Abstract
The role of root systems in drought tolerance is a subject of very limited information compared with above-ground responses. Adjustments to the ability of roots to supply water relative to shoot transpiration demand is proposed as a major means for woody perennial plants to tolerate drought, and is often expressed as changes in the ratios of leaf to root area (AL:AR). Seasonal root proliferation in a directed manner could increase the water supply function of roots independent of total root area (AR) and represents a mechanism whereby water supply to demand could be increased. To address this issue, seasonal root proliferation, stomatal conductance (gs) and whole root system hydraulic conductance (kr) were investigated for a drought-tolerant grape root system (Vitis berlandieri×V. rupestris cv. 1103P) and a non-drought-tolerant root system (Vitis riparia×V. rupestris cv. 101-14Mgt), upon which had been grafted the same drought-sensitive clone of Vitis vinifera cv. Merlot. Leaf water potentials (ψL) for Merlot grafted onto the 1103P root system (–0.91±0.02 MPa) were +0.15 MPa higher than Merlot on 101-14Mgt (–1.06±0.03 MPa) during spring, but dropped by approximately –0.4 MPa from spring to autumn, and were significantly lower by –0.15 MPa (–1.43±0.02 MPa) than for Merlot on 101-14Mgt (at –1.28±0.02 MPa). Surprisingly, gs of Merlot on the drought-tolerant root system (1103P) was less down-regulated and canopies maintained evaporative fluxes ranging from 35–20 mmol vine−1 s−1 during the diurnal peak from spring to autumn, respectively, three times greater than those measured for Merlot on the drought-sensitive rootstock 101-14Mgt. The drought-tolerant root system grew more roots at depth during the warm summer dry period, and the whole root system conductance (kr) increased from 0.004 to 0.009 kg MPa−1 s−1 during that same time period. The changes in kr could not be explained by xylem anatomy or conductivity changes of individual root segments. Thus, the manner in which drought tolerance was conveyed to the drought-sensitive clone appeared to arise from deep root proliferation during the hottest and driest part of the season, rather than through changes in xylem structure, xylem density or stomatal regulation. This information can be useful to growers on a site-specific basis in selecting rootstocks for grape clonal material (scions) grafted to them.
Keywords: Drought tolerance, grape, root hydraulic conductance, root hydraulic conductivity, root water relations, stomatal conductance, Vitis vinifera
Introduction
Hydraulic limitations of water supply to leaves of woody plants is still the subject of some debate. At issue is not necessarily where key points of flow restriction occur, as much as the relative role each physical limitation plays in the control of hydraulic conductance through the soil–plant–atmosphere continuum for different species and in diverse environments (Oren et al., 2001; Addington et al., 2006). Models of woody plant hydraulic conductance (Sperry et al., 1998, 2002; Williams et al., 2001) are consistent with respect to how resistances to water transport in the soil–plant–atmosphere continuum act to optimize water use in a given environment. These models are generally based on stomata acting as the major control point for limiting water loss and thus regulating undesirable negative pressures, while hydraulic resistances at the soil–rhizosphere interface (Newman, 1969), root and stem xylem anatomical properties (Sperry, 1995; Davis et al., 1999; Hacke et al., 2004), and differences in the extent of root surface area versus leaf surface area (Jackson et al., 2000) comprise the main factors that limit water supply versus demand. These modelling exercises predict that adjustment of of root surface area and leaf surface area is one of the major architectural features that can moderate water demand with respect to supply, but empirical data to verify this prediction for roots are not readily available.
There are two generally recognized points of weakness where the hydraulic continuum can more easily break for woody plants. The first concerns loss of contact between the rhizosphere and soil-matrix as soils dry and water potential gradients steepen. The second concerns the cavitation of xylem elements as stem water potentials exceed a critical value (Ψcrit) (Milburn, 1973). When canopy water demand exceeds that of the integrated capacity of the entire conduction pathway to supply water, or the ability of the hydraulic equipment of the plant to limit water loss (Sperry et al., 2002), catastrophic or ‘runaway’ xylem cavitation ensues (Sperry et al., 1988; Tyree and Sperry, 1988), resulting in lethal desiccation. In the short-term, stomata clearly serve to regulate water loss instantaneously (Jones, 1998), and ease negative water potential pressures that would lead to catastrophic xylem cavitation. But many other physiological adjustments (like canopy leaf area modification) require longer time periods to effectively alter water demand with respect to supply (Givinish, 1986).
A conceptual feature of the above models with respect to longer-term adjustments to moderate water demand with respect to supply is that root system size (or conduction capacity) is often viewed as remaining static during a season while major adjustments may occur at the leaf level (Fordyce et al., 1997; Hacke et al., 2000; Vilagrosa et al., 2003). For example, Sperry et al. (2002) approach the thesis ‘that stomatal regulation and longer-term leaf area regulation of gas exchange is necessary to preserve hydraulic continuity of the soil-leaf continuum’. Although we do not disagree with the thesis, we simply note that little information exists concerning root response to drying soils. Williams et al. (2001), for example, pointed out that there were ‘important uncertainties that need to be resolved’ with respect to understanding the hydraulic continuum that ‘concern seasonal dynamics of root growth and rooting depth, especially in response to developing drought…’. Root system properties alone may comprise a key element of plant sensitivity to drought stress (Jackson et al., 2000) and reports have indicated that root xylem water potential may often operate near its hydraulic limitation (Alder et al., 1996; Hacke and Sauter, 1996; Domec et al., 2006). In order to examine the hypothesis that root growth dynamics might serve to moderate the water supply and demand equation on a seasonal basis, root growth was examined with depth, root system hydraulic conductance, root anatomical features, and stomatal conductance (gs) for a drought-sensitive clone of grape (scion) grafted onto two rootstocks that differ in drought tolerance (Carbonneau, 1985) and growth dynamics (Carbonneau, 1985; Bauerle et al., 2007).
Materials and methods
Field site
The experiment was carried out in a 1.05 hectare vineyard situated in Oakville, Napa Valley (California) (38° 25′ N 122° 24′ W). The Oakville region averages 830 mm of precipitation annually and has a mean annual temperature of 14.3 °C (CIMIS, 2007, http://wwwcimis.water.ca.gov/cimis/). The vines (13 years old) were trained to a bilateral cordon with vertical shoot positioning (VSP). Rows were oriented SE to NW, with 2.4×2.2 m between and within row vine spacing respectively. Water was withheld in order to restrict new leaf area production during the period of fruit growth and veraison (ripening) beginning in 2002. In order to achieve this restriction, irrigation amounts were regulated at 40% of the estimated evapotranspiration demand (ETc) as calculated from the Penmann–Monteith relationship and adjusted using a grape crop coefficient (Kc) and evapouration from a Class A pan (Pritchard, 1992). ETc amounts were calculated and water applied bi-weekly.
