Abstract
Water stress reduces endogenous cytokinin (CK) content and may inhibit CK production. Maintenance of endogenous CK levels by genetic transformation with ipt in leaves and roots undergoing senescence may promote stress tolerance. This study was designed to determine the physiological effects of ipt expression on immature and mature leaves and in roots for plants exposed to different levels of water stress for creeping bentgrass (Agrostis stolonifera). Plants containing the ipt gene, encoding the enzyme adenine isopentenyl phosphotransferase for CK synthesis ligated to a senescence-activated promoter (SAG12), and wild-type ‘Penncross’ (WT) were grown hydroponically in a growth chamber and exposed to water stress by weekly additions of polyethylene glycol 8000 to reduce the growing solution osmotic potential from –0.05 to –0.3, –0.5, –0.7, –1.0, and –1.4 MPa. Immature and mature leaves and roots of SAG12-ipt creeping bentgrass were evaluated for ipt expression, CK content, leaf relative water content (RWC), chlorophyll content (Chl), photochemical efficiency (FvFm), osmotic adjustment (OA), photosynthesis rate (Pn), stomatal conductance (gs), transpiration (E), water use efficiency (WUE), carbon isotope discrimination (Δ), and root viability. Expression of ipt was detected in all plant parts and a higher CK content, primarily in the form of isopentyladenine (iPa), was found in SAG12-ipt plants but not in the WT plants under water stress. Immature leaves exhibited higher iPa and OA at all treatment levels. Mature leaves of SAG12-ipt plants maintained higher OA, Pn, Chl, WUE, and Δ, whereas gs and E were relatively unaffected compared to the WT. Roots of SAG12-ipt plants had higher levels of iPa and greater root viability than the WT. The results demonstrate that expression of ipt enhanced the tolerance of creeping bentgrass to water stress, which could be attributed to the positive effects on osmotic adjustment, efficient water use, and maintaining higher photosynthetic rate primarily for mature leaves, as well as increased root viability.
Keywords: Cytokinins, drought stress, osmotic stress, SAG12-ipt, senescence
Introduction
A decline in water quality or availability for irrigation frequently disrupts the osmotic environment of the root zone and can lead to whole-plant water or osmotic stress. Typical symptoms of water stress include stomatal closure, leaf desiccation, leaf senescence, inhibition of photosynthesis, growth restriction, and root death, as well as other overall plant stress resistance mechanisms. Physiological damage caused by water stress and stress signalling are closely associated with the endogenous level and balance of hormones (Davies et al., 1994; Yang et al., 2002). Cytokinin (CK) synthesis and transport are typically inhibited whereas degradation is promoted under water stress, all of which have been associated with growth inhibition and a decline in stress tolerance (Yang et al., 2002; Kudoyarova et al., 2006). It is well accepted that natural or stress-induced leaf senescence is related to a decline in CK content in various plant species (Naqvi, 1995). CK involvement in delaying leaf senescence has been shown by the exogenous application of CK (Richmond and Lang, 1957; Badenoch-Jones et al., 1996; Liu and Huang, 2002; Okamoto et al., 2010) and by increasing endogenous production of CK through transgenic modification of CK biosynthesis genes or genes regulating CK degradation pathways (Naqvi, 1995).
The CK biosynthesis gene ipt encodes for the enzyme isopentyl transferase, which catalyses the rate-limiting first step in de novo CK biosynthesis and promotes the formation of isopentenyladenosine-5'-monophosphate (iPa) (Akiyoshi et al., 1984; Barry et al., 1984; McGraw, 1987). The different forms of endogenous ipt genes are expressed at relatively low levels in the control condition and during drought stress in several plant species and are highly organ, developmental, or cell type-specific and are down-regulated during stress (Vyroubalova et al., 2009). Generally, it has been demonstrated that ipt expression increases endogenous CK or maintains CK levels under stress conditions, thereby delaying leaf senescence and promoting stress resistance in several plant species, such as Arabidopsis (Arabidopsis thaliana) (Medford et al., 1989; Zhang et al., 2000), lettuce (Lactuca sativa) (McCabe et al., 2001), tobacco (Nicotiana tabacum) (Rivero et al., 2007), petunia (Petunia×hybrida) (Clarke et al., 2004), tall fescue (Festuca arundinacea) (Hu et al., 2005), and creeping bentgrass (Agrostis stolonifera) (Xu et al., 2009; Merewitz et al., 2010). However, the physiological mechanisms of CK regulation of plant tolerance to water stress remain less well-documented than other water stress regulators such as abscisic acid (Bray, 1993; Kramer and Boyle, 1995; Chaves et al., 2003; Marrion-Poll and Leung, 2006). Analysis of the various organs of different developmental stages of ipt transgenic plants with different growth habits and stress resistance mechanisms is critical for further the documentation of the effects of CK on water stress tolerance and the effects of water stress on CK changes.
Previous studies have used senescence activated promoters, such as SAG12 and SARK to auto-regulate or control ipt expression to prevent over-production of CK, which may occur with constitutive expression (Medford et al., 1989; Gan and Amasino 1995; Morris, 1995; Rivero et al., 2007; Verdonk, et al., 2008). Transformation of tobacco (Nicotiana tabacum) with ipt, regulated by a senescence-inducible promoter, resulted in significant improvement in drought tolerance, attributed to the delay in leaf senescence and increases in photosynthesis rates and antioxidant activities (Rivero et al., 2007, 2009). Leaf senescence of mature leaves may be a mechanism for drought survival in order to reduce the surface area for transpiration and to redirect energy reserves to reproductive systems in annual crops where a high yield of the reproductive organs is desirable (Chaves, 2003). However, the maintenance of older leaves by the avoidance of senescence can be beneficial for additional energy produced by maintaining a greater amount of photosynthetic leaves as was found in tobacco (Rivero et al., 2007). The much reduced growth rate of mature leaves largely prevents them from being a sink to draw nutrients and energy from immature leaves or roots that could alternately have gone towards drought survival (Khan, 1981).
For perennial grasses, delaying leaf senescence and maintaining physiological function of both immature and mature leaves under water stress is important for plant biomass production in forage and for the aesthetic appearance in turfgrasses and may improve drought tolerance. Previously, it has been demonstrated that expression of ipt in 11 creeping bentgrass transgenic lines maintained higher levels of CK, increased leaf chlorophyll content (Chl), and higher root to shoot ratios after 14 d of drought stress relative to the WT (Merewitz et al., 2010). Cytokinins also regulate many other processes, including stomatal opening, photosynthesis, water relations, and root growth. However, how elevated CK in a perennial grass species such as creeping bentgrass may alter those processes related to water stress tolerance is not clear. Furthermore, limited information is available about the root growth characteristics of ipt plants, which is an important factor influencing water uptake under water stress. In the current study, physiological responses of a typical SAG12-ipt transgenic line (S41) that exhibited superior drought tolerance compared with the wild-type ‘Penncross’ of creeping bentgrass were examined in an attempt to elucidate the physiological changes associated with increased CK in the SAG12-ipt transgenic plants in both the shoots and roots under water stress. The specific objectives of the study were to determine ipt gene expression patterns in leaves and roots during the progression of water stress and to examine the physiological effects of ipt expression on immature and mature leaves and in roots for creeping bentgrass exposed to water stress. Physiological assessment focused on leaf senescence (chlorophyll content), water relations (relative water content, osmotic adjustment, stomatal conductance, transpiration, and water use efficiency), and photosynthetic activities (photochemical efficiency and net photosynthetic rate). In addition, since enhanced rooting in the SAG12-ipt plants was previously reported, the aim here was therefore to evaluate whether the enhanced rooting was due to delayed root senescence or increased root viability.
