Abstract
The stomata of conifers display very little short-term response to changes in atmospheric CO2 concentration (Ca), whereas the stomatal responses of angiosperms to Ca increase in response to water stress. This behaviour of angiosperm stomata appears to be dependent on foliar levels of abscisic acid (ABAf). Here two alternative explanations for the stomatal insensitivity of conifers to Ca are tested: that conifers have either low ABAf or a higher or absent threshold for ABA-induced sensitivity. The responsiveness of stomatal conductance (gs) to a sequence of transitions in Ca (386, 100, and 600 μmol mol−1) was recorded over a range of ABAf in an angiosperm and two divergent conifer species. The different ABA levels were induced by a mild drought cycle. Although the angiosperm and conifer species showed similar proportional increases in ABAf following drought, conifer stomata remained insensitive to changes in Ca whereas angiosperm stomata showed enhanced sensitivity with increasing ABAf. The conifers, however, had much higher ABAf prior to drought than the angiosperm species, suggesting that non-sensitivity to Ca in these conifers was due to an absent or inactive response/signalling pathway rather than insufficient ABAf.
Keywords: Abscisic acid, angiosperm, carbon dioxide, conifer, drought, stomata, stomatal conductance
Introduction
Environmentally responsive stomata are a prerequisite for the function of leaves on land. The turgor pressure of guard cells, responding to environmental and physiological signals (Schroeder et al., 2001), modulates stomatal aperture thereby regulating leaf water loss and carbon dioxide assimilation. Stomatal conductivity to the diffusion of gases (gs) responds to atmospheric carbon dioxide concentration (Ca) across a wide diversity of angiosperm species (Linsbauer, 1917; Morison, 1985). This instantaneous response involves stomatal opening at low Ca and stomatal closure at high Ca, thus altering gs. The signal for the stomatal response to Ca remains enigmatic, though it appears to originate in the mesophyll (Mott et al., 2008).
The stomatal response to Ca, particularly stomatal closure in response to increasing Ca, is an important process in angiosperms because it provides a basis for the optimization of water use during photosynthesis (Farquhar and Sharkey, 1982). As a central component of water use optimization, the stomatal response to Ca has significant agricultural as well as ecological implications. The response of gs to changes in Ca is therefore of immense significance to plants (Miller-Rushing et al., 2009) and for atmospheric water balance (Betts et al., 2007) as Ca continues to rise.
Although instantaneous stomatal responses to Ca have been recorded from a number of C3 and C4 angiosperm herbs and trees (Morison, 1987), the stomatal sensitivity of individual plants to changes in Ca can vary considerably depending on growth conditions and endogenous chemical signals (Raschke and Hedrich, 1985; Talbott et al., 1996). Variation in stomatal response to Ca in intact leaves has been attributed to numerous physiological factors including abscisic acid (ABA) level (Raschke, 1975; Dubbe et al., 1978; Eamus and Narayan, 1989), humidity or vapour pressure deficit (VPD) (Bunce, 1998; Talbott et al., 2003), photosynthetic light response (Wong et al., 1978; Messinger et al., 2006), and levels of the ABA biosynthetic precursor zeaxanthin (Zhu et al., 1998; Seo and Koshiba, 2002).
ABA was first demonstrated as an important regulator of stomatal responses to Ca in the asteraceous herb Xanthium strumarium L. (Raschke, 1975). Raschke (1975) found that the stomata of leaves from well-watered plants suspended in pure water did not respond to perturbations in Ca; however, following the addition of exogenous ABA to the transpiration stream, stomata were significantly sensitized to changes in Ca. ABA-induced sensitivity of stomata to Ca and the resulting effects on transpiration, assimilation, and water-use efficiency were quantified in five angiosperm species by Dubbe et al. (1978). The requirement of ABA for a stomatal response to Ca has subsequently been confirmed in many other angiosperm species including trees (Ridolfi et al., 1996) and herbs (Eamus and Narayan, 1989; Bunce, 1998; Leymarie et al., 1998). Current consensus recognizes ABA as a key modulator of stomatal sensitivity to Ca, with recent biochemical studies demonstrating that changes in Ca alter internal guard cell Ca2+ concentration (Young et al., 2006), with ABA enhancing the sensitivity of guard cell anion channels and pumps to cytosolic Ca2+ concentration (Siegel et al., 2009).
Recently, Brodribb et al. (2009) identified a broad phylogenetic pattern in the sensitivity of stomata to Ca, in which non-angiosperms (conifers, ferns, and a lycopod) showed very weak or absent responses to changing Ca. A similar lack of stomatal response to Ca in conifers was reported in a few Picea and Pinus species (Beadle et al., 1979; Morison and Jarvis, 1983) as well as in long-term elevated CO2 studies (Medlyn et al., 2001). The physiological basis of this phylogenetic variation in stomatal sensitivity to Ca is unknown. Here two alternative explanations for stomatal insensitivity to Ca in conifers are investigated. The first is that conifer stomata are insensitive to Ca at all normal levels of endogenous foliar ABA (ABAf) due to an absent or very high threshold for ABA-induced stomatal sensitivity to Ca. The second alternative is that conifers have low levels of ABAf and therefore may not differ from angiosperms in stomatal responses to Ca when the ABA level is increased. Using mild drought treatments as an effective way of causing natural variations in ABAf and thereby natural increases in stomatal sensitivity to Ca, the relationship between stomatal sensitivity to Ca and ABAf is examined in two conifers and an angiosperm species.