Plant material
Two rootstocks Vitis berlandieri×V. rupestris cv. 1103P (1103P) and V. riparia×V. rupestris cv. 101-14Mgt (101-14Mgt) were examined. Rootstock 101-14Mgt confers lower growth to the grafted grapevine cultivar (Wolpert et al., 2002; Bauerle et al., 2008b) and is, and is classified as highly drought susceptible (Carbonneau, 1985). Rootstock 1103P, on the other hand, confers much higher growth to its scion, and is classified as highly drought resistant (Carbonneau, 1985). Both rootstocks were grafted to the identical drought-sensitive clone (scion) of Vitis vinifera cv. Merlot.
Root growth dynamics
In April 2002, 120 clear cellulose acetate butyrate (CAB) root observation tubes (minirhizotrons) were installed at an angle of 30° from the vertical through the drip irrigation zone and a second minirhizotron was placed about 60 cm from the trunk on the opposite side of the vine in an area that was not irrigated. Tubes were 1.5 m in length, 6 cm outside diameter and had a viewing area of 0.0192 m2. They were maintained in a light- and watertight condition by plugging the ends of the tubes protruding above ground and covering them with white aluminium heat shields. From 2003 to 2006 digital images were acquired every two weeks during the growing season, and once a month after leaf fall and before bud break, using a specially designed digital imaging camera (BTC-2, Bartz Technology, Santa Barbara, CA). Root images were acquired using software designed for root observation capture (ICAP v.4.1; Bartz Technology, Santa Barbara, CA). Images were analysed using Win RhizoTron MF software (Regents Inc. Quebec, Canada). Root births were estimated by calculating the date midway between the date when a root was first observed and the previous date of observation (Comas et al., 2000). Roots transecting more than one minirhizotron observation window were only counted once.
Leaf area measurements
Vine leaf area (AL) was estimated using the specific leaf weight (SLW, area per gram dry mass) of 20 leaves from each of the four vines per rootstock that were excavated. Leaves were removed from vines used for measuring root hydraulic resistance on 21 June 2006 and 19 September 2006. All leaves were removed at the petiole base, placed in an ice chest and returned to the laboratory. Leaf area for the 20 leaves was obtained using a planimeter (Li-Cor Inc. Model LI-3000, Lincoln, Nebraska, USA). After leaf area was measured leaves were dried to constant weight at 65 °C, to obtain SLW. All of the remaining leaves were similarly dried to obtain whole canopy dry biomass and thus, an estimate of total leaf area. Fruit biomass was also separated and dried at 65 °C in a forced air oven until constant weight was achieved.
Leaf gas exchange measurements
Leaf gas-exchange measurements were conducted on mature, fully expanded canopy leaves with an open-path gas-exchange system (Li-Cor 6400, Lincoln, Nebraska, USA). Measurements were made on fully exposed leaves just following the stage of pea-size berry development (21 June), and again just prior to harvest (19 September) in six vines per rootstock. Photosynthetic rate (A), transpiration rate (E), and stomatal conductance (gs) were measured diurnally between sunrise and sunset, the first measurement was taken at predawn and then every 2 h from 08.00 h to 19.00 h. Maximum stomatal conductance (gmax) at ambient CO2 concentration (382 ppm) was observed between 10.30 and 12.00 h. To obtain an estimate of whole canopy transpiration rate (Ec, mmol H2O vine−1 s−1), a weighted mean average was used of
| (1) |
where E is the maximum instantaneous leaf transpiration rate measured during the day, Si is the proportion of leaf area with respect to the vine area in each of eight canopy sectors, and αi is the proportion of E corresponding to each of the eight sectors as defined by Escalona et al. (2003). In their experiment, they measured the leaf gas interchange and the leaf area in eight canopy sectors of the vine, αi represented the proportion of the measured gs and A related to the maximum measurement in the canopy.
Leaf water status
The diurnal course of leaf water potential starting at predawn and following the same schedule as leaf gas exchange measurements, was monitored on 20 June and 11 September 2006 in six vines per rootstock using a pressure chamber (Soil Moisture Inc., Santa Barbara, CA). The 4th, 5th, or 6th leaf was selected on a randomly chosen cane, and the petiole cut free from the cane with a razor blade. The leaf was simultaneously placed in a plastic bag that had been charged to approximately 20000 with anthropogenic derived CO2 and H2O (Turner and Long, 1980), and immediately inserted into the pressure chamber. Leaf water potential (ΨPD or ΨL) was measured within 1–2 min of cutting the leaf from the vine by slowly pressurizing the chamber until sap emerged from the cut end of the petiole.
Root hydraulic conductances and coarse root morphology
The hydraulic resistance of the intact root system was measured using a high pressure flow meter (HPFM; Tyree et al., 1995). Four vines of each rootstock were measured starting on 21 June and five vines were measured starting on 19 September 2006 between 06.00 h and 10.00 h, solar time. In both cases, measurements were completed in four days. In order to avoid pressure anomalies caused by cavitated xylem, the root was completely re-hydrated during the night by irrigating soils around each vine to field capacity the day before measuring. The trunks were cleanly cut approximately 6 cm above the swelling of the graft union using a pair of razor-sharp shears in order to make a quick single cut. The cut trunks were level and did not reveal any signs of crushing or cracking. Bark and the underlying cambial tissue (phloem) were removed to avoid any non-xylematic flow to roots during measurements. This also exposed a clean smooth surface that facilitated attaching the HPFM collar quickly and in a manner that precluded any leakage that might interfere with measurements of root hydraulic conductance (kr). Immediately after attaching the HPFM to the cut trunk, the hydraulic resistance of the root was measured using the transient mode of measurement (Tyree et al. 1995; Bogeat-Triboulot et al., 2002). The HPFM system forces distilled and degassed water through the cut trunk into the root system under increasing pressure. Pressure was increased from 0 kPa to 500 kPa at a constant rate of 0.5 kPa s−1. The water flow (ϕ) was plotted against the pressure (P), and k was calculated as the slope of this plot,
| (2) |
Following the first measurement, the HPFM was disconnected, the trunk was cut just below the grafting union, bark and cambium removed, and a new hydraulic resistance measurement was taken. Leaf area specific hydraulic conductance (kL) of the whole root/leaf surface system was obtained by expressing the hydraulic conductance of the root system with respect to the leaf surface area of each individual vine (kr/AL).
