Summary
The renin-angiotensin system (RAS), in addition to its endocrine functions, plays a role within individual tissues such as the brain. The brain RAS is thought to control blood pressure through effects on fluid intake, vasopressin release and sympathetic nerve activity (SNA), and may regulate metabolism through mechanisms which remain undefined. We used a double-transgenic mouse model that exhibits brain-specific RAS activity to examine mechanisms contributing to fluid and energy homeostasis. The mice exhibit high fluid turnover through increased adrenal steroids, which is corrected by adrenalectomy and attenuated by mineralocorticoid receptor blockade. They are also hyperphagic but lean because of a marked increase in body temperature and metabolic rate, mediated by increased SNA and suppression of the circulating RAS. β-adrenergic blockade or restoration of circulating angiotensin-II, but not adrenalectomy, normalized metabolic rate. Our data point to contrasting mechanisms by which the brain RAS regulates fluid intake and energy expenditure.
Introduction
Despite a growing body of evidence supporting the existence of local, tissue-level expression and activity of the renin-angiotensin system (RAS), there persists a general lack of appreciation for the roles of these autocrine/paracrine systems in both normal physiology and in pathophysiological states. Indeed, major roles for the RAS in the vasculature, heart, and kidney in the development and maintenance of hypertension and its sequelae have been reported (Paul et al., 2006).
The brain, like several peripheral organ systems, expresses all components of the RAS, and generates angiotensin peptides locally. There is evidence to support the hypothesis that angiotensin peptides may be utilized as neurotransmitters, acting in certain cardiovascular-control substructures of the brain (reviewed in Grobe et al., 2008, Ferguson et al., 2001, and Johnson 1985). Through autocrine/paracrine signaling and acting as a neurotransmitter, the brain RAS is thought to control blood pressure (Bickerton and Buckley, 1961), fluid balance (Booth, 1968, Daniels et al., 1969, and Epstein et al., 1970), learning, memory and anxiety (reviewed in Wright et al., 2008), and metabolic function (Porter et al., 2003, and Porter and Potratz, 2004). Abnormalities of the brain RAS leading to its over-activity have been implicated in several forms of genetic and experimental hypertension (Veerasingham and Raizada, 2003). Despite the recognition that the brain RAS is involved in these processes, the efferent mechanisms mediating these functions remain unclear.
To examine the functions of the brain RAS, we developed a double-transgenic mouse model expressing human renin controlled by the neuron-specific synapsin promoter (“sR”) and the human angiotensinogen gene controlled by its own promoter (“A”) (Sakai et al., 2007). Due to the strict species-specificity of the renin-mediated cleavage of angiotensinogen, the human angiotensinogen protein is only cleaved into angiotensin I in the presence of human renin, so, despite a widespread expression of human angiotensinogen, RAS hyperactivity only occurs in tissues where both transgenes are active. Double-transgenic “sRA” mice exhibit RAS hyperactivity only in the brain regions where angiotensinogen is normally expressed, and thus this model may phenocopy hypertension caused by increased brain RAS activity. Using this unique sRA animal model, we have identified divergent efferent signaling mechanisms that mediate the marked hydromineral and metabolic consequences of increased brain RAS activity. The physiological relevance of our model was confirmed by showing similar metabolic consequences in an independent experimental model of brain RAS-mediated hypertension.
Results
sRA Mice Exhibit Polydipsia and Polyuria
sRA mice exhibit robust polydipsia and polyuria (Figure 1A–B). Remarkably, they consume the equivalent of their own body mass of fluids, per day, when offered tap water and 0.15 M NaCl in a two-bottle choice paradigm. To examine whether the elevated intake of fluids in sRA mice was dependent upon the concentration of the NaCl solutions offered, we performed a NaCl preference-aversion experiment by observing 24 hour intakes of water and a range of hypotonic to hypertonic NaCl drink solutions (Figure 1B). sRA mice displayed preference-aversion behavior that was indistinguishable from control mice, but total fluid intake remained greatly elevated in sRA mice across all solute concentrations. Total sodium intake from both food and drink solutions was similar between sRA and control mice at hypotonic and hypertonic concentrations, but sodium intake was greatly increased when sRA mice were offered isotonic (0.15 M) NaCl. The elevation in sodium intake when offered at an isotonic concentration may represent a taste-dependent or a post-ingestive effect, but does not fit the classical definition of an increased sodium appetite because the sRA mice did not consume excess sodium when presented at unpalatable, hypertonic concentrations. In addition, sRA mice consistently produced a large volume of dilute urine across all solute concentrations. Urine sodium (UNaC) and potassium (UKC) concentrations, and urine osmolality were significantly suppressed in sRA mice (Figure 1B, Figure S1). Renal sympathetic nerve activity (RSNA) was markedly elevated (Figure 1C), perhaps as a compensatory mechanism to retain sodium in the face of an exaggerated diuresis. Indeed, sRA mice exhibit an 8% increase in hematocrit suggesting that they may be dehydrated (Figure 1D). This is unlikely to be due to increased hematopoiesis, as renal erythropoietin expression is not elevated in sRA mice (P=0.42). Although serum osmolality and serum potassium concentrations are normal in sRA mice, serum sodium concentrations are significantly decreased (Figure 1E), thus suggesting sRA mice exhibit selective, chronic hyponatremia. Interestingly, there is increased lethality associated with the sRA genotype, as only 1-in-3 sRA pups survive until weaning, suggesting neonatal sRA mice may have difficulty retaining sodium (Figure 1F).
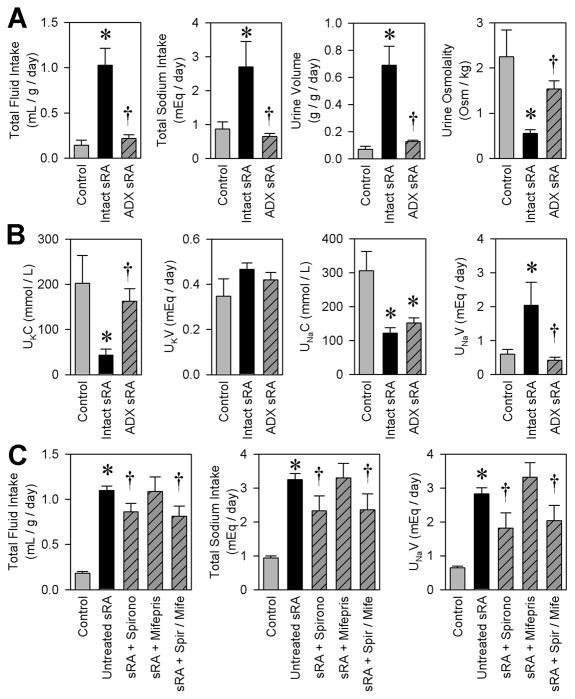
Figure 1. Mice with brain renin-angiotensin system hyperactivity (sRA mice) exhibit potent hydromineral phenotypes.