Materials and methods
Plant material
Three species were selected for comparison, the ruderal angiosperm herb Senecio minimus Poir. (Asteraceae) acting as an angiosperm control, and two phylogenetically and functionally divergent conifer trees, the relatively slow-growing Callitris rhomboidea R.Br (Cupressaceae) and the fast-growing species Pinus radiata D.Don (Pinaceae). These three species had similar stomatal anatomy with only minimal encryption in the conifer species, a trait believed to have little effect on gas exchange (Roth-Nebelsick et al., 2009).
Senecio minimus seedlings at the second full leaf stage were collected from within their natural range and grown individually in 1.3 l pots containing an 8:2:1 mix of composted pine bark, coarse river sand, and peat moss with added slow release fertilizer. Callitris rhomboidea individuals were grown from seed collected from within their natural range and potted in 2.0 l pots. They were ∼2 years of age at the time of the experiment. Pinus radiata individuals (grown from commercially available seed of composite provenance) were 1 year of age at the time of the experiment and were also potted in 2.0 l pots.
Growth conditions and drought treatment
All plants were grown under controlled glasshouse conditions for at least 8 weeks prior to measurements. Growth conditions were 16 h days at 20 °C/15 °C day/night temperatures, receiving a maximum quantum photosynthetic photon flux density (PPFD) of 1300 μmol quanta m−2 s−1. Natural light was supplemented by sodium vapour lamps to ensure a minimum 300–500 μmol quanta m−2 s−1 at the leaf surface throughout the day period. Relative humidity was maintained at 50% by a dehumidifier coupled to a humidity probe. Maintaining a constant air temperature and relative humidity restricted VPD to a narrow range, meaning that measured transpiration (E) was closely related to stomatal conductance. Plants were watered daily to full pot capacity when not under drought conditions. Measurements of ABAf and gas exchange were carried out over a cycle where plants were initially well watered, then mildly droughted by withholding water and monitoring gas exchange (see below) until E fell to <25% of maximum. The plants were then rewatered and maintained at soil capacity. Leaf water potential (Ψleaf) was measured over drought cycles using a Scholander pressure chamber on single leaves (S. minimus), small shoots (C. rhomboidea), or fascicles (P. radiata), excised, and immediately wrapped in damp paper towel then aluminium foil, and finally bagged.
ABA extraction, purification, and GC-MS-MS quantification
ABAf was quantified from between 1 g and 2 g of tissue from a single fully expanded leaf (S. minimus), small, scale-leaved, terminal branch (C. rhomboidea), or 8–10 needles (P. radiata). Extraction, purification, and gas chromatography–tandem mass spectrometry (GC-MS-MS) quantification of ABAf were performed according to the methods of Jager et al. (2008) with the following modifications. After pre-conditioning, the Sep-Pak® C18 cartridge (Waters, Milford, USA) was washed with 15 ml of 20% (v/v) methanol in 0.4% (v/v) acetic acid and ABA was eluted with 15 ml of 45% (v/v) methanol in 0.4% (v/v) acetic acid. The eluate was taken up in 400 μl of methanol and methylated with 750 μl of a 1:10 dilution of (trimethylsilyl)diazomethane in diethyl ether for 30 min, following which ABA was taken up in 2×100 μl washes of diethyl ether, each time reduced to dryness under a nitrogen stream. The sample was then resuspended in 50 μl of chloroform prior to GC-MS-MS analysis. The ions monitored in the GC-MS-MS system were MS1 m z−1 190, MS3 m z−1 162 (endogenous ABA), and MS1 m z−1 194, MS3 m z−1 166 (internal standard [2H4]ABA). The ratio of endogenous ion intensity to internal standard ion intensity was calculated. The product of this ratio and the amount of internal standard added was divided by the fresh weight of the tissue sample and adjusted for aliquot volume to determine ABAf. All values are expressed in terms of leaf fresh weight.
Diurnal foliar ABA concentration post-drought
Three individuals of S. minimus and C. rhomboidea were used in a preliminary exploration of diurnal ABAf following mild drought recovery to determine the time of maximum and minimum ABAf in both species. Diurnal variation in ABAf was measured in S. minimus and C. rhomboidea on the day immediately following rewatering.
All pots were triple bagged to eliminate evaporative water loss from the soil and daily watering was withheld. Plants were weighed on a precision balance (±0.01 g, Mettler-Toledo XS6002S, Switzerland) between 12:00 h and 13:00 h, and transpiration (g s−1) was recorded on each successive day following the initiation of drought. Drought continued until plants reached the above-described drought conditions. All three individuals of each species arrived at the prescribed level of drought on the same day and were rewatered at 05:00 h the following morning. On the day of rewatering, tissue was removed for ABA quantification from a single cohort of leaves at a similar stem height across all individuals to reduce the effect of age-related gradients in ABAf (Soar et al., 2004; Valdés et al., 2004). Tissue was collected from all individuals at hourly intervals from 08:00 h to 13:00 h then at 90 min intervals until 17:30 h.
Leaf gas exchange measurements and the drought cycle
Three individuals from each species were used to determine the stomatal sensitivity to Ca under varying ABAf over the course of a mild drought cycle.