Materials and methods
Plant material and growth conditions
Transgenic creeping bentgrass plants were produced by the Agrobacterium tumefaciens transformation method as described previously (Merewitz et al., 2010; Xu et al., 2009; Xing et al., 2010). Plant material included the wild-type cultivar ‘Penncross’ (WT) and the SAG12-ipt transgenic line (S41) which performed well under drought stress in our previous drought study (Merewitz et al., 2010). A hydroponic growth method was used for uniformity of water stress imposition in order to reduce the potential confounding effects of nutrient deficiencies and to eliminate root damage during sampling, which may occur with soil-based water stress imposition. All plant lines were grown in a hydroponic system within a large walk-in controlled environment growth chamber with conditions set to maintain a 12 h photoperiod, 50% relative humidity, 500 μmol m−2 s−1 photosynthetic photon flux density, and a day/night temperature of 23/20 °C. Stock solutions (1000×) of the following nutrient solutions were prepared and diluted into Hoagland's nutrient solution: ammonium sulphate ((NH4)2SO4, 71.361 g l−1), potassium nitrate (KNO3, 27.3 g l−1), calcium nitrate (Ca(NO3)2.4H2O, 127.521 g l−1), potassium phosphate (KH2PO4, 68.045 g l−1), potassium sulphate (K2SO4, 43.568 g−1), magnesium sulphate (MgSO4.7H2O, 199.65 g l−1), disodium ethylenediaminetetraacetate (Fe(EDTA)Na, 14.684 g l−1), Micronutrients (H3BO3, 1.43 g l−1, MnCl2.4H2O, 0.91 g l−1, ZnSO4.H2O, 0.11 g l−1, CuSO4, 0.04 g −1, (NH4)Mo7 O24.4H2O, 0.01 g l−1). Plants were suspended within 1.27 cm holes in Styrofoam boards that fit within plastic bins (54×42×14 cm in height) that floated on the growth media. The hydroponic solution was aerated with a tube inserted into the solution through the Styrofoam connected to a pump (115 V, 60 Hz, Tetra® Blacksburg, VA). Solution pH was monitored and adjusted as needed every other day and the solution was changed on a weekly basis. Plants were not clipped to allow for adequate stem development for the separation of new and old leaves.
PEG-induced water stress
The osmotic potential of the growth solution was decreased in a stepwise manner for the imposition of water stress by weekly additions of increasing volumes of polyethylene glycol 8000 (PEG-8000). This chemical has been used in previous studies to impose water stress to plants and the large MW of 8000 to prevent root uptake of PEG (Lagerwerff et al., 1961; Janes, 1974). The nutrient solution osmotic potential was approximately –0.05 MPa due to the presence of the nutrient salts. PEG-8000 was added to bring the osmotic potential of the solution to approximately –0.3, –0.5, –0.7, –1.0, and –1.4 MPa. The osmotic potential of the growing solution was monitored by running a sample of the solution into a vapour pressure osmometer (Vapro5520, Wescor, Inc. Logan, UT) after each addition of PEG-8000 was fully dissolved and at each treatment level as described in Burlyn (1983).
Physiological analysis
Leaves were separated based on relative stem position, with the youngest two leaves considered immature and the remaining leaves considered mature. Grass plants were hand trimmed twice a week prior to PEG treatment. Approximately 10 d before PEG treatment the plants were left uncut to allow for more vertical growth for the separation of immature and mature leaves. The plants were not trimmed during the PEG treatment. Overall turf performance was evaluated by visually rating turf quality (TQ). Turf quality was visually rated every 2 d based on leaf wilting, turf uniformity, colour, and density on a scale of 1 to 9 with 1 being brown and desiccated turf, 6 being the minimal acceptable level, and 9 being a green and dense turf (Turgeon, 2008).
Relative water content (RWC) of leaves was measured as an indicator of leaf hydration status. Leaf RWC was calculated based on fresh (FW), turgid (TW), and dry weights (DW) of approximately 0.1 g of leaf samples using the following formula: (FW–DW)/(TW–DW)×100. Leaf FW was determined on a mass balance immediately after being excised from the plants. Turgid weights were determined after soaking the leaves in deionized water for 12 h in a closed Petri dish at 4 °C and weighed immediately after being blotted dry. Leaves were then dried in an 80 °C oven for at least 72 h prior to weighing for DW (Barrs and Weatherley, 1962).
Leaf chlorophyll content (Chl) and photochemical efficiency (Fv/Fm) were measured to evaluate leaf senescence. Total Chl was extracted in the dark for 72 h in dimethyl sulphoxide. The absorbance of the leaf extract was measured at 663 nm and 645 nm with a spectrophotometer (Spectronic Genesys 2; Spectronic Instruments, Rochester, NY, USA). Chl was calculated using the formula described in Arnon (1949). Fv/Fm was evaluated as a ratio of the variable fluorescence (Fv) to the maximal fluorescence (Fm) value determined using a chlorophyll fluorescence meter (Fim 1500; Dynamax, Houston, TX, USA). Leaf clips were used to adapt individual leaves to darkness for 30 min prior to reading the Fv/Fm ratio with the fluorescence meter. Two subsamples were taken per plant at each sampling day.
Osmotic adjustment (OA) was determined by measuring the osmotic potential of leaf sap of fully rehydrated leaves. Leaves samples were allowed to soak in deionized water overnight, blotted dry, placed into micro-centrifuge tubes, frozen in liquid nitrogen, and stored at –20 °C until further analysis. Thawed leaves were then immediately ground with a micro-pestle to express the leaf sap. The sap was then inserted into osmometer (Wescor, Inc., Logan, UT) for the determination of the osmolality (mmol kg−1). Osmolality was converted to osmotic potential and OA was then calculated as the difference between the well-watered control and drought-stressed plants (Blum, 1988).
Roots were washed free of PEG nutrient solution for CK extraction and root viability measurements. Root CK content was determined in the same way as for leaves, but were bulked due to difficulties in separating younger and older roots. Root viability was estimated by measuring the activity of dehydrogenase by using the triphenyltetrazolium chloride (TTC) reduction technique (Knievel, 1973; McMichael and Burke, 1994). The activity was based on the dry weight of the root sample, determined after drying in an 80 °C oven for at least 72 h.
Net photosynthetic rate (Pn), stomatal conductance (gs), and transpiration rate (E) were measured using a leaf chamber (6 cm2) of an infrared gas exchange analyser (Li-Cor 6400, Li-Cor, Inc., Lincoln, NE). Detached leaf samples of approximately 5–10 leaves were immediately placed in the leaf chamber, which provided red and blue light from an LED source (665 nm and 470 nm ranges), 400 μl l−1 CO2. Leaf area of the Pn sample was measured with Digimizer software (MedCalc Software bvba; Mariakerke, Belgium) using scanned digital images of the fresh leaf samples. Pn, gs, and E values were converted from the 6 cm2 area of the leaf chamber to the actual Pn, gs, and E values based on the measured leaf area. Instantaneous water use efficiency (WUE) was calculated as a molar ratio of Pn to E (μmol CO2 m−2 s−1 mmol−1 H2O m−2 s−1).
Carbon isotope discrimination (Δ) analysis has been shown to be negatively correlated to WUE, and used widely to estimate WUE in various plant species (Johnson and Basset, 1991). The Δ value was measured as described previously (DaCosta and Huang, 2006). Briefly, leaf tissue samples were separated based on leaf position and maturity, ground to a fine powder, passed through a 45-mesh screen, and sent to the Stable Isotope Ratio Facility for Environmental Research (University of Utah, Salt Lake City) for the measurement of carbon isotopic composition (Smedley et al., 1991; Ebdon et al., 1998). The Δ (per mil ‰) value was calculated using the equation (δa–δp)/(1+δp) where δp was the C isotopic composition of the plant sample and δa=28‰ the standard C isotopic composition of the source air (Farquhar and Richards, 1984; Farquhar et al., 1989).
