou4f2-positive cells in the neuroblast layer of RESTfx/fx;Six3-Cre retinas than in that of RESTfx/+ littermates (Figure 6A 5D). To compare the number of RGCs in wild-type and REST mutant retinas, we counted the number of Pou4f2-positive and Isl1-positive RGCs at E14 and E15.5. We found a 12–25% increase in Pou4f2-positive cells and 6–17% increase in Isl1-positive cells in REST mutant retinas compared to wild-type littermates (Table S2). Furthermore, we did not detect an increase in Pou4f2-positive or Isl1-positive cells in the neuroblast layer between E11 and E13. The results suggested that loss of REST did not cause premature cell cycle exit in mitotic RPCs but rather caused an upregulation in the expression of key transcription factors that are required for RGC differentiation to commence during in a very narrow developmental window.
Figure 6.

Upregulation of Pou4f2 and Isl1 in proliferating RPCs of retinas from REST mutant mice. Images of E15.5 Restfx/+ and RESTfx/fx;Six3-Cre retinas. Pou4f2 expression (A, B) and Isl1 expression (C, D). Arrowheads in panels B and D point out the boundaries of dorsal region within the neuroblast layer where Pou4f2 (B), and to a lesser extent, Isl1 (D) are upregulated relative to Pou4f2 and Isl1 expression in the neuroblast layer of Restfx/+ control retinas (A and C, respectively). Arrowheads in panels B and D point to the region within the neuroblast layer where Pou4f2 and Isl1 are upregulated. (E–J) Co-expression of Pou4f2 and BrdU-positive RPCs. Scale bars in panels C and I: 100 um. D<>V, dorsal-ventral axis; GCL, ganglion cell layer; NBL, neuroblast layer.
We always observed more Pou4f2-positive cells than Isl1-positive cells in the neuroblast layer of RESTfx/fx;Six3-Cre retinas (Figures 6B, 5D). Notably, Pou4f2, but not Isl1, has been shown to be a direct target of REST (Mortazavi et al., 2006; Johnson et al., 2007). At E15.5, the neuroblast layer contains both proliferating RPCs and committed RGCs that have exited the cell cycle and are expressing Isl1 and Pou4f2 (Mu et al., 2008; Pan et al., 2008). To distinguish proliferating from nonproliferating cells, E15.5 retinas were co-labeled with anti-Pou4f2 and anti-BrdU antibodies. We found a substantial number of Pou4f2-positive cells were co-labeled with BrdU in RESTfx/fx:Six3-Cre retinas whereas fewer were co-labeled in control retinas (Figures 5E 5J ; Pou4f2-positive/Brdu-positive, 8.18%1.50% versus 4.04%0.86%, n=3, P<0.01). Besides Pou4f2, other REST target genes known to be expressed in the retina, including Pou4f1 and Rtn1, were not upregulated in the neuroblast layer of RESTfx/fx;Six3-Cre retinas (data not shown). Aberrant expression of Pou4f2 and Isl1 in retinas of REST mutants did not cause increased cell death (data not shown).
Aberrant expression of Pou4f2 and Isl1 in proliferating RPCs of REST mutant retinas does not require Atoh7
Atoh7 is required for the expression of Pou4f2 and Isl1 and for RGCs to commit to an RGC fate (Brown et al., 2001; Wang et al., 2001; Mu et al., 2005, 2008; Pan et al., 2008). However, Atoh7 is not a target of REST, and Atoh7 expression was not upregulated in RPCs of REST mutant retinas (Figure S2). Because Pou4f2 is a direct target of REST, its aberrant expression in REST mutants might be the direct result of chromatin de-repression at the Pou4f2 locus. The aberrant upregulation of Isl1, however, must occur through a less direct mechanism because Isl1 is not known to be a direct target of REST. In both cases, we expected that aberrant expression would be independent of the presence of Atoh7 and that REST would act as a repressor in RPCs to prevent the Atoh7-independent expression of Pou4f2 and Isl1.