A portable infrared gas analyser (Li-6400, Li-Cor Biosciences, Lincoln, NE, USA) was used to measure gs (mol m−2 s−1) over a sequence of transitions in Ca. Other variables within the leaf chamber of the Li-6400 were standardized during measurements (leaf temperature was maintained at 20 °C, PPFD at 1000 μmol quanta m−2 s−1, and VPD automatically set at 1.3 kPa). Automatic setting of VPD resulted in small variations in air flow (±50 ml min−1); however, during all measurements, major differences between inlet air VPD and the automatically set VPD were eliminated by manual adjustment of inlet air diverted through a desiccant column, thereby minimizing fluctuations in air flow. Leaf chamber Ca was controlled for the duration of all measurements by a gas injection system (Li-6400-01, Li-Cor Biosciences) regulating the concentration of CO2 in the air supply line. At the start of measurements a single leaf (S. minimus) or collection of small terminal branches (C. rhomboidea) or needles (P. radiata) were arranged in the leaf chamber so that there were no leaves or stems overlapping. Leaves were allowed to equilibrate for 20 min in the chamber at current ambient Ca (386 μmol mol−1), after which Ca was lowered to 100 μmol mol−1 for 20 min then increased to 600 μmol mol−1 for 20 min. Twenty minutes was sufficient time to establish a new stomatal steady state with <1% change in gs per minute according to the dynamic responses in all three species (Brodribb et al., 2009). During gas exchange measurements, gs, assimilation (A), and leaf environmental traits were logged every 2 min. Following gas exchange measurement, all logged data were standardized against leaf area in the chamber.
On the day prior to the commencement of the drought cycle, gas exchange measurements were made twice on different leaves of the same individual at 09:30 h and 15:00 h for S. minimus or at both 12:30 h and 15:30 h for C. rhomboidea and P. radiata. The times at which gas exchange measurements were made were determined from the periods of maximum and minimum ABAf from the post-drought diurnal ABAf flux experiment to ensure maximum variation in ABAf for each species. Tissue was harvested for ABAf quantification 30 min into each gas exchange measurement cycle from a leaf, fascicle or branchlet adjacent to that undergoing gas exchange measurement.
Initial gas exchange measurements and drought commencement were undertaken on a separate day for each individual over no more than 3 d for each species. At the initiation of the drought treatment, plants were triple bagged and water was withheld. Plant water loss was determined gravimetrically as described above and, once the prescribed level of drought had been reached, individuals were rewatered at 05:00 h the following day. Assessment of stomatal sensitivity to Ca was only possible after rewatering and drought recovery when Ψleaf and gs had increased to sufficient levels to allow gas exchange measurements to take place while ABAf remained relatively high. When plants were experiencing drought, the strong interaction between gs and Ψleaf made determining stomatal sensitivity to Ca impossible. Following rewatering, twice-daily assessment of Ca sensitivity and ABAf was undertaken in all individuals on the first day, then a single individual per species was assessed over 4 d following drought recovery, to track stomatal sensitivity to Ca over a natural decline in ABAf. Stomatal sensitivity was calculated as the slope of the linear regression between absolute values of gs and Ca over the range 100–600 μmol mol−1 CO2.
Statistical analysis
ABAf and A data presented for both pre-drought and post-drought conditions are means and standard errors of means of three replicates per species. Means were compared using paired sample t-tests. One-way analyses of variance were used to assay differences between stomatal sensitivities at different sampling times over the drought cycle. A two-parameter single exponential rise to maximum curve was fitted to the sensitivity data of S. minimus over the ABAf using SigmaPlot for Windows Version 8.02 (2002).
Results
Diurnal foliar ABA concentration post-drought
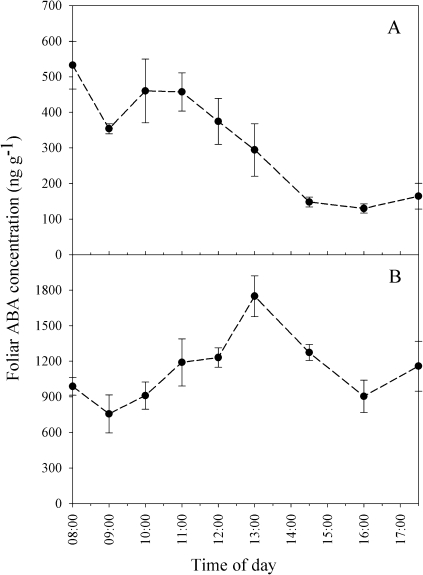
Distinctive patterns were observed in both the conifer and angiosperm species. In the angiosperm S. minimus, peak post-rewatering ABAf (mean maximum 450 ng g−1) occurred between 10:00 h and 11:00 h on the day following rewatering, dropping to lower values of ∼120 ng g−1 after 14:30 h (Fig. 1A). In contrast, post-rewatering ABAf in the conifer C. rhomboidea peaked at ∼1700 ng g−1 by 13:00 h the day after rewatering, with relatively lower values, ∼900 ng g−1, measured at the start and end of the sampling period (Fig. 1B). The pattern of ABAf post-drought in the conifer P. radiata was assumed to be the same as the diurnal pattern observed in C. rhomboidea, as the trend in C. rhomboidea was very similar to that of ABA flux to the leaves observed in stressed Pinus sylvestris L. individuals by Jackson et al. (1995) using radioimmunoassay ABA quantification methods.
Fig. 1.
Time course of foliar ABA concentration for S. minimus (A) and C. rhomboidea (B) on the day immediately following recovery from mild drought. Data points are means ±SE (n=3). Plants were rewatered at 05:00 h.