Endogenous CK content was measured by extraction and quantification by an indirect enzyme linked immunosorbent assay (ELISA) method described in Setter et al. (2001) with modifications (Wang et al., 2003). Samples were extracted in 80% (v/v) methanol and purified with reverse phase C18 columns. Hydrophilic contaminants were removed and the CK were eluted with 30% methanol. Total CK was calculated as the sum of the contents of iPa, zeatin riboside (ZR), and dihydrozeatin riboside (DHZR).
Gene expression analysis
Total RNA was extracted from leaves using the RNeasy Plant Mini Kit method according to the manufacturer's protocol (Qiagen Inc., Valencia, CA). The DNA-free protocol was used prior to reverse transcription-polymerase chain reaction (RT-PCR) analysis to eliminate contamination (Am1906, Ambion, Inc., Austin, TX). Gel electrophoresis and absorbance at 260/280 nm was performed to ensure RNA integrity. RT-PCR was performed on 2 μg of each RNA extract on illustra ready-to go RT-PCR beads (27-9266-01, GE Healthcare, Piscataway, NJ) with a Taq polymerase enzyme (R001-A, Takara, Madison, WI) set to program rt-pcr50 of the GeneAmp PCR system (9700, Applied Biosystems, Inc., Foster City, CA). PCR control was used to test for contamination.
Experimental design and statistical analysis
The experimental design was a split-plot design with irrigation treatment as the main plots and plant materials as the sub-plots, with four replicates for each irrigation treatment and grass material. Effects of watering treatment, plant materials, and corresponding interactions were determined by analysis of variance according to the general linear model procedure of SAS (Version 9.0; SAS Institute, Cary, NC). Differences between watering treatments and plant means were separated by Fisher's protected least significance difference (LSD) test at the 0.05 probability level.
Results
RT-PCR analysis of ipt expression
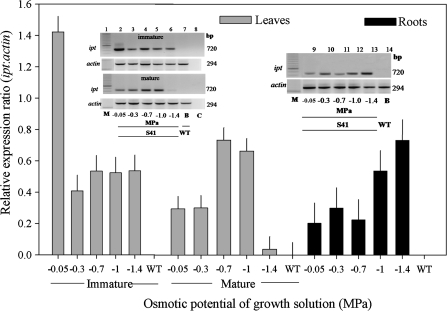
No expression of ipt was detected in the WT line in either leaves or roots. The expression of ipt was detected in immature leaves of SAG12-ipt transgenic plants in all PEG treatments, and the transcript level remained relative constant throughout the PEG treatments; ipt was detected in transgenic plants without PEG treatment (at 0 MPa) (Fig. 1). In mature leaves of transgenic plants, there was a gradual increase in ipt transcript abundance in PEG treatments from 0 to –0.7 MPa and then a decline at higher level of PEG-induced water stress (–1.4 MPa). The ipt expression was detected in roots of SAG12-ipt plants in all treatments and transcript abundance increased with increasing levels of PEG-induced water stress, with the ipt transcript levels approximately three times higher at –1.4 MPa than at 0 MPa.
Fig. 1.
RT-PCR gel images (inset) and relative expression ratio (bar graph) of ipt to the actin internal control in immature leaves, mature leaves, and roots of SAG12-ipt creeping bentgrass plants (line S41; lanes 2–8) and wild-type ‘Penncross’ (WT) exposed to PEG-induced water stress at various growing solution osmotic potentials (–0.05 to –1.4 MPa). C, PCR control, M, molecular weight marker, BP, base pair. Significance at P ≤0.05 is indicated by mean standard error bars (n=3).
CK content of immature leaves
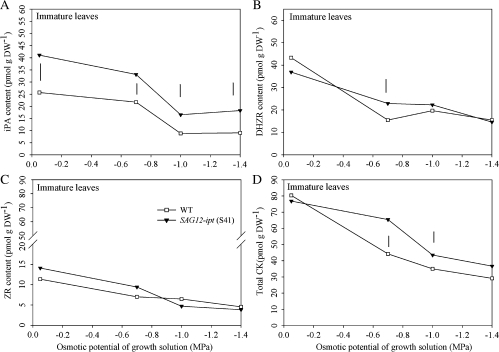
The iPA content in immature leaves was significantly higher in SAG12-ipt plants than in the WT at 0 MPa and during the PEG treatments (Fig. 2). The iPA content declined at –1.4 MPa in all plants, but to a greater extent in WT (65%) than in SAG12-ipt plants (24%) (Fig. 2A). DHZR content declined significantly in both WT and transgenic plants during PEG treatment (Fig. 2B). A significantly higher DHZR content in the transgenic plants was detected only at –0.7 MPa, which was 7% higher than in the WT. ZR content was not different between the WT and SAG12-ipt plants under the control conditions or throughout the duration of PEG treatment (Fig. 2C). PEG-induced drought stress reduced the ZR content of immature leaves by an average of 71% for both lines. Total CK content (including iPA, ZR, and DHZR) did not differ between the plant lines at the control (0 MPa) level, however, after the osmotic potential of the growth solution was reduced from –0.7 to –1.4 MPa, significant differences were observed. For instance, at –0.7 and –1.4 MPa, total CK in WT plants was reduced by 45% and 64%, respectively, whereas that in the transgenic plants was reduced by 14% and 52%, respectively (Fig. 2D).
Fig. 2.
(A) Isopentyl adenine (iPa) (B) dihydrozeatin riboside (DHZR), (C) zeatin riboside (ZR), and (D) total cytokinin content (CK) (sum of iPa, DHZR, and ZR) of immature leaves (top two leaves not fully expanded) of SAG12-ipt creeping bentgrass (line S41) and wild-type ‘Penncross’ (WT) exposed to PEG-induced water stress at various growing solution osmotic potentials (0 to –1.4 MPa). Vertical bars indicate LSD values where significant differences were detected (P ≤0.05) for comparison between plant lines at a given osmotic potential of the growth solution.
CK content of mature leaves
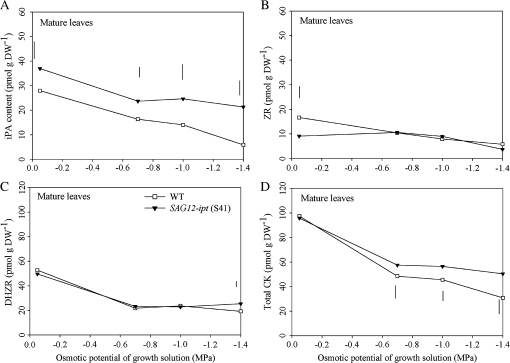
iPA content of mature leaves was greater in the transgenic plants compared to WT at all levels of PEG treatment (Fig. 3A). iPA content declined with PEG-induced water stress, but the rate of iPA loss was slower in the SAG12-ipt plants than in WT. For instance, at –1.4 MPa iPA declined by 57% and 13% relative to their respective control level in WT and SAG12-ipt plants, respectively. Differences in DHZR content between plant lines were detected only at –1.4 MPa, which was 10% greater in the transgenic plants than the WT (Fig. 3B). ZR content in mature leaves was not statistically different between plant lines at any PEG treatment levels (Fig. 3C). Total CK in mature leaves did not differ between plant lines in the control condition, but was maintained at a significantly higher level in the transgenic plants than in the WT at –0.7, –1.0, and –1.4 MPa, which was approximately 25% higher level at –1.4 MPa (Fig. 3D).
