Abstract
As reported in our previous studies, dithiaden (an antagonist of histamine H1-receptor, used clinically as an anti-allergic or anti-emetic drug) in a concentration range of 5×10−5–10−4 M decreased the production of reactive oxygen species by phagocytes. In this study we investigated the influence of dithiaden on nitric oxide (NO) production by LPS-stimulated macrophages.
The cell viability in the presence of 10−4–5×10−5 M dithiaden was evaluated by an ATP-test. RAW 264.7 cells (2.5×106/well) were preincubated with dithiaden for 60 mins and subsequently stimulated with 0.1 µg/ml of bacterial lipopolysaccharide. After incubating for 24 hours the NO production was determined spectrophotometrically using Griess reaction as a concentration of nitrites (the end product of NO metabolism) accumulated in the cell supernatants. The expression of inducible nitric oxide synthase (iNOS) in cell-lysates was evaluated using Western blot analysis. Scavenging properties of dithiaden against NO were evaluated amperometrically.
Our data demonstrate that dithiaden in the concentration of 5×10−5 M (approved by ATP test as non toxic) caused a significant decrease in the accumulation of nitrites, and in addition, this decline was followed by a marked reduction of iNOS protein expression. Amperometrical analysis did not show any scavenging properties of dithiaden against NO.
From this data it can be suggested that the inhibition effect of dithiaden on macrophage NO production is caused exclusively by the suppression of iNOS protein expression.
Keywords: dithiaden, nitric oxide, RAW 264.7 cells, lipopolysaccharide, nitrites, inducible nitric oxide synthase
Abbreviations
- ATP
adenosine triphosphate
- DMEM
Dulbecco's Modified Eagle's Medium
- FBS
fetal bovine serum
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NO
nitric oxide
- PBS
phosphate buffer salt
- SDS
sodium dodecyl sulfate
Introduction
H1-antihistamine dithiaden (4[3-dimethylamino (propylidiene)]-4,9dihydrothieno-(2,3b)benzo-[e]thiepin) is a member of the 1st class of histamine H1-receptor blockers and is used clinically as an anti-allergic or anti-emetic drug (De Vos, 1999). The suppresive effects of this group of drugs on histamine secretion from mast cells and basophils are well known. Experiments suggested that the chemical structure of H1-antihistamines, namely positively charged lipophilic molecules, allows them to associate with the cell membrane. Moreover, they are able to inhibit the activity of calcium-dependent enzymes, affect the calcium mobilization and the discharge of intracellular calcium stores as being responsible for various effects including histamine secretion, eicosanoids or reactive oxygen metabolites generation (Church, 2001; Lullmann et al., 1980). The production of reactive oxygen and nitrogen species by phagocytes belongs to the important microbicidal mechanisms of fight against pathogenic microorganisms, bacterias, tumor cells etc., in the process of inflammation. Normal inflammatory responses are self-limited by a process that involves the downregulations of pro-inflammatory proteins and the upregulations of anti-inflammatory proteins (Lawrence et al., 2002). But chronic inflammation accompanied by a rise in oxidative stress with an overproduction of reactive oxygen and nitrogen species could contribute to the pathogenesis of many inflammatory diseases, such as bronchitis or rheumatoid arthritis. Whereas the effect of dithiaden on the production of reactive oxygen species is relatively well described (Kralova et al., 2006; Nosal et al., 2002; Nosal et al., 2006), the information about the role of dithiaden on reactive nitrogen species generation is insufficient. Therefore, the present study investigated the effects of dithiaden on nitric oxide (NO) production by murine macrophages like cells RAW 264.7 stimulated by lipopolysaccharide and also tries to detect the direct scavenging properties of dithiaden against NO in a cell free system.
Materials and methods
Materials
Dithiaden (Zentiva, Czech Rep.) was dissolved in distilled water and the stock solution 3×10−3M was stored in −20°C. Lipopolysaccharide (LPS) from Escherichia coli serotype 0111:B4 (Sigma-Aldrich, USA) was dissolved in PBS and the stock solution 1mg/ml was stored in −20°C. Other chemicals were purchased from local distributors.