Trends in E and Ψleaf over the drought cycle
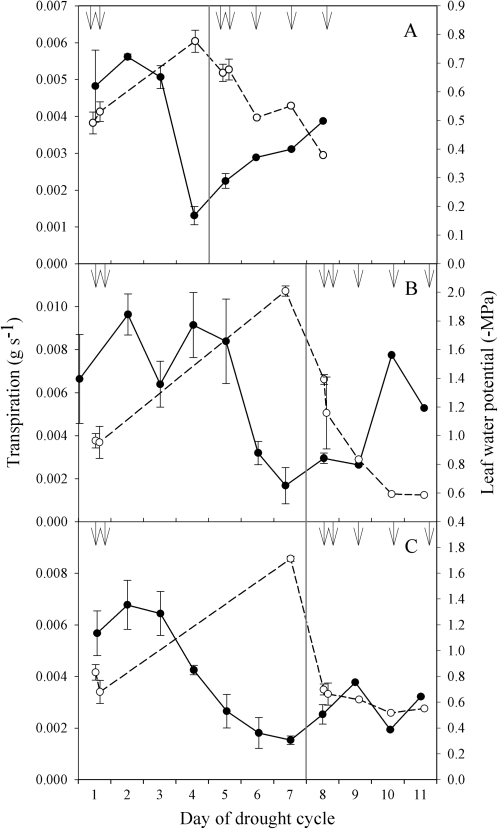
Prior to drought stress all species had relatively high midday E (>0.005 g s−1) and this level was maintained for 3 d (S. minimus and P. radiata) to 5 d (C. rhomboidea) after water was withheld (Fig. 2). Drought caused stomatal closure in all species, reducing E to <25% of initial E over 3–5 d depending on the species (Fig. 2). Minimum Ψleaf at 25% initial E was different for each species, ranging from between –0.7 MPa and –0.9 MPa in S. minimus, between –1.9 MPa and –2.1 MPa in C. rhomboidea, and between –1.7 MPa and –1.8 MPa in P. radiata (Fig. 2).
Fig. 2.
Transpiration (solid line) and leaf water potential (Ψleaf) (dashed line) over the course of a mild drought cycle in S. minimus (A), C. rhomboidea (B), and P. radiata (C). Data points represent means ±SE (n=3) for all days except the final three when only one individual was represented. Water was withheld from day 1; rewatering occurred at the vertical line. Arrows mark times at which both ABA level and stomatal conductance were measured.
Following rewatering, a full recovery of Ψleaf to pre-drought levels took between 1 d and 2 d in all species, after which Ψleaf remained similar to or higher than pre-drought Ψleaf (Fig. 2). Following recovery from drought, E in S. minimus individuals increased to levels similar to pre-drought conditions by the fourth day (Fig. 2A). Transpiration in C. rhomboidea remained low for 2 d following drought recovery, after which E returned to pre-drought levels (Fig. 2B). However, in P. radiata E only recovered slightly, remaining low, between 0.002 g s−1 and 0.003 g s−1, over the 4 d post-drought recovery (Fig. 2C).
Stomatal sensitivity to Ca and ABAf
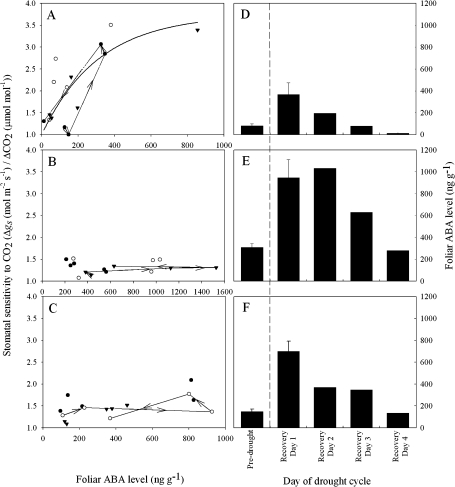
The pattern of stomatal sensitivity to Ca (measured over the range of 100–600 μmol mol−1 CO2), as ABAf levels varied over the drought cycle, was noticeably different in the angiosperm S. minimus from that in the two conifer species (Fig. 3).
Fig. 3.
Stomatal sensitivity to CO2 relative to foliar ABA level in S. minimus (A), C. rhomboidea (B), and P. radiata (C). Three individuals are represented by different symbols, with a representative individual from each species linked with arrows indicating the transition from two initial well-watered states, two states on the initial day following rewatering, and a subsequent day post-drought recovery. A significant regression (R2=0.57; P <0.01) described the sensitivity of S. minimus stomata to Ca [Ca = 2.745 (1-e-0.0032ABAf)] as indicated by the solid line, although neither conifer species presented a significant relationship. Mean foliar ABA level ±SE (n=3) for pre-drought, and the 4 d post-drought recovery are also shown for S. minimus (D), C. rhomboidea (E), and P. radiata (F). Vertical dashed lines separate pre-drought unstressed ABA levels from levels following drought recovery.
Following rewatering, the highest recorded ABA levels in all species occurred on the first day, after which levels gradually declined over the following 3 d (Fig. 3D–F). ABAf increased on average at least 4-fold in all species; however, the pre-drought baseline ABAf varied between species, with S. minimus displaying a relatively low pre-drought ABAf (75 ng g−1) compared with the two conifers C. rhomboidea (320 ng g−1) and P. radiata (190 ng g−1) (Fig. 3D–F).