Upon completion of HPFM measurements, the immediate portion of the root system was excavated and at least two 20 cm segments of each first order root emerging from the trunk was cleanly cut. Two other root segments of at least 0.5 cm diameter and between 15 cm and 20 cm in length were also cleanly cut from each root system, wrapped in moist paper towels, placed in plastic bags on ice in a cooler where they were transported to the laboratory. The number and cross-sectional area of the first order (NR, no. trunk−1), of the framework roots emerging from the trunk (ACR, cm2 root−1) and the roostock trunk diameter (Td, mm) measured right below the beginning of the callus swelling were recorded for the excavated roots. Once in the laboratory, the maximum hydraulic conductivity in one root fragment per size category for each excavated root system (Kr) was measured using the gravimetric method (Sperry et al., 1988). Root segments were previously flushed using a 0.4 MPa water source. The hydraulic conductance (K) was measured gravimetrically by connecting the xylem segment to a low-pressure water source (approximately 9.8 10−4 MPa) and registering the weight of the flowing water every second on a balance until a steady-state was reached. This process was repeated until K no longer changed after flushing, and this point was determined to be the maximum conductance (Kmax).
Root vessel length distribution
The other root fragments excised were used to define vessel length distribution in the root xylem. They were first flushed with deionized water to refill the xylem and avoid the presence of embolisms after which they were vacuum-infused (Rhodorsil RTV-141; Rhodia, USA) with a red silicon-based pigment (Silastic LSPRD11; Dow Corning; (André, 1998). Each sample was dried for 45 min in a 65 °C oven and a thin cross-sectional disc of about 2.5 mm thickness was cut every 2 cm along the root fragment. Discs were set out in order starting from the silicon dye injection point to the end of the root fragment. The number of silicon-filled vessels in the initial section (Nsfv0) and every 2 cm thereafter (NsfvL) were counted using a dissecting microscope. The fraction of silicon-filled vessels over Nsfv0 was calculated, and vessel length distribution for each root sample was calculated using an exponential decay function (Sperry et al., 2005):
| (3) |
where L is the length of the individual segment and α is a coefficient representing the rate of disappearance (extinction) of dyed cross-sectional vessel elements. Data were fitted using the PROC NLIN procedure in SAS 9.1 (P ≤0.0001). The best fit coefficient of extinction (α) (Cohen et al., 2003) was obtained for each silicon-injected sample and a mean α value was obtained for each rootstock and data were subjected to one-way ANOVA. The proportion of conduits (PLC) between two lengths of root (L1, L2) was obtained as follows (Sperry et al., 2005):
| (4) |
Finally, the maximum vessel length (Lm) and the most common vessel length (L0) were determined for both rootstocks according to Cohen et al. (2003):
| (5) |
and
| (6) |
Data analysis
The vines were in a previously established experiment (Bauerle et al., 2007) consisting of a completely randomized block design with three levels of irrigation and two rootstock cultivars within six blocks. Only one irrigation level was used in the present experiment. Four of the six blocks (randomly selected) were used for leaf area, fruit weights, and root hydraulic resistance properties on account of the amount of labour involved in acquiring the data. Six blocks were used for gas exchange and water potential measurements. All six blocks were also used for the root growth investigations. All data were subjected to ANOVA using a randomized complete blocks design with four or six blocks depending on the measurements taken.
Results
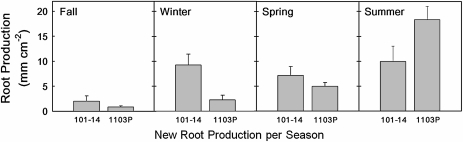
The 1103P root system produced a much larger fraction of new roots during the hot dry season (Fig. 1; Bauerle et al., 2008b). The 101-14Mgt root system on the other hand produced nearly 3-fold more of its new roots during the period of shoot dormancy and of low water stress (season ×rootstock interaction: P=0.002; Fig. 1). As summer progressed, root production by the 1103P root system shifted to greater depths where more than 40% of new roots were produced below 60 cm, whereas less than 20% of new roots and fewer roots overall were produced below 60 cm for the 101-14Mgt rootstock during August (Bauerle et al., 2008b).
Fig. 1.
Seasonal root production (±1 SE) for root systems of V. berlandieri×V. rupestris cv. 1103P and V. riparia×V. rupestris cv. 101-14Mgt (season×root system interaction: P=0.002). Data represent total root length produced cm−2 of observational window over three month periods for the years, 2003–2005. Each season corresponded to the following months: Autumn, September–November (significance of difference between 1103P and 101-14Mgt, P=0.328); winter, December–February (P=0.009), spring, March–May (P=0.230), and summer, June–August (P=0.032). (Bauerle et al., 2008b). Reprinted with the permission of The New Phytologist (Chesire England UK).
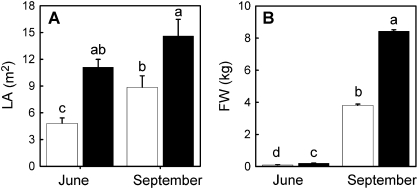
Twice as much leaf area was produced by Vitis vinifera cv. Merlot on the 1103P root system compared with 101-14Mgt (Fig. 2A). Both canopies had leaf area removed during the summer as a normal management practice. The vines were hedged twice, once in June and once in July, and leaves were manually removed from the fruiting zone in mid-July. The amount of leaf surface area removed from the vines by the above practices during the 2006 growing season were 2.24±0.24 m2 per vine for Merlot on 1103P and 0.81±0.11 m2 per vine for Merlot on 101-14Mgt. When transpiration rates were normalized to the canopy scale, Ec for Merlot growing on the 1103P rootstock exceeded that of 101-14Mgt by nearly 2-fold in both June and in September (Fig. 3C, F). This occurred in spite of the fact that the canopies on the 1103P rootstock had higher leaf area (Fig. 2A; P ≤0.05) and thus a larger fraction of their leaf area in more densely shaded portions of the canopy where transpiration rates were decidedly lower. A difference that emerged between the two rootstocks was that Merlot grafted onto 1103P sustained higher photosynthetic carbon assimilation rates compared to 101-14Mgt during the time period between approximately 09.00 h and 10.00 h when VPD was still below about 2 kPa (Fig. 4; P ≤0.05).
Fig. 2.
Shown for spring (June) and autumn (September) are square metres of leaf area, AL (A) and fruit weight, FW (B) per vine for Vitis vinifera cv. Merlot grafted onto V. rupestris×V. riparia cv. 101-14Mgt (open bars) and V. berlandieri ×V. rupestris cv. 1103P (shaded bars) root systems. Each value is the mean of five vines of the same rootstock for each sampling date. Bars represent the standard error. Means that do not share the same lower case letter are significantly different (P ≤0.05).