To determine whether this was the case, we generated Atoh7G/G;RESTfx/fx;Six3-Cre mice and examined Pou4f2 and Isl1 expression in developing retinas at different embryonic times. At E13.5, many Pou4f2- and Isl1-positive RPCs were detected in the neuroblast layer of Atoh7G/G;RESTfx/fx;Six3-Cre retinas, while, as expected, very few Pou4f2- and Isl1-positive cells were seen in Atoh7G/G retinas (Table S2; Figures 7A–7E). A small fraction of Pou4f2- and Isl1-positive RPCs in Atoh7-REST double mutants were mitotically active as shown by co-labeling with BrdU (Figures 6C1, 6F1, arrowheads). Despite their significant upregulation in the neuroblast layer, only a few Pou4f2-positive cells were detected in the nascent ganglion cell layer of the retina, suggesting that these cells were defective in their ability to migrate and differentiate into mature RGCs. Consistent with this observation, many dying cells were observed in the ganglion cell layer of Atoh7G/G;RESTfx/fx;Six3-Cre retinas (Figure 7I, arrowheads). The results suggested that Pou4f2- and Isl1-positive cells were defective in their ability to differentiate into RGCs and could not survive. In addition, retinas from adult Atoh7 G/G;RESTfx/fx;Six3-Cre mice displayed more severe mis-patterned retinal than that seen in RESTfx/fx;Six3-Cre retinas (Figure S3), but unlike RESTfx/fx;Six3-Cre retinas, the double mutants did not have more RGC cells (data not shown).
Figure 7.

Restored expression of Atoh7-downstream RGC genes Pou4f2 and Isl1 in Atoh7G/G;RESTfx/fx;Six3-Cre double mutant retinas. (A–C) Pou4f2 expression in E13.5 RESTfx/+ (A), RESTfx/+;Atoh7G/G (B), and RESTfx/fx;Atoh7G/G;Six3-Cre retinas (C), and its co-expression with BrdU-labeled RPCs in E14.5 retinas (A1–C1). (D–F) Isl1 expression in E13.5 RESTfx/+ (D), RESTfx/+;Atoh7G/G (E), and RESTfx/fx;Atoh7G/G;Six3-Cre (F) retinas, and its co-expression with BrdU-labeled RPCs in E14.5 retinas (D1–F1). Note that a dorsal to ventral descending gradient is seen in panels C, F, C1, F1. Arrowheads in panels A1–F1 point to mitotically active RPCs. (G–I) TUNEL assay for cell death. Arrowheads in panel G-I point to dying cells. Scale bars in panels A and A1: 100 um. V<>D, ventral-dorsal axis.
We used qRT-PCR analysis to compare the expression levels of other Atoh7-dependent genes, including Pou4f2, Isl1, Stmn2, GAP43, and Persyn. Among these, Isl1 and GAP43 are not known targets of REST but Pou4f2, Stmn2, and Persyn harbor RE1 in their regulatory regions. We compared the relative expression levels of these genes in E14.5 retinas of REST mutant embryos and heterozygous controls, and in Atoh7-REST double mutant embryos and Atoh7 mutant embryos. The expression levels of all the genes were upregulated to varying degrees (Figure 8). These data supported the hypothesis that removing REST in developing retinas caused an upregulation of RGC genes and triggered an Atoh7-independent program to initiate RGC gene expression.
Figure 8.

Upregulation of Atoh7-downstream RGC genes in REST mutant retinas. qRT-PCR analysis of Atoh7-dependent genes Pou4f2, Isl1, Stmn2, GAP43, and Persyn were performed using retinas from 4 different genotypes. The y-axis is the relative transcript level using GAPDH as internal control. Note that Isl1 and GAP43 are not direct targets of REST. The sequences of the PCR primers are shown in Materials and Methods (n=3, *: p<0.05; **: p<0.01).
Expression of RGC genes downstream of Pou4f2 and Isl1 in Atoh7-REST double mutant retinas
We have proposed a gene regulatory network for RGC development that has as its central feature four hierarchical tiers of transcription factors (Mu et al., 2008). The most downstream of these tiers includes the T-box-containing transcription factor Eomes (also called Tbr2) along with several proteins associated with the differentiation and maintenance of RGCs and their axons (Mu et al., 2005; Mao et al., 2008a). To determine the extent to which this gene regulatory network was operable in Atoh7-REST double-mutant retinas, we examined the expression of a set of RGC genes downstream of Pou4f2 and Isl1 in Atoh7G/G;RESTfx/fx;Six3-Cre retinas from E15.5 embryos. Eomes, whose expression depends on both Pou4f2 and Isl1, was expressed in a subset of RGCs located in the ganglion cell layer of RESTfx/+ wild-type retinas (Figure 8A). In Atoh7G/G;RESTfx/fx;Six3-Cre retinas, many Eomes-positive cells were detected in the neuroblast layer in a dorsal-to-ventral descending gradient similar to what was observed for Pou4f2 and Isl1 expression (Figure 9A1). Likewise, GAP43, another gene downstream of Pou4f2 and Isl1, was activated in the neuroblast layer of Atoh7G/G;RESTfx/fx;Six3-Cre retinas, although at less than wild-type levels (Figures 8D, 8D1). Neither Eomes nor Gap43 was expressed at significant levels in Atoh7-mutant retinas. However, other RGC-expressed genes, including Stmn2, Persyn, TUJ1, and GSK3®, were not noticeably upregulated in the neuroblast layer of Atoh7G/G;RESTfx/fx;Six3-Cre retinas (Figures 9, B9C1, 9E 9F1 ). Together, these results indicated that some but not all of the genes downstream of Pou4f2 and Isl1 are activated in the NBL in Atoh7-REST double-mutant retinas. This suggests that the RGC gene regulatory network is partially but not fully restored in Atoh7-mutant retinas when the repressive functions of REST are alleviated.