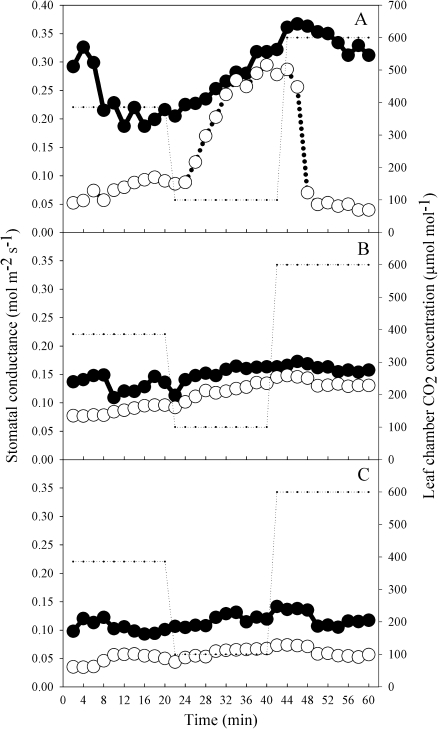
In S. minimus, stomatal sensitivity to Ca was weak when ABAf was comparatively low prior to drought stress (Fig. 3A). Stomatal sensitivity was highest in leaves immediately recovered from mild drought, when ABAf was ∼4 times higher than in pre-drought conditions (Fig. 3A). On the subsequent days following recovery from drought stress, stomatal sensitivity to Ca declined in parallel with ABAf, although with some variability (Fig. 3A). The relationship between stomatal sensitivity of S. minimus to Ca and ABAf was curvilinear, apparently saturating at ABAf >500 ng g−1 (Fig. 3A). In contrast, the two conifer species C. rhomboidea and P. radiata showed little sensitivity to Ca despite a substantial enhancement of ABAf in leaves of droughted plants (Fig. 3B, C). In both conifer species the stomatal sensitivity to Ca was not significantly affected by increasing ABAf and was no different from the stomatal sensitivity prior to drought (P >0.05) (Fig. 3A–C). The two conifer species displayed a similar lack of stomatal response to Ca prior to and following drought (Fig. 4). Only the angiosperm S. minimus showed a pronounced change in the dynamics of the stomatal response to Ca following drought, when ABAf was high (Fig. 4).
Fig. 4.
Time-courses showing the responses of stomatal conductance (gs) to step changes in CO2 concentration (small dotted line) in a single representative individual of S. minimus (A), C. rhomboidea (B), and P. radiata (C) in the morning prior to the commencement of drought (filled circles) and the morning immediately following recovery from mild drought stress (open circles).
The effect of drought on A
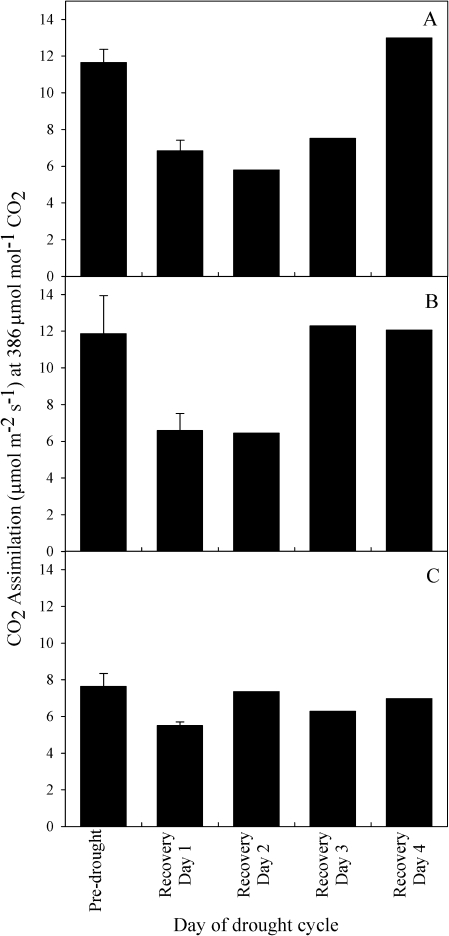
Prior to the drought treatment, individuals of S. minimus and C. rhomboidea both had similar mean A (11.65±0.72 and 11.86±2.08 μmol m−2 s−1, respectively) while P. radiata individuals had a slightly lower mean A (7.6±0.71 μmol m−2 s−1) (Fig. 5). Mild drought stress reduced mean A in all species; in S. minimus and C. rhomboidea mean A was reduced by a similar degree (41% and 46%, respectively), while in P. radiata mean A was only reduced by 27% (Fig. 5). This reduction in mean A recovered in both conifer species by the third day following rewatering, and by the fourth day in the angiosperm S. minimus (Fig. 5).
Fig. 5.
Mean assimilation of CO2 at ambient (386 μmol mol−1) CO2 ±SE over the mild drought cycle including prior to the drought, and the 4 d post-drought recovery in S. minimus (A), C. rhomboidea (B), and P. radiata (C). (n=3 for pre-drought and recovery day 1, n=1 for the remaining days following drought recovery).
Discussion
The stomata of the two conifer species C. rhomboidea and P. radiata were found to be insensitive to changes in Ca in spite of >4-fold increases in ABAf induced by mild drought treatment (Fig. 3). This lack of an ABA-induced stomatal sensitivity in the two phylogenetically and ecologically disparate conifer species suggests a common state for conifers in general, strongly contrasting with the response of the representative angiosperm S. minimus in which stomatal sensitivity was largely dependent on ABAf following recovery from drought (Fig. 3A, D). The stomatal responsiveness of S. minimus to Ca increased with levels of ABAf, similar to that previously reported in the excised or ABA-injected leaves of other angiosperm species (Raschke, 1975; Dubbe et al., 1978; Raschke and Hedrich, 1985; Ridolfi et al., 1996; Bunce, 1998; Leymarie et al., 1998).
