Abstract
Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) were studied by exposing plants to six salinity levels (0–500 mM NaCl range) for 70 d. Salt stress was administered either by pre-mixing of the calculated amount of NaCl with the potting mix before seeds were planted or by the gradual increase of NaCl levels in the irrigation water. For both methods, the optimal plant growth and biomass was achieved between 100 mM and 200 mM NaCl, suggesting that quinoa possess a very efficient system to adjust osmotically for abrupt increases in NaCl stress. Up to 95% of osmotic adjustment in old leaves and between 80% and 85% of osmotic adjustment in young leaves was achieved by means of accumulation of inorganic ions (Na+, K+, and Cl–) at these NaCl levels, whilst the contribution of organic osmolytes was very limited. Consistently higher K+ and lower Na+ levels were found in young, as compared with old leaves, for all salinity treatments. The shoot sap K+ progressively increased with increased salinity in old leaves; this is interpreted as evidence for the important role of free K+ in leaf osmotic adjustment under saline conditions. A 5-fold increase in salinity level (from 100 mM to 500 mM) resulted in only a 50% increase in the sap Na+ content, suggesting either a very strict control of xylem Na+ loading or an efficient Na+ removal from leaves. A very strong correlation between NaCl-induced K+ and H+ fluxes was observed in quinoa root, suggesting that a rapid NaCl-induced activation of H+-ATPase is needed to restore otherwise depolarized membrane potential and prevent further K+ leak from the cytosol. Taken together, this work emphasizes the role of inorganic ions for osmotic adjustment in halophytes and calls for more in-depth studies of the mechanisms of vacuolar Na+ sequestration, control of Na+ and K+ xylem loading, and their transport to the shoot.
Keywords: Halophyte, ion loading, membrane transport, osmotic adjustment, potassium, salt stress, sequestration, sodium
Introduction
Salinity is a major environmental issue affecting crop production around the world. Up to 7% of the total land surface is saline (Flowers and Yeo, 1995; Munns, 2002), and about one-third of the world's irrigated land suffers from secondary-induced salinization (Flowers and Yeo, 1995). The economic penalties are in the billion dollar range. As the salinity problem cannot be solved solely via remedial land management, salt-tolerant crops, able to remove excessive salt from the soil and lower the water table at the same time, may contribute significantly to reducing this economical burden. The key to plant engineering for salt tolerance lies in a better understanding of the underlying physiological mechanisms of plant adaptive responses. In addition, the genetic potential of existing salt-tolerant species should be more fully utilized. One of these species is quinoa.
Quinoa (Chenopodium quinoa Willd.) is a seed crop that has been cultivated in the Andean region for thousands of years (Jacobsen et al., 2003). It is an annual broad-leaved plant, 1–2 m tall with deep penetrating roots and can be cultivated from sea level up to an altitude of 3800 m. The plant shows a remarkable tolerance to a wide range of abiotic stresses such as frost, salinity, and drought, as well as an ability to grow on marginal soils (Maughan et al., 2009; Jacobsen et al., 2009). Some varieties can grow in salt concentrations similar to those found in seawater (40 dS m−1) and even higher (Jacobsen et al., 2001), well above the threshold for any known crop species. The physiological mechanisms behind this remarkable abiotic stress tolerance remain unclear. Despite an apparent interest from agronomists towards quinoa (Jensen et al., 2000; Jacobsen et al., 2003; Sanchez et al., 2003; Trognitz, 2003), the underlying physiological mechanisms conferring this tolerance are still not understood. This is specifically true for salinity tolerance. Is quinoa's ability to tolerate high salinity levels related to its superior ability to exclude Na+ from uptake, or is it a result of a remarkable tissue tolerance? Is this tolerance encoded in the roots or shoots? How is the osmotic adjustment in root and leaf tissues achieved? Does quinoa need to invest in the production of compatible solutes for this purpose (e.g. Bohnert and Shen, 1999; Hasegawa et al., 2000), or can the osmotic adjustment be achieved by accumulation and sequestration of inorganic ions only? What is more critical: osmotolerance or specific ion toxicity?
In glycophytes, active Na+ removal from the cytosol is mediated by the SOS1 plasma membrane Na+/H+ exchanger (Blumwald et al., 2000) and is considered to be an important trait conferring salinity tolerance (Hasegawa et al., 2000). Recently, Maughan et al. (2009) have cloned and characterized two homoeologous SOS1 loci in quinoa, but the extent to which SOS1-mediated Na+ extrusion from cytosol contributes to salinity tolerance in quinoa remains to be answered.
Rosa et al. (2009) have recently studied temperature effects on enzyme activities involved in sucrose–starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa seedlings. These authors interpreted the observed changes in cotyledon source–sink relations in salt-treated plants in the light of requirements to supply soluble sugars and proline for osmotic adjustment. They did not, however, address the role of inorganic osmolytes for osmotic adjustment in tissues in their work. Ruffino et al. (2010) mention that at the cotyledonous stage, high adaptability to soil salinity is due to improved metabolic control based on ion absorption, osmolyte accumulation, and osmotic adjustment. So it seems that quinoa does invest in production of compatible solutes without relying predominantly on inorganic ions, as one would expect for halophytic species (e.g. Flowers and Colmer, 2008). Is it really the case?
The aim of this work was to quantify the role of inorganic ions for osmotic adjustment in quinoa, and investigate the relative contribution of root K+ retention, vacuolar Na+ sequestration, control of Na+ and K+ xylem loading, and their transport to the shoot in salinity tolerance in quinoa.