Figure 9.
Upregulation of RGC genes downstream of Pou4f2 and Isl1 in Atoh7-REST double mutant retinas. (A–C1) Immunostaining. (D–F1) In situ hybridization. Eomes (A, A1) and GAP43 (D, D1) expression was detected in the neuroblast layer in Atoh7G/G;RESTfx/fx;Six3-Cre retinas. The expression of other downstream RGC genes, including TUJ1 (B, B1), GSK-3® (C, C1), Persyn (E, E1), and Stmn2 (F, F1), was not detectable in the neuroblast layer but was detectable in the ganglion cell layer. (G) Scale bars in panels A1 and D: 200um. NBL, neuroblast layer.
Upregulation of Neurod1 in REST-deleted retinas and fewer RGCs specified in Atoh7-Neurod1-REST triple-mutant retinas than in Atoh7-REST double mutant retinas
Previous investigations have shown that a few RGCs still remain in Atoh7-null adult retinas (Lin et al., 2004; Moshiri et al., 2008). These RGCs form in retinal peripheral rim of the developing retina by unknown mechanisms. In earlier work, we replaced Atoh7 with another proneural bHLH gene, Neurod1, and found that the Atoh7Neurod1 allele replaced Atoh7’s function in restoring RGC formation, albeit not completely (Mao et al., 2008b). This was a somewhat surprising result since endogenous Neurod1 has not been shown to function in RGC formation in mice (Liu et al., 2008; Pennesi et al., 2003; Morrow et al., 1999). It is therefore possible that Neurod1 might play a role in RGC formation during retinal development, independent of Atoh7. Moreover, Neurod1 is a direct target of REST (Mortazavi et al., 2006; Johnson et al., 2007; Otto et al., 2007), suggesting that REST inactivation in proliferating RPCs of Atoh7-null retinas will result in upregulation of Neurod1 expression. If so, this could subsequently contribute to the aberrant activation of Pou4f2 and Isl1.
We detected a 60.4% increase in Neurod1-expressing RPCs in RESTfx/fx: Six3-Cre retinas compared with RPCs of heterozygous controls, and a 15.3% increase in RPCs of Atoh7G/G: RESTfx/fx: Six3-Cre retinas compared with RPCs of Atoh7G/G retinas (Figure S4). This upregulation might contribute to the observed increases in Pou4f2 and Isl1 expression, and it further suggested a role for Neurod1 in RGC formation. The results prompted us to determine whether Atoh7-Neurod1-REST triple-mutant retinas would lead to a loss of the up-regulation of Pou4f2 that are observed in Atoh7-REST double-mutant retinas. We interbred Atoh7G/+, Neurod1+/−, and RESTfx/+ mice to generate Atoh7G/G: Neurod1−/−: RESTfx/fx; Six3-Cre embryoss. To determine the number of specified RGCs, we compared Pou4f2 expression between Atoh7G/G;RESTfx/+, Atoh7G/G;RESTfx/fx; Six3-Cre, and Atoh7G/G;Neurod1−/−;RESTfx/fx; Six3-Cre retinas. We found that the aberrantly up-regulated Pou4f2 in Atoh7G/G: RESTfx/fx; Six3-Cre retinas was reduced in the triple-mutant retinas (Figure 10). These results were consistent with the hypothesis that Neurod1 contributes to the Atoh7-independent program for RGC development and may be required for the formation of the small population of RGCs that form in the absence of Atoh7.
Figure 10.