There are four possible explanations that could account for the lack of stomatal sensitivity to Ca in conifers: (i) that ABA levels in conifers were too low to enhance sensitivity to Ca; (ii) that photosynthesis was severely down-regulated or damaged by drought in the conifer species; (iii) that the stomatal response to Ca in conifers is entirely absent; or (iv) that ABA has a more limited physiological role in coniferous species compared with angiosperms. The first three explanations are unlikely, for reasons discussed below.
The first explanation, that ABA levels in the conifers were insufficient to enable stomatal sensitivity to changes in Ca, is the most unlikely explanation. In this study both conifer species contained high levels of ABAf and showed a similar 4-fold increase in ABAf as a result of mild drought stress compared with the angiosperm species (Fig. 3). To date all angiosperm species reported are either always sensitive to Ca or show sensitivity induced by increases in ABAf, unlike the two conifer species in this study (Raschke and Hedrich, 1985).
The possibility of reduced stomatal sensitivity in the conifer species due to a reduced photosynthetic capacity prior to or caused by mild drought is also unlikely (Fig. 5). The mesophyll plays a significant role in regulating the stomatal sensitivity to Ca in angiosperms (Mott et al., 2008) with photosynthesis probably acting as a transducer, possibly from the mesophyll through a vapour phase (Sibbernsen and Mott, 2010). In all species the mild drought treatment that caused significant increases in ABAf resulted in similar mild reductions in A (Fig. 5). The stomata of S. minimus were most sensitive to Ca on the day immediately following drought recovery (Fig. 3A, D) when A in this species was 41% lower than on the day before the initiation of drought (Fig. 5). These results validate ABA as the primary cause of increased stomatal sensitivity to Ca in S. minimus, and that the reduced sensitivity of the two conifer species was not due to an initially very low A or a damaged photosynthetic system as a result of the mild drought (Fig. 5).
The third explanation that the pathway responsible for the Ca response is entirely absent in conifers and hence the sensitization of stomata by ABA never occurs is also unlikely. Small increases in gs observed when conifer stomata are exposed to low Ca (Brodribb et al., 2009) as well as an increased sensitivity of conifer stomata to Ca under increasing VPD (Bunce, 2007) suggest that the stomatal response to Ca in conifers is not entirely absent. Bunce (2007) reported that the stomatal insensitivity of the conifer Picea sitchensis (Bong.) to decreasing Ca (from 380 μmol mol−1 to 100 μmol mol−1) was reversed to a significant but small degree by increasing VPD above ∼1.4 kPa. This reported increase in sensitivity at high VPD was, however, much smaller than the relative increases in sensitivity observed in the angiosperm Helianthus annuus L. in which gs at 100 μmol mol−1 CO2 returned to maximum levels regardless of the VPD exposure (Bunce, 2007). The small increase in stomatal sensitivity to Ca in conifers when exposed to high VPD suggests that conifer stomata have the potential to respond to changes in Ca, but that this response is constrained by the lack of another regulating signal. The strong interaction between the two stomatal signals, ABA and Ca, in angiosperms is evident and suggests that the angiosperm-like regulation of stomatal control by ABA is absent in the two coniferous species.
A diminished role for ABA in conifers?
The possibility of a missing or inactive signalling pathway in the stomata of conifers leads to the final explanation for the contrast between the angiosperm and two conifer species: a fundamental difference in stomatal control by ABA between the two lineages. A limited number of studies have investigated ABAf in conifers using physicochemical methods (Kraus and Ziegler, 1993; Hoffman et al., 1999; Kong et al., 2009). These studies all indicate that ABAf in conifers, including Pinaceae (Kraus and Ziegler, 1993; Kong et al., 2009) and Taxaceae (Hoffman et al., 1999) species, were typically >400 ng g−1 fresh weight under well-watered conditions. This is in contrast to angiosperm species under similar unstressed conditions for which ABAf is typically <100 ng g−1. The large differential between conifers and angiosperms was also observed in this study, where mean ABAf in unstressed C. rhomboidea was 320 ng g−1, and in P. radiata 190 ng g−1, compared with mean levels of 75 ng g−1, in S. minimus (Fig. 3D–F).
The link between ABAf and stomatal conductance has been widely documented in angiosperms from detached leaves (Raschke and Hedrich, 1985) as well as in ABA biosynthetic mutants fed ABA solutions (Pugliesi et al., 1994). In all cases increasing ABAf had the effect of closing stomata. Murphy and Ferrell (1982) found in the conifer Pseudotsuga menziesii (Mirb.) Franco a very weak relationship between ABAf and E, apparent only in early summer. In the present study, values of E (Fig. 2A–C) and gs (Fig. 4A–C) prior to drought were similarly high in the angiosperm and two conifer species despite the relatively high ABAf in the two conifers (Fig. 3D–F). Additionally, by the fourth day following drought recovery ABAf had returned to pre-drought levels in all species (Fig. 3D–F) but this recovery did not correspond to a full recovery in E (Fig. 2). Recovery of ABAf (Henson, 1981; Liu et al., 2001) and xylem ABA level (Loewenstein and Pallardy, 2002) to pre-drought levels prior to the recovery of E or gs has been recorded in a number of angiosperm species and highlights a transient role for ABA in the control of stomatal conductance following drought. The E, gs, and ABAf results from this study point to the possibility of ABA levels in conifers having less of a control over stomatal aperture, a conclusion recently suggested in a study on conifer gas exchange recovery over a drought cycle by Brodribb and Cochard (2009).