Fig. 3.
(A) Isopentyladenine (iPa) (B) dihydrozeatin riboside (DHZR), (C) zeatin riboside (ZR), and (D) total cytokinin content (CK) (sum of iPa, DHZR, and ZR) of mature (fully expanded) leaves of SAG12-ipt creeping bentgrass (line S41) and wild-type ‘Penncross’ (WT) exposed to PEG-induced water stress at various growing solution osmotic potentials (–0.05 to –1.4 MPa). Vertical bars indicate LSD values where significant differences were detected (P ≤0.05) for comparison between plant lines at a given osmotic potential of the growth solution.
Leaf water status
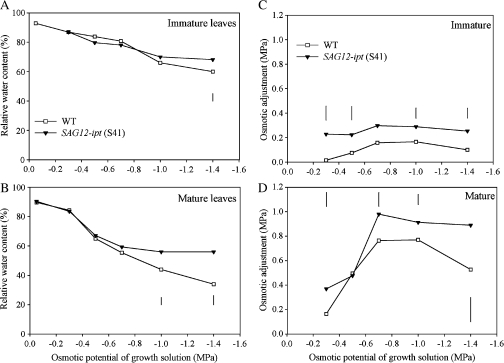
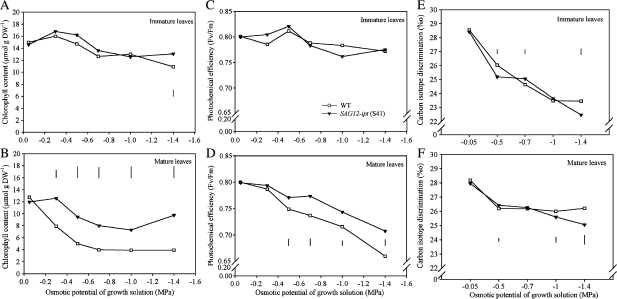
RWC of both immature and mature leaves did not differ between plant lines under the control conditions, but decreased with increasing severity of PEG-induced drought stress (Fig. 4). The transgenic plants maintained significantly higher RWC than WT in immature leaves at –1.4 MPa and in mature leaves at –1.0 and –1.4 MPa. Osmotic adjustment (OA) increased with increasing levels of PEG-induced water stress and was significantly higher in SAG12-ipt plants than in the WT in both immature and mature leaves in most of the PEG treatments (Fig. 5).
Fig. 4.
Relative water content (RWC, %) of (A) immature (top two leaves not fully expanded) and (B) mature (fully expanded) and osmotic adjustment (OA), calculated as the difference in osmotic potential at between fully rehydrated control and PEG-induced water stress treated leaves, of (C) immature (top two leaves not fully expanded) and (D) mature (fully expanded) leaves of SAG12-ipt creeping bentgrass (line S41) and wild-type ‘Penncross’ (WT) exposed to PEG-induced water stress at various growing solution osmotic potentials (–0.05 to –1.4 MPa). Vertical bars indicate LSD values where significant differences were detected (P ≤0.05) for comparison between plant lines at a given osmotic potential of the growth solution.
Fig. 5.
(A, B) Total chlorophyll content (Chl), (C, D) photochemical efficiency (FvFm), and (E, F) carbon isotope discrimination of immature (top two leaves not fully expanded) and mature leaves of SAG12-ipt creeping bentgrass (line S41) and wild-type ‘Penncross’ (WT) exposed to PEG-induced water stress at various growing solution osmotic potentials (–0.05 to –1.4 MPa). Vertical bars indicate LSD values where significant differences were detected (P ≤0.05) for comparison between plant lines at a given osmotic potential of the growth solution.
Photosynthetic parameters and carbon isotope discrimination
The transgenic line and WT did not differ for Chl content in both immature and mature leaves under the control conditions (Fig. 6). Chl content was significantly greater in the transgenic plants than the WT at –1.4 MPa for immature leaves and under all levels of PEG-induced water stress for the mature leaves.
Fig. 6.
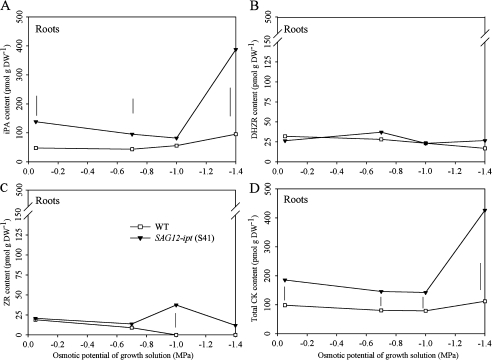
(A) Isopentyl adenine (iPa) (B) dihydrozeatin riboside (DHZR), (C) zeatin riboside (ZR), and (D) total cytokinin content (CK) (sum of iPa, DHZR, and ZR) of roots of SAG12-ipt creeping bentgrass (line S41) and wild-type ‘Penncross’ (WT) exposed to PEG-induced water stress at various growing solution osmotic potentials (–0.05 to –1.4 MPa). Vertical bars indicate LSD values where significant differences were detected (P ≤0.05) for comparison between plant lines at a given osmotic potential of the growth solution.
Leaf photochemical efficiency (FvFm) declined in both immature and mature leaves in response to increasing severity of PEG-induced water stress (Fig. 7). Immature leaf Fv/Fm did not differ significantly between the WT and SAG12-ipt plants under control or PEG treatments. In mature leaves, FvFm was significantly higher in SAG12-ipt plants from –0.5 to –1.4 MPa during the PEG treatment (Fig. 7).
Fig. 7.
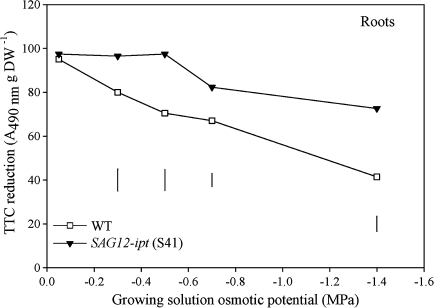
Root viability, as measured by the triphenyl tetrazolium chloride (TTC) reduction method and the absorbance (A) at 490 nm, of SAG12-ipt creeping bentgrass (line S41) and wild-type ‘Penncross’ (WT) exposed to PEG-induced water stress at various growing solution osmotic potentials (–0.05 to –1.4 MPa). Vertical bars indicate LSD values where significant differences were detected (P ≤0.05) for comparison between plant lines at a given osmotic potential of the growth solution.
Plant lines differed in net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (E), and water use efficiency (WUE, calculated as Pn/E ratio) of both immature and mature leaves at –0.7 and –1.0 MPa, but not in the control or other PEG treatment levels (Table 1). Values for Pn were significantly higher for the transgenic plants compared to the WT at –0.7 and –1.0 in immature leaves, and at –0.7 MPa for mature leaves. Mature leaves of transgenic plants had higher gs than the WT at the –0.7 MPa treatment, but did not differ from the WT for transpiration rate. The WUE of transgenic plants were significantly greater than the WT for immature leaves at –0.7 MPa and for mature leaves at –0.7 and –1.0 MPa. In response to PEG treatment, carbon discrimination ratio (Δ) declined in both WT and transgenic plants, however, transgenic plants had lower Δ values in immature leaves at –1.4 MPa and in mature leaves at –1.0 and –1.4 MPa treatment (Fig. 8).
Table 1.