Materials and methods
Plant material and growth conditions
A daylength-neutral quinoa (C. quinoa Willd.) cv. 5206 was used in the experiments. The cultivar has been bred and selected at the University of Copenhagen from material originating from a cross between southern Chilean and Peruvian lines. Seeds were propagated in the field in Denmark. Plants were grown in a University of Tasmania glasshouse between November 2007 and May 2008 in 10.0 l black plastic pots, with two plants per pot. The standard potting mix (70% composted pine bark; 20% coarse sand; 10% sphagnum peat; Limil at 1.8 kg m−3; and dolomite at 1.8 kg m−3) was used. Plant nutrient balance was maintained by adding the slow release Osmocote Plus™ fertilizer (at 6 kg m−3) plus ferrous sulphate (at 500 g m−3). Plants were grown under ambient light in a temperature-controlled glasshouse (between 19 °C and 26 °C and average humidity at ∼65%) at the University of Tasmania (Hobart, Australia).
Salinity treatment and agronomic assessment
Salinity stress was administered to plants in two different ways. Method 1 used a calculated amount of NaCl salt pre-mixed with the potting mix before seeds were planted. The amount of salt added ranged from 1.8 g l−1 to 9.2 g l−1 to create a desirable range of NaCl concentration (e.g. between 100 mM and 500 mM in the saturated paste extract from the soil). Plants were watered on a daily basis (either hand watering or using overhead irrigation) in a way that ensured that no excess leaching from the pots occurred. A saucer was placed under each pot to retain salt and other nutrients. In this way, quinoa seeds were exposed to salinity from the very first moments of imbibition, thus mimicking field conditions.
In another series of experiments (Method 2), NaCl was added to pots directly with irrigation water. Seedlings were established under control conditions (no salt) until 10 d old, and NaCl treatment was then applied in 40 mM d−1 increments until the desired concentration was reached.
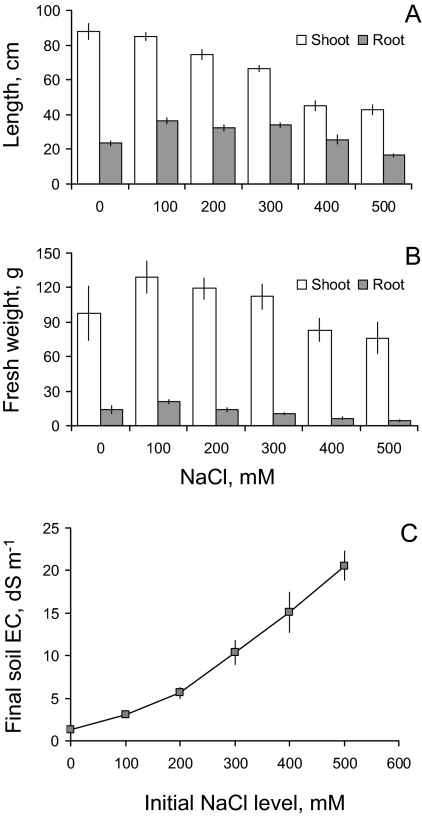
It should be noted that, as plants were grown in a pot of limited size, some NaCl was removed from the pot by transpiring plants (in Method 1) and transported to the shoots. This resulted in a gradual decline of soil NaCl levels over the duration of the experiment. However, NaCl stress was still substantial; even a few months after salt was added to the pot, ∼50% of the salt initially added was still present (Fig. 2C). This difference would be much less during earlier stages of the trial, making a comparison between both methods possible.
Fig. 2.
Agronomic characteristics of quinoa plants grown at various salinity levels. NaCl salt was pre-mixed with the soil before seeds were planted (Method 1). (A) Root and shoot length; (B) root and shoot fresh weight. Mean ±SE (n=6). (C) Soil electrical conductivity (EC) data at the end of the experiment using Method 1. Mean ±SE (n=6). Although some salt was removed from the pot (both as a result of accumulation in plants and due to some minor leaching), the EC values remained sufficiently high.
The overall duration of salinity treatments in both experiments was ∼70 d, after which plants were removed from pots, their shoot, root length, and biomass measured, and appropriate leaf samples collected for analysis. The pots were arranged in a randomized complete block design, with six replicates (pots) per treatment.
Chlorophyll fluorescence measurements
The maximum photochemical efficiency of photosystem II (PSII; chlorophyll fluorescence Fv/Fm ratio) was measured in dark-adapted quinoa leaves using an OS-30p (Optics-Sciences Inc., USA) portable pulse-modulated fluorometer following a previously described protocol (Smethurst and Shabala, 2003).
Leaf sap Na+ and K+ content
Young (third from the tip) and old (the lowest non-senescing leaf at the base) quinoa leaves were sampled, wrapped in aluminium foil, and immediately frozen at –18 °C. Close to the time of measurements, frozen samples were thawed and hand squeezed to extract all the sap as described in Cuin et al. (2009). The collected sample was thoroughly mixed, diluted by a factor of 50, and measured for its K+ and Na+ concentration using flame photometry (Corning 410C, Essex, UK).
Sap osmolality measurements
Quinoa leaf sap was extracted as described above, and the osmolality of the undiluted sap was measured using a vapour pressure osmometer (Vapro; Wescor Inc., Logan, UT, USA).