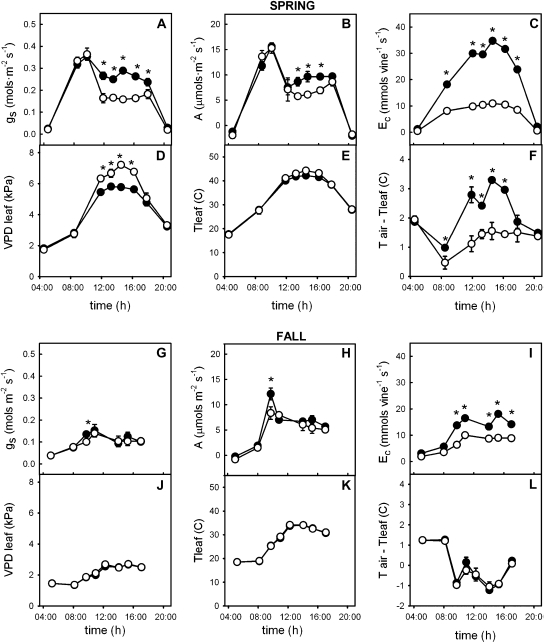
Fig. 3.
Diurnal courses of leaf stomatal conductance (gs, mmol m−2 s−1), net photosynthetic rate (A, μmol CO2 m−2 s−1), canopy transpiration rate once scaled with the leaf area values (Ec, mmol vine−1 s−1), leaf to air vapour pressure deficit (VPDleaf, kPa), leaf temperature (Tleaf, C) and leaf temperature-air temperature diference (Tair–Tleaf, C) on 21 June (A, B, C, D, E, F) and 19 September (G, H, I, J) for V. vinifera cv. Merlot grafted onto V. berlandieri×V. rupestris cv. 1103P (closed symbols) and, V. riparia×V. rupestris cv. 101-14Mgt (open symbols) rootstocks. Each value is the mean of observations from six vines. Vertical bars represent the standard error (P ≤0.05) and an asterisk denotes a significant difference (P ≤0.05) between rootstocks. Errors smaller than the symbols are hidden.
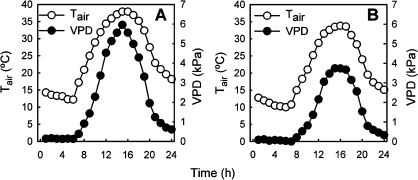
Fig. 4.
Shown are diurnal courses of air temperature (T, open symbols) and vapour pressure deficit (VPD, closed symbols) for 21 June (A) and 19 September 2006 (B) for a vineyard in Oakville California, Napa Valley USA.
Patterns of daily stomatal conductance (gs) for Merlot on the two rootstocks differed. In June, stomatal conductance (Fig. 3A) of Merlot on both root systems rose during the early morning hours to rates generally considered to be high (see Padgett-Johnson et al., 2000). After 10.00 h, during a time when vapour pressure deficits increased from less than 1 kPa to greater than 5 kPa (Fig. 4), gs decreased. Stomatal conductance then remained steady throughout the afternoon and was statistically significantly lower for Merlot on the 101-14Mgt rootstock at 166.5±24.5 compared with 260.6±52.4 for Merlot growing on 1103P (Fig. 3A; P ≤0.05). The cost in carbon gain after stomata began to restrict water loss was approximately a 52.2% reduction in photosynthetic carbon assimilation rates on average for Merlot on 101-14Mgt while it was only reduced by 33.9% on average for Merlot growing on the 1103P rootstock (Fig. 3B).
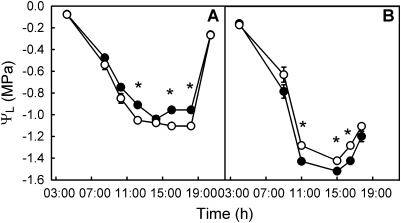
Predawn leaf water potentials (ΨPD) for each rootstock were not statistically significantly different at both time periods and this was true throughout the season (P=0.812 in June and P=0.557 in September, (Fig. 5). In June, ΨL dropped gradually during the early morning hours until it went below approximately –0.90 MPa. It subsequently stabilized at approximately –0.99±0.08 MPa for Merlot on rootstock 1103P and –1.09±0.08 MPa for Merlot growing on 101-14Mgt when stomatal conductance became more restricted (Fig. 3A). During the summer as soils dried these levels dropped to –1.44±0.095 MPa and –1.32±0.125 MPa for 1103P and 101-14Mgt rootstocks, respectively, in spite of the fact that vines received approximately 40–80 l of irrigation water weekly depending on evapotranspiration demand. These apparently steady-state levels of leaf water potential measured at midday (12.00–16.00 h) differed between the two root systems (P ≤0.05). Thus, at the beginning of summer (20 June) 1103P sustained midday leaf water potentials of approximately 0.1–0.2 MPa less negative than 101-14Mgt, whereas in autumn the reverse was true; on 11 September ΨL for Merlot on rootstock 1103P was about –0.2 MPa more negative than 101-14Mgt (Fig. 5).
Fig. 5.
Diurnal course of leaf water potential (ΨL) for V. vinifera cv. Merlot growing on V. berlandieri×V. rupestris cv. 1103P (closed symbols) and V. riparia×V. rupestris cv. 101-14Mgt (open symbols). Shown are the mean values for six vines of each rootstock and sampling date, June (A) and September (B). Vertical bars represent the standard error and an asterisk indicates significant differences between rootstocks (P ≤0.05). Errors smaller than the symbols are hidden.
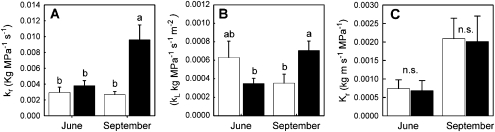
In June, whole root hydraulic conductance (kr) was similar for the two rootstocks. Rootstock 101-14Mgt was 2.92×10−3 ± 6.89×10−4 kg MPa−1 s−1 (mean ±SE, n=4) and not statistically significantly different from that of rootstock 1103P at 3.81×10−3 ± 6.23×10−4 (P=0.379; Fig. 6A). The resistance to water transport for the graft union of 101-14Mgt was 28.1±14.3% as a fraction of the total resistance of the root system plus the graft union, while it was 34.4±11.8% for 1103P. The hydraulic conductance of the graft union did not differ between the two rootstocks in June (at 1.37×10−2 ± 5.85 10−3 for 101-14Mgt and 1.2×10−2 ± 5.35×10−3 for 1103P, P=0.887) or in September (at 3.71×10−2 ± 6.00×10−3 for 101-14Mgt and 2.45×10−2 ± 4.62×10-3, P=0.695). Thus, while the graft union did represent a relatively large proportion of the total root resistance to water transport (Rr), there were no indications that this represented a substantial difference between the two rootstock scion systems. Hydraulic conductance (kr) remained constant during the summer dry season for the 101-14Mgt root system (P=0.4082), while, in contrast to 101-14Mgt, kr for the 1103P root system increased more than 2-fold during the summer dry period (P=0.017; Fig. 6A).