(A) Ad libitum 24-hour intake volumes for tap water and 0.15 M NaCl solutions (Control N=6, sRA N=3). (B) NaCl preference, total fluid intake, total sodium intake, urine volume, urine sodium concentration (UNaC), and urine osmolality from sRA and control littermate mice offered ad libitum access to varying concentrations of NaCl drink solution, distilled water, and standard chow (Control N=6, sRA N=6). (C) Renal sympathetic nerve activity (RSNA) (Males only; Control N=7, sRA N=6). (D) Blood hematocrit (Control N=9, sRA N=8). (E) Serum osmolality (Control N=10, sRA N=6), potassium concentration, and sodium concentration (Control N=17, sRA N=15). (F) Analysis of pup survival to birth and to weaning. (G) Plasma arginine vasopressin (AVP) concentrations at baseline and following a 4-hour water restriction (N=4 for all). (H) Total 24-hour sodium intake, preference for 0.15 M NaCl, fluid intake and urine volume after treatment with conivaptan (Saline N=5, Conivaptan N=6). * P<0.05 vs. control, or sRA+Saline. All data are mean ± SEM. See also Figure S1.
Dehydration and brain angiotensin-II are known stimuli for the release of arginine vasopressin (AVP) and the brain RAS is known to regulate AVP synthesis. Female sRA mice demonstrated a large elevation in plasma AVP at baseline (Figure 1G). Whereas male sRA mice did not exhibit increased AVP at baseline, a brief period (4 hours) of water restriction markedly increased plasma AVP in sRA but not control mice. We next tested whether increased AVP is responsible for the elevated hydromineral turnover by treating sRA mice with conivaptan, a non-peptide V1A/V2 receptor antagonist (Vaprisol, 10 mg/kg/day for 3 days). AVP receptor blockade suppressed sodium intake and preference for 0.15 M NaCl, but had no effect on total fluid intake or urine output (Figure 1H). Conivaptan also had no effect on serum electrolytes (not shown). This suggests that AVP has selective effects on intake behaviors in sRA mice.
Elevated Adrenal Steroids Cause Polydipsia and Polyuria
Adrenal hormones, particularly steroids, are suggested to play a role in food intake, metabolic expenditure, and fluid/electrolyte balance (Mastorakos et al., 2004). The adrenal glands are hypertrophied, and steroid levels are elevated in urine from sRA mice (Table 1). We performed bilateral complete adrenalectomy (ADX) to examine if adrenal steroids are mechanistically involved in the polydipsia exhibited by sRA mice. Four weeks after complete removal of adrenal tissue, steroids were no longer detectable in urine. ADX completely normalized total fluid and sodium intake, and urine volume and osmolality (Figure 2A). ADX normalized urine potassium concentration, and whereas it had no effect on urine sodium concentration, it completely normalized daily sodium loss (Figure 2B). We treated a separate cohort of male mice with high doses of either the mineralocorticoid receptor antagonist, spironolactone, and/or the progesterone/glucocorticoid receptor antagonist, mifepristone (RU-486) for four days to assess the importance of these signaling pathways. Spironolactone treatment, with or without co-treatment by mifepristone, significantly blunted total fluid and sodium intake, and sodium loss (Figure 2C), though the magnitude of the effect was small in comparison to full ADX, possibly due to dose or duration of treatment. In contrast, mifepristone treatment had no effects on these endpoints. These data support a role for adrenal steroids and mineralocorticoid receptor signaling in the hydromineral phenotypes of the sRA mouse, though other non-mineralocorticoid, adrenal-derived hormones (such as various catecholamines) may also be involved.
Table 1.
Tissue masses and endocrine measures in adult sRA mice.
| Control | sRA | t-test | ||
|---|---|---|---|---|
| Anatomical Measures | ||||
| Body Mass | (g) | 28.53 ± 0.96 (6) | 23.94 ± 1.18 (7) | P=0.013 |
| Nose-Anus | (cm) | 9.20 ± 0.13 (6) | 9.03 ± 0.16 (7) | P=0.295 |
| Organ Masses | ||||
| Heart | (mg) | 112.0 ± 4.7 (6) | 113.6 ± 10.3 (7) | P=0.731 |
| (mg/g) | 12.15 ± 0.34 | 12.50 ± 0.91 | P=0.742 | |
| Lungs | (mg) | 182.7 ± 9.2 (6) | 136.8 ± 3.6 (7) | P<0.001 |
| (mg/g) | 19.85 ± 0.93 | 15.17 ± 0.38 | P<0.001 | |
| Liver | (mg) | 1285.5 ± 75.6 (6) | 1053.4 ± 62.5 (7) | P=0.051 |
| (mg/g) | 139.3 ± 6.4 | 116.4 ± 5.5 | P=0.019 | |
| Kidney | (mg) | 170.2 ± 7.4 (6) | 118.1 ± 8.2 (7) | P<0.001 |
| (mg/g) | 18.47 ± 0.60 | 13.03 ± 0.74 | P<0.001 | |
| Adrenal Gland | (mg) | 2.7 ± 0.3 (9) | 3.8 ± 0.3 (10) | P=0.032 |
| (mg/kg) | 83 ± 10 | 166 ± 15 | P<0.001 | |
| Adipose Masses | ||||
| Interscapular BAT | (mg) | 61.1 ± 5.2 (6) | 48.0 ± 4.1 (7) | P=0.073 |
| (mg/g) | 6.64 ± 0.57 | 5.28 ± 0.37 | P=0.063 | |
| Peri-Genital WAT | (mg) | 284.0 ± 27.8 (6) | 151.4 ± 21.2 (7) | P=0.003 |
| (mg/g) | 30.73 ± 2.63 | 16.60 ± 2.03 | P=0.001 | |
| Peri-Renal WAT | (mg) | 77.8 ± 16.7 (6) | 48.5 ± 10.5 (7) | P=0.154 |
| (mg/g) | 8.49 ± 1.87 | 5.41 ± 1.19 | P=0.179 | |
| Mesenteric WAT | (mg) | 394.1 ± 25.4 (6) | 304.9 ± 9.5 (7) | P=0.002 |
| (mg/g) | 43.00 ± 3.20 | 33.75 ± 0.75 | P=0.014 | |
| Endocrine Measures | ||||
| Serum Total T3 | ng/mL | 12.29 ± 1.41 (9) | 14.43 ± 3.47 (8) | P=0.847 |
| Serum Total T4 | μg/dL | 6.33 ± 1.39 (4) | 5.11 ± 1.11 (4) | P=0.520 |
| Serum Leptin | ng/mL | 1.16 ± 0.69 (3) | 1.39 ± 0.41 (3) | P=0.788 |
| Plasma Insulin (Fasted) | ng/mL | 0.218 ± 0.026 (8) | 0.174 ± 0.011 (8) | P=0.148 |
| Blood Glucose (Fasted) | mg/dL | 123.6 ± 10.6 (11) | 137.3 ± 9.8 (8) | P=0.182 |
| Plasma ACTH | ng/mL | 5.474 ± 2.235 (6) | 1.423 ± 0.689 (5) | P=0.052 |
| Urinary Aldosterone | ng/mL | 23.04 ± 1.59 (8) | 20.00 ± 1.82 (8) | P=0.231 |
| ng/day | 30.63 ± 4.11 | 65.99 ± 9.20 | P=0.003 | |
| Urinary Corticosterone | ng/mL | 1.15 ± 0.03 (8) | 1.61 ± 0.10 (8) | P<0.001 |
| ng/day | 1.63 ± 0.27 | 6.37 ± 1.37 | P=0.004 | |
See also Figure S2.
Figure 2. Adrenal hormones are necessary for the hydromineral phenotypes of sRA mice.
