Abstract
Background
The flowers of Inula japonica (Inulae Flos) have long been used in traditional medicine for the treatment of inflammatory diseases. In the present study, we investigated the anti-inflammatory properties of Inulae Flos Extract (IFE).
Methods
The anti-inflammatory effects of IFE against nitric oxide (NO), PGE2, TNF-α, and IL-6 release, as well as NF-κB and MAP kinase activation were evaluated in RAW 264.7 cells.
Results
IFE inhibited the production of NO and the expression of inducible nitric oxide synthase (iNOS) in LPS-stimulated RAW264.7 cells. In addition, IFE reduced the release of pro-inflammatory cytokines, such as TNF-α and IL-6. Furthermore, IFE inhibited the NF-κB activation induced by LPS, which was associated with the abrogation of IκB-α degradation and subsequent decreases in nuclear p65 and p50 levels. Moreover, the phosphorylation of ERK, JNK, and p38 MAP kinases in LPS-stimulated RAW 264.7 cells was suppressed by IFE in a dose-dependent manner.
Conclusion
These results suggest that the anti-inflammation activities of IFE might be attributed to the inhibition of NO, iNOS and cytokine expression through the down-regulation of NF-κB activation via suppression of IκBα and MAP kinase phosphorylation in macrophages.
Keywords: Inula japonica, Inulae Flos Extract (IFE), Nitric oxide (NO), iNOS, Cytokine, NF-κB, MAP kinase
INTRODUCTION
Inflammation is the host response to infection and injury that results in the production of a variety of pro-inflammatory cytokines including TNF-α and IL-6. Also, PGE2 and NO, which are synthesized by cyclooxygenase (COX-2) and inducible nitric oxide synthase (iNOS) respectively, are known to mediate inflammatory reaction. However, if left un-controlled, the inflammatory mediators become involved in the pathogenesis of many inflammatory disorders (1).
Expression of these inflammatory mediators can be regulated by activation of the transcription factor nuclear factor kappa-B (NF-κB), which plays a critical role in regulating the expression of various genes, including cytokines, iNOS and COX-2 (2). NF-κB exists in an active form associated with regulatory protein, which is known as inhibitory protein of NF-κB (IκB). Upon stimulation by various inflammatory stimuli including LPS, the IκB kinase (IKK) phosphorylates IκB, inducing its ubiquitination and degradation. NF-κB is then free to translocate to the nucleus where it facilitates the transcription of many genes, including pro-inflammation mediators such as iNOS, COX-2, TNF-α and cytokines (3,4). Because of its ubiquitous role in the pathogenesis of inflammatory gene expression, NF-κB is a current target for treatment of various diseases (5).
The use of herbal therapy or alternative medicine is becoming an increasingly attractive approach for the treatment of various inflammatory disorders. The genus Inula consists of more than one hundred species and is found mainly in Mediterranean regions (6). The flowers of Inula japonica T. and Inula britannica L. have long been used in traditional Chinese medicine for the treatment of digestive disorders, bronchitis and inflammation (7). Here we decided to explore the mechanism underlying the anti-inflammatory effects of Inula japonica. To examine if the ethanol extract of flowers of Inula japonica (Inulae Flos Extract, IFE) influences the inflammatory mediators, we investigated the anti-inflammatory functions of IFE in RAW 264.7 cells following LPS stimulation. In addition, we examined the LPS- induced DNA binding activity of NF-κB and the protein levels of its p50 and 65 subunits.
MATERIALS AND METHODS
Plant materials
The dried flowers of Inula japonica (Inulae Flos) were purchased from Omniherb (Youngchun, Korea). The Inulae Flos was extracted with ethanol at a ratio of 1:10 (w/v) and then refluxed for 24 h at 70℃. Following extraction, the solutions were filtered and the solvents were evaporated under vacuum at 40℃ (Eyela, Tokyo, Japan), after which they were freeze-dried to obtain the concentrated extract (yield 8%, w/w). The IFE was dissolved in dimethyl sulfoxide (DMSO) and diluted in the medium so that the final concentration of DMSO was less than 0.25% v/v. A control consisting of DMSO alone was also run in all cases.