The reduced stomatal response to ABA in conifers raises the question of how these plants function so effectively. One alternative that needs investigation is raised by the fact that stomata of conifers, unlike angiosperms, operate similarly to ferns with a large safety margin between Ψleaf at 50% closure and Ψleaf at the point of hydraulic failure (Brodribb and Holbrook, 2004; Brodribb and Cochard, 2009). This relatively large Ψleaf safety margin means cavitation and repair are very rare in vessel-less conifers compared with angiosperms, and hence the utility of ABA as a means of enhancing embolism repair (Lovisolo et al., 2008) is also very limited.
Conclusion
The combination of a lack of ABA-induced stomatal response during drought (Brodribb and Cochard, 2009), stomatal insensitivity to Ca despite increases in ABAf, and high levels of pre-drought ABA suggests that conifers have a relatively weak biochemical and physiological response to ABA. These differences between conifers and well-studied angiosperm species suggest missing or inactive biochemical pathways in the guard cells of conifers, placing an emphasis on the need for re-evaluation of generalizations about the role of ABA in plants.
Acknowledgments
We thank Ian Cummings for tending glasshouse experiments, Noel Davies for running samples on GC-MS, and two anonymous reviewers for their helpful comments. This project was funded by ARC Discovery grants to TJB and GJJ.
Glossary
Abbreviations
- A
CO2 assimilation rate
- ABA
abscisic acid
- ABAf
foliar abscisic acid level
- Ca
atmospheric carbon dioxide concentration
- E
transpiration
- gs
stomatal conductivity to the diffusion of gases
- PPFD
photosynthetic photon flux density
- Ψleaf
leaf water potential
- VPD
vapour pressure deficit
References
- Beadle CL, Jarvis PG, Neilson RE. Leaf conductance as related to xylem water potential and carbon dioxide concentration in Sitka spruce. Physiologia Plantarum. 1979;45:158–166. [Google Scholar]
- Betts RA, Boucher O, Collins M, et al. Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature. 2007;448:1037–1041. doi: 10.1038/nature06045. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Cochard H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytologist. 2004;162:663–670. doi: 10.1111/j.1469-8137.2004.01060.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Feild TS. Evolution of stomatal responsiveness to CO2and optimization of water-use efficiency among land plants. New Phytologist. 2009;183:839–847. doi: 10.1111/j.1469-8137.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- Bunce JA. Effects of humidity on short-term responses of stomatal conductance to an increase in carbon dioxide concentration. Plant, Cell and Environment. 1998;21:115–120. [Google Scholar]
- Bunce JA. Low carbon dioxide concentrations can reverse stomatal closure during water stress. Physiologia Plantarum. 2007;130:552–559. [Google Scholar]
- Dubbe DR, Farquhar GD, Raschke K. Effect of abscisic acid on the gain of the feedback loop involving carbon dioxide and stomata. Plant Physiology. 1978;62:413–417. doi: 10.1104/pp.62.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamus D, Narayan AD. The influence of prior water stress and abscisic acid foliar spraying on stomatal responses to carbon dioxide, IAA, ABA and calcium in leaves of Solanum melongena. Journal of Experimental Botany. 1989;40:573–580. [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology. 1982;33:317–345. [Google Scholar]
- Henson IE. Abscisic acid and after-effects of water stress in pearl millet (Pennisetum americanum (L.) Leeke) Plant Science Letters. 1981;21:129–135. [Google Scholar]
- Hoffman A, Shock C, Feibert E. Taxane and ABA production in yew under different soil water regimes. Hortscience. 1999;34:882–885. [Google Scholar]
- Jackson GE, Irvine J, Grace J, Khalil AAM. Abscisic acid concentrations and fluxes in droughted conifer saplings. Plant, Cell and Environment. 1995;18:13–22. [Google Scholar]
- Jager CE, Symons GM, Ross JJ, Reid JB. Do brassinosteroids mediate the water stress response? Physiologia Plantarum. 2008;133:417–425. doi: 10.1111/j.1399-3054.2008.01057.x. [DOI] [PubMed] [Google Scholar]
- Kong L, Abrams SR, Owen SJ, Van Niejenhuis A, Von Aderkas P. Dynamic changes in concentrations of auxin, cytokinin, ABA and selected metabolites in multiple genotypes of Douglas-fir (Pseudotsuga menziesii) during a growing season. Tree Physiology. 2009;29:183–190. doi: 10.1093/treephys/tpn009. [DOI] [PubMed] [Google Scholar]
- Kraus M, Ziegler H. Quantitative analysis of abscisic acid in needles of Abies alba Mill. by electron capture gas chromatography. Trees. 1993;7:175–181. [Google Scholar]
- Leymarie J, Lascéve G, Vavasseur A. Interaction of stomatal responses to ABA and CO2 in Arabidopsis thaliana. Australian Journal of Plant Physiology. 1998;25:785–791. [Google Scholar]
- Linsbauer K. Beiträge zur Kenntnis der Spaltöffnungsbewegung. Flora. 1917;109:100–143. [Google Scholar]
- Liu L, McDonald AJS, Stadenberg I, Davies WJ. Stomatal and leaf growth responses to partial drying of root tips in willow. Tree Physiology. 2001;21:765–770. doi: 10.1093/treephys/21.11.765. [DOI] [PubMed] [Google Scholar]