Fig. 6.
(A) Root hydraulic conductance (kr, kg H2O MPa−1 s−1) and (B) canopy specific root hydraulic conductance (kL, kg H2O MPa−1 s−1 m−2) in spring (June) and autumn (September) for V. vinifera cv. Merlot grafted onto on to V. rupestris×V. riparia cv. 101-14Mgt (open bars) and V. berlandieri×V. rupestris cv. 1103P (shaded bars). (C) Hydraulic conductivity (Kr, kg H2O m s−1 MPa−1) for individual root segments. Each value is the mean of five vines of each and sampling date. Vertical bars represent the standard error. Different lower case letters indicate statistically significant differences between rootstocks (P ≤0.05).
The leaf area specific hydraulic conductance (kL), measured here with respect to the total leaf area in the canopy (kg MPa−1 s−1 m−2), changed during the summer dry period for Merlot growing on the 1103P root system while it did not change for Merlot on 101-14Mgt (Fig. 6B). In the beginning of summer (21 June) kL for 1103P was 0.001±3.4855×10−4 which was not significantly different from that of Merlot on 101-14Mgt at 0.001±1.762×10−4 (P=0.179; Fig. 6B). Three months later, on 19 September, kL of Merlot on 1103P was nearly two times greater (P=0.047) in spite of the fact that leaf area was sustained at a constant level (Fig. 2A).
The conductivity of root segments (Kr) increased from June to September (Fig. 6C). For rootstock 101-14Mgt, it increased from 7.24±2.19×10−4 kg m MPa−1 s−1 (mean SE, n=15) to 20.92±5.91×10−4 kg m MPa−1 s−1 and for rootstock 1103P it increased from 7.06±2.60 10−4 to 19.48±4.71 10−4 kg m MPa−1 s−1. While the increase from June to September was statistically significant (P <0.05), the difference between rootstocks in the same season (month) was not.
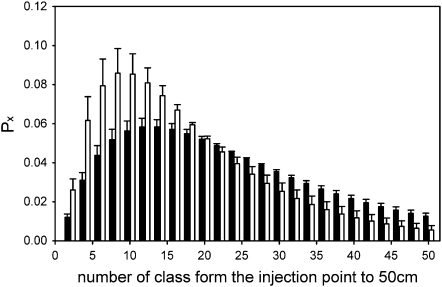
The frequency distribution of vessels (Px) in each of 25 length classes, calculated as the number of vessel-ends in each 2 cm root fragment from the injection point to the end of root segment, differed between 1103P and 101-14Mgt (Fig. 7). For all vessel length classes included in the length intervals between classes 3 through 8 (e.g. 6–16 cm) and between classes 15 through 25 (e.g. 30–50 cm), the proportion of vessel-ends expressed as a fraction of the initial (Nsfv0) was different for 101-14Mgt versus 1103P. In the shorter vessel length interval, a proportion of 0.56±0.07 were included for 101-14Mgt, while only 0.37±0.04 were detected between 6 cm and 16 cm for 1103P. For the proportion of vessels found in the longer length interval of 30–50 cm, it was greater for 1103P at 0.25±0.02 compared with 101-14Mgt at 0.15±0.04 (Fig. 7).
Fig. 7.
Frequency distribution of conduits (Px) in each of the 25, 2 cm length classes from a silicon injection point, representing the proximal end with respect to the vine trunk, up to 50 cm distal to the trunk for roots of Vitis vinifera cv. Merlot grafted onto V. rupestris×V. riparia cv. 101-14Mgt (open bars) and V. berlandieri×V. rupestris cv. 1103P (shaded bars).
These differences in vessel length distribution (Px) resulted in an estimated most common vessel length (L0) and a maximum vessel length (Lm) that were both statistically significantly longer for 1103P than for 101-14Mgt (Table 1). On the one hand, when number of vessels (NV) and mean lumen diameter (dL) were measured for 1 mm2 of root cross-section, no significant differences were found between rootstocks (Table 1). On the other hand, root cross-sectional area was significantly higher for 1103P than for 101-14Mgt, even though first order root cross-sectional area (ACR) was the same for both rootstocks, the number of first order roots (NR) was significantly greater in 1103P, at 22.44±2.47 per vine, compared with 101-14Mgt where it was 15.00±1.26, and also trunk diameter (Td) was significantly bigger for 1103P (Table 1).
Table 1.
Most common vessel length L0 (m), maximum vessel length Lm (m), number of vessels per cross-sectional area of root xylem (NV, no. mm−2), xylem vessel lumen diameter (dL, mm), number of first order roots (NR, no. trunk−1), mean cross-sectional area of framework roots emerging from the trunk (ACR, cm2 root), and mean roostock trunk diameter (Td, mm) for plants of Vitis vinifera cv. Merlot grafted onto V. riparia×V. rupestris cv. 101-14Mgt or V. Berlandieri×V.rupestris cv. 1103P root systems Each value is the mean from five plants for xylem measurements and rootstock trunk diameter and nine plants for root morphology measurements within the same rootstock and sampling date ± the corresponding standard error. Statistically significant differences between rootstocks are indicated by values within the same row that do not share the same lowercase letter (P ≤0.05).
| Rootstock property | Root system |
|
| V. riparia×V. rupestris cv. 101-14Mgt | V. berlandieri×V. rupestris cv. 1103P | |
| L0 (m) | 0.09±0.01 b | 0.13±0.01 a |
| Lm (m) | 0.52±0.06 b | 0.70±0.04 a |
| NV (number mm−2) | 27.92±3.60 a | 27.33±3.27 a |
| dL (mm) | 0.09±0.01 a | 0.11±0.02 a |
| NR (number trunk−1) | 15.00±1.26 b | 22.44±2.47 a |
| ACR (cm2 root−1) | 1.80±0.26 a | 1.86±0.22 a |
| Td (mm) | 42.53±0.91 b | 65.19±0.86 a |
Discussion
Woody plants respond to water stress in a number of important ways. These responses range in temporal scale from being extremely rapid and reversible, like stomatal closure or accumulation and compartmentation of osmotically active solutes, to growth and development of permanent phenotypic structures and hydraulic linkages between them. In the later case, changes of canopy transpiration demand and/or root water supply (for example, AL:AR) in order to moderate the demand for water with respect to supply, and the production of xylem elements less vulnerable to embolism are two responses deemed to have high importance for tolerating more negative water potential gradients or restricted water supply (Sperry et al., 2002). Long-term adjustments to root system size with respect to soil water capacity have been reported (Hacke et al., 2000), and inferences have also been drawn from comparative observations: Jackson et al. (2000), for example, have shown that differences in rooting depth patterns exist with respect to the world's major plant biomes (Canadell et al., 1996), with plants of xeric environments having deeper root-depth distributions than plants in more humid environments. In this report, it is shown that short-term below-ground root growth during drought stress by a woody plant (growth) increased water conductance. Such adjustments to AR have not been documented (Williams et al., 2001). Recent investigations of root response have demonstrated a high sensitivity of root xylem to embolism (Alder et al., 1996) and suggest a signalling function (Jackson et al., 2000). Others have indicated the internal redistribution of water within the root system of woody perennial plants (xylem refilling) may play an important short-term functional role in drought resistance (Smart et al., 2005; Bauerle et al., 2008a).