Cell culture
RAW 264.7 cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and then cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100µg/ml streptomycin and 100µM MEM non-essential amino acid solution. In all experiments, cells were grown to 80~90% confluence and then subjected to no more than 10 cell passages.
Measurement of cell viability
Cell viability was assessed using the CellTiter 96 Aqueous One kit (Promega, Madison, WI, USA). Briefly, RAW 264.7 cells were seeded onto a 96-well plate at 5×104 cells/well. After incubation with various concentration of IFE, 20µl of 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTS), which is converted to a formazan product by metabolically active cells, was added to each well. After 2 h of incubation, the optical densities at 490 nm were measured using a microplate reader (Tecan System, San Jose, CA, USA).
Measurement of nitric oxide
RAW 264.7 cells (5×105 cells/well) were seeded onto a 24-well culture plate at 37℃ for overnight in medium. The cells were preincubated with various concentrations of IFE for 24 h. NO production was then monitored by measuring nitrite levels in the culture media using Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine dihydrochloride and 2.5% phosphoric acid). The absorbance was measured at 570 nm after incubation for 10 min. The nitrite levels in the samples were calculated from a standard curve generated using known concentrations of sodium nitrite.
Measurement of PGE2
PGE2 production was measured in culture medium to determine the inhibitory activity of IFE against COX-2. Briefly, RAW 264.7 cells were plated in 24-well plates at a density of 5×105/ml in DMEM and then pretreated with IFE for 1 h, after which they were stimulated with LPS for 24 h. All reactions were stopped by centrifugation at 3,000 rpm g at 4℃ for 5 min. The supernatant and cell pellets were immediately frozen in liquid N2 and stored at -80℃ for further analysis. PGE2 was determined using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Enzyme-linked immunosorbent assay (ELISA)
The RAW 264.7 cells were plated in a 24-well culture dish at a density of 5×105 cells/well and then incubated with IFE in the presence or absence of LPS for 24 h. The supernatants of cell cultures with or without treatment with IFE were used to measure the TNF-α and IL-6 levels using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The concentrations of TNF-α and IL-6 in the samples were calculated from a standard curve developed using a known concentration of recombinant TNF-α and IL-6.
Western blot analysis
After activation with LPS, RAW 264.7 cells (7×105) were washed once with 10 mM PBS (pH 7.4) containing 150 mM NaCl and then lysed in PBS containing 0.1% SDS and 10 mM β-mercaptoethanol. The lysates containing 30µg of protein were applied to 10% SDS-polyacrylamide gels. The proteins were then transferred to nitrocellulose membranes in 20% methanol/25 mM Tris/192 mM glycine. Next, the membranes were blocked with 5% non-fat dry milk in TTBS (25 mM Tris-HCl, 150 mM NaCl, and 0.2% Tween-20) and then probed with various primary antibodies. After 1 h of incubation followed by three washes, the membranes were incubated for 1 h with a secondary HRP-conjugated antibody. The protein bands were then visualized using an ECL system.
Luciferase assay
A NF-κB luciferase plasmid was transfected into RAW 264.7 cells using Lipofectamine Plus™ Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations. Cells were pretreated with IFE for 30 min and then stimulated with LPS (200 ng/ml) for 6 h. Cell extracts were then prepared and assayed for luciferase activity using a luminometer (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Statistical analysis
All values shown represent the arithmetic mean±S.D. One-way ANOVA was used to determine the statistical significance.
RESULTS
Suppression of LPS-induced NO production and expression of iNOS by IFE
The effects of IFE concentration (12.5, 25 and 50µg/ml) on cell viability were assessed by an MTS assay and there was no significant change in cell viability observed in response to these concentrations (data not shown).