Seed germination experiments
Quinoa seeds were germinated in Petri dishes on wet filter paper at 25±1 °C in the dark for 7 d. Seeds were exposed to six levels of osmotic stress (osmolality range 0–0.9 Osm kg−1) using two types of osmotica: ionic (NaCl) and non-ionic [mannitol or polyethylene glycol (PEG)]. For NaCl treatment, seed germination was tested at five salt concentrations (100, 200, 300, 400, and 500 mM) and compared with control (distilled water). Seeds were also germinated in non-ionic solutions isotonic to the above concentrations using either mannitol (up to 300 mM NaCl equivalent) or PEG6000 (for the two highest concentrations). The final osmolalities of the prepared solutions were checked using a Wescor Vapro osmometer (Wescor Inc., Logan, UT, USA). Each treatment was replicated four times, with 25 seeds used in each replication. The number of germinated seeds was counted every day, and the germination test lasted 7 d.
Electrophysiology
Net K+ and H+ fluxes were measured non-invasively using a microelectrode ion flux measuring (MIFE) technique (UTas Innovation Ltd, Hobart, Australia). Specific details on microelectrode fabrication and calibration are given in Shabala and Shabala (2002), and the principles of the MIFE measurements are comprehensively described in Shabala et al. (2006). Electrodes were equilibrated in an appropriate set of standards (0.2–1 mM for K+; pH 4.6–7.8 for H+). Electrodes with a response of <50 mV per decade and correlation <0.999 were discarded.
Quinoa seeds were surface sterilized by immersion in 1% commercial bleach for 15 min, washed thoroughly under flowing distilled water, and then germinated in vertically aligned Petri dishes on a Pythogel medium as described elsewhere (Tegg et al., 2005). Seedlings were used for flux measurements after day 4, when their average root length was ∼30–40 mm. Seedlings were taken out of the incubation chamber and mounted in a 5 ml chamber filled with a basic salt medium (BSM) bath solution (0.2 mM KCl, 0.1 mM CaCl2, pH 5.6, unbuffered). K+ and H+ microelectrodes were mounted on a multimanipulator providing three-dimensional positioning. Net ion fluxes were measured in the mature (10 mm from the tip) zone. Electrodes were positioned 50 μm above the root surface, with their tips ∼3 μm apart. During measurement, electrodes were moved in a square-wave manner between 50 μm and 90 μm at a frequency of 0.1 Hz by a computerized stepper motor as described elsewhere (Shabala et al., 2006). Ion fluxes were measured in steady conditions for ∼5–20 min to make sure that no oscillations were present. Then the desired NaCl concentration was added to the bath solution, and transient ion flux kinetics were measured for another 20–30 min. The effect of ionic strength of electrode characteristics (Shabala et al., 2006) was accounted for by including an appropriate concentration of NaCl in each K+ and pH standard.
Results
Growth responses, germination, and photosynthetic characteristics
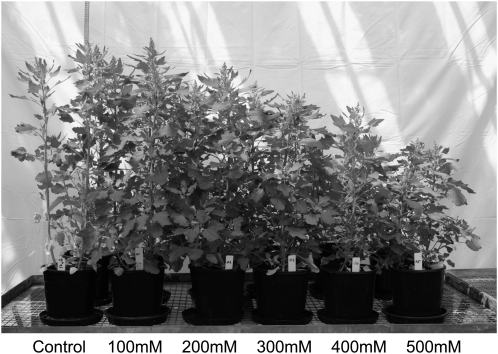
Quinoa plants responded to salinity treatment in a manner typical of halophytes (Fig. 1). The optimal plant growth was achieved at NaCl concentrations of ∼100 mM, regardless of whether salt stress was administered abruptly (e.g. seeds planted directly in a saline medium; Fig. 2) or by a gradual increase in NaCl levels (data not shown). The maximum shoot biomass was obtained for 100 mM NaCl treatment in both cases. Even the most severe salinity treatment (500 mM; roughly equivalent to seawater) caused a relatively minor (∼20%) and barely significant (at P <0.05) reduction in shoot fresh weight (Fig. 2B). Much more pronounced was the NaCl effect on shoot length, with a nearly 50% reduction observed for the most severe (500 mM) treatment. Nonetheless, even in this case, plants were able to produce some viable seeds (data not shown).
Fig. 1.
Quinoa plants (genotype 5206) grown at various NaCl levels for 70 d.
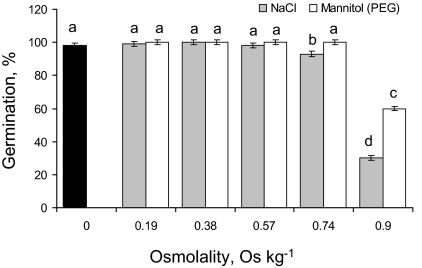
A significant (at P <0.05) effect of salinity on seed germination was obvious only for concentrations >400 mM (Fig. 3, filled bars). Comparison between isotonic NaCl and mannitol or PEG solutions suggests that specific ion toxicity is the major reason for the observed decline in seed germination (Fig. 3). Indeed, at the highest concentration (500 mM NaCl; equivalent to 0.9 Osm kg−1) seed germination was only 24±1.5%, while for isotonic PEG treatment quinoa germination was 60±2% (significant at P <0.001; Fig. 3).
Fig. 3.
Percentage of germinated quinoa seeds after 7 d of treatment with various concentrations of NaCl, isotonic mannitol, or PEG solutions. Mean ±SE (n=4). Different lowercase letters indicate a significant difference at P <0.05.
No significant (P <0.05) effect of salinity on leaf photosynthetic performance was observed for the entire salinity range, with Fv/Fm values (maximum photochemical efficiency of PSII estimated by chlorophyll fluorescence measurements) being >0.8 for all treatment (data not show).