Net photosynthesis rate (Pn), stomatal conductance (gs), transpiration rate (E), and instantaneous water use efficiency (WUE) of immature and mature leaves of wild-type ‘Penncross’ (WT) and SAG12-ipt (S41) plants exposed to PEG-induced drought stress via reduced osmotic potential of the growing solution (–0.7 and –1.0 MPa)
Values followed by the same letter are not significantly different based on Fisher's protected LSD test (P ≤0.05) between plant lines at a given osmotic potential.
| Leaf age | MPa | Plant | Pn | gs | E | WUE |
| (CO2 μmol m−2 s−1) | (mmol) | (mmol H2O m−2 s−1) | (μmol mmol−1) | |||
| Immature | –0.7 | WT | 5.35 b | 13.96 a | 3.31 a | 1.62 b |
| S41 | 5.66 a | 12.62 a | 2.84 a | 1.99 a | ||
| LSD | 0.29 | 0.70 | 1.00 | 0.31 | ||
| –1.0 | WT | 1.41 b | 2.05 a | 0.71 a | 1.98 a | |
| S41 | 1.74 a | 2.93 a | 0.79 a | 2.21 a | ||
| LSD | 0.27 | 1.30 | 0.30 | 0.50 | ||
| Mature | –0.7 | WT | 2.83 b | 6.32 b | 2.26 a | 1.25 b |
| S41 | 3.82 a | 8.52 a | 2.37 a | 1.61 a | ||
| LSD | 0.42 | 0.40 | 0.60 | 0.30 | ||
| –1.0 | WT | 0.29 a | 1.86 a | 0.55 a | 0.52 b | |
| S41 | 0.26 a | 1.97 a | 0.38 a | 0.68 a | ||
| LSD | 0.31 | 0.18 | 0.29 | 0.15 |
CK content of roots and root viability
Root iPa content increased in response to PEG treatment to approximately 400% of the control level in transgenic plants and 100% in the WT at –1.4 MPa (Fig. 9). Roots of transgenic plants were significantly higher than the WT roots under control and PEG treatment, and the difference was most pronounced at –1.4 MPa. Root DHZR content was unchanged during PEG treatment and no significant differences in DHZR content were detected between the WT and transgenic plants. ZR content of the transgenic plants was significantly higher at –1.0 and –1.4 MPa, as ZR content of the WT declined to zero in these treatments while it was maintained in the transgenic plants.
Root viability determined by TTC reduction decreased relative to the control level in response to PEG treatment in both plant lines (Fig. 10). The reduction in root viability was greater for the WT plants, since the average rate of TTC reduction was reduced to 40% of the control level in the WT and by approximately 25% in the transgenic plants at –1.4 MPa. Root viability did not differ between the plant lines under the control conditions, but were significantly greater in the transgenic plants than the WT during PEG treatment.
Discussion
PEG-induced water stress through lowering the osmotic potential of the growth solution (from 0 to –1.4 MPa) caused physiological damage in both the leaves and roots of the WT and SAG12-ipt transgenic plants; however, the transgenic plants exhibited better tolerance to the PEG-induced water stress, as demonstrated by the maintenance of higher FvFm, Chl content, RWC, Pn, gs, and CK content, particularly in mature leaves, and the greater viability and CK production of roots. The same transgenic line has previously been reported to exhibit superior drought tolerance compared with the WT when plants were subjected to soil drying by withholding irrigation (Merewitz et al., 2010). The results, in combination with our previous study, demonstrated that expression of the ipt gene in creeping bentgrass was effective in improving plant tolerance to water stress.
The expression of ipt was detected in immature and mature leaves as well as in roots under all levels of PEG-induced water stress in transgenic creeping bentgrass. PEG-induced water stress could have evoked an initial senescence response, which activated the SAG12 promoter for expression of ipt in immature and mature leaves and roots of SAG12-ipt plants. Drought has been shown to cause expression of ipt in a previous study (Clarke et al., 2004). Expression of SAG12-ipt was also detected under non-stressed conditions in this study and similar findings were reported previously in other plant species, such as in petunia (Petunia×hybrida) (Clarke et al. 2004), maize (Zea mays) (Robson et al., 2004), and tobacco (Rivero et al., 2007), which has been attributed to natural senescence-induced expression. Expression under non-stressed conditions has also been attributed to expression of the endogenous ipt genes, since several ipt genes have recently been isolated from species such as Arabidopsis (Sakamoto et al., 2006), petunia (sho gene; Zubko et al., 2002), rice (Oryza sativa L., 10 forms of ipt; Sakamoto et al., 2006), and maize (ipt1,ipt2, ipt4–8; Brugière et al., 2008). Presumptively, the high level of ipt expression observed here under control conditions and in immature leaves of creeping bentgrass could be due to a combination of these factors; however, it seems more likely that it was induced by natural senescence since perennial grass leaves have a relatively high rate of leaf turnover due to the perennial growth habit.
The expression of ipt under different levels of PEG-induced water stress was associated with the elevated total CK content in immature and mature leaves and roots of SAG12-ipt plants relative to the WT plants. The enhancement of total CK accumulation was primarily due to increased levels of iPa, which is expected since the immediate end-product of the reaction catalysed by ipt is iPA (Medford et al., 1989). In immature leaves, the higher ipt expression level was generally associated with higher levels of iPa and total CK content. Conversely, mature leaves had relatively low ipt expression in the non-stressed condition in conjunction with high CK content but during moderate to severe drought stress, higher expression was accompanied by moderate to low levels of CK. Thus, it is worth noting that ipt expression was not always closely correlated to CK content most likely due to drought damage or other factors affecting post-transcription processes and CK abundance. The results suggest that there may be differences in auto-regulation of the SAG12 promoter and post-trancription processes such as those regulating post-transcription modifications, transcript stability, and translation rates between immature and mature leaves. In addition, CK abundance caused by differences in CK destination, transport activity, and CK degradation due to cellular drought damage may contribute to this CK accumulation difference relative to expression levels between immature and mature leaves. The abundance and activities of cytokinin oxidase (Motyka et al., 1996) and antioxidant enzymes (Synkova et al., 2006) may contribute to these differences, since iPa is the preferred substrate for cytokinin oxidase (Redig et al., 1997) and ipt plants have exhibited differential elevation of antioxidant transcripts and enzyme activity in different plant organs of tobacco (Rivero et al., 2007). However, more research is needed to elucidate post-transcriptional phenomenon of ipt and how it associates with CK content between immature and mature leaves of creeping bentgrass.