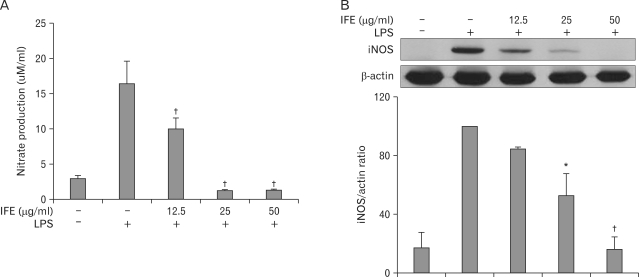
Because NO is the major inflammatory marker produced through the synthesis of iNOS during inflammation, we examined the effects of IFE on NO and iNOS expression in LPS-stimulated RAW 264.7 cells. To analyze the NO production, RAW 264.7 cells were pretreated with IFE for 1 h prior to stimulation with LPS (200 ng/ml). Following 24 h of LPS stimulation, the levels of NO in the culture media were determined. As shown in Fig. 1A, LPS stimulation resulted in a marked induction of NO production when compared to the untreated cells. However, pretreatment of IFE resulted in a marked reduced production of NO caused by LPS stimulation. We next examined the effect of IFE on iNOS expression. The significant induction of iNOS protein by LPS stimulation was inhibited by IFE pretreatment in a dose-dependent manner (Fig. 1B). These results showed that IFE inhibited NO production by the decreased iNOS protein expression.
Figure 1.
Effect of IFE on LPS-induced NO production and iNOS expression in RAW 264.7 cells. Cells were pretreated with different concentrations of IFE for 1 h and then stimulated with LPS (200 ng/ml) for 24 h. The levels of nitrite were measured in the culture media by Griess reagents (A). Cells were harvested and the cell lysates were prepared as described in the experimental procedure. The protein levels of iNOS were measured by Western blot analysis using antibody against iNOS. Quantification of band intensities from three independent results was determined by densitometric analysis. The values were expressed as a percentage of maximal band intensity in the LPS-treated cells, which was set to 100% (B). Values are expressed as means±S.D. of three different samples. *p<0.01, †p<0.001, compared with the control value.
Effect of IFE on the LPS-induced production of PGE2 and expression of COX-2
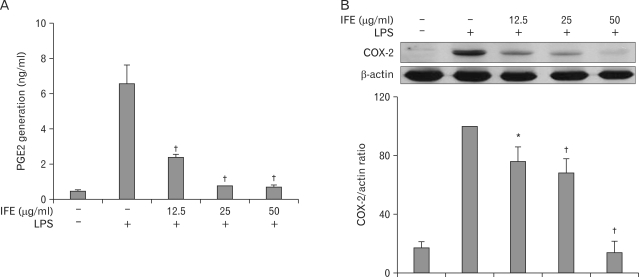
We examined the effects of IFE on PGE2 production and COX-2 protein expression following LPS stimulation in RAW 264.7 cells. Briefly, the cells were pretreated with IFE for 1 h and then stimulated with LPS. After incubation for 24 h, the production of PGE2 from the culture supernatant was measured using ELISA. As shown in Fig. 2A, the amount of PGE2 in the culture supernatant increased with LPS stimulation, and this increase was reduced by treatment with IFE. To further elucidate the effects of IFE, we evaluated the COX-2 protein levels using Western blot analysis. COX-2 protein was not detected in the absence of LPS treatment, but the levels of COX-2 were significantly upregulated after LPS exposure. As shown in Fig. 2B, IFE elicited a concentration- dependent inhibition of LPS-stimulated COX-2 protein.
Figure 2.
Effect of IFE on PGE2 generation and COX-2 expression in LPS-treated RAW 264.7 cells. Cells were pretreated with different concentrations of IFE for 1 h and then stimulated with LPS (200 ng/ml) for 24 h. After incubation, the PGE2 levels in the cultured media were measured using an ELISA kit (A). Cells were harvested and equal amounts of cell extracts were subjected to Western blot analysis using antibody against COX-2. Quantification of band intensities from three independent results was determined by densitometric analysis. The values were expressed as a percentage of maximal band intensity in the LPS-treated cells, which was set to 100% (B). Values are expressed as the means±S.D. of three different samples. *p<0.01, †p<0.001, when compared with the control value.