During the three years of root observations conducted in this investigation, it was found the drought-tolerant root system (1103P) had an enhanced ability to produce roots during summer drought (Fig. 1). Most of these roots were produced at depths greater than 60 cm where moisture was available (Bauerle et al., 2008b). Seasonal adjustments of AR with respect to AL provides information on the relative importance of root system growth during water limitations. Direct experimental observation of roots of woody perennials is extremely challenging and limited experimental information exists in this area. We found that whole root system water conductance (kr), measured using the high pressure flow meter (HPFM) approach (Tyree et al., 1995; Tsuda and Tyree, 2000) increased during the period of drought for the root system that confers drought tolerance. The change in kr observed for the drought-tolerant root system was nearly 2-fold in September compared to June, while kr remained constant for the drought-sensitive root system (Fig. 6A). Increased root growth during this same time frame provided the most reasonable explanation for the change in kr since other water-conducting properties of the root system remained constant during the same period (Table 1).
One reason that might explain an increase in kr during the period of observation would be an increase in xylary conduction pathways. This would increase conductivity (Kr) of individual framework roots, or roots of greater than one year of age, by growing and producing new conduction pathways. But it was found that although Kr increased during the period of investigation, it increased in each root system by the same magnitude (Fig. 6C). Thus, changes in root hydraulic conductivity measured in sections of framework roots could not explain the difference observed in whole root system conductance. Zwieniecki et al. (2003) have shown that root water absorption occurs primarily in the apical ends of roots. Thus, root proliferation should increase conductance to water supply as new roots and their branching connections increase. Conductance of water by the whole root system would also increase (Comstock and Mencuccini, 1998), and this is what was observed.
Restriction of stomatal conductance (gs) to maintain a positive balance between carbon uptake and water loss is one of the first responses to water stress in field-grown grapevines (Schultz, 2003), but did not appear to play a role in relative drought tolerance of the two root systems studied. The magnitude of plant gs depends on the hydraulic conductivity of the entire soil–leaf pathway (Sperry and Pockman, 1993; Nardini and Salleo, 2003), but the signalling mechanisms involved in gs regulation are still the subject of some debate. Grapevine stomata have been shown to respond to chemical signals like ABA synthesized as soils dry (Tardieu and Simonneau, 1998), as well as to decreasing leaf water potential (Schultz, 2003). Diurnal restriction of gs by Merlot grafted onto both 101-14 Mgt and 1103P rootstocks (Fig. 3) was observed, but the degree of control and apparent signal differed. In June, when water was still readily available, the maximum gs (gmax) was achieved mid-morning, and was the same for Merlot on both rootstocks. But, corresponding with a decline in ΨL, gs was more strongly restricted on the 101-14Mgt root system compared with 1103P (Fig. 3A). Under the assumption that signal perception by the Merlot clonal tissue grafted onto the two rootstocks is the same, this indicated that signal strength was fundamentally different in nature between the two rootstocks. Measured values of kr in June were not significantly different for both rootstocks, suggesting that a non-hydraulic signal was strongly acting on stomata in vines on 101-14Mgt. This calls into question the results of Schultz (2003) in as much as the two cultivars he examined (Syrah, anisohydric and Garnacha, isohydric) were apparently grafted onto two different ‘V. rupestris’ rootstocks (Schultz, 2003), and our V. rupestris rootstocks conferred anisohydric-like stomatal behaviour on the Merlot clone in one case (1103P), while conferring isohydric-like behaviour in the other (101-14Mgt).
The significantly greater leaf area of vines grafted onto 1103P in addition to its higher gs, resulted in substantially larger canopy water use (Ec) for vines on this root system (Fig. 3C, F). If the low degree of stomatal regulation demonstrated by vines grafted onto 1103P at the beginning of the growing cycle had been maintained throughout the summer drought period from June to September when no rain, high temperatures (T), net radiation (Rn), and VPD were registered, ΨL may have exceeded critical values (Ψcrit) and suffered severe hydraulic failure. No evidence to support this hypothesis was observed from the current or previous seasons and late in the season vines on 1103P still maintained higher gs (Fig. 3D) and Ec (Fig. 3F) than vines on 101-14Mgt before midday. These results, taken together with the observation of root production at depth during the dry season, can be important to practitioners. The probable involvement of rootstocks in drought tolerance has long been recognized (Carbonneau, 1985), but information based on water relations and stomatal behaviour of own-rooted Vitis species differs from these earlier classifications (Padgett-Johnson et al., 2003). Much less information exists concerning drought-tolerance mechanisms of grape rootstock/scion combinations. Our results concerning a drought-sensitive scion, have implications for rootstock selection at xeric sites with deep (geologic) water sources where the 1103P rootstock may be superior, and for depth of irrigation, where deeper irrigations may be more effective for 1103P rootstock/scion combinations.
Our data indicated that the increased kr, of 1103P was a consequence of new root production in as much as the change in kr occurred during the growing season and not as a consequence of root system size gained over the 11 years that the vines had established permanent root structures in this environment. Thicker first order (framework) roots measured for 1103P (Table 1) will contain more vessels per cross-sectional area, resulting in a greater number of parallel water-conducting pathways within the root system. From the Ohm's law analogy this would increase kr, since it allows for a higher number of redundant water paths from soil to leaves. The number of first order roots was also statistically significantly higher for 1103P than for 101-14 Mgt (Table 1) but this did not result in a greater whole root conductance in June while it was substantially greater for 1103P in September (Fig. 6). Thus, new root production was the most likely reason why Merlot on rootstock 1103P maintained better hydraulic supply and thus supported higher evaporative fluxes (Fig. 3C, F).