RAW 264.7 cells
A murine leukaemic macrophage-like RAW 264.7 cells (ATCC, USA) were grown in plastic culture flasks in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with heat-inactivated 10% fetal bovine serum (FBS), gentamycin, glucose and NaHCO3 in a CO2 incubator (5% CO2 and 95% of air humidity) at 37°C. Cells were seeded at an initial density of 2.5×106 cells/well in a 6-well tissue culture plates and preincubated with dithiaden for 60 min. Cells were then stimulated with LPS (0.1 µg/ml) and incubated 24 h. Non-treated cells were used as the control. Cell supernatants were used for the determination of nitrite concentration, cells were used for the measurement of ATP content and iNOS protein expression.
ATP test of cell viability
The viability of RAW 264.7 cells was tested by the commercial ATP cellular kit (Biothema, Sweden). Cells were incubated for 24 hours with 10−4 and 5×10−5 M dithiaden, supernatant was removed and cells were lyzed by Somatic cell ATP releasing reagent (Sigma Aldrich, USA). The volume of 50 µl of lyzate were mixed with a 20 µl ATP reagent SL containing D-luciferin, luciferase and stabilizers. Intracellular ATP contents were determined luminometrically using luminometer Orion II. (Berthold Detection Systems GmbH, Germany).
Determination of nitrite production by cells
Detection of accumulated nitrites (NO2 −) in the cell supernatants was performed using the Griess reagent as described previously (Migliorini et al., 1991). 150 µl of the cell supernatant was incubated with 150 µl Griess reagent (Sigma-Aldrich, USA) for 15 min in a dark at room temperature and the absorbance was measured at 546 nm on a Spectra Rainbow UV/Vis microplate reader (SLT Tecan, Germany). The concentrations of nitrites were derived by regression analysis using serial dilutions of sodium nitrite as a standard.
Determination of iNOS protein expression by cells
Cells were lysed with 1% SDS lysing buffer with the addition of 1% phenylmethanesulphonylfluoride. Protein volume in cell samples was determined using commercial BCA protein assay (Pierce, USA), proteins (22 µg) were separated by 7.5% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane in a buffer containing Tris-glycine and 20% methanol. Protein was labeled using mouse antibody (1:1000) specific to iNOS (Becton – Dickinson, USA) and HRP-conjugated goat anti-mouse antibody (1:2000) (ECL™ Anti-mouse IgG, Amersham, Biosciences, USA), subsequently immunoreactive bands were visualised using ECL detection reagent (Detection reagents kit, Pierce, USA).
Amperometrical detection of NO scavenging
The measurement of NO scavenging was performed amperometrically using ISO-NO Mark II NO meter (World Precision Instruments, Inc. USA). The measurement was practised using NO standard solutions prepared with pure NO gas. Preparation of 2 mM NO stock solution was described earlier (Feelisch and Kelm, 1991). 1 µl of NO stock solution was injected either into the 10 ml of PBS in a glass vial or into the 10 ml of PBS with the addition of 5×10−5 M dithiaden and the signal was measured for 10 min. Then the integrals of control curve and sample curve were calculated and the scavenging activity of the dithiaden was evaluated.
Statistical evaluation
Data are expressed as the mean ± standard error of the mean (SEM) of at least 3 independent experiments, that were run in duplicates. Results were analysed by Student's two-tailed t-test using Statistica software (StatSoft, USA), values below 0.05 (*) were considered statistically significant.
Results
Nitrite production was dependent on the activating state of RAW 264.7 cells. While only very low concentrations of nitrites (0.5–2 µM) were detected in non-stimulated cells, the cells stimulated with 0.1 µg/ml LPS generated 28.2 ± 2.52 µM (mean ± SEM) of nitrites. The value of nitrite accumulation after LPS-stimulation was used as a positive control.
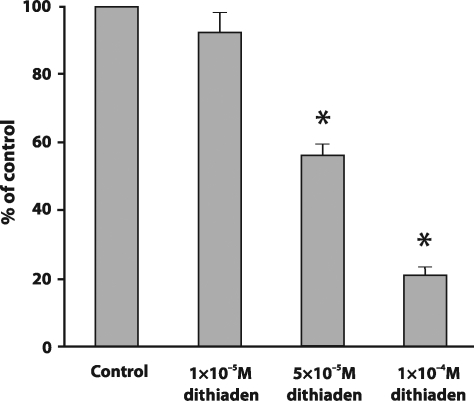
Figure 1 demonstrates that the lowest concentration of dithiaden (10−5 M) did not exert ability to modulate nitrite production in cells stimulated with LPS. 5×10−5 M dithiaden significantly decreased the nitrite concentration (56.06 ± 3.34% of positive control level). Dithiaden in concentration 10−4 M also affected the nitrite accumulation (20.93 ± 2.19% of positive control level) in LPS-stimulated cells. However, this concentration of dithiaden also caused a significant decline in intracellular ATP concentrations (data not shown) and exerted a strong cytotoxic effect on RAW 264.7 after 24 hours of incubation. Therefore, the 5×10−5 M concentration of dithiaden which proved to be non toxic but still effective was used for the following experiments with cells.