Effect of the IFE on cytokine release
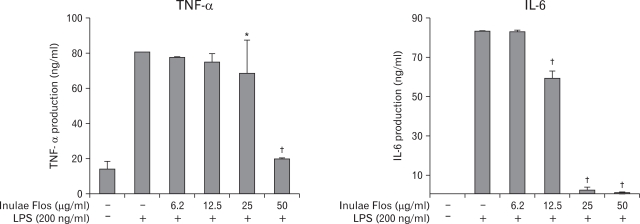
To analyze the mechanism of anti-inflammation by IFE, we next determined the effect of IFE on LPS-induced production of proinflammatory cytokines including TNF-α and IL-6. Fig. 3 demonstrated that unstimulated RAW 264.7 cells cultured for 24 h produced a negligible quantity of cytokines. However, the levels of TNF-α and IL-6 were increased in the culture supernatant of the LPS-stimulated cells, whereas pretreatment with IFE resulted in a decrease in cytokine production.
Figure 3.
Effects of IFE on cytokine release in LPS-treated RAW 264.7 cells. Cells were pretreated with different concentrations of IFE for 1 h and then treated with LPS (200 ng/ml) for 24 h. The cytokine levels in the culture media were measured using an ELISA kit as described in the experimental procedures. Values are expressed as the means±S.D. of three independent experiments. *p<0.05, †p<0.001, when compared with the control value.
Effect of IFE on LPS-stimulated NF-κB activity
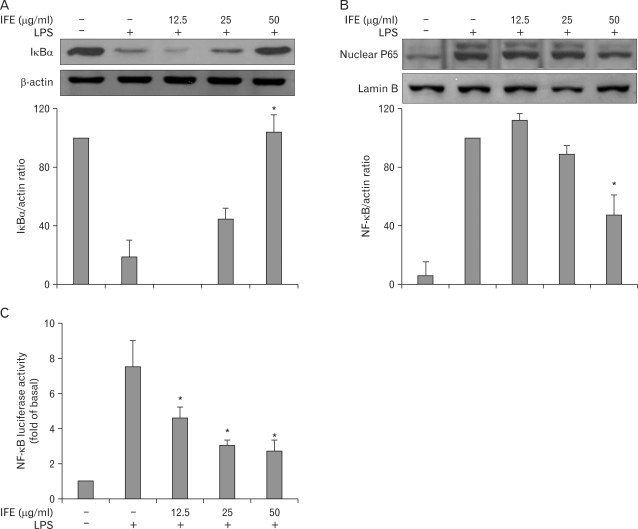
The transcription factor, NF-κB, is a pleiotropic regulator of many genes involved in immune and inflammatory responses, including leukocyte adhesion molecules (8). In response to external stimuli, C-bound IκB is phosphorylated, leading to degradation by the ubiquitin-dependent proteasomal system. This leaves NF-κB dimers free to translocate to the nucleus. Therefore, we examined the effects of IFE on LPS-stimulated IκB degradation. One hour of pretreatment with IFE followed by treatment with LPS for 30 min suppressed the LPS-stimulated IκB degradation in a dose-dependent fashion (Fig. 4A). Next, we investigated the translocation of the NF-κB subunit p65 from the cytosol to the nucleus using Western blot analysis. LPS stimulation caused p65 translocation from the cytosol to the nucleus, while IFE inhibited this translocation (Fig. 4B). These results suggest that IFE represses NF-κB translocation by inhibiting IκB degradation.
Figure 4.
Effect of IFE on NF-κB activation in RAW 264.7 cells. Cells were pretreated with different concentrations of IFE for 1 h and then stimulated with LPS (200 ng/ml) for 30 min. Total cellular proteins and nuclear extracts were prepared for Western blot analysis of NF-κB p65 and IκBα proteins. Quantification of band intensities from three independent results was determined by densitometric analysis. The values were expressed as a percentage of maximal band intensity in the LPS-treated cells, which was set to 100% (A, B). Cells were transiently transfected with the pNF-κB-Luc plasmid using a lipofectamine method. Cells were pretreated with different concentrations of IFE for 1 h and then further stimulated with LPS (200 ng/ml) for 6 h. The cells were then harvested and the luciferase activities were determined using a Promega luciferase assay system and a luminometer (C). Data represent the mean±S.D. of three different samples. *p<0.001, when compared with the LPS-treated group.