Ionic and osmotic relations in shoots
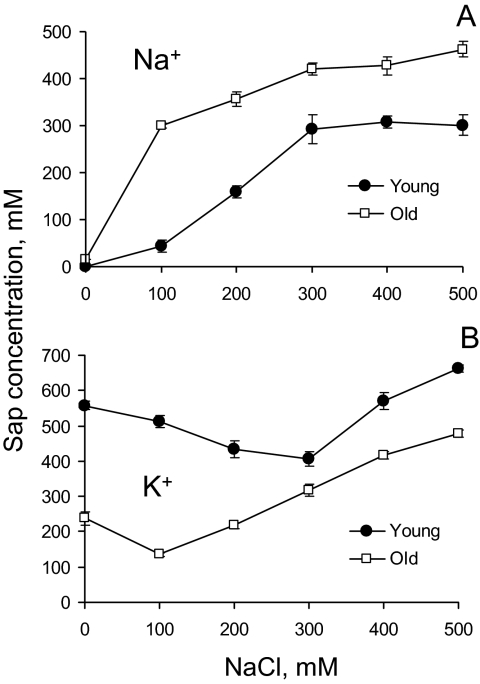
A substantial difference was found between sap K+ and Na+ concentrations in young and old leaves over the entire range of salinity treatments. As expected, an increase in the external NaCl level caused a progressive increase in the amount of Na+ accumulated in plant leaves (Fig. 4A); this increase was more pronounced in old leaves. Interestingly, the largest difference between leaf Na+ content in old and young leaves was for 100 mM NaCl treatment (∼7.5-fold; Fig. 4A), while for external NaCl concentrations >300 mM this difference was only 1.5-fold.
Fig. 4.
Ion content in the shoot sap (measured by squeezing frozen and thawed leaf samples) from young and old leaves from plants grown at various NaCl levels. Mean ±SE (n=5). The difference between old and young leaves is significant at any time at P <0.01.
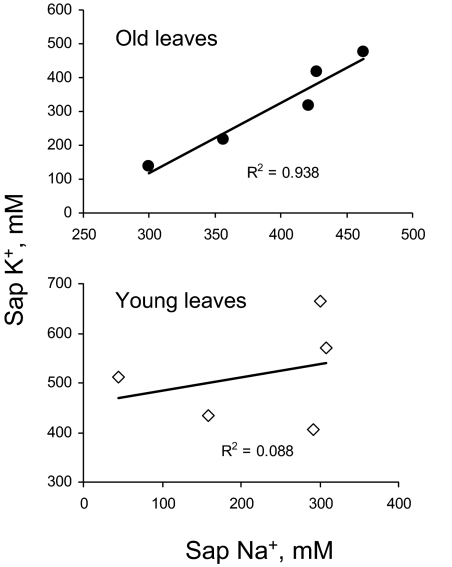
In contrast, leaf sap K+ content was significantly (P <0.05) higher in young leaves (Fig. 4B). No clear trend for salinity impact on sap K+ level in young leaves was observed. As a result, the sap K/Na ratio was much higher in young compared with old leaves (Table 1). In old leaves, increased external NaCl concentrations caused a progressive increase in sap K+ in old leaves (Fig. 4B), with a >3-fold difference between 100 mM and 500 mM treatments. As a result, a strong positive correlation was found between sap K+ and Na+ content in old leaves (r=0.97; Fig. 5A) while no significant correlation (P <0.05) was found in young leaves (Fig. 5B).
Table 1.
Potassium to sodium ratio in old and young leaves of quinoa plants grown at various external NaCl levels (calculated from the original data shown in Fig. 4)
| External NaCl (mM) | Old leaves | Young leaves | Young/old (%) |
| 0 | 15.9 | 558 | 3517 |
| 100 | 0.5 | 11.6 | 2567 |
| 200 | 0.6 | 2.8 | 451 |
| 300 | 0.8 | 1.4 | 183 |
| 400 | 1.0 | 1.9 | 191 |
| 500 | 1.0 | 2.2 | 214 |
Fig. 5.
Correlation between shoot K+ and Na+ sap concentration in young and old quinoa leaves grown at various NaCl levels in glasshouse experiments. Mean ±SE (n=5).
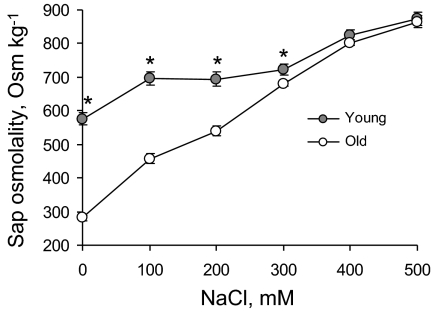
Increasing NaCl levels resulted in a progressive increase in leaf sap osmolality (Fig. 6). This increase was much steeper in old compared with young leaves. A 2-fold difference in sap osmolality was measured for low NaCl levels, while at the highest NaCl treatment (500 mM), no significant (P <0.05) difference in osmolality was found between old and young leaves (Fig. 6).
Fig. 6.
The dose dependency of the leaf sap osmolality in old and young quinoa leaves exposed to various levels of NaCl. Mean ±SE (n=5). * indicates a significant difference between young and old leaves at P <0.05.