(A) Total 24-hour fluid intake, sodium intake, urine volume, and urine osmolality when offered ad libitum access to tap water and 0.15 M NaCl (Control N=7, Intact sRA N=5, ADX sRA N=4). (B) Urine potassium concentration (UKC), total daily potassium loss in the urine (UKV), urine sodium concentration (UNaC), and total daily sodium loss in the urine (UNaV). (C) Total 24-hour fluid intake, sodium intake, and total daily sodium loss (UNaV) following spironolactone (30 mg/kg/day) and/or mifepristone (50 mg/kg/day) treatment (Males only; Control N=15, Untreated sRA N=15, Spironolactone N=5, Mifepristone N=4, Combined N=6). * P<0.05 vs. control. † P<0.05 vs. intact/untreated sRA. All data are mean ± SEM. See also Table S1.
sRA Mice Exhibit Increased Energy Expenditure and Thermogenesis
Concurrent with polydipsia and polyuria, adult sRA mice exhibit a greatly reduced body mass regardless of sex (Figure 3A). This reduction is not observed until weaning (Figure 3B). MRI revealed that adult sRA mice exhibit a profound reduction in both subcutaneous and visceral adiposity (Figure 3C). There was a 20% reduction in adipocyte cross-sectional area within interscapular brown, peri-genital white, and peri-renal white adipose pads (Figure 3D). Despite reduced fat mass at baseline (Table 1), sRA mice gain weight when fed a high fat diet (Figure S2).
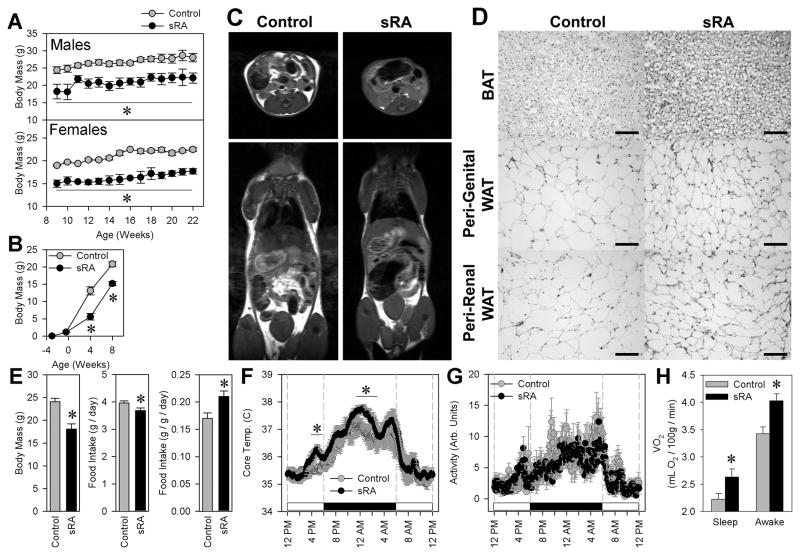
Figure 3. sRA mice are small and lean, primarily due to a large increase in activity-independent energy expenditure.
(A) Body masses of male and female mice from 9–22 weeks of age (Control males N=9, females N=6, sRA males N=6, females N=4). (B) Body masses of mice at day 17 of gestation (Control N=15, sRA N=8), and 4-weeks (Control N=9, sRA N=4), and 8-weeks (Control N=17, sRA N=9) after birth. (C) MRI scans of adult male mice. (D) Hematoxylin & Eosin stained sections of interscapular brown adipose (BAT), and peri-genital and peri-renal white adipose (WAT) from adult mice. Scale bars = 100 microns. (E) Body masses, total 24-hour ad libitum intake of standard chow, and food intake data normalized to body mass (Control N=12, sRA N=8). (F) Core body temperatures determined by telemetric probes (N=6 for all). (G) Spontaneous physical activity determined by telemetric probes. (H) Metabolic rate, determined by indirect calorimetry (N=8 for all). * P<0.05 vs. control. All data are mean ± SEM. See also Figure S3.
To identify the mechanism causing the lean phenotype, we examined both food intake and energy expenditure. Over seven consecutive days in metabolism cages, sRA mice consumed 7% less food than littermate controls. However, since the body masses of sRA mice were reduced by 25%, they exhibited hyperphagia and increased fecal output when the data were normalized to body mass (Figure 3E, S3). Telemetry revealed an approximate 1°C elevation of core body temperature during multiple portions of the light-dark cycle (Figure 3F). Importantly, this was not correlated with any genotype-related differences in locomotor activity (Figure 3G), thus providing evidence that their elevated energy expenditure is due to an elevation in activity-independent thermogenesis. Oxygen consumption at thermoneutrality was elevated by 16% in sRA mice aged 9–12 weeks, during both sleep and stationary wakefulness (Figure 3H). The increased metabolic rate in sRA mice was evident in other cohorts ranging from 15 to 36 weeks of age (N>60 in both groups). There was no change in preferred ambient temperature, suggesting a normal thermoneutral set-point (Figure S3). sRA mice did not exhibit signs of anxiety (Figure S3).
Unlike hydromineral phenotypes, bilateral ADX had no effect on body mass, metabolic rate, food intake or fecal output of sRA mice (Figure 4A, Table S1). Mineralocorticoid or glucocorticoid receptor antagonists similarly had no effect on these endpoints. sRA mice exhibited a normal body length, normal fasting blood glucose and plasma insulin levels, and normal levels of triiodothyronine (T3) and thyroxine (T4) suggesting growth hormone and thyroid hormone signaling are not mechanistically involved in the increased metabolism observed in sRA mice (Table 1).
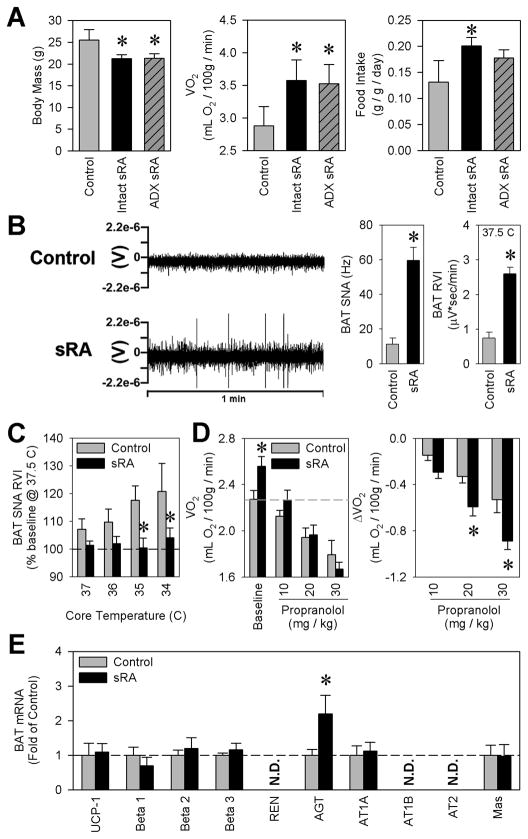
Figure 4. Elevated sympathetic nerve activity contributes to the metabolic phenotypes of sRA mice.
(A) Body masses, metabolic rate, and food intake four weeks following bilateral adrenalectomy (ADX) (Control N=7, Intact sRA N=5, ADX sRA N=4). (B) Example tracings from sympathetic nerves innervating interscapular brown adipose tissue (BAT), and quantification of BAT sympathetic nerve activity (SNA) by both frequency analysis and integrated voltage (RVI) with core temperatures maintained at 37.5°C (Males only; N=6 for all). (C) BAT SNA RVI from mice with core temperatures decreased to 34°C (N=6 for all). (D) Metabolic rate, determined by indirect calorimetry, following graded doses of propranolol (Control N=9, sRA N=10). (E) Gene expression profile from interscapular brown adipose tissue (BAT) (N=4 for all). * P<0.05 vs. control. All data are mean ± SEM. See also Figure S4.