The inhibitory effects of IFE on NF-κB activation were further examined using an NF-κB-Luc vector. The NF-κB-Luc reporter vector contained four copies of the NF-κB binding site for determination of NF-κB-driven transactivation. Luciferase activity was increased after LPS stimulation, while pretreatment with IFE dose-dependently inhibited this increase (Fig. 4C). These results suggest that IFE inhibits LPS-stimulated NF-κB activation, leading to inhibition of the expression of iNOS, COX-2 and cytokines.
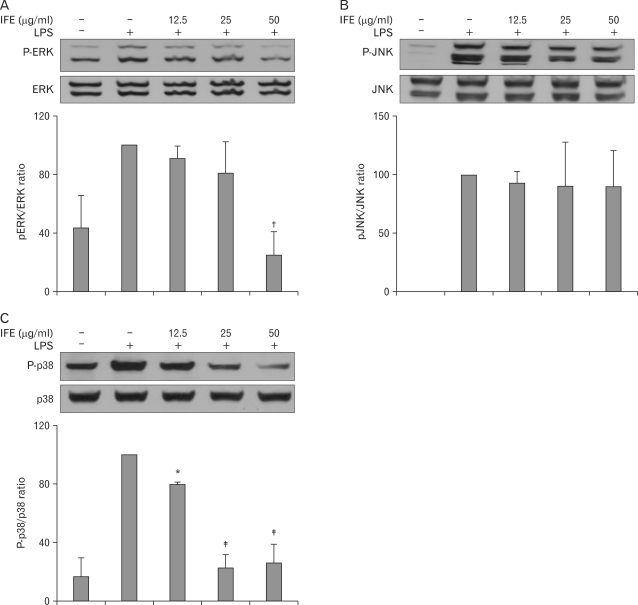
Effect of IFE on MAP kinases
MAP kinases are known to be important for the activation of NF-κB. To determine if the inhibition of NK-κB activation by IFE is mediated through the MAP kinase pathway, we examined the effects of IFE on the LPS-stimulated phosphorylation of ERK, JNK and p38 MAP kinases in RAW 264.7 cells. As shown in Fig. 5, LPS clearly stimulated an increase in the level of activation of ERK, JNK and p38 MAP kinases in untreated cells. However, the induced activities of ERK and p38 MAP kinases were inhibited by pretreatment with IFE. These results suggest that the phosphorylation of ERK and p38 MAP kinase may be involved in the inhibitory effect on LPS- stimulated NF-κB binding in RAW 264.7 cells.
Figure 5.
Effect of IFE on LPS-stimulated MAPK activation in RAW 264.7 cells. Cells were pretreated with different concentrations of IFE for 1 h and then were incubated with LPS (200 ng/ml) for 30 min. Whole cell lysates were analyzed for ERK (A), JNK (B) and p38 (C) phosphorylation by Western blot analysis. Quantification of band intensities from three independent results was determined by densitometric analysis. The values were expressed as a percentage of maximal band intensity in the LPS-treated cells, which was set to 100%. Data represent the mean±S.D. of three different samples. *p<0.05, †p<0.01, ‡p<0.001, when compared with the LPS-treated group.
DISCUSSION
The use of herbal medicine is becoming an increasingly attractive approach for the treatment of various inflammatory disorders. Inula japonica, a well known traditional medicinal herb, possesses diverse biological activities and pharmacological function such as hypoglycemic and hypolipidemic activities (9). Although the anti-inflammatory activities of active compounds isolated from close species (Inula britannica L. and Inula viscosa) have been demonstrated in vitro and vivo (10-14), those of Inula japonica have not been reported. In this study we demonstrated that IFE inhibits the inflammatory mediators and the active compounds are different from those isolated from Inula japonica or close species (6,15,16) (paper preparation).