Atoh7-Neurod1-REST triple-mutant retinas contains fewer Pou4f2-postive RGCs than do Atoh7-REST double-mutant retinas. (A–C) Pou4f2 expression in E13.5 Atoh7G/G RESTfx/+ (A), Atoh7G/G;RESTfx/fx;Six3-Cre (B), and Atoh7G/G; Neurod1lz/lz;RESTfx/fx;Six3-Cre retinas (C). Scale bars in panels C: 100 um.
Discussion
Investigations of the biological role of REST in neurogenesis have been hampered by a lack of readily accessible in vivo models. The mouse retina is particularly suited for use in addressing the role of REST in neurogenesis and sensory neuron development in the context of an intact animal. Extensive knowledge of the major regulatory processes that control neural development in the retina provides a framework into which information gained on REST can be integrated.
Our study establishes for the first time an important in vivo role for REST in mammalian retinogenesis. By conditionally deleting REST in the developing retina, we uncovered the existence of a novel RGC gene expression program that operates independently of the proneural bHLH gene Atoh7. The Atoh7-independent program activates the expression of Pou4f2 and Isl1, two early expressing transcription factors required for commitment to an RGC fate. Remarkably, the expression of these regulatory genes, which is normally restricted to differentiating RGCs that have exited the cell cycle, could be induced in mitotically active progenitors once REST suppression was relieved. Moreover, RGC numbers increased significantly in the REST-deleted retinas of developing embryos and postnatal mice further suggests that REST plays an important role in suppressing RGC differentiation in actively dividing RPCs. In Atoh7-REST double mutant retinas, Pou4f2, Isl1, and some but not all downstream genes in the RGC gene regulatory network were activated. However, RGCs that formed in the Atoh7-REST double mutant retinas were abnormal and did not survive. This indicates that Atoh7 is required for normal RGC development even when REST suppression is relieved. As is the case in other neuronal progenitors, REST-mediated gene repression in RPCs most likely functions to prevent premature RGC differentiation and ensure that proper spatiotemporal expression patterns are maintained in both mitotically active RPCs and newly committed RGCs (Su et al., 2004; Ballas et al., 2005). De-repression of REST likely relieves the repressive chromatin in RGC genes, which subsequently allows the access of Atoh7 and other transcriptional activators to initiate the expression of RGC genes.
Residual RGCs are always observed in Atoh7-mutant retinas, indicating that a few RGCs can form independently of Atoh7 (Lin et al., 2004; Moshiri et al., 2008). Our analysis of Atoh7-Neurod1-Rest triple-mutant retinas suggests that Neurod1 regulates an Atoh7-independent pathway for RGC fate specification. Because only a few RGCs appeared to develop by this pathway, their loss would be difficult to detect in Neurod1-null retinas (Pennesi et al., 2003). Neurod1 has been shown to function in the formation of amacrine cells and photoreceptor cells, and in the maintenance and survival of photoreceptor cells in postnatal life (Inoue et al., 2002; Liu et al., 2008; Ochocinska and Hitchcock, 2009). Conversely, Atoh7 is likely to have other roles in the developing retina besides specifying RGC fate because many postmitotic Atoh7-expressing RPCs give rise to other retinal cell types (Yang et al., 2003). Atoh7, Neurod1, and other proneural bHLH genes are thus likely to have multiple functions in the developing retina that cannot be identified by simple analysis of single-gene knockouts.
REST-mediated suppression appears to be a highly conserved mechanism that has evolved in vertebrates to repress the expression of genes associated with neuronal differentiation (Chen et al., 1998; Coulson, 2005; Majumder, 2006). Because it is a potent transcriptional repressor, REST might have originally functioned more broadly to repress terminal differentiation genes in actively dividing cells (Majumder, 2006). In neural progenitors, REST’s suppressive function is likely to be intertwined with gene regulatory networks involved in neuronal differentiation. This appears to be the case in RPCs, where RGC genes repressed by REST are activated by Atoh7-independent and Atoh7-dependent mechanisms. The existence of the Atoh7-independent process was unexpected but it might be necessary for fine-tuning the production of RGCs from RPCs. REST is an important epigenetic factor, suggesting that RGC genes are dynamically regulated through REST-mediated epigenetic mechanisms. However, it is presently unclear how REST responds to the local environment to engage with or disengage itself from RE1 elements within RGC gene regulatory regions.