Vessel-length distribution is a fundamental parameter in determining the hydraulic conductance for long-distance transport elements of plants (Zimmermann and Jeje, 1981). The most common vessel lengths (L0) for roots of 101-14Mgt (at 9±1 cm) and 1103P (at 13±1 cm) were within a range very similar to that estimated for stems of an unidentified cultivar of grape at 13±5 cm (Sperry et al., 2005). This finding indicated that vessel length in stems of grape may, in general, be somewhat conserved in proximal roots, even though significant differences were found in most common vessel length (L0) and maximum vessel length (Lm) for 1103P roots compared with 101-14Mgt (Table 1). Hydraulic resistance in xylem conduits is largely determined by lumen resistance, which is known to increase with length (Zimmermann and Jeje, 1981) and by intervessel hydraulic resistance. The latter has been found to represent approximately 50% of the total conduit resistance (Sperry et al., 2005). A higher proportion of shorter vessels measured for 101-14Mgt, in contrast with 1103P (Fig. 7; Table 1), would technically decrease kr in 101-14Mgt as both rootstocks showed the same cross-sectional area for a single vessel and the same vessel density in xylem cross-sectional area. This may help to explain why 1103P and 101-14Mgt had the same whole root kr in spring, thus the relative size, or conducting capacity of each root with respect to the major resistances to water transport was the same (Fig. 6C).
Acknowledgments
The authors gratefully acknowledge the expert assistance of Sam Metcalf and financial support from the Institut de Recerca i Tecnologia Agroalimentàries that allowed Maria del Mar Alsina to conduct this study. Further financial support was provided by the USDA Western Viticulture Consortium under agreement number 05-34360-15800 to DR Smart and the American Vineyard Foundation under agreement number V301 to DR Smart.
Appendix
| Abbreviation | Parameter (units) |
| A | Net photosynthetic rate (mmol CO2 m−2 s−1) |
| AL | Leaf area (m2) |
| AR | Root area (m2) |
| ACR | Mean cross-sectional area of the trunk (cm2) |
| dL | Xylem lumen diameter (mm) |
| E | Leaf transpiration rate (mmol H2O m−2 s−1) |
| Ec | Canopy transpiration rate (mmol H2O vine−1 s−1) |
| Ea | Ambient vapour pressure (kPa) |
| Es | Saturation vapour pressure (kPa) |
| Kc | Crop coefficient |
| gs | Stomatal conductance to water vapour (mmol m−2 s−1) |
| gmax | Maximum stomatal conductance to water vapour (mmol m−2 s−1) |
| kR | Root hydraulic conductance (kg MPa−1 s−1) |
| kL | Leaf specific root hydraulic conductance (kg MPa−1 s−1 m−2) |
| Kr | Hydraulic conductivity of excised root segments (kg m MPa−1 s−1) |
| L0 | Most common vessel length (m) |
| Lm | Maximum vessel length (m) |
| NV | Number of vessels per cross-sectional area of root xylem (no. mm−2) |
| NR | Number of first order roots (no. trunk−1) |
| Nsfv | Number of silicon filled vessels per root cross-section |
| Px | Frequency distribution of vessels in specific length classes (%) |
| Td | Roostock trunk diameter (mm) |
| Tleaf | Leaf temperature (°C) |
| Ψcrit | Stem water potential at which xylem cavitation ensues (MPa) |
| ΨLmin | Leaf water potential at which stomata begin to close (MPa) |
| ΨPD | Pre-dawn leaf water potential (MPa) |
| ΨL | Midday leaf water potential (MPa) |
| VPD | Air vapour pressure deficit (kPa). |
| VPDleaf | Leaf to air vapour pressure deficit (kPa) |
References
- Addington RN, Donovan LA, Mitchell RJ, Vose JM, Pecot SD, Jack SB, Hacke UG, Sperry JS, Oren R. Adjustments in hydraulic architecture of Pinus palustris maintain similar stomatal conductance in xeric and mesic habitats. Plant, Cell and Environment. 2006;29:535–545. doi: 10.1111/j.1365-3040.2005.01430.x. [DOI] [PubMed] [Google Scholar]
- Alder NN, Sperry JS, Pockmanm WT. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia. 1996;105:293–301. doi: 10.1007/BF00328731. [DOI] [PubMed] [Google Scholar]
- André J. A study of the vascular organization of bamboos (Poaceae, Bambuseae) using a microcasting method. IAWA Journal. 1998;19:265–278. [Google Scholar]
- Bauerle TL, Eissenstat DM, Granett J, Gardner DM, Smart DR. Consequences of grape phylloxera infestations on root survivorship and root age structure in a California vineyard. Plant, Cell and Environment. 2007;30:786–795. doi: 10.1111/j.1365-3040.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- Bauerle TL, Richards JH, Smart DR, Eissenstat DM. Importance of internal hydraulic redistribution for prolonging the lifespan of roots in dry soil. Plant, Cell and Environment. 2008a;31:177–186. doi: 10.1111/j.1365-3040.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- Bauerle TL, Smart DR, Bauerle W, Stockert CM, Eissenstat DM. Root foraging in response to heterogeneous soil moisture in two grapevines that differ in potential growth rate. New Phytologist. 2008b;179:857–866. doi: 10.1111/j.1469-8137.2008.02489.x. [DOI] [PubMed] [Google Scholar]
- Bogeat-Triboulot MB, Martin R, Chatelet D, Cochard H. Hydraulic conductance of root and shoot measured with the transient and dynamic modes of the high-pressure flowmeter. Annals of Forestry Science. 2002;59:389–396. [Google Scholar]
- Canadell J, Jackson RB, Ehleringer JB, Mooney HA, Sala OE, Schulze ED. Maximum rooting depth of vegetation types at the global scale. Oecologia. 1996;108:583–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- Carbonneau A. The early selection of grapevine rootstocks for resistance to drought conditions. American Journal of Enology and Viticulture. 1985;36:195–198. [Google Scholar]
- Cohen S, Bennink J, Tyree M. Air method measurements of apple vessel length distributions with improved apparatus and theory. Journal of Experimental Botany. 2003;54:1889–1897. doi: 10.1093/jxb/erg202. [DOI] [PubMed] [Google Scholar]
- Comas LH, Eissenstat DM, Lakso AN. Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytologist. 2000;147:171–178. [Google Scholar]
- Comstock J, Mencuccini M. Control of stomatal conductance by leaf water potential in Hymenoclea salsola (TandG.), a desert subshrub. Plant, Cell and Environment. 1998;21:1029–1038. [Google Scholar]
- Davis SD, Ewers FW, Wood J, Reeves JB, Kolb KJ. Differential susceptibility to xylem cavitation among three pairs of Ceanothus species in the Transverse Mountain Ranges of southern California. Ecoscience. 1999;6:180–186. [Google Scholar]