Elevated Sympathetic Nervous Activity Increases Metabolic Rate
We next measured the electrical activity of the sympathetic nerves (SNA) innervating the interscapular brown adipose tissue (BAT) and the peri-genital white adipose tissue. sRA mice exhibited a remarkable elevation in BAT SNA (Figure 4B). Unlike controls, sRA mice were incapable of increasing BAT SNA more than 2–3% to stepwise decreases in core temperature (Figure 4C). Similar trends in SNA were observed in peri-genital white adipose tissue (Figure S4).
We next sought to determine if elevated SNA mediated the elevation in basal metabolic rate. Acute delivery of the β-adrenergic receptor antagonist, propranolol, resulted in large reductions in metabolic rate (Figure 4D). sRA mice exhibited a robust dose-dependent response to propranolol, compared to controls (group × dose interaction P=0.017), indicating increased metabolic sensitivity to β-adrenergic blockade and dependency upon β-adrenergic signaling. Interestingly, expression of uncoupling protein-1 (UCP-1) mRNA, the typical effector of β-adrenergic signaling, and surrogate marker for sympathetic tone, was not increased in BAT from sRA mice (Figure 4E). The reason for this discrepancy was not immediately obvious, as the expression of β-adrenergic receptors was normal. Interestingly, expression of endogenous mouse angiotensinogen, also a known sympathetic- and cAMP-sensitive gene, was significantly up-regulated in BAT. This prompted us to measure other components of the RAS in BAT. There was no change in the expression of angiotensin (Ang) II type 1A (AT1A) or Ang-(1–7) (a.k.a. Mas) receptors. Mouse renin, AT1B and Ang II type 2 (AT2) receptor mRNAs were not detected (Figure 4E).
Elevated Brain and Decreased Circulating RAS Increases Metabolic Rate
Whereas Ang II levels were unchanged in whole-brain homogenates (Control 35±7 vs sRA 34±16 pg Ang II/mg protein), a significant elevation in Ang II was noted in the combined anteroventral third ventricle (AV3V) and hypothalamic region of the brain (Control 5.2±0.7 vs sRA 10.5±0.2 pg Ang II/mg protein, P=0.002) (Figure 5A). This highlights the regional localization of elevated Ang in sRA mice previously identified by immunohistochemistry (Sakai et al., 2007). In contrast, RAS peptides were significantly suppressed in the circulation (Figure 5B), likely the result of suppressed renal renin gene expression (Figure 5C). We hypothesize that this is the result of chronic hypertension in sRA mice (Figure 5D). We next chronically infused mice with Ang II to test whether RAS replacement/supplementation would be sufficient to normalize the metabolic phenotype. Chronic infusion of a non-pressor dose of Ang II resulted in a decrease in metabolic rate back to the baseline level of control mice and a significant shift in respiratory quotient toward the increased utilization of carbohydrates (Figure 5E). These data suggest that the circulating RAS plays a mechanistic role in metabolic regulation.
Figure 5. Suppression of the peripheral RAS contributes to the metabolic phenotypes of sRA mice.

(A) Angiotensin II in whole-brain homogenate (Control N=5, sRA N=7), cortex (Control N=4, sRA N=4), combined anteroventral third ventricle (AV3V) and hypothalamic regions (Control N=4, sRA N=3), and brain stem (Control N=4, sRA N=3). (B) Plasma RAS peptides (N=8 for all). (C) Renal murine renin mRNA expression (N=4 for all). (D) Mean arterial blood pressure determined by carotid artery cannula during anesthesia (Control N=7, sRA N=6), and in conscious, freely-moving mice by radiotelemetry during dark and light phases (Control N=4, sRA N=4). (E) Metabolic rate and respiratory quotient following angiotensin II treatment (100 ng/kg/min, s.c., for 8 weeks. Males only; Control saline, Ang II N=4 each. sRA saline N=7, sRA Ang II N=3). * P<0.05 vs. control. † P≤0.05 vs. saline-treated sRA. All data are mean ± SEM.
Elevated Metabolic Rate in DOCA-salt Treated C57BL/6J Mice
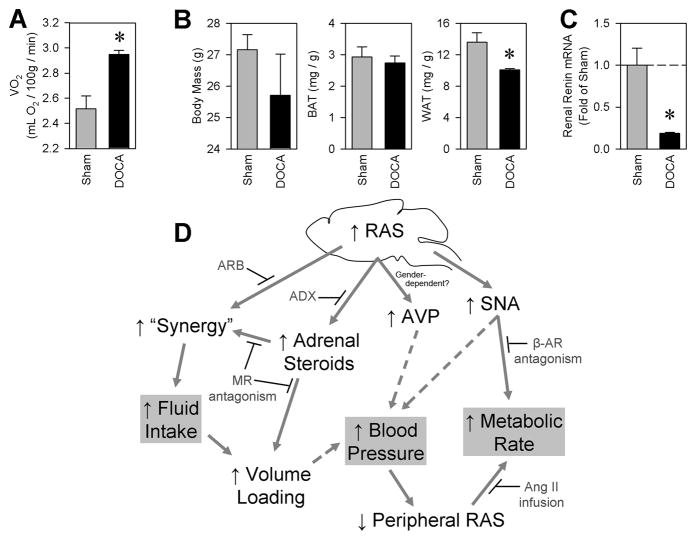
In order to establish the physiological relevance of the sRA mouse model, we examined metabolic parameters in a well-established independent model of hypertension. The deoxycorticosterone acetate (DOCA)-salt model of hypertension is known to be dependent upon increased brain RAS activity and characterized by decreased circulating renin (Itaya et al., 1986). Consequently, we hypothesized that DOCA-salt mice should exhibit metabolic alterations similar to those of sRA mice. We treated adult non-transgenic C57BL/6J mice with subcutaneous DOCA and ad libitum 0.15 M NaCl (DOCA-salt treatment) for three weeks. Consistent with our observations in sRA mice, DOCA salt-treated C57BL/6J mice exhibited a large increase in basal metabolic rate (Figure 6A). This was accompanied by a modest decrease in body mass, a trend toward decreased interscapular BAT mass, and a significant reduction in peri-genital WAT mass (Figure 6B). DOCA-salt-treated mice, like sRA, exhibited decreased renal renin expression (Figure 6C). Similar effects of DOCA were observed in female mice (data not shown). These data demonstrate that sRA mice parallel the phenotypes of the DOCA-salt model of hypertension (moderate hypertension, high metabolic rate leading to weight loss and decreased adiposity), likely through both an elevation in brain RAS activity and a decrease in circulating RAS activity, thereby underscoring the physiological relevance of the sRA mouse model.
Figure 6. DOCA-salt treated C57BL/6J mice exhibit similar phenotypes to sRA mice, and a working model.
Adult C57BL/6J male mice were treated for 3 weeks with DOCA-salt (N=3 for all). (A) Metabolic rate determined by indirect calorimetry. (B) Body masses, interscapular brown adipose, and peri-genital white adipose tissue masses. (C) Renal murine renin mRNA expression. (* P<0.05 vs. sham. All data are mean ± SEM) (D) A working model for the efferent mechanisms mediating the elevated fluid turnover, hypertension, and elevated metabolic rate in sRA mice. Interventions supporting this model include adrenalectomy (ADX), spironolactone-mediated blockade of mineralocorticoid receptors (MR antagonism), intracerebroventricular blockade of angiotensin receptors (ARB, from Sakai et al., 2007), propranolol-mediated blockade of β-adrenergic signaling (β-AR antagonism), and chronic subcutaneous angiotensin II supplementation (Ang II infusion).