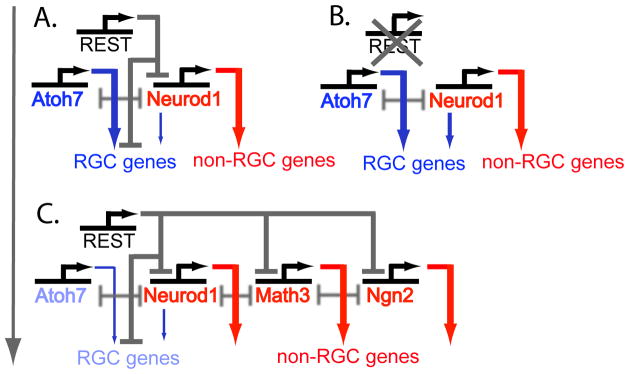
REST clearly has more general functions in regulating neuronal gene expression. Upon neuronal differentiation, REST is released from chromatin-repressed RE1 sites in the regulatory regions of neuronal genes and is rapidly degraded at the G2 phase (Ballas et al., 2005; Guardavaccaro et al., 2008; Westbrook et al., 2008), a time when several proneural bHLH genes begin to be expressed and when subpopulations of progenitors, including RPCs, make the decision to exit the cell cycle and adopt a competency state (Ballas et al., 2005; Lunyak and Rosenfeld, 2005). Several proneural bHLH genes, including Math3, Neurod1, and Ngn2, contain distinct RE1 sites and are direct targets of REST (Mortazavi et al., 2006; Johnson et al., 2007; Otto et al., 2007). In retinogenesis, each of these proneural bHLH genes has evolved specialized functions that are essential for regulating the specification of individual retinal cell fates (Hatakeyama and Kageyama, 2004). REST is known to occupy distinct RE1 elements and repress downstream targets in a cell-type-specific manner (Bruce et al., 2009). The release of REST-mediated repression and the subsequent degradation of REST at the G2 phase must relieve the chromatin-repressed state of different proneural bHLH genes at different times and in different subpopulations of RPCs as retinogenesis proceeds, hence enabling those RPCs to adopt a particular competency state (Fig. 11). This model implies that REST functions as a key regulatory link between extrinsic signals and intrinsic gene regulatory programs to advance RPCs to particular cell fates (Figure 11). Besides REST repression, proneural bHLH genes have been shown to repress one another’s expression, thus adding another layer of complexity to the regulatory mechanisms controlling RPC competency states (Mu et al., 2005; Le et al., 2006). REST and the proneural bHLH genes that REST represses may constitute a double-negative gate to regulate downstream outputs in different RPC lineages as has been observed for other developmental gene regulatory networks (Figure 11; Revilla-i-Domingo et al., 2007). This model could be tested by using lineage-specific promoter-Cre constructs or by specifically deleting RE1 sites associated with the different proneural bHLH genes that control the sequential formation of the retinal cell types.
Figure 11.
Model for REST function in retinal cell fate determination. (A) In the early stages of retinal development (E12–E16), REST suppresses RGC genes in RPCs to prevent premature activation and maintain an appropriate balance of proliferating RPCs and differentiating RGCs. REST also regulates the expression of some proneural bHLH genes, such as Neurod1, to fine-tune the expression of downstream target genes. Neurod1, and perhaps other bHLH factors, appear to have supplementary roles in regulating RGC gene expression. (B) In the absence of REST, additional RGCs are formed as the result of de-repression of RGC gene expression in RPCs, as well as de-repression of Neurod1, which then supplements Atoh7 in upregulating RGC genes. (C) In later stages of retinal development (E16 onwards), the retinal environment is no longer conducive to production of RGCs. REST may respond to local environmental signals dynamically to differentially bind to distinct RE1 sites within different bHLH genes and repress expression levels in a lineage- or cell-specific manner. The expression level of a particular bHLH gene within a particular RPC will influence the competency state and the decision about when to commence differentiation. Gray arrow on the left represents the developmental progression.
Our study highlights the complexity associated with gene regulatory networks operating in the developing retina. We predict that other transcriptional regulators involved in RGC formation will be identified as downstream targets of REST, thereby reinforcing REST’s place at a distinct node in the gene regulatory network controlling RGC development. Our study raises questions as to how redundant mechanisms for RGC formation arose and why they persist. The conditional floxed REST allele will be a valuable tool for addressing these questions, and more generally, for investigating the role of REST in neurogenesis.
Supplementary Material
Supplementary Figure 1. REST inactivation does not affect the genesis of the seven retinal cell types in P18 adult retinas. (A, B) Chx10, pan-bipolar cell marker. (C, D) Pax6, amacrine cell marker. (E, F) Calbindin, horizontal cell marker. (A-D). M-Opsin and S-Opsin, cone photoreceptor markers. (G, H) Sox9, Müller glial cell marker. GFAP, reactive gliosis marker. Scale bar in panel H, 50um.