Ion flux kinetics
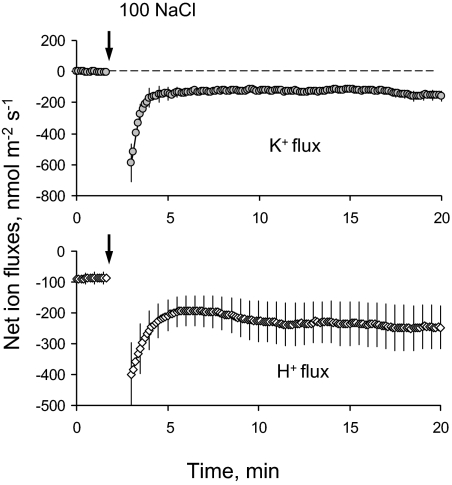
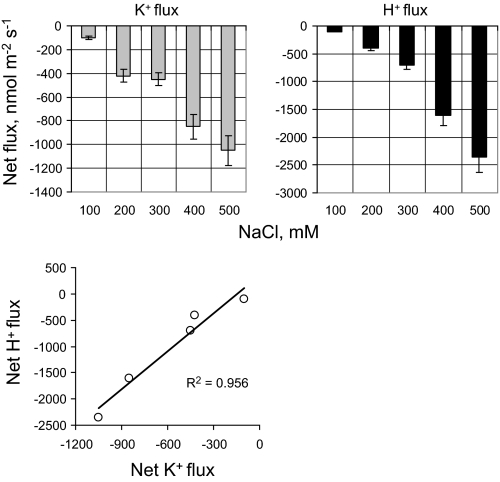
Net fluxes of K+ and H+ were measured in the root zone of 4-day-old quinoa seedlings. Similar to previous observations for other species (reviewed in Shabala and Cuin, 2008), the onset of the salt stress caused a rapid and massive net K+ efflux from the root (Fig. 7A). This K+ efflux was accompanied by increased efflux in net H+ (Fig. 7B), resulting in a very strong positive correlation (r=0.98; Fig. 8) between fluxes of these two ions. The observed efflux of each ion showed a clear dose dependency on the external NaCl concentrations (Fig. 8).
Fig. 7.
Typical transient kinetics of net K+ and H+ fluxes measured from roots of 4-day-old quinoa seedlings in response to 100 mM NaCl treatment. Mean ±SE (n=5). For all MIFE experiments, the sign convention is ‘influx positive’.
Fig. 8.
The dose dependency of NaCl-induced net ion fluxes in 4-day-old quinoa roots. Steady-state K+ and H+ fluxes were measured 1 h after onset of acute salt stress. Mean ±SE (n=5).
Discussion
Osmotolerance versus specific ion toxicity
The importance of separating osmotic stress and sodium toxicity has been repeatedly stressed by many authors (e.g. Munns and Tester, 2008). There is also an ongoing debate in the literature as to what is considered to be more ‘natural’ to plants: being exposed to an abrupt salt stress (NaCl ‘up-shock’), or to have salinity levels increased incrementally. Proponents of the latter approach usually argue that, in many cases, we are dealing with transient salinity resulting from the raising of water tables and, hence, the salinity build-up in the rhizosphere is thought to be a gradual process. As a result, a large number of researchers have adopted this gradual increase approach in their studies (e.g. Munns et al., 2000; Rivelli et al., 2002). While it may indeed be true for many situations, the seeds of many native plants are thought to have an inherent ability to germinate in soils/conditions that are already highly saline. Also, the ability of a plant to be able to handle an abrupt saline ‘up-shock’ may be important when saline water is used for irrigation purposes. Therefore, some researchers and breeders advocate direct seed planting into saline media (Meixue Zhou, personal communication).
The results reported here show that, in the case of quinoa, there is not much difference in plant agronomic and growth responses to these two different ways of applying the salt stress. Optimal plant growth and biomass was achieved at NaCl levels between 100 mM and 200 mM NaCl. Hence, quinoa appears to have a very efficient system to adjust osmotically to abrupt NaCl stress. According to Munns (2002), the time scales for the osmotic and specific ionic component of salinity stress differ significantly, with the osmotic component dominating over the first several weeks. This was not demonstrated previously (Jensen et al., 2000; Jacobsen et al., 2009). Interestingly, however, a comparison of seed germination ability in isotonic NaCl/PEG solution suggests that most probably Na or other specific ion toxicity is more detrimental to quinoa plants compared with the osmotic component of salt stress; this is illustrated by the ∼2-fold difference at the highest treatment (0.9 Osm kg−1 which is equivalent to 500 mM NaCl). This high salinity tolerance of quinoa was explained by the existence of a significant gradient in the distribution of potentially toxic (Na and Cl) and non-toxic essential (K, Mg, Ca, P, and S) elements across the seed coat of salt-treated plants, as well as in the embryo (Koyro and Eisa, 2008). Hence, it may be suggested that, once the seed's ability to exclude toxic Na+ from the developing embryo fails, ion toxicity occurs, and seeds becomes unviable. This process appears to be unrelated to the osmotic component of salt stress.
The specific details of the above control of compartmentalization of toxic and essential nutrients, specifically Na+ and K+, in germinating seed warrant some further investigation. Earlier it was shown that significant changes in the profiles of net ion fluxes occurred from the surface of germinating wheat seed as early as 1 h after seed imbibition (Shabala et al., 2000). The evidence was presented that this process was not related to some general loss of membrane integrity but originated from activities of plasma membrane transporters. It will be interesting to follow-up this issue in the future in an attempt to understand what specific Na+ and K+ transport systems are activated in germinating quinoa seeds, and see what the time sequence of this activation is.