Discussion
From the data presented here, we conclude that a diverse, but specific, set of efferent signaling modalities are activated by brain RAS hyperactivity, and combinations of these signals converge to elicit the robust alterations in fluid and energy balance in the sRA mouse model. A schematic representation of these pathways is illustrated in Figure 6D. While adrenal steroids are absolutely necessary for the maintenance of polydipsia and polyuria in these animals, these hormones are dispensable for the increased energy expenditure of sRA mice. In contrast, the metabolic phenotypes of sRA mice are the result of elevated sympathetic nerve activity and a suppression of the peripheral RAS. Further validation for this mechanism comes from the observation that the metabolic phenotypes of sRA mice are replicated in an independent model of neurogenic hypertension caused by an elevated brain and depressed circulating RAS.
Hydromineral regulation by the brain RAS
The hydromineral phenotypes of sRA mice are the result of a combination of brain RAS signaling and peripheral steroids. We previously demonstrated the dependence of the polydipsia in these animals upon both brain AT1 stimulation and hyperactivity of the RAS within the subfornical organ (Sakai et al., 2007), and here we have demonstrated the necessity of adrenal steroids in these behaviors. This synergy of adrenal steroids and brain Ang has previously been documented in rats, as co-administration of peripheral steroids and brain Ang II synergize to elicit robust drinking responses (Epstein 1982, Fluharty and Epstein 1983, Johnson et al., 2003, Krause and Sakai, 2007). This overlap of thirst mechanisms between mice and rats is important to note, as many investigators have previously documented that while peripheral Ang peptides are potent dipsogens in rats, and both species exhibit robust responses to intra- and extracellular dehydration, mice are largely insensitive to peripheral Ang (Hoshishima et al., 1962, Kobayashi et al., 1979, Rowland, 1986, Rowland and Fregly, 1988). Presumably the synergy between brain angiotensinergic signaling and adrenal steroids is mediated through mineralocorticoid regulation of brain AT1 receptors (Thornton et al., 2007). Others have demonstrated binding sites for adrenal steroids in brain regions involved in water and sodium intake (Birmingham et al., 1979, Gerlach and McEwen, 1972, McEwen et al., 1986); and interference with brain mineralocorticoid receptor activation (McEwen et al., 1986) or gene expression (Sakai et al., 2000) has inhibitory effects on hydromineral phenotypes in rats. Thus, the determination here that ADX, and more specifically mineralocorticoid receptor antagonism, attenuates the hydromineral phenotypes of the sRA mouse supports a similar mechanism in mice. Additional studies will nevertheless be required to directly test if the responses to mineralocorticoids are mediated within the brain.
The mechanism by which adrenal steroids are elevated in sRA mice is unclear. ACTH, vasopressin, interleukin-6 and tumor necrosis factor-α all participate in the regulation of corticosterone levels (Tanoue et al., 2004, and Judd et al., 2000), while circulating and local adrenal RAS activity, potassium, corticotropin, catecholamines, and prostaglandins all contribute to the regulation of aldosterone levels (Willenberg et al., 2008). Vasopressin is known to regulate steroid production through modulation of ACTH levels, but plasma ACTH levels were depressed in sRA mice (Table 1). We considered the possibility that ectopic expression of the hREN transgene may cause local RAS hyperactivity in the adrenal gland. We previously reported that hREN was not detected in the adrenal glands of sR mice (Morimoto et al., 2002). Nevertheless we examined adrenal expression of human and mouse renin by realtime RT-PCR, and determined that while the sR construct is expressed, its level of expression is 1000-fold lower its expression in the brain, and 30-fold lower than expression of endogenous mouse renin in the adrenal gland (Table S2). These data rule out ectopic expression of hREN in the adrenal gland as a cause for adrenal hypertrophy and elevated steroid levels in sRA mice. We also considered the possibility that there may be altered circadian rhythms in the adrenal gland of sRA mice. Doi, et al. (2010) recently reported that expression of 3β-hydroxyl-steroid dehydrogenase type 6 (Hsd3b6) but not Hsd3b1 is regulated by the circadian clock. Deficiency of the core clock components Cryptochrome-1 and -2 resulted in aldosterone-dependent hypertension through markedly increased expression of Hsd3b6. Unlike their study, we found no evidence for altered Hsd3b6 or Hsd3b1 expression in adrenal gland (Table S2). This is consistent with other data endpoints suggesting a retention of circadian rhythms in sRA mice (Figures 3F, 3G, 5D). Sympathetic stimulation of adrenal glands may also play a role in the elevated adrenal steroid production. Preliminary studies utilizing the α-adrenergic antagonist, prazosin, suggest no effect on steroid levels, although it was previously reported that sympathetic regulation of adrenal steroid production is mediated through β-adrenergic (and possibly dopaminergic) stimulation of cAMP signaling (Pratt et al., 1985, Bugajski et al., 1991, Missale et al., 1990, Ehrhart-Bornstein et al., 1995), and thus catecholaminergic signaling may represent the primary stimulus for the elevated steroid production in sRA mice.
Metabolic regulation by the brain RAS
The metabolic phenotypes of sRA mice are dependent upon a combination of elevated sympathetic nerve activity and suppression of the peripheral RAS, as blockade of β-adrenergic signaling or supplementation with subcutaneous angiotensin II both corrected the elevation in basal metabolic rate in sRA mice. The decrease in renal renin expression and circulating Ang was at first puzzling given the remarkable increase in RSNA. Indeed, renal renin production is regulated through many inputs, including endothelin-1, Ang II, mechanical stretch, inflammatory cytokines, α- and β-adrenergic stimulation, salt, and renal perfusion pressure (Pan and Gross, 2005). We hypothesize that the chronic hypertension in this model is the primary cause for decreased renal renin production in the sRA mouse model, just as it appears to be in the DOCA-salt model (Goodwin et al., 1969, Makrides et al., 1988).
Previous investigators have demonstrated that global knockout of the genes encoding renin (Takahashi et al., 2007), angiotensinogen (Massiera et al., 2001), angiotensin converting enzyme (Jayasooriya et al., 2008), AT1A receptors (Kouyama et al., 2005), AT2 receptors (Yvan-Charvet et al., 2005), or the Mas receptor (Santos et al., 2008) result in lean phenotypes and/or resistance to weight gain. Thus, we originally hypothesized that sRA mice, with increased RAS activity in the brain, might exhibit weight gain when compared to control littermates. Discovering that the sRA mice are lean and exhibit many of the characteristics of mouse models of global RAS knockout, we investigated the activity of the circulating RAS. We determined that two indexes of circulating RAS activity (circulating Ang II and renal renin expression) were greatly suppressed. Consequently, from the standpoint of the circulating RAS, our model phenocopies models of RAS deficiency or blockade. Importantly, we also documented an inverse correlation between circulating RAS activity and metabolic rate in the DOCA-salt model, a well documented experimental model of neurogenic hypertension (Itaya et al., 1986). The determination that hyperactivity of the brain RAS and hypoactivity of the peripheral RAS results in a negative energy balance is also in agreement with previous findings from studies in rats utilizing brain Ang infusion (Porter et al., 2003, Porter and Potratz 2004), peripheral pharmacological inhibition of Ang converting enzyme (ACE) (Weisinger et al., 2008, Santos et al., 2008, Carter et al., 2004), and peripheral pharmacological antagonism of AT1 receptors (Zorad et al., 2006). Weight loss in humans in response to ACE inhibition (Beevers 1984) or AT1 receptor antagonism (Shimabukuro et al., 2007) has also been reported previously. Interestingly, double transgenic mice from our laboratory which exhibit RAS hyperactivity throughout the body including the brain, and therefore do not have a suppressed circulating RAS (Merrill et al., 1996), exhibit normal body masses (25–27 weeks of age; control 31.2±1.2g N=9; double transgenic 29.7±1.3g, N=5, P=0.46). It appears that regardless of mechanism (genetic deletion, pharmacological inhibition or antagonism, feedback inhibition, or suppression due to elevated brain RAS activity), suppression of the peripheral RAS elicits increased metabolic rate, altered adipose development, and generally a negative energy balance.