Supplemental Figure 2. Real-time quantification RT-PCR of RGC Atoh7 expression in the retinas of REST heterozygous control and REST mutant mice. Note that no change in expression level can be detected in three separate experiments. Each experimental sample contains four retinas of the same genotype from the same littermates.
Supplementary Figure 3. Atoh7G/G;RESTfx/fx;Six3-Cre retinas develop severe mis-patterned retinas. Retinal histology of a P20 Atoh7G/+;RESTfx/+ control (A), Atoh7G/G;RESTfx/+ (B), and
Acknowledgments
We especially thank our colleague, Xiuqian Mu, for providing his expertise on REST RE1 sites in RGC genes. We thank Yas Furuta for providing the Six3-Cre transgenic mice, Jan Parker-Thornburg and the Genetically Engineered Mouse Facility at The University of Texas M. D. Anderson Cancer Center for generating the floxed REST-mouse, and Ming-Jer Tsai (Baylor College of Medicine) for Neurod1 mice. We acknowledge the M. D. Anderson Cancer Center DNA Analysis Facility for DNA sequencing. The Genetically Engineered Mouse Facility and DNA Analysis Facility are supported in part by a National Cancer Institute Cancer Center Core Grant (CA016672). The work was supported by grants to W.H.K. from the National Eye Institute (EY011930 and EY010608-139005) and from the Robert A. Welch Foundation (G-0010), and grant to M.C.B. from the National Institutes of Health (GM081627)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akagi T, Inoue T, Miyoshi G, Bessho Y, Takahashi M, Lee JE, Guillemot F, Kageyama R. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J Biol Chem. 2004;279:28492–28498. doi: 10.1074/jbc.M400871200. [DOI] [PubMed] [Google Scholar]
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009;25:45–69. doi: 10.1146/annurev.cellbio.042308.113259. [DOI] [PubMed] [Google Scholar]
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Gottgens B, Buckley NJ. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci USA. 2004;101:10458–10463. doi: 10.1073/pnas.0401827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AW, López-Contreras AJ, Flicek P, Down TA, Dhami P, Dillon SC, Koch CM, Langford CF, Dunham I, Andrews RM, Vetrie D. Functional diversity for REST (NRSF) is defined by in vivo binding affinity hierarchies at the DNA sequence level. Genome Research. 2009;19:994–1005. doi: 10.1101/gr.089086.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Poggi L, Harris WA. Lineage in the vertebrate retina. Trends Neurosci. 2006;29:563–570. doi: 10.1016/j.tins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- Chen ZF, Paquette AJ, Anderson DJ. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet. 1998;20:136–142. doi: 10.1038/2431. [DOI] [PubMed] [Google Scholar]
- Chong JA, Tapia-Ramirez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MF, Kraner SD, Mandel G. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- Coulson JM. Transcriptional regulation: cancer, neurons, and the REST. Curr Biol. 2005;15:R665–668. doi: 10.1016/j.cub.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Farley FW, Sorioano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (Flipper) mice. Genesis. 2000;28:106–100. [PubMed] [Google Scholar]
- Furuta Y, Lagutin O, Hogan BL, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–132. [PubMed] [Google Scholar]
- Gan L, Wang SW, Huang Z, Klein WH. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev Biol. 1999;210:469–480. doi: 10.1006/dbio.1999.9280. [DOI] [PubMed] [Google Scholar]
- George SHL, Gertsenstein M, Vintersten K, Korets-Smith E, Murphy J, Stevens ME, Haigh JJ, Nagy A. Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4455–4460. doi: 10.1073/pnas.0609277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardavaccaro D, Frescas D, Dorrello NV, Peschiaroli A, Multani AS, Cardozo T, Lasorella A, Iavarone A, Chang S, Hernando E, Michele Pagano M. Control of chromosome stability by the ®-TrCP REST Mad2 axis. Nature. 2008;452:365–369. doi: 10.1038/nature06641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007;21:367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- Hernandez J, Matter-Sadzinski L, Skowronska-Krawczyk D, Chiodini F, Alliod C, Ballivet M, Matter JM. Highly conserved sequences mediate the dynamic interplay of basic helix-loop-helix proteins regulating retinogenesis. J Biol Chem. 2007;282:37894–37905. doi: 10.1074/jbc.M703616200. [DOI] [PubMed] [Google Scholar]