The mechanisms of CK regulation of drought tolerance are not yet fully clear. It is known that CK stimulates stomatal opening, which could help with carbon absorption for photosynthesis, but may cause leaf desiccation under drought stress due to water loss (Chernya'dev, 2005). More recently, it has been found that the timing of increased CK content, the form of CK present, and the balance of hormones may be more critical in determining stomatal responses during drought stress, particularly with ipt plants (Pospisilova et al., 2000, 2005). In the current study, immature leaves of the transgenic plants had higher Pn under moderate levels of PEG-induced water stress (–0.7 and –1.0) but gs and E did not differ from the WT plants. In addition, mature leaves of the transgenic plants had a lower Δ value relative to the WT throughout drought treatment at –0.5, –0.7, and –1.4 MPa. iPa content, total CK content, and Pn were generally higher compared with the WT during the PEG treatment, but there were few differences in gs or E in leaves of transgenic plants; the elevated CK may have affected Pn through other mechanisms besides stomatal regulation. Similar results were found in water-stressed ipt tobacco since stomatal conductance was largely the same between ipt and WT plants (Rivero et al., 2009). They concluded that maintenance of photosynthesis rates in ipt tobacco was possibly due to non-stomatal traits such as increased photorespiration, which promotes photosynthesis under drought stress by providing RUBP and other beneficial metabolites (Wingler et al., 2000). This was evident to Rivero et al. (2009) by the increased level of transcripts coding for enzymes in the photorespiration pathway, increased metabolites generated by photorespiration, greater antioxidant content, and an increase in the CO2 compensation point in ipt plants compared with the WT. These mechanisms may also be a factor in SAG12-ipt creeping bentgrass observed indirectly in this study as higher Pn in the mature leaves of the transgenic plants, increases in chlorophyll content, and greater Fv/Fm. In addition, differences in Pn contributed to differences in WUE between plants lines. This is in conjunction with the differences in Δ values between the WT and transgenic plants, as transgenic plants had lower Δ than the WT during most of the PEG treatments. In Kentucky bluegrass (Poa pratensis), higher values of WUE have been associated with less wilting, greater TQ, and superior water relations under drought stress (Ebdon and Kopp, 2004). In several cool season grasses, low Δ was associated with higher instantaneous water use efficiency; plants with low Δ had higher water potential, solute potential, and turgor pressure, exhibiting a better capacity for growth under drought stress (Johnson and Basset, 1991). Thus, most likely the ipt gene and increased CK may enhance metabolic activities that may promote photosynthetic activity without increasing stomatal conductance or transpiration rate, thereby increasing water use efficiency, especially in immature leaves. CK involvement in regulating Pn, gs, E, WUE, and Δ under drought stress deserves additional investigation since increases in CK is not always associated with increases in gs as previously proposed and some studies have shown a decrease in gs in response to elevated CK (Pospisilova et al., 1997, 1998).
Our results show that CK may have some involvement in regulating water relations and OA, as SAG12-ipt plants had better OA and retained more water, particularly in mature leaves. However, it is not clear whether this is a direct, primary result of altered OA and water status or a secondary effect of improved water status due to increased overall physiological activities or photosynthetic efficiency. Recent literature in the role of CK in regulating osmotic adjustment is conflicting. In transgenic potato that accumulate CKs (Solanum tuberosum), RWC and OA seemed to be unaffected by CK levels (Siffel et al., 1992; Popsisilova et al., 2000). Conversely, ipt plants have also been shown to have enhanced levels of osmolytes such as proline, which would typically have an effect on OA. The changes in proline in this study were not always associated with reduced wilting of ipt plants (Thomas et al., 1995). Thus, due to the complexities of comparing different plant species under different promoters expressing ipt, a clear role of CK in regulating water relations is still not fully elucidated. It has been suggested that CK may cause a slight osmotic response similar to a small degree of salt stress to allow increased tolerance via adaption for subsequent stress such as drought (Thomas et al., 1995; Pospisilova et al., 2000). Our results seem to be consistent with this explanation since CK levels were higher and OA was increased even under a low level of water stress (–0.3 MPa), and, consistently, the transgenic plants, compared to WT, in both immature and mature leaves, even when differences in other parameters such as RWC or Fv/Fm were not yet observed.
Effects of ipt expression on the physiological responses to PEG-induced water stress varied between mature and immature leaves. Generally, the positive effects of ipt expression on the physiological parameters examined here were more pronounced for mature leaves, whereas fewer differences occurred for immature leaves between the transgenic and WT plants. Significant differences between SAG12-ipt and WT in Pn, WUE, and Δ in immature leaves were observed earlier during moderate water stress (–0.7 and –1.0 MPa), whereas differences between SAG12-ipt and WT in Chl, FvFm, E, RWC, and OA in immature leaves, were not observed until more severe water stress levels were reached (–1.0 to –1.4 MPa). In mature leaves, differences between SAG12-ipt and WT in most of these parameters started at approximately –0.5 MPa. Senescence was delayed in mature leaves of SAG12-ipt plants compared with mature leaves of WT, indicated by maintenance of higher Chl and FvFm during water stress. Delay in leaf senescence is significant for plants to adapt to water stress, as the first symptoms of senescence are seen as a loss of chlorophyll and chloroplasts as the plant degrades leaf mesophyll cells for nutrient remobilization (Lim et al., 2007). The results of this study show that the expression of the SAG12-ipt in creeping bentgrass at highly detectable levels was sufficient to maintain levels of CK in immature and mature leaves and increase root CK of creeping bentgrass subjected to water stress. It may be assumed that enough CK was produced to overcome degradation of the free forms of CK by cytokinin oxidases, which are up-regulated in the drought response in most plant organs (Vyroubalova et al., 2009).
Root ipt expression analysis revealed a gradual increase in transcript abundance with the severity of PEG-induced water stress, which corresponded to an increase in CK levels at –1.4 MPa osmotic potential. Despite the increase in ipt transcription during moderate (–0.5 to –1.0 MPa) water stress, a corresponding increase in root CK content during moderate stress was not observed, which could be due to sustained CK transport to the leaves. However, in roots exposed to –1.4 MPa PEG treatment, a sharp increase in CK was observed, which could be due to the interruption of CK transport from roots to other parts of a plant under severe water stress. A decline of root CK content during moderate water stress followed by an accumulation of CK in the roots during severe water stress was also found in SAG12-ipt tobacco (Havlova et al., 2008). The results of the current study suggest that this could be due to reduced CK transport activity as opposed to a decline in root viability, as SAG12-ipt root viability was significantly higher at –1.4 MPa relative to the WT plants. Other work has also suggested that CK accumulates in roots under severe drought stress due to reduced xylem transport of CK or possibly due to a decrease in CKX activity (Novakova et al., 2007). Kudoyarova et al. (2006) reported that loading of CK into the xylem was decreased in tomato plants exposed to dry soils. Similar results were found in response to heat stress, SAG12-ipt bentgrass exhibited increased iPa in the roots by approximately ten times the amount in the roots at optimal temperature of 20 °C, whereas the leaves had a less dramatic increase in iPa content in response to temperature, however, leaf iPa content was maintained at higher levels in the transgenic lines compared with the WT. Similar to our results, a less dramatic change was observed for the other forms of CK such as ZR and DHZR compared with iPa in response to temperature and the transgene in this study (Xu et al., 2009).
Limited information is available about the role of CK in root senescence and mortality under drought-stressed conditions. Our results indicate that increased endogenous CK in the SAG12-ipt plants was associated with higher root viability. The results show that CK may be involved in regulating root mortality, since SAG12-ipt plants had increased root viability under water-stress conditions. Promoting root growth and viability were previously reported in a study with exogenous applications of different forms of CKs on creeping bentgrass exposed to heat stress (Zhang and Ervin, 2008). The mechanisms for root survival of water stress associated with ipt expression are not known. It is possible that the delay in leaf senescence and the maintenance of higher photosynthesis of the SAG12-ipt plants under water stress could have maintained adequate carbon available to transport to the roots to maintain root growth or survival. Alternatively, CK may directly be involved in the regulation of root mortality; however, this is not yet clear. Previous research has indicated CK may play a direct role in root stress signalling by stimulation of root antioxidants to reduce root lipid peroxidation, which increased root viability (Liu and Huang, 2002). Programmed cell death (PCD), primarily of root tips, is caused by abiotic stress such as drought and may be mediated by reactive oxygen species (ROS) accumulation that causes an endoplasmic reticulum (ER) signal to cause PCD (Duan et al., 2010). Since ipt plants may maintain more antioxidants due to the presence of increased CK, there may be less of a signal sent to the ER, to reduce PCD of the roots under drought for increased root viability. Recent work by Vyroubalova et al. (2009) has more explicitly shown that CKs most likely do not have a direct function in drought signalling, since CK changes occur slowly and the reduced growth rate is not caused by decreased active CK or increased CK oxidation, but rather due to changes in abscisic acid. They concluded that enhanced CK content by transgenic methods may be the best method for the creation of cultivars with increased drought tolerance by promoting root growth under stress. Future work will analyse antioxidant enzyme responses in SAG12-ipt creeping bentgrass under drought stress. Our results indicate that increased endogenous CK may help delay drought-induced root mortality; however, the exact mechanism deserves more attention, especially the effects of increased CK on root tip viability under water stress.