Conclusions, implications, and future directions
Significant evidence supports the existence of a local RAS within the brain of rodents and humans (reviewed in Grobe et al., 2008). Evidence also directly supports the function of the brain RAS in the physiological and pathophysiological regulation of fluid balance and energy metabolism, though the mechanisms mediating these phenotypes were largely unknown. Here we have demonstrated robust hydromineral and metabolic consequences of brain RAS hyperactivity in mice, and identified the efferent signaling mechanisms involved in the modulation of these endpoints. From these findings, we hypothesize selective participation by fore-/mid-brain versus hindbrain regions in the regulation of hydromineral and metabolic endpoints. Further, we hypothesize the involvement of altered brain RAS signaling in the hydromineral and metabolic consequences of various high- and low-renin forms of hypertension and the metabolic syndrome.
Experimental Procedures
Animals
Double-transgenic sRA mice were generated as previously described (Sakai et al., 2007). C57BL/6J mice expressing human renin via the synapsin promoter (sR mice) were crossed with mice expressing human angiotensinogen via its own promoter (A mice). A closely related mouse model (sRAflox) was also used, in which the A strain is replaced by a human angiotensinogen construct in which exon 2 of the gene is flanked by LoxP sequences (Aflox). Just as with A mice, Aflox mice are phenotypically normal, unless human renin is also present. sRAflox mice have the same metabolic, blood pressure, and hydromineral phenotypes as sRA mice, and thus, sRA and sRAflox lines were used interchangeably. MRI imaging, histological examinations, high fat diet, radiotelemetric core temperature and activity, quantitative RT-PCR from adipose and renal samples, plasma hormones were determined only with the sRA line. Sympathetic nerve recordings, Ang II replacement, and propranolol treatment were performed in the sRAflox line. Baseline metabolic endpoints were examined using both lines. Single-transgenic (sR, A and Aflox) and non-transgenic littermate mice are phenotypically normal for every endpoint examined, and have thus been combined into a single control group for all studies. Both sexes of mice were utilized for all endpoints, with the exceptions that only males were used for steroid antagonist injections, sympathetic nerve activity recordings, and angiotensin II infusions. All animals were housed in shoebox-style forced-air cages, with ad libitum access to standard chow and water at all times, unless otherwise noted. Lighting was maintained on a standard 12:12 hr cycle, and housing and experimental rooms were maintained between 23–25°C and 10–40% humidity. All procedures performed herein were approved by the University of Iowa’s Institutional Animal Care and Use Committee.
MRI
Adipose imaging by magnetic resonance was performed as previously described (Morgan et al., 2008, Rahmouni et al., 2008). Mice were anesthetized using a mixture of ketamine and xylazine (87.5 & 12.5 mg/kg, i.p.), and images were captured in the axial and coronal planes with a Varian Unity/Inova 4.7 T small-bore MRI system (Varian, Palo Alto, CA), using a T1-weighted fast spin-echo sequence (TR/TE = 625/12 ms) with in-plane resolution of 0.13 × 0.25 mm2 and slice thickness of 1 mm.
Metabolic Cages
Mice were acclimated to Nalgene (Rochester, NY) single-mouse sized metabolism cages for at least two days before any studies were performed. All animals received ad libitum access to powdered chow (Harlan Teklad, NIH-31 modified 6% mouse/rat diet), water, and NaCl drink solutions (0.00 to 0.50 M, for two-bottle choice tests). Bottle positions were alternated daily during two-bottle choice paradigms, to account for side biases.
Metabolic Rate
Animals were placed into a water-jacketed two liter beaker (Ace Glass, Vineland, NJ) maintained at 30°C, and room air was drawn through the chamber at 300 mL/min (R2 flow control, AEI). Air samples were dried by passage through two successive columns of CaSO4 (Drierite, Arcos) dessicant, then analyzed for CO2 (CD-31, AEI) and O2 (S-3A/II, AEI) content. Data were recorded and analyzed using a PowerLab (ADInstruments) and associated Chart software on a PC computer.
Telemetric Blood Pressure
Mice were anesthetized with ketamine/xylazine, and a radiotelemetric blood pressure probe (DSI, Model TA11PA-C10) was implanted into the common carotid artery, as previously (Halabi et al., 2008). The telemeter was within the abdomen, and animals were allowed to recover for two days. Blood pressure and heart rate data were collected for 30 seconds every 5 minutes and recorded using the Dataquest program (DSI) on a PC computer. Data were collapsed within animal across two days of recording for statistical analyses.
Telemetric Core Temperature
Animals were anesthetized using ketamine/xylazine. A midline incision through the abdominal wall was made, and a radiotelemetric probe (Model TA10TA-F20, DSI) was inserted. The abdominal wall and the overlying skin were separately sutured shut, and singly-housed animals were allowed one week of recovery. Following recovery, the home cages of the animals were placed on top of receiver platforms (DSI). Core temperature and physical activity data (30 seconds every 5 minutes) were recorded using the associated Dataquest program (DSI) on a PC computer. Individual time-points from five consecutive days were collapsed within each animal for statistical analyses.
Hormone Assays
Urine collected during metabolism cage studies, serum (trunk blood collected into clean microcentrifuge tubes allowed to stand 5 minutes before a 5 minute centrifugation at 5,000 × g), and plasma (400 μL trunk blood collected into a microcentrifuge tube containing 50 μL 0.5 M EDTA, gently mixed, then immediately centrifuged for 5 minutes at 5,000 × g) were analyzed for various hormones. Hormones were analyzed by selective ELISAs, as per the manufacturer’s instructions, including urine corticosterone and aldosterone (Cayman Chemical), serum total triiodothyronine (T3) and thyroxine (T4) (Alpco), plasma leptin (Crystal Chem), plasma insulin (Alpco), and plasma ACTH (Bachem). Blood glucose levels were determined using an Accu-Check meter (Roche).