- Hufnagel RB, Le TT, Riesenberg AL, Brown NL. Neurog2 controls the leading edge of neurogenesis in the mammalian retina. Dev Biol. 2010;340:490–503. doi: 10.1016/j.ydbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Honjo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Müller glial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28:249–262. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Reh RA. Strategies for retinal repair: cell replacement and regeneration. Prog Brain Res. 2009;175:23–31. doi: 10.1016/S0079-6123(09)17502-7. [DOI] [PubMed] [Google Scholar]
- Le TT, Wroblewski E, Patel S, Riesenberg AN, Brown NL. Math5 is required for both early retinal differentiation and cell cycle progression. Dev Biol. 2006;295:764–768. doi: 10.1016/j.ydbio.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chai YG, Hersh LB. Expression patterns of mouse repressor element-1 silencing transcription factor 4 (REST4) and its possible function in neuroblastoma. J Mol Neurosci. 2000;15:205–214. doi: 10.1385/JMN:15:3:205. [DOI] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Liu H, Etter P, Hayes S, Jones I, Nelson B, Hartman B, Forrest D, Reh TA. NeuroD1 regulates expression of thyroid hormone receptor 2 and cone opsins in the developing mouse retina. J Neurosci. 2008;16:749–756. doi: 10.1523/JNEUROSCI.4832-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Lunyak VV, Rosenfeld MG. No rest for REST: REST/NRSF regulation of neurogenesis. Cell. 2005;121:499–501. doi: 10.1016/j.cell.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Majumder S. REST in good times and bad: roles in tumor suppressor and oncogenic activates. Cell Cycle. 2006;5:1929–1935. doi: 10.4161/cc.5.17.2982. [DOI] [PubMed] [Google Scholar]
- Mao CA, Kiyama T, Pan P, Furuta Y, Hadjantonakis AK, Klein WH. Eomesodermin, a target gene of Pou4f2, is required for retinal ganglion cell and optic nerve development in the mouse. Development. 2008a;135:271–280. doi: 10.1242/dev.009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CA, Wang SW, Pan P, Klein WH. Rewiring the retinal ganglion cell gene regulatory network: Neurod1 promotes retinal ganglion cell fate in the absence of Math5. Development. 2008b;135:3379–3388. doi: 10.1242/dev.024612. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Chen E, Thompson L, Garcia ST, Myers RM, Wold B. Comparative genomics modeling of the NRSF/REST repressor network: From single conserved sites to genome-wide repertoire. Genome Res. 2006;16:1208–1221. doi: 10.1101/gr.4997306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri A, Gonzalez E, Tagawa K, Maeda H, Wang M, Frishman LJ, Wang SW. Near complete loss of retinal ganglion cells in the math5/brn3b double knockout elicits severe reductions of other cell types during retinal development. Dev Biol. 2008;316:214–227. doi: 10.1016/j.ydbio.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Klein WH. Gene regulatory networks and the development of retinal ganglion cells. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. Cambridge, MA: MIT Press; 2008. pp. 321–332. [Google Scholar]
- Mu X, Beremand PD, Zhao S, Pershad R, Sun H, Scarpa A, Liang S, Thomas TL, Klein WH. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development. 2004;131:1197–1210. doi: 10.1242/dev.01010. [DOI] [PubMed] [Google Scholar]
- Mu X, Fu X, Beremand PD, Thomas TL, Klein WH. Gene regulatory logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc Natl Acad Sci USA. 2008;105:6942–6947. doi: 10.1073/pnas.0802627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Fu X, Sun H, Beremand PD, Thomas TL, Klein WH. A gene network downstream of transcription factor Math5 regulates retinal progenitor cell competence and ganglion cell fate. Dev Biol. 2005;280:467–481. doi: 10.1016/j.ydbio.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Gumuscu B, Hartman BH, Reh TA. Notch activity is downregulated just prior to retinal ganglion cell differentiation. Dev Neurosci. 2006;281:128–141. doi: 10.1159/000090759. [DOI] [PubMed] [Google Scholar]