Osmotic adjustment and the relative contribution of organic and inorganic osmolytes
Given the high energy cost of de novo synthesis of organic osmolytes (Raven, 1985), it is much more advantageous for the plant to use sodium as a metabolically cheap osmoticum for the purposes of osmotic adjustment, assuming it does not interfere with cell metabolism. This appears to be the case for quinoa. The simple model calculations show that >95% of cell osmotic turgor in old leaves, and between 80% and 100% of cell turgor in young leaves, may be adjusted by means of accumulation of Na+ and K+ only (Table 2). The rest may be attributed to chloride. Although the actual Cl– in quinoa leaf sap samples was not measured in this work, it is generally accepted that its content is ∼1.2 times that for Na+ (see James et al., 2006). This is more than enough to add the few remaining percentage points required for 100% osmotic adjustment. Thus, it appears that in both young and old quinoa leaves the full osmotic adjustment may be achieved by means of inorganic osmolytes only.
Table 2.
Relative contribution of K+ and Na+ to the total shoot sap osmolality in quinoa leaves grown at various salinity levels
| NaCl (mM) | Old leaves |
Young leaves |
||||
| Na+K (mOsm) | Measured (mOsm) | % measured | Na+K (mOsm) | Measured (mOsm) | % measured | |
| 0 | 253 | 281 | 90 | 559 | 575 | 97 |
| 100 | 436 | 458 | 95 | 556 | 696 | 80 |
| 200 | 575 | 540 | 107 | 593 | 694 | 85 |
| 300 | 740 | 681 | 109 | 697 | 722 | 96 |
| 400 | 845 | 800 | 106 | 878 | 825 | 106 |
| 500 | 938 | 865 | 108 | 964 | 873 | 110 |
The relative contribution of organic versus inorganic osmolytes is still highly disputed in the literature. A plant's ability to accumulate compatible solutes is frequently cited as a key putative mechanism for increasing the yield of crops subjected to osmotic stress, and compatible solutes have been a ‘hot topic’ for some time (Bohnert and Shen, 1999; Hasegawa et al., 2000), with numerous genetic engineering attempts being made to manipulate the biosynthesis pathway of compatible solutes in order to enhance salt tolerance (e.g. Chen and Murata, 2002).
A stress-induced increase in the total level of soluble sugars, proline, and glycinebetaine was reported for quinoa (Jacobsen et al., 2007, 2009; Ruffino et al., 2010). Glycinebetaine and other betaine derivatives have long been recognized as major osmolytes in species of the Chenopodiaceae family (Flowers and Colmer, 2008). However, the reported levels for both proline and glycinebetaine (7–13 mM and 3–6 mM, respectively) appear to be far too low to contribute significantly to cell osmotic adjustment (Ruffino et al., 2010). Indeed, these two compatible solutes may account for only ∼3% of the total osmolality values measured in the present experiments (Fig. 6; Table 2). This is consistent with previous observations in glycophytes (Bohnert and Shen, 1999) and suggests an indirect role for compatible solutes in plant osmotic adjustment. At the same time, turgor adjustment by inorganic ions has been previously shown in other halophytes (Inan et al., 2004) and in salt-tolerant characeae (Bisson and Kirst, 1980). Earlier it was shown that physiologically relevant concentrations of free amino acids (Cuin and Shabala, 2007), as well glycinebetaine (Cuin and Shabala, 2005), are efficient in controlling K+ transport across the plasma membrane, thus contributing to cell osmotic adjustment indirectly, via potassium retention. The present observations of very high K+ levels in quinoa leaf sap (400–650 mM in young leaves; Fig. 4) are consistent with this suggestion.
Consistently higher K+ and lower Na+ levels were found in young as compared with old leaves, for all salinity treatments (Fig. 4); this resulted in much higher sap K/Na ratios in young leaves (Table 1). Protecting young developing leaves from excessive amounts of Na+ has long been considered as a key attribute of Na+ compartmentalization at the whole-plant level in many species (Munns, 2002). It appears that quinoa is no exception to this rule. As a result, at no salinity level was the maximum photochemical efficiency of PSII (measured as the Fv/Fm chlorophyll fluorescence ratio from dark-adapted leaves) significantly (P <0.05) reduced (data not shown). At the same time, to drive the leaf expansion growth, young leaves posses much higher osmolalities (Fig. 6). This is consistent with evidence of the importance of maintaining the K/Na ratio for salinity tolerance (reviewed in Shabala and Cuin, 2008).
Interestingly, a 5-fold increase in salinity level (from 100 mM to 500 mM) resulted in only a 50% increase in the sap Na+ content in old leaves (Fig. 4). This may suggest either a very strict control of xylem Na+ loading, or an efficient mechanism for Na removal from leaves. Both these mechanisms are often discussed as key determinants of salinity tolerance in various plant species (Munns and Tester, 2008). Given the lack of research on this topic in halophytes, it will be interesting to reveal the contribution of the specific ion transporters (e.g. SKOR or HKT) to this process in quinoa in the future.
One more aspect deserves further comment. The shoot sap K+ progressively increased with increased salinity in old leaves (Fig. 4). To some extent, this was a ‘counterintuitive’ observation, given the numerous reports on salinity-induced K+ deficiency in various species (Flowers and Hajibagheri, 2001; Genc et al., 2007). However, all the above reports deal with glycophyte species, while halophytes are known to keep their K+ concentration steady or increasing with salinity (Bisson and Kirst, 1980; Inan et al., 2004). Also, it should be kept in mind that the present data report sap K+ concentrations analysed by the freeze–thaw method. This sap is composed of vacuolar, cytosolic, and apoplastic free K+. This differs significantly from the total K+ measured by acid digestion that also includes structural K+ used in a variety of organic molecules. A comprehensive comparison between two methods, acid digestion versus the freeze–thaw method, was recently published (Cuin et al., 2009). In that work, 23 of 25 durum varieties showed a statistically significant (P <0.05) sap K+ increase under saline conditions, while the total potassium content had sharply declined. Thus, the present quinoa data are consistent with these observations. Given the fact that most of the sap K+ measured originates from the vacuole, these data may be interpreted as evidence for the important role of free K+ in leaf osmotic adjustment under saline conditions.