Sympathetic Nerve Activity
Sympathetic nerve recordings were performed as previously described (Morgan et al., 2008, Rahmouni et al., 2008). Mice were anesthetized using ketamine/xylazine, and instrumented with a colonic temperature probe. The left carotid artery and jugular veins were cannulated, and the brown adipose pad was exposed. A sympathetic nerve innervating the brown adipose was isolated and suspended on a 36-gauge platinum-iridium electrode and secured in place with silicone gel (World Precision Instruments). Electrodes were attached to a high-impedance probe (HIP-511, Grass Instruments), and the nerve signal was amplified (0.5–1.0×105 for RSNA, 5.5×106 for BAT, 6.0×105 for WAT) with a Grass P5 AC preamplifier, filtered at a 100- and 1000-Hz cutoff, and routed to a resetting voltage integrator (model B600c, University of Iowa Bioengineering). Data were recorded and analyzed using a PowerLab unit and associated Chart software (ADInstruments) on a Macintosh computer.
Gene Expression
Total RNA was extracted using TriReagent (Molecular Research Center). RNA was then treated with DNase-I (Fermentas), and cDNA generated by reverse transcriptase using Superscript III (Invitrogen). Realtime PCR was performed using primer/probe sets from Applied Biosystems, and gene expression was determined by the Livak method (Livak and Schmittgen, 2001).
RAS Peptide Analyses
After isolation from whole blood as above, 150 μL of plasma was diluted with 15 μL of methanol and rapidly frozen at −80°C. Plasma samples were then analyzed by HPLC as previously described (Cassis et al., 2004, Daugherty et al., 2004). Tissues were snap frozen in liquid nitrogen, homogenized in 0.1 N HCl containing 0.5 mM o-phenanthroline and 0.1 mM pepstatin. After centrifugation (20,000 × g, 20 min, 4°C), the supernatant was placed at −20°C overnight, and centrifugation was repeated the next day. The supernatant was diluted (1:1) with 0.1% orthophosphoric acid, and stored at 4°C for 6 hours before centrifugation a final time at 20,000 × g (4°C). The supernatant was diluted with 0.02% orthophosphoric acid before applying to C18 minicolumns. Tissue extracts and plasma were then purified by passage through a C18 column. Angiotensin peptides were then resolved using an HPLC system with ultraviolet detection (254 nm). The mobile phase at time 0 was 20% buffer A (0.05% trifluoroacetic acid), 80% buffer B (100% acetonitrile). Gradient elution was achieved by increasing the concentration of buffer B to 40% over 30 minutes, followed by 100% buffer B for 15 minutes to clean the column. With each set of plasma samples, a set of angiotensin standards (Ang II, Ang-(2–8), Ang-(3–8), Ang-(4–8), Ang-(5–8); 5 μg/ml each; Sigma) was injected onto the system to define angiotensin peptide retention times. A buffer blank was injected and collected intermittently to check for carryover of peptides across individual runs. Fractions (0.5 mL) from the HPLC were collected, evaporated to dryness, and reconstituted for Ang II RIA.
Bilateral Adrenalectomy (ADX)
Adult sRA and littermate control mice, between 20–30 weeks of age, underwent bilateral ADX. For two days preceding and one day following surgery, mice were injected daily with 20 μg dexamethasone, i.p. Mice were anesthetized with ketamine/xylazine. The adrenals were exposed through bilateral dorsal incisions, isolated by silk suture and removed by cautery, and the muscle wall and skin were closed separately with silk suture. Mice were then housed singly, with ad libitum access to food, tap water, and 0.15 M NaCl for four weeks. Four control and four sRA mice survived to the end of the experiment following ADX (44% and 36% survival after ADX, respectively).
DOCA-Salt Model
Ten week old wildtype C57BL/6J mice were anesthetized with ketamine/xylazine and a pellet of deoxycorticosterone acetate (DOCA, 50 mg × 21 days, Innovative Research of America) was implanted subcutaneously. Notably, animals were not uninephrectomized for this study. Animals were allowed ad libitum access to chow, tap water, and 0.15 mM NaCl for four weeks.
Statistical Analyses
Data were analyzed by 1- or 2-way ANOVA (with/without repeated measures), with P<0.05 considered significant. Bonferroni post-hoc analyses were used when main effects reached significance. Non-parametric analyses were utilized (Mann-Whitney, Friedman’s ANOVA) were utilized when data failed normality or equal variance tests.
Supplementary Material
Acknowledgments
The authors would like to thank Victoria L. English, Mark S. Blumberg, Andrew J. Gall, Sara A. Romig-Martin, Ralph F. Johnson, Judith A. Herlein, Brian D. Fink, William I. Sivitz, Ella J. Born, Deborah R. Davis, and Vickie L. Akers for assistance/input on this project. This work was supported through Institutional T32 Post-Doctoral Fellowships funded by the NIH (JLG, CLG), a Post-Doctoral Fellowship in Physiological Genomics from the American Physiological Society (JLG), a K99/R00 Pathway to Independence Award (JLG, HL098276), Pre-Doctoral (DX, 0910035G) and Post-Doctoral (HL, 0825813G) Fellowships from the American Heart Association, a Post-Doctoral Fellowship from the Japan Society for the Promotion of Science (KS), and through research support from the National Institutes of Health (HL048058, HL061446, HL084207 to CDS; HL014388, DK066086, and MH080241 to AKJ; HL073085 to LAC; HL084207 to KR and ALM). We also gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beevers DG. Enalapril in essential hypertension: a comparative study with propranolol. Enalapril in hypertension study group (UK) Br J Clin Pharmacol. 1984;18:51–56. doi: 10.1111/j.1365-2125.1984.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickerton RK, Buckley JP. Evidence for a central mechanism in angiotensin-induced hypertension. Proc Soc Exp Biol Med. 1961;106:834–837. [Google Scholar]
- Birmingham MK, Stumpf WE, Sar M. Nuclear localization of aldosterone in rat brain cells assessed by autoradiography. Experientia. 1979;35:1240–1241. doi: 10.1007/BF01963313. [DOI] [PubMed] [Google Scholar]
- Booth DA. Mechanism of action of norepinephrine in eliciting an eating response on injection into the rat hypothalamus. J Pharmacol Exp Ther. 1968;160:336–348. [PubMed] [Google Scholar]
- Bugajski J, Turoń M, Gadek-Michalska A, Borycz JA. Catecholaminergic regulation of the hypothalamic-pituitary-adrenocortical activity. J Physiol Pharmacol. 1991;42:93–103. [PubMed] [Google Scholar]
- Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, Sonntag WE, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Huang J, Gong MC, Daugherty A. Role of metabolism and receptor responsiveness in the attenuated responses to Angiotensin II in mice compared to rats. Regul Pept. 2004;117:107–116. doi: 10.1016/j.regpep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Daniels AE, Ogden E, Vernikos-Danellis J. Effects of centrally administered angiotensin II in the unanesthetized rat. Physiologist. 1969;12:205. [Google Scholar]
- Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GTJ, Okamura H. Salt- Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- Epstein A. Mineralocorticoids and cerebral angiotensin may act together to produce sodium appetite. Peptides. 1982;3:493–494. doi: 10.1016/0196-9781(82)90113-9. [DOI] [PubMed] [Google Scholar]
- Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the rain of the rat. J Physiol. 1970;210:457–474. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Bornstein SR, González-Hernández J, Holst JJ, Waterman MR, Scherbaum WA. Sympathoadrenal regulation of adrenocortical steroidogenesis. Endocr Res. 1995;21:13–24. doi: 10.3109/07435809509030417. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 2001;226:85–96. doi: 10.1177/153537020122600205. [DOI] [PubMed] [Google Scholar]