Free radical NO is produced by iNOS and its overproduction has been implicated in the pathology of a variety of inflammatory disorders, including septic shock (17). A change in the NO level through the inhibition of iNOS enzyme activity or iNOS induction provides a method of assessing the effects of these agents on the inflammation process. In this study, stimulation of macrophages with LPS led to a significant increase in the levels of NO/iNOS as well as PEG2/COX-2 in macrophages, while IFE attenuated such increases (Fig. 1 and 2).
Macrophages release TNF-α, IL-1, IL-6 and other inflammatory mediators in response to pathologic stimuli. TNF-α elicits a number of physiological effects, such as septic shock and inflammation (18). IL-6 is an endogenous mediator of LPS-induced fever. The results of this study showed that IFE significantly inhibits the release of TNF-α and IL-6 (Fig. 3).
NF-κB is an important factor regulating the expression of inflammation-associated mediators, such as iNOS, COX-2 and TNF-α, which contain NF-κB binding motifs within their promoters (19). Many anti-inflammatory agents exhibit their potency by suppressing NF-κB signaling (20-22). In this study, we demonstrated that the molecular mechanism by which IFE inhibits the expression of these inflammatory mediators appeared to involve inhibition of NF-κB activation via blocking LPS-stimulated IκBκ degradation and translocation of the NF-κB p65 protein (Fig. 4). Therefore, NF-κB-targeted therapeutics might be effective at treating inflammatory diseases since a variety of pharmacologic agents have been reported to inhibit one or more activation steps in the signaling pathway (23).
MAP kinases are a group of signaling molecules that also appear to play a critical role in inflammatory processes (24). Several studies have shown that activation of MAP kinases is important in the regulation of NO production via control of the activation of NF-κB (25,26). In agreement with these previous observations, the LPS-stimulated RAW cells caused phosphorylation of the ERK, JNK and p38 kinases. Treatment with IFE was found to significantly inhibit ERK and p38 phosphorylation in LPS-stimulated RAW 264.7 cells (Fig. 5), suggesting that MAP kinases are involved in the inhibition of LPS-stimulated NF-κB binding by IFE in RAW 264.7 cells.
In conclusion, the results of the present study provide the first evidence that IFE inhibits LPS-induced NO, PGE2 and cytokine (TNF-α and IL-6) production. These inhibitory effects of IFE were found to be associated with NF-κB and MAP kinase signaling, suggesting a possible approach to the treatment of inflammatory diseases.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Oriental Medicine R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (B0800023).
Footnotes
The authors have no financial conflict of interest.
References
- 1.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–831. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6:441–448. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 3.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67:668–673. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- 4.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480-481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geng HM, Zhang DQ, Zha JP, Qi JL. Simultaneous HPLC determination of five flavonoids in flos inulae. Chromatographia. 2007;66:271–275. [Google Scholar]
- 7.Liu S, Liu H, Yan W, Zhang L, Bai N, Ho CT. Studies on 1-O-acetylbritannilactone and its derivative, (2-O-butyloxime-3-phenyl)-propionyl-1-O-acetylbritannilactone ester. Bioorg Med Chem Lett. 2004;14:1101–1104. doi: 10.1016/j.bmcl.2003.12.078. [DOI] [PubMed] [Google Scholar]
- 8.Grilli M, Chiu JJ, Lenardo MJ. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 9.Shan JJ, Yang M, Ren JW. Anti-diabetic and hypolipidemic effects of aqueous-extract from the flower of Inula japonica in alloxan-induced diabetic mice. Biol Pharm Bull. 2006;29:455–459. doi: 10.1248/bpb.29.455. [DOI] [PubMed] [Google Scholar]