Root ion fluxes
It is widely accepted by now that the salinity tolerance of plants is attributed to their ability to maintain an optimal cytosolic K/Na ratio (reviewed by Shabala and Cuin, 2008). Previously a strong positive correlation between root K+ retention ability and salinity tolerance in barley was reported (Chen et al., 2007a, b; Shabala and Cuin, 2008). In this study, a substantial K+ efflux was observed (Fig. 7). For 100 mM NaCl treatment, the peak value reached −600 nmol m−2 s−1; even steady-state values were at the –100 nmol m−2 s−1 level. This is comparable with tolerant barley varieties (Chen et al., 2007b). Given the fact that halophytes are classified as ‘tolerant’, by definition, one would expect much less K+ efflux.
Several possible explanations can be put forward. First, among 14 quinoa varieties screened, genotype 5206 was one of the most salt sensitive (S-EJ, unpublished). Thus, some overlap in physiological responses between the most tolerant barley genotypes and the most sensitive quinoa genotypes can be expected. Secondly, and most likely, is that in quinoa, Na+ is taken up by roots and transported to the shoot to be used for osmotic adjustment purposes and to drive expansion growth (see above). To provide a charge balance, some cations have to be expelled from the cytosol, and potassium is widely understood to be used for such charge balancing (Marschner, 1995).
There also appears to be some inconsistency between the sap concentration data (Fig. 4) and the K+ efflux measured by MIFE (Fig. 7). If the shoots accumulate K+, there cannot be sustained K+ efflux from the root. Thus, the observed efflux cannot be a symptom of salt stress pathology and must be transient. This issue should be addressed in future experiments by comparing long- and short-term effects of salt stress on K+ fluxes from quinoa roots.
Another interesting observation was that there was a very strong correlation between K+ and H+ fluxes observed in the present experiments (Fig. 8). Given a strong inhibition of NaCl-induced H+ flux by vanadate, a known blocker of the plasma membrane H+-ATPase pump, these results may be interpreted as evidence for the rapid NaCl-induced activation of H+-ATPase needed to restore otherwise depolarized membrane potential and thereby prevent any further K+ leak from the cytosol (data not shown).
The pump is the energizing element for both turgor up-regulation and sodium exclusion from the cytoplasm, and an NaCl-induced increase in plasma membrane H+-ATPase activity has been reported for many halophytic species (Yao et al., 1992; Vera-Estrella et al., 1999; Beilby and Shepherd, 2001). In the light of the existing correlation between the intrinsic level of H+-ATPase activity and salinity tolerance in some species (Chen et al., 2007a), elucidation of the specific mechanisms of H+-ATPase regulation under saline conditions in quinoa warrants further investigation.
In conclusion, this work emphasizes the role of inorganic ions for osmotic adjustment in halophytes and calls for more in-depth studies of the mechanisms of vacuolar Na+ sequestration, control of Na+ and K+ xylem loading, and their transport to the shoot.
Acknowledgments
We are grateful to Alex Mackay for his technical assistance in preparation of this manuscript. Supported by the Australian Research Council Discovery and Linkage grants to Associate Professor Sergey Shabala.
References
- Beilby MJ, Shepherd VA. Modeling the current–voltage characteristics of charophyte membranes. II. The effect of salinity on membranes of Lamprothamnium papulosum. Journal of Membrane Biology. 2001;181:77–89. doi: 10.1007/pl00020977. [DOI] [PubMed] [Google Scholar]
- Bisson MA, Kirst GO. Lamprothamnium, a euryhaline charophyte. 1. Osmotic relations and membrane-potential at steady-state. Journal of Experimental Botany. 1980;31:1223–1235. [Google Scholar]
- Blumwald E. Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology. 2000;12:431–434. doi: 10.1016/s0955-0674(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Bohnert HJ, Shen B. Transformation and compatible solutes. Scientia Horticulturae. 1999;78:237–260. [Google Scholar]
- Chen THH, Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Current Opinion in Plant Biology. 2002;5:250–257. doi: 10.1016/s1369-5266(02)00255-8. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Pottosin, Cuin TA, et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology. 2007b;145:1714–1725. doi: 10.1104/pp.107.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Zhou MX, Newman IA, Mendham NJ, Zhang GP, Shabala S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Functional Plant Biology. 2007a;34:150–162. doi: 10.1071/FP06237. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Shabala S. Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant and Cell Physiology. 2005;46:1924–1933. doi: 10.1093/pcp/pci205. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Shabala S. Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta. 2007;225:753–761. doi: 10.1007/s00425-006-0386-x. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S. Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Functional Plant Biology. 2009;36:1110–1119. doi: 10.1071/FP09051. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Hajibagheri MA. Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant and Soil. 2001;231:1–9. [Google Scholar]
- Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants: where next? Australian Journal of Plant Physiology. 1995;22:875–884. [Google Scholar]