- Fluharty SJ, Epstein AN. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. Synergistic interaction with systemic mineralocorticoids. Behavioral Neuroscience. 1983;97:746–758. doi: 10.1037//0735-7044.97.5.746. [DOI] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS. Rat brain binds adrenal steroid hormone: Radioautography of hippocampus with corticosterone. Science. 1972;175:1133–1136. doi: 10.1126/science.175.4026.1133. [DOI] [PubMed] [Google Scholar]
- Goodwin FJ, Knowlton AI, Laragh JH. Absence of renin suppression by deoxycorticosterone acetate in rats. Am J Physiol. 1969;216:1476–1480. doi: 10.1152/ajplegacy.1969.216.6.1476. [DOI] [PubMed] [Google Scholar]
- Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact hypothesis, or fantasy. Physiology. 2008;23:187–193. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshishima K, Yokoyama S, Seto K. Taste sensitivity in various strains of mice. Am J Physiol. 1962;202:1200–1204. doi: 10.1152/ajplegacy.1962.202.6.1200. [DOI] [PubMed] [Google Scholar]
- Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol. 1986;251:H261–H268. doi: 10.1152/ajpheart.1986.251.2.H261. [DOI] [PubMed] [Google Scholar]
- Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, Egan GF, McKinley MJ, Rodger PD, Sinclair AJ, Wark JD, Weisinger HS, Jois M, Weisinger RS. Mice lacking angiotensin converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci U S A. 2008;105:6531–6536. doi: 10.1073/pnas.0802690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AK. The periventricular anteroventral third ventricle (AV3V): its relationship with the subfornical organ and neural systems involved in maintaining body fluid homeostasis. Brain Res Bull. 1985;15:595–601. doi: 10.1016/0361-9230(85)90209-6. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Beltz TG, Thunhorst RL, Johnson AK. Investigations on the physiological controls of water and saline intake in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R394–R403. doi: 10.1152/ajpregu.00130.2003. [DOI] [PubMed] [Google Scholar]
- Judd AM, Call GB, Barney M, McIlmoil CJ, Balls AG, Adams A, Oliveira GK. Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Ann N Y Acad Sci. 2000;917:628–637. doi: 10.1111/j.1749-6632.2000.tb05428.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Uemura H, Wada M, Takei Y. Ecological adaptation of angiotensin-induced thirst mechanism in tetrapods. Gen Comp Endocrinol. 1979;38:93–104. doi: 10.1016/0016-6480(79)90093-5. [DOI] [PubMed] [Google Scholar]
- Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005;146:3481–3489. doi: 10.1210/en.2005-0003. [DOI] [PubMed] [Google Scholar]
- Krause EG, Sakai RR. Richter and sodium appetite: From adrenalectomy to molecular biology. Appetite. 2007;49:353–367. doi: 10.1016/j.appet.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Makrides SC, Mulinari R, Zannis VI, Gavras H. Regulation of renin gene expression in hypertensive rats. Hypertension. 1988;12:405–410. doi: 10.1161/01.hyp.12.4.405. [DOI] [PubMed] [Google Scholar]
- Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, Fukamizu A, Negrel R, Ailhaud G, Teboul M. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220–5225. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Zapanti E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: the role of corticotropin releasing hormone. Nutr Neurosci. 2004;7:271–280. doi: 10.1080/10284150400020516. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lambdin LT, Rainbow TC, De Nicola AF. Aldosterone effects on salt appetite in adrenalectomized rats. Neuroendocrinology. 1986;43:38–43. doi: 10.1159/000124506. [DOI] [PubMed] [Google Scholar]
- Merrill DC, Thompson MW, Carney CL, Granwehr BP, Schlager G, Robillard JE, Sigmund CD. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Lombardi C, Sigala S, Spano PF. Dopaminergic regulation of aldosterone secretion. Biochemical mechanisms and pharmacology. Am J Hypertens. 1990;3:93S–95S. doi: 10.1093/ajh/3.6.93s. [DOI] [PubMed] [Google Scholar]
- Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1730–R1736. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S, Cassell MD, Sigmund CD. Glia- and neuron-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem. 2002;277:33235–33241. doi: 10.1074/jbc.M204309200. [DOI] [PubMed] [Google Scholar]
- Pan L, Gross KW. Transcriptional regulation of renin: an update. Hypertension. 2005;45:3–8. doi: 10.1161/01.HYP.0000149717.55920.45. [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R422–R428. doi: 10.1152/ajpregu.00537.2003. [DOI] [PubMed] [Google Scholar]
- Porter JP, Anderson JM, Robison RJ, Phillips AC. Effect of central angiotensin II on body weight gain in young rats. Brain Res. 2003;959:20–28. doi: 10.1016/s0006-8993(02)03676-4. [DOI] [PubMed] [Google Scholar]
- Pratt JH, Turner DA, McAteer JA, Henry DP. Beta-adrenergic stimulation of aldosterone production by rat adrenal capsular explants. Endocrinology. 1985;117:1189–1194. doi: 10.1210/endo-117-3-1189. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118:1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE. Comparative physiological psychology of feeding and salt appetite in rodents. Nutrition and Behavior. 1986;3:27–42. [Google Scholar]
- Rowland NE, Fregly MJ. Characteristics of thirst and sodium appetite in mice (Mus musculus) Behav Neurosci. 1988;102:969–974. doi: 10.1037//0735-7044.102.6.969. [DOI] [PubMed] [Google Scholar]
- Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai RR, McEwen BS, Fluharty SJ, Ma LY. The amygdala: Site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney International. 2000;57:1337–1345. doi: 10.1046/j.1523-1755.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Santos EL, de Picoli Souza K, Guimarães PB, Reis FC, Silva SM, Costa-Neto CM, Luz J, Pesquero JB. Effect of angiotensin converting enzyme inhibitor enalapril on body weight and composition in young rats. Int Immunopharmacol. 2008;8:247–253. doi: 10.1016/j.intimp.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Santos SH, Fernandes LR, Mario EG, Ferreira AV, Pôrto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 2008;57:340–347. doi: 10.2337/db07-0953. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Tanaka H, Shimabukuro T. Effects of telmisartan on fat distribution in individuals with the metabolic syndrome. J Hypertens. 2007;25:841–848. doi: 10.1097/HJH.0b013e3280287a83. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007;6:506–512. doi: 10.1016/j.cmet.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu TA, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton SN, Omouessi ST, Falconetti C. Mineralocorticoid modulation of central angiotensin-induced neuronal activity, water intake and sodium appetite. Braz J Med Biol Res. 2007;40:699–705. doi: 10.1590/s0100-879x2007000500014. [DOI] [PubMed] [Google Scholar]
- Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisinger HS, Begg DP, Egan GF, Jayasooriya AP, Lie F, Mathai ML, Sinclair AJ, Wark JD, Weisinger RS. Angiotensin converting enzyme inhibition from birth reduces body weight and body fat in Sprague-Dawley rats. Physiol Behav. 2008;93:820–825. doi: 10.1016/j.physbeh.2007.11.046. [DOI] [PubMed] [Google Scholar]
- Willenberg HS, Schinner S, Ansurudeen I. New mechanisms to control aldosterone synthesis. Horm Metab Res. 2008;40:435–441. doi: 10.1055/s-2008-1065336. [DOI] [PubMed] [Google Scholar]
- Wright JW, Yamamoto BJ, Harding JW. Angiotensin receptor subtype mediated physiologies and behaviors: new discoveries and clinical targets. Prog Neurobiol. 2008;84:157–181. doi: 10.1016/j.pneurobio.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–999. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- Zorad S, Dou JT, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.