- Domec J-C, Scholz FG, Bucci SJ, Meinzer FC, Goldstein G, Villalobos-Vega R. Diurnal and seasonal variation in root xylem embolism in neotropical savanna woody species: impact on stomatal control of plant water status. Plant, Cell and Environment. 2006;29:26–35. doi: 10.1111/j.1365-3040.2005.01397.x. [DOI] [PubMed] [Google Scholar]
- Escalona JM, Flexas J, Bota J, Medrano H. Distribution of leaf photosynthesis and transpiration within grapevine canopies under different drought conditions. Vitis. 2003;42:57–64. [Google Scholar]
- Fordyce IR, Duff G, Eamus D. The water relations of Allosyncarpia ternata (Myrtaceae) at contrasting sites in the monsoonal tropics of Northern Australia. Australian Journal of Botany. 1997;45:259–274. [Google Scholar]
- Givinish TT. On the economy of plant and function. 1986. Cambridge, UK. [Google Scholar]
- Hacke U, Sauter JJ. Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia. 1996;105:435–439. doi: 10.1007/BF00330005. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Ewers BE, Ellsworth DS, Schafer KVR, Oren R. Influence of soil porosity on water use in Pinus taeda. Oecologia. 2000;124:495–505. doi: 10.1007/PL00008875. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. Analysis of circular bordered pit function. II. Gymnosperm tracheids with torus-margo pit membranes. American Journal of Botany. 2004;91:386–400. doi: 10.3732/ajb.91.3.386. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Sperry JS, Dawson TE. Root water uptake and transport: using physiological processes in global predictions. Trends in Plant Science. 2000;5:482–488. doi: 10.1016/s1360-1385(00)01766-0. [DOI] [PubMed] [Google Scholar]
- Jones H. Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany. 1998;49:387–398. [Google Scholar]
- Milburn JA. Cavitation studies on whole Ricinus plants by acoustic detection. Planta. 1973;112:333–342. doi: 10.1007/BF00390306. [DOI] [PubMed] [Google Scholar]
- Nardini A, Salleo S. Effects of the experimental blockage of the major veins on hydraulics and gas exchange of Prunus laurocerasus L. leaves. Journal of Experimental Botany. 2003;54:1213–1219. doi: 10.1093/jxb/erg130. [DOI] [PubMed] [Google Scholar]
- Newman EI. Resistance to water flow in soil and plant. I. Soil resistance in relation to amounts of root: theoretical estimates. Journal of Applied Ecology. 1969;6:1–12. [Google Scholar]
- Oren R, Sperry JS, Ewers BE, Pataki DE, Phillips N, Megonigal JP. Sensitivity of mean canopy stomatal conductance to vapour pressure deficit in a flooded Taxodium distichum L. forest: hydraulic and non-hydraulic effects. Oecologia. 2001;126:21–29. doi: 10.1007/s004420000497. [DOI] [PubMed] [Google Scholar]
- Padgett-Johnson M, Williams LE, Walker MA. The influence of Vitis riparia rootstock on water relations and gas exchange of Vitis vinifera cv. Carignane scion under non-irrigated conditions. American Journal of Enology and Viticulture. 2000;51:137–143. [Google Scholar]
- Pritchard TL. A volume balance approach to quality wine grape irrigation. In: Walker MA, Kliewer WM, editors. Viticultural practices. USA: University of California, Davis; 1992. pp. 12–23. [Google Scholar]
- Schultz HR. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant, Cell and Environment. 2003;26:1393–1405. [Google Scholar]
- Smart DR, Carlisle E, Goebal M, Núñez BA. Transverse hydraulic redistribution by a grapevine. Plant, Cell and Environment. 2005;28:157–166. [Google Scholar]
- Sperry JS. Limitations on stem water transport and their consequences. In: Gartner BL, editor. Plant stems. Physiology and functional morphology. Corvallis, USA: Academic Press; 1995. [Google Scholar]
- Sperry JS, Adler FR, Campbell GS, Comstock JP. Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant, Cell and Environment. 1998;21:347–359. [Google Scholar]
- Sperry JS, Donelly JR, Tyree MT. A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell and Environment. 1988;11:35–40. [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP. Water deficits and hydraulic limits to leaf water supply. Plant, Cell and Environment. 2002;25:251–263. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Wheeler JK. Comparative analysis of end wall resistivity in xylem conduits. Plant, Cell and Environment. 2005;28:456–465. [Google Scholar]
- Sperry JS, Pockman WT. Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis. Plant, Cell and Environment. 1993;16:279–287. [Google Scholar]
- Tardieu F, Simonneau T. Variability among species of stomatal control under fluctuating soil water status and evapourative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany. 1998;49:419–432. [Google Scholar]
- Tsuda M, Tyree MT. Plant hydraulic conductance measured by the high pressure flow meter in crop plants. Journal of Experimental Botany. 2000;51:823–828. [PubMed] [Google Scholar]
- Turner NC, Long MJ. Errors arising from rapid water loss in the measurement of leaf water potential by the pressure chamber technique. Functional Plant Biology. 1980;1:527–537. [Google Scholar]
- Tyree MT, Patiño S, Benink J, Alexander J. Dynamic measurements of root hydraulic conductance using a high pressure flowmeter in the laboratory and field. Journal of Experimental Botany. 1995;46:83–94. [Google Scholar]
- Tyree MT, Sperry JS. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model. Plant Physiology. 1988;88:574–580. doi: 10.1104/pp.88.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilagrosa A, Bellot J, Vallejo VR, Gil-Pelegrin E. Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-occurring Mediterranean shrubs during an intense drought. Journal of Experimental Botany. 2003;54:2015–2024. doi: 10.1093/jxb/erg221. [DOI] [PubMed] [Google Scholar]
- Williams M, Bond BJ, Ryan MG. Evaluating different soil and plant hydraulic constraints on tree function using a model and sap flow data from ponderosa pine. Plant, Cell and Environment. 2001;24:670–690. [Google Scholar]
- Wolpert J, Walker M, Bettiga L, Bianchi M, Hirschfelt D, McGourty G, Smith R, Weber E. Field evaluation of winegrape rootstocks. Napa, CA: American Vineyard Foundation; 2002. [Google Scholar]
- Zimmermann MH, Jeje AA. Vessel-length distribution in stems of some American woody plants. Canada Journal of Botany. 1981;59:1882–1892. [Google Scholar]
- Zwieniecki M, Thompson M, Holbrook NM. Understanding the hydraulics of porous pipes: tradeoffs between water uptake and root length utilization. Journal of Plant Growth Regulation. 2003;21:315–323. [Google Scholar]