In conclusion, SAG12-ipt transformation of creeping bentgrass resulted in increases in CK accumulation in the leaves and roots and in the overall plant tolerance to water stress. The physiological effects of ipt expression on improving plant tolerance to water stress were reflected by an enhancement in OA, photosynthetic characteristics, water use efficiency, delay in leaf senescence, and maintenance of higher root viability under water stress in creeping bentgrass. Future work will aim to identify the mechanisms associated with CK regulation of photosynthesis, OA, and root viability in SAG12-ipt creeping bentgrass during water stress. In addition, the interaction of CK derived from SAG12-ipt with other stress regulation hormones, such as ABA, auxin, and ethylene may deserve further investigation.
Acknowledgments
The authors wish to thank the OJ Noer Foundation and Rutgers Center of Turfgrass Science for funding support for this research.
References
- Akiyoshi D, Klee H, Amasino R, Nester EW, Gordon MP. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proceedings of the National Academy of Sciences. 1984;81:5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949;24:1–13. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenoch-Jones J, Parker CW, Letham DS, Singh S. Effect of cytokinins supplied via the xylem at multiples of endogenous concentrations on transpiration and senescence in de-rooted seedlings of oat and wheat. Plant, Cell and Environment. 1996;19:504–516. [Google Scholar]
- Barry GF, Rogers SG, Fraley RT, Brand L. Identification of a cloned cytokinin biosynthetic gene. Proceedings of the National Academy of Sciences, USA. 1984;81:4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs HD, Weatherley PE. A re-examination of the relative turgidity techniques for estimating water deficits in leaves. Australian Journal of Biological Science. 1962;15:413–428. [Google Scholar]
- Blum A. Plant breeding for stress environments. Boca Raton, FL: CRC Press; 1988. [Google Scholar]
- Bray EA. Molecular responses to water deficit. Plant Physiology. 1993;103:1035–1040. doi: 10.1104/pp.103.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugière N, Humbert S, Rizzo N, Bohn J, Habben JE. A member of the maize isopentenyl transferase gene family, Zea mays isopentenyl transferase 2 (ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development. Plant Molecular Biology. 2008;67:215–229. doi: 10.1007/s11103-008-9312-x. [DOI] [PubMed] [Google Scholar]
- Burlyn EM. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiology. 1983;72:66–70. doi: 10.1104/pp.72.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought: from genes to whole plant. Functional Plant Biology. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Chernyad'ev I. Effect of water stress on the photosynthetic apparatus of plants and the protective role of cytokinins: a review. Applied Biochemistry and Microbiology. 2005;41:115–128. [PubMed] [Google Scholar]
- Clark DG, Dervinis C, Barrett JE. Drought-induced leaf senescence and horticultural performance of transgenic PSAG12-ipt petunias. Journal of the American Society for Horticultural Science. 2004;129:93–99. [Google Scholar]
- DaCosta M, Huang B. Deficit irrigation effects on water use characteristics of bentgrass species. Crop Science. 2006;46:1779–1786. [Google Scholar]
- Davies WJ, Tardieu F, Trejo C. How do chemical signals work in plants that grow in drying soil? Plant Physiology. 1994;104:309–314. doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Zhang W, Li B, Wang Y, Li K, Sodmergen Han C, Zhang Y, Li X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytologist. 2010;186:681–695. doi: 10.1111/j.1469-8137.2010.03207.x. [DOI] [PubMed] [Google Scholar]
- Ebdon JS, Kopp KL. Relationships between water use efficiency, carbon isotope discrimination, and turf performance in genotypes of kentucky bluegrass during drought. Crop Science. 2004;44:1754–1762. [Google Scholar]
- Ebdon JS, Petrovic AM, Dawson TE. Relationship between carbon isotope discrimination, water use efficiency, and evapotranspiration in Kentucky bluegrass. Crop Science. 1998;38:157–162. [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlated with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology. 1984;11:539–552. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- Gan SS, Amasino RM. Inhibition of leaf senescence by auto-regulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- Havlova M, Dobrev PI, Motyka V, Storchova H, Libus J, Dobra J, Malbeck J, Gaudinova A, Vankova R. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant, Cell and Environment. 2008;31:341–353. doi: 10.1111/j.1365-3040.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jia W, Wang J, Zhang Y, Yang L, Lin Z. Transgenic tall fescue containing the Agrobacterium tumefaciens ipt gene shows enhanced cold tolerance. 2005. Plant Cell Reports. 2005;23:705–709. doi: 10.1007/s00299-004-0863-2. [DOI] [PubMed] [Google Scholar]
- Janes B. The effect of molecular size, concentration in nutrient solution, and exposure time on the amount and distribution of polyethylene glycol in pepper plants. Plant Physiology. 1974;54:226–230. doi: 10.1104/pp.54.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC, Basset LM. Carbon isotope discrimination and water use efficiency in four cool-season grasses. Crop Science. 1991;31:157–162. [Google Scholar]
- Khan AA. Effect of leaf position and plant age on the translocation of 14C-assimilates in onion. Journal of Agricultural Science. 1981;96:451–455. [Google Scholar]
- Knievel DP. Procedure for estimating ratio of living to dead root dry matter in the root core sample. Crop Science. 1973;13:124–126. [Google Scholar]
- Kramer PJ, Boyer JS. Water relations of plants and soils. New York, NY: Academic Press, Inc; 1995. [Google Scholar]
- Kudoyarova GR, Vysotskaya LB, Cherkozyanova A, Dodd IC. Effects of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. Journal of Experimental Botany. 2006;58:161–168. doi: 10.1093/jxb/erl116. [DOI] [PubMed] [Google Scholar]
- Lagerwerff JV, Ogata G, Eagle HE. Control of osmotic pressure of culture solutions with polyethylene glycol. Science. 1961;133:1486–1487. doi: 10.1126/science.133.3463.1486. [DOI] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Nam HG. Leaf senescence. Annual Review of Plant Biology. 2007;58:115–136. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- Liu X, Huang B. Cytokinin effects on creeping bentgrass response to heat stress. II. Leaf senescence and antioxidant metabolism. Crop Science. 2002;42:466–472. [Google Scholar]
- Marrion-Poll A, Leung J. Abscisic acid synthesis, metabolism, and signal transduction. In: Hedden P, Thomas TG, editors. Plant hormone signalling. Annual Plant Reviews. Oxford: Blackwell Publishing. Ltd; 2006. pp. 1–35. [Google Scholar]
- McCabe MS, Garratt LC, Schepers F, Jordi WJRM, Stoopen GM, Davelaar E, Hans J, Van Rhijn A, Power JB, Davey MR. Effects of PSAG12-IPT gene expression on development and senescence in transgenic lettuce. Plant Physiology. 2001;127:505–516. [PMC free article] [PubMed] [Google Scholar]
- McMichael BL, Burke JJ. Metabolic activity of cotton roots in response to temperature. Environmental and Experimental Botany. 1994;34:201–206. [Google Scholar]
- McGraw BA. Cytokinin biosynthesis and metabolism. In: Davies PJ, editor. Plant hormones and their role in plant growth and development. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 76–93. [Google Scholar]