- Genc Y, McDonald GK, Tester M. Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant, Cell and Environment. 2007;30:1486–1498. doi: 10.1111/j.1365-3040.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annual Review of Plant Biology. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Inan G, Zhang Q, Li PH, et al. Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiology. 2004;135:1718–1737. doi: 10.1104/pp.104.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Liu F, Jensen CR. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.) Scientia Horticulturae. 2009;122:281–287. [Google Scholar]
- Jacobsen SE, Quispe H, Mujica A. Scientists and farmer–partners in research for the 21st century. 2001. Quinoa: an alternative crop for saline soils in the Andes. CIP Program Report 1999–2000, 403–408. [Google Scholar]
- Jacobsen SE, Monteros C, Corcuera LJ, Bravo LA, Christiansen JL, Mujica A. Frost resistance mechanisms in quinoa (Chenopodium quinoa Willd.) European Journal of Agronomy. 2007;26:471–475. [Google Scholar]
- Jacobsen SE, Mujica A, Jensen CR. The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Reviews International. 2003;19:99–109. [Google Scholar]
- James RA, Munns R, von Caemmerer S, Trejo C, Miller C, Condon T. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+, and Cl– in salt-affected barley and durum wheat. Plant, Cell and Environment. 2006;29:2185–2197. doi: 10.1111/j.1365-3040.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Jensen CR, Jacobsen SE, Andersen MN, Núńez N, Andersen SD, Rasmussen L, Mogensen VO. Leaf gas exchange and water relation characteristics of field quinoa (Chenopodium quinoa Willd.) during soil drying. European Journal of Agronomy. 2000;13:11–25. [Google Scholar]
- Koyro HW, Eisa SS. Effect of salinity on composition, viability and germination of seeds of Chenopodium quinoa Willd. Plant and Soil. 2008;302:79–90. [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Maughan PJ, Turner TB, Coleman CE, Elzinga DB, Jellen EN, Morales JA, Udall JA, Fairbanks DJ, Bonifacio A. Characterization of Salt Overly Sensitive 1 (SOS 1) gene homoeologs in quinoa (Chenopodium quinoa Wilid.) Genome. 2009;52:647–657. doi: 10.1139/G09-041. [DOI] [PubMed] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant, Cell and Environment. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Munns R, Hare RA, James RA, Rebetzke GJ. Genetic variation for improving the salt tolerance of durum wheat. Australian Journal of Agricultural Research. 2000;51:69–74. [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Raven JA. Regulation of pH and generation of osmolarity in vascular plants: a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytologist. 1985;101:25–77. doi: 10.1111/j.1469-8137.1985.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Rivelli AR, James RA, Munns R, Condon AG. Effect of salinity on water relations and growth of wheat genotypes with contrasting sodium uptake. Functional Plant Biology. 2002;29:1065–1074. doi: 10.1071/PP01154. [DOI] [PubMed] [Google Scholar]
- Rosa M, Hilal M, Gonzalez JA, Prado FE. Low-temperature effect on enzyme activities involved in sucrose–starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa (Chenopodium quinoa Willd.) seedlings. Plant Physiology and Biochemistry. 2009;47:300–307. doi: 10.1016/j.plaphy.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Ruffino AMC, Rosa M, Hilal M, Gonzalez JA, Prado FE. The role of cotyledon metabolism in the establishment of quinoa (Chenopodium quinoa) seedlings growing under salinity. Plant and Soil. 2010;326:213–224. [Google Scholar]
- Sanchez HB, Lemeur R, Van Damme P, Jacobsen SE. Ecophysiological analysis of drought and salinity stress of quinoa (Chenopodium quinoa Willd.) Food Reviews International. 2003;19:111–119. [Google Scholar]
- Shabala L, Ross T, McMeekin T, Shabala S. Non-invasive microelectrode ion flux measurements to study adaptive responses of microorganisms to the environment. FEMS Microbiology Reviews. 2006;30:472–486. doi: 10.1111/j.1574-6976.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. Potassium transport and plant salt tolerance. Physiologia Plantarum. 2008;133:651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- Shabala S, Newman I, Wilson S, Clark R. Nutrient uptake patterns over the surface of germinating wheat seeds. Australian Journal of Plant Physiology. 2000;27:89–97. [Google Scholar]
- Shabala S, Shabala L. Kinetics of net H+, Ca2+, K+, Na+, NH4+, and Cl– fluxes associated with post-chilling recovery of plasma membrane transporters in Zea mays leaf and root tissues. Physiologia Plantarum. 2002;114:47–56. doi: 10.1046/j.0031-9317.2001.1140108.x. [DOI] [PubMed] [Google Scholar]
- Smethurst CF, Shabala S. Screening methods for waterlogging tolerance in lucerne: comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Functional Plant Biology. 2003;30:335–343. doi: 10.1071/FP02192. [DOI] [PubMed] [Google Scholar]
- Tegg RS, Melian L, Wilson CR, Shabala S. Plant cell growth and ion flux responses to the streptomycete phytotoxin thaxtomin A: calcium and hydrogen flux patterns revealed by the non-invasive MIFE technique. Plant and Cell Physiology. 2005;46:638–648. doi: 10.1093/pcp/pci069. [DOI] [PubMed] [Google Scholar]
- Trognitz BR. Prospects of breeding quinoa for tolerance to abiotic stress. Food Reviews International. 2003;19:129–137. [Google Scholar]
- Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O. Salt stress in Mesembryanthemum crystallinum L cell suspensions activates adaptive mechanisms similar to those observed in the whole plant. Planta. 1999;207:426–435. doi: 10.1007/s004250050501. [DOI] [PubMed] [Google Scholar]
- Yao X, Bisson MA, Brzezicki LJ. ATP-driven proton pumping in two species of Chara differing in salt tolerance. Plant, Cell and Environment. 1992;15:199–210. [Google Scholar]