- 10.Han M, Wen JK, Zheng B, Zhang DQ. Acetylbritannilatone suppresses NO and PGE2 synthesis in RAW 264.7 macrophages through the inhibition of iNOS and COX-2 gene expression. Life Sci. 2004;75:675–684. doi: 10.1016/j.lfs.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Hernández V, del Carmen Recio M, Máñez S, Prieto JM, Giner RM, Ríos JL. A mechanistic approach to the in vivo anti-inflammatory activity of sesquiterpenoid compounds isolated from Inula viscosa. Planta Med. 2001;67:726–731. doi: 10.1055/s-2001-18342. [DOI] [PubMed] [Google Scholar]
- 12.Hernández V, Máñez S, Recio MC, Giner RM, Ríos JL. Anti-inflammatory profile of dehydrocostic acid, a novel sesquiterpene acid with a pharmacophoric conjugated diene. Eur J Pharm Sci. 2005;26:162–169. doi: 10.1016/j.ejps.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Hernández V, Recio MC, Máñez S, Giner RM, Ríos JL. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007;81:480–488. doi: 10.1016/j.lfs.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Whan Han J, Gon Lee B, Kee Kim Y, Woo Yoon J, Kyoung Jin H, Hong S, Young Lee H, Ro Lee K, Woo Lee H. Ergolide, sesquiterpene lactone from Inula britannica, inhibits inducible nitric oxide synthase and cyclo-oxygenase-2 expression in RAW 264.7 macrophages through the inactivation of NF-kappaB. Br J Pharmacol. 2001;133:503–512. doi: 10.1038/sj.bjp.0704099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu NJ, Zhao YM, Zhang YZ, Li YF. Japonicins A and B from the flowers of Inula japonica. J Asian Nat Prod Res. 2006;8:385–390. doi: 10.1080/10286020500034832. [DOI] [PubMed] [Google Scholar]
- 16.Bai N, Lai CS, He K, Zhou Z, Zhang L, Quan Z, Zhu N, Zheng QY, Pan MH, Ho CT. Sesquiterpene lactones from Inula britannica and their cytotoxic and apoptotic effects on human cancer cell lines. J Nat Prod. 2006;69:531–535. doi: 10.1021/np050437q. [DOI] [PubMed] [Google Scholar]
- 17.Cheng PY, Lee YM, Wu YS, Chang TW, Jin JS, Yen MH. Protective effect of baicalein against endotoxic shock in rats in vivo and in vitro. Biochem Pharmacol. 2007;73:793–804. doi: 10.1016/j.bcp.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7:93–124. [PubMed] [Google Scholar]
- 19.Thanos D, Maniatis T. Identification of the rel family members required for virus induction of the human beta interferon gene. Mol Cell Biol. 1995;15:152–164. doi: 10.1128/mcb.15.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, Sung JM, Jang Y, Chung N, Hwang KC, Kim TW. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545:192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 21.Choi MS, Lee SH, Cho HS, Kim Y, Yun YP, Jung HY, Jung JK, Lee BC, Pyo HB, Hong JT. Inhibitory effect of obovatol on nitric oxide production and activation of NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. Eur J Pharmacol. 2007;556:181–189. doi: 10.1016/j.ejphar.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 22.Choi HJ, Eun JS, Park YR, Kim DK, Li R, Moon WS, Park JM, Kim HS, Cho NP, Cho SD, Soh Y. Ikarisoside A inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated RAW 264.7 cells via p38 kinase and nuclear factor-kappaB signaling pathways. Eur J Pharmacol. 2008;601:171–178. doi: 10.1016/j.ejphar.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 23.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31(1 Suppl):S105–S111. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- 24.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Kim DH, Baek SH, Lee HJ, Kim MR, Kwon HJ, Lee CH. Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of NF-kappaB and p38 MAP kinase activity in LPS-stimulated RAW 264.7 cells. Biochem Pharmacol. 2006;71:1198–1205. doi: 10.1016/j.bcp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Suh SJ, Chung TW, Son MJ, Kim SH, Moon TC, Son KH, Kim HP, Chang HW, Kim CH. The naturally occurring biflavonoid, ochnaflavone, inhibits LPS-induced iNOS expression, which is mediated by ERK1/2 via NF-kappaB regulation, in RAW264.7 cells. Arch Biochem Biophys. 2006;447:136–146. doi: 10.1016/j.abb.2006.01.016. [DOI] [PubMed] [Google Scholar]