- Loewenstein NJ, Pallardy SG. Influence of a drying cycle on post-drought xylem sap abscisic acid and stomatal responses in young temperate deciduous angiosperms. New Phytologist. 2002;156:351–361. doi: 10.1046/j.1469-8137.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- Lovisolo C, Perrone I, Hartung W, Schubert A. An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytologist. 2008;180:642–651. doi: 10.1111/j.1469-8137.2008.02592.x. [DOI] [PubMed] [Google Scholar]
- Medlyn BE, Barton CVM, Broadmeadow MSJ, et al. Stomatal conductance of forest species after long-term exposure to elevated CO2concentration: a synthesis. New Phytologist. 2001;149:247–264. doi: 10.1046/j.1469-8137.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- Messinger SM, Buckley TN, Mott KA. Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiology. 2006;140:771–778. doi: 10.1104/pp.105.073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Rushing AJ, Primack RB, Templer PH, Rathbone S, Mukunda S. Long-term relationships among atmospheric CO2, stomata, and intrinsic water use efficiency in individual trees. American Journal of Botany. 2009;96:1779–1786. doi: 10.3732/ajb.0800410. [DOI] [PubMed] [Google Scholar]
- Morison JIL. Sensitivity of stomata and water use efficiency to high CO2. Plant, Cell and Environment. 1985;8:467–474. [Google Scholar]
- Morison JIL. Intercellular CO2 concentration and stomatal response to CO2. In: Zeiger E, Farquhar GD, Cowan IR, editors. Stomatal function. Stanford, CA: Stanford University Press; 1987. pp. 229–252. [Google Scholar]
- Morison JIL, Jarvis PG. Direct and indirect effects of light on stomata. I. In Scots pine and Sitka spruce. Plant, Cell and Environment. 1983;6:95–101. [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC. The role of the mesophyll in stomatal responses to light and CO2. Plant, Cell and Environment. 2008;31:1299–1306. doi: 10.1111/j.1365-3040.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- Pugliesi C, Fambrini M, Vernieri P, Baroncelli S. Characterization of a wilty sunflower (Helianthus annuus L.) mutant: I. Abscisic acid content, light–dark changes in the stomatal conductance and genetic analysis. Journal of Experimental Botany. 1994;45:533–538. [Google Scholar]
- Raschke K. Simultaneous requirement of carbon dioxide and abscisic acid for stomatal closing in Xanthium strumarium L. Planta. 1975;125:243–259. doi: 10.1007/BF00385601. [DOI] [PubMed] [Google Scholar]
- Raschke K, Hedrich R. Simultaneous and independent effects of abscisic acid on stomata and the photosynthetic apparatus in whole leaves. Planta. 1985;163:105–118. doi: 10.1007/BF00395904. [DOI] [PubMed] [Google Scholar]
- Ridolfi M, Fauveau ML, Label P, Garrec JP, Dreyer E. Responses to water stress in an ABA-unresponsive hybrid poplar (Populus koreana×trichocarpa cv. Peace) New Phytologist. 1996;134:445–454. [Google Scholar]
- Roth-Nebelsick A, Hassiotou F, Veneklaas EJ. Stomatal crypts have small effects on transpiration: a numerical model analysis. Plant Physiology. 2009;151:2018–2027. doi: 10.1104/pp.109.146969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends in Plant Science. 2002;7:41–48. doi: 10.1016/s1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- Sibbernsen E, Mott KA. Stomatal responses to flooding of the intercellular air spaces suggest a vapor-phase signal between the mesophyll and the guard cells. Plant Physiology. 2010;153:1435–1442. doi: 10.1104/pp.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, Yang Y, Nishimura N, Wang A, Schroeder JI. Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K+channels in Arabidopsis guard cells. The Plant Journal. 2009;59:207–220. doi: 10.1111/j.1365-313X.2009.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soar CJ, Speirs J, Maffei SM, Loveys BR. Gradients in stomatal conductance, xylem sap ABA and bulk leaf ABA along canes of Vitis vinifera cv. Shiraz: molecular and physiological studies investigating their source. Functional Plant Biology. 2004;31:659–669. doi: 10.1071/FP03238. [DOI] [PubMed] [Google Scholar]
- Talbott LD, Rahveh E, Zeiger E. Relative humidity is a key factor in the acclimation of the stomatal response to CO2. Journal of Experimental Botany. 2003;54:2141–2147. doi: 10.1093/jxb/erg215. [DOI] [PubMed] [Google Scholar]
- Talbott LD, Srivastava A, Zeiger E. Stomata from growth-chamber-grown Vicia faba have an enhanced sensitivity to CO2. Plant, Cell and Environment. 1996;19:1188–1194. doi: 10.1111/j.1365-3040.1996.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Valdés AE, Centeno ML, Fernández B. Age-related changes in the hormonal status of Pinus radiata needle fascicle meristems. Plant Science. 2004;167:373–378. [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. Leaf conductance in relation to assimilation in Eucalyptus pauciflora Sieb. ex Spreng. Plant Physiology. 1978;62:670–674. doi: 10.1104/pp.62.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. CO2signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2insensitivity of the gca2 mutant. Proceedings of the National Academy of Sciences, USA. 2006;103:7506–7511. doi: 10.1073/pnas.0602225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Talbott LD, Jin X, Zeiger E. The stomatal response to CO2is linked to changes in guard cell zeaxanthin. Plant, Cell and Environment. 1998;21:813–820. [Google Scholar]