- Medford JI, Horgan R, El-Sawi Z, Klee HJ. Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. The Plant Cell. 1989;1:403–413. doi: 10.1105/tpc.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merewitz E, Gianfagna T, Huang B. Effects of SAG12-ipt and HSP18.2-ipt. expression on cytokinin production, root growth and leaf senescence in creeping bentgrass exposed to drought stress. Journal of the American Society for Horticultural Science. 2010;135:230–239. [Google Scholar]
- Morris RO. Genes specifying auxin and cytokinin biosynthesis in prokaryotes. In: Davies PJ, editor. Plant hormones physiology, biochemistry, and molecular biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 318–339. [Google Scholar]
- Motyka V, Faiss M, Strnad M, Kaminek M, Schmulling T. Changes in cytokinin content and cytokinin oxidase activity in response to de-repression of ipt gene transcription in transgenic tobacco calli and plants. Plant Physiology. 1996;112:1035–1043. doi: 10.1104/pp.112.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi SSM. Plant/crop hormones under stressful conditions. In: Pessarakli M, editor. Handbook of plant and crop physiology. New York, NY: Marcel Dekker Inc; 1995. pp. 645–660. [Google Scholar]
- Novakova M, Dobrev P, Motyka V, et al. Cytokinin function in drought stress response and subsequent recovery. In: Xu Z, Li J, Xue Y, Yang W, editors. Proceedings of the 11th IAPTC& B Congress, August 31–18, 2006. Beijing: China; 2007. pp. 171–174. [Google Scholar]
- Okamoto M, Tatematsu K, Matsui A, et al. Genome-wide analysis of endogenous abscisic acid-mediated transcription in dry and imbibed seeds of arabidopsis using tiling arrays. The Plant Journal. 2010;62:39–51. doi: 10.1111/j.1365-313X.2010.04135.x. [DOI] [PubMed] [Google Scholar]
- Pospíšilová J, Čatský J, Šesták Z. Photosynthesis in plants cultivated in vitro. In: Pessarakli M, editor. Handbook of photosynthesis. New York, Basel, Hong Kong: Marcel Dekker; 1997. pp. 525–540. [Google Scholar]
- Pospisilova J, Synkova H, Machackova I, Catsky J. Photosynthesis in different types of transgenic tobacco plants with elevated cytokinin content. Biologia Plantarum. 1998;40:81–89. [Google Scholar]
- Pospisilova J, Synkova H, Rulcova J. Cytokinins and water stress. Biologia Plantarum. 2000;43:321–328. [Google Scholar]
- Pospisilova J, Vagner M, Malbeck J, Travnickola A, Batkova P. Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biologia Plantarum. 2005;49:533–540. [Google Scholar]
- Redig P, Motyka V, Van Onckelen HA, Kaminek M. Regulation of cytokinin oxidase activity in tobacco callus expressing the T-DNA ipt gene. Physiologia Plantarum. 1997;99:89–96. [Google Scholar]
- Richmond AE, Lang A. Effect of kinetin on protein content and survival of detached Xanthium leaves. Science. 1957;125:650–651. [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proceedings of the National Academy of Sciences, USA. 2007;104:19631–19636. doi: 10.1073/pnas.0709453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Shulaev V, Blumwald E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiology. 2009;150:1530–1540. doi: 10.1104/pp.109.139378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PRH, Donnison IS, Wang K, Frame B, Pegg SE, Thomas A, Thomas H. Leaf senescence is delayed in maize expressing the Agrobacterium IPT gene under the control of a novel maize senescence-enhanced promoter. Plant Biotechnology Journal. 2004;2:101–112. doi: 10.1046/j.1467-7652.2004.00054.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakakibara H, Kojima M, Yamamoto H, Nagasaki Y, Inukai Y, Sato Y, Matsuoka M. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiology. 2006;142:54–62. doi: 10.1104/pp.106.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Flannigan BA, Melkonian J. Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisic acid, and cytokinins. Crop Science. 2001;41:1530–1540. [Google Scholar]
- Šiffel P, Šindelková E, Durchan M, Zajícová M. Photosynthetic characteristics of Solanum tuberosum L. plants transformed by Agrobacterium strains. 1. Pigment apparatus. Photosynthetica. 1992;27:441–447. [Google Scholar]
- Smedley MP, Dawson TE, Comstock JP, Donovan LA. Seasonal carbon isotope discrimination in a grassland community. Oecologia. 1991;85:314–320. doi: 10.1007/BF00320605. [DOI] [PubMed] [Google Scholar]
- Synkova H, Semoradova S, Schnablova R, Witters E, Husak M, Valcke R. Cytokinin-induced activity of antioxidant enzymes in transgenic Pssu-ipt tobacco during plant ontogeny. Biology Plantarum. 2006;50:31–41. [Google Scholar]
- Thomas JC, Smigocki AC, Bohnert HJ. Light-induced expression of ipt from Agrobacterium tumefaciens results in cytokinin accumulation and osmotic stress symptoms in transgenic tobacco. Plant Molecular Biology. 1995;27:225–235. doi: 10.1007/BF00020179. [DOI] [PubMed] [Google Scholar]
- Turgeon AJ. Turfgrass management. 8th edn. Upper Saddle River, NJ: Pearson Prentice Hall; 2008. [Google Scholar]
- Verdonk JC, Shibuya K, Loucas HM, Colquhoun TA, Underwood BA, Clark DG. Flower-specific expression of the Agrobacterium tumefaciens isopentenyltransferase gene results in radial expansion of floral organs in Petunia hybrida. Plant Biotechnology Journal. 2008;6:694–701. doi: 10.1111/j.1467-7652.2008.00349.x. [DOI] [PubMed] [Google Scholar]
- Vyroubalová S, Václavíková K, Turecková V, Novák O, Smehilová M, Hluska T, Ohnoutková L, Frébort I, Galuszka P. Characterization of new maize genes putatively involved in cytokinin metabolism and their expression during osmotic stress in relation to cytokinin levels. Plant Physiology. 2009;151:433–447. doi: 10.1104/pp.109.142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Huang B, Xu Q. Effects of abscisic acid on drought response of kentucky bluegrass. Journal of the American Society of Horticultural Science. 2003;128:36–41. [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC. Photorespiration: metabolic pathways and their role in stress protection. Philosophical Transactions of the Royal Society of Biological Sciences. 2000;355:1517–1529. doi: 10.1098/rstb.2000.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Xu Y, Tian J, Gianfagna T, Huang B. Suppression of shade or heat-induced leaf senescence in creeping bentgrass through transformation with the ipt gene for cytokinin synthesis. Journal of the American Society for Horticulture Science. 2010;134:602–609. [Google Scholar]
- Xu Y, Tian J, Gianfagna T, Huang B. Effects of SAG12-ipt expression on cytokinin production, growth and senescence of creeping bentgrass (A. stolonifera L.) under heat stress. Plant Growth Regulation. 2009;57:281–291. [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q, Liu L. Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta. 2002;215:645–652. doi: 10.1007/s00425-002-0789-2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ervin EH. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Science. 2008;48:364–370. [Google Scholar]
- Zhang J, Van Toai T, Huynh L, Preiszner J. Development of flooding-tolerant arabidposis by autoregulated cytokinin production. Molecular Breeding. 2000;6:135–144. [Google Scholar]
- Zubko E, Adams CJ, Machaekova U, Malbeck J, Scollan C, Meyer P. Activation tagging identifies a gene from Petunia hybrida responsible for the production of active cytokinins in plants. The Plant Journal. 2002;29:797–808. doi: 10.1046/j.1365-313x.2002.01256.x. [DOI] [PubMed] [Google Scholar]