- Ochocinska MJ, Hitchcock PF. NeuroD regulated proliferation of photoreceptor progenitors in the retina of zebrafish. Mech Dev. 2009;126:128–141. doi: 10.1016/j.mod.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SJ, McCorkle SR, Hover J, Conaco C, Han JJ, Impey S, Yochum GS, Dunn JJ, Goodman RH, Mandel G. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci. 2007;27:6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm K, Belluardo N, Metsis M, Timmusk T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Deng M, Xie X, Gan L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development. 2008;135:1981–1990. doi: 10.1242/dev.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Cho JH, Yang Z, Wu SH, Zhang J, Wu SM, Tsai MJ. BETA2/NeuroD1 null mice: a new model for transcription factor-dependent photoreceptor degeneration. J Neurosci. 2003;15:453–461. doi: 10.1523/JNEUROSCI.23-02-00453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron M, Harris WA. Determination of vertebrate retinal progenitor cell fate by the Notch pathway and basic helix-loop-helix transcription factors. Cell Mol Life Sci. 2000;57:215–223. doi: 10.1007/PL00000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla-i-Domingo R, Oliveri P, Davidson EH. A missing link in the sea urchin embryo gene regulatory network: hesC and the double-negative specification of micromeres. Proc Natl Acad Sci USA. 2007;104:12383–12388. doi: 10.1073/pnas.0705324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenberg AN, Liu Z, Kopan R, Brown NL. Rbpj cell autonomous regulation of retinal ganglion cell and cone photoreceptor fates in the mouse retina. J Neurosci. 2009;29:12865–12877. doi: 10.1523/JNEUROSCI.3382-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- Shimojo M, Paquette AJ, Anderson DJ, Hersh LB. Protein kinase A regulates cholinergic gene expression in PC12 cells: REST4 silences the silencing activity of neuron-restrictive silencer factor/REST. Mol Cell Biol. 1999;19:6788–6795. doi: 10.1128/mcb.19.10.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Parker A, Wikaningrum R, Coleman M. Combined immunohistochemical labeling and in situ hybridization to colocalize mRNA and protein in tissue sections. In: Darby IA, editor. Methods in Molecular Biology. Vol. 123. Totowa, NJ: Humana Press; 2000. pp. 165–175. In Situ Hybridization Protocols. [DOI] [PubMed] [Google Scholar]
- Souren M, Martinez-Morales JR, Makri P, Wittbrodt B, Wittbrodt J. A global survey identifies novel upstream components of the Ath5 neurogenic network. Genome Biol. 2009;10:R92. doi: 10.1186/gb-2009-10-9-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Kameoka S, Lentz S, Majumder S. Activation of REST/NRSF target genes in neural stem cells is sufficient to cause neuronal differentiation. Mol Cell Biol. 2004;24:8018–8025. doi: 10.1128/MCB.24.18.8018-8025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YM, Cooper M, Finch S, Lin HH, Chen ZF, Williams BP, Buckley NJ. Rest-mediated regulation of extracellular matrix is crucial for neural development. PLoS One. 2008;3:e3656. doi: 10.1371/journal.pone.0003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WW, Nguyen TT, Shi Y, Barton MC. p53-targeted LSD1 functions in repression of chromatin structure and transcription in vivo. Mol Cell Biol. 2008;28:5139–5146. doi: 10.1128/MCB.00287-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Kameoka S, Gopalakrishnan V, Aldape KD, Pan ZZ, Lang FF, Majumder S. Conversion of myoblasts to physiologically active neuronal phenotype. Genes Dev. 2004;18:889–900. doi: 10.1101/gad.1179004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, Liang A, Leng Y, Maehr R, She Y, Harper JW, Elledge SJ. SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature. 2008;452:370–374. doi: 10.1038/nature06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willardsen MI, Suli A, Pan Y, March-Armstrong N, Chien CB, El-Hodiri H, Brown NL, Moors KB, Vetter ML. Temporal regulation of Ath5 gene expression during eye development. Dev Biol. 2009;326:471–481. doi: 10.1016/j.ydbio.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. REST inactivation does not affect the genesis of the seven retinal cell types in P18 adult retinas. (A, B) Chx10, pan-bipolar cell marker. (C, D) Pax6, amacrine cell marker. (E, F) Calbindin, horizontal cell marker. (A-D). M-Opsin and S-Opsin, cone photoreceptor markers. (G, H) Sox9, Müller glial cell marker. GFAP, reactive gliosis marker. Scale bar in panel H, 50um.
Supplemental Figure 2. Real-time quantification RT-PCR of RGC Atoh7 expression in the retinas of REST heterozygous control and REST mutant mice. Note that no change in expression level can be detected in three separate experiments. Each experimental sample contains four retinas of the same genotype from the same littermates.
Supplementary Figure 3. Atoh7G/G;RESTfx/fx;Six3-Cre retinas develop severe mis-patterned retinas. Retinal histology of a P20 Atoh7G/+;RESTfx/+ control (A), Atoh7G/G;RESTfx/+ (B), and