Figure 1.
The effect of dithiaden on the concentration of nitrites in the supernatant collected from RAW 264.7 cells stimulated by 0.1 µM LPS after a 24 hour incubation period at 37°C in comparison with non-treated cells (control). Data are expressed as percentages of the control. Values represent mean ± SEM, n=3. Asterisks show significant differences as compared to control, p<0.05 was considered as statistically significant as compared to control.
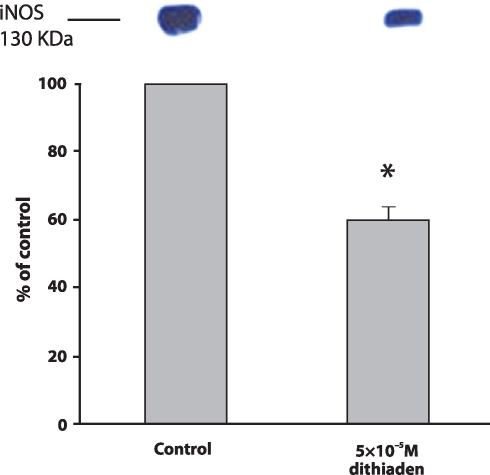
The possibilty that the decrease in nitrite concentration was induced by a suppressioon of iNOS protein expression was verified in subsequent experiments. Figure 2 shows that 5×10−5 M dithiaden inhibited the iNOS protein levels in cell lysates to 59.88 ± 4.03% of positive control level (cells stimulated with LPS).
Figure 2.
The expression of iNOS in the cell lysates from RAW 264.7 cells stimulated by 0.1 µM LPS after 24 hour incubation with 5×10−5 M dithiaden at 37°C in comparison with non-treated cells (control). Data are expressed as percentages of the control. Values represent mean ± SEM, n=3. Asterisks show significant differences as compared to control, p<0.05 was considered as statistically significant as compared to control.
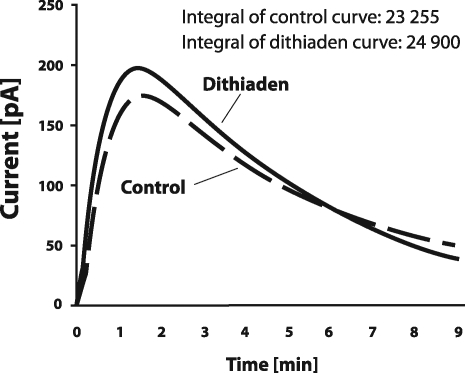
Dithiaden was further tested for scavenging NO by direct amperometric analysis using a carbon electrode selective for NO. Dithiaden in the 5×10−5 M concentration did not approve any scavenging properties against nitric oxide in PBS as shown in Figure 3.
Figure 3.
The effect of 5×10−5 M dithiaden against nitric oxide (NO) evaluated amperometrically. The electrochemical signal was induced by the addition of the NO stock solution as described in the text. Data are expressed as mean ± SEM, n = 3.
Discussion
Althought the number of recently published results (Jancinova et al., 2006; Kralova et al., 2006; Nosal et al., 2002; Nosal et al., 2005; Nosal et al., 2006) have demonstrated that dithiaden and other H1-antihistamines play a very important role in the regulation of reactive oxygen species production and also in the regulation of myeloperoxidase activity (Kralova et al., 2008), any information about the effect of H1-antihistamines on the generation of reactive nitrogen species is yet to be determined. We consequently investigated the role of dithiaden in the regulation of nitric oxide production in macrophages. Detection of iNOS protein expression and nitrite concentration are reliable methods for verifying NO production by cells. NO is generated by several types of cells in an L-arginine – pathway in the presence of nitric oxide synthases (NOS) (Moncada and Higgs, 1993; Palmer et al., 1987). iNOS is mainly expressed in macrophages in response to inflammatory stimuli, e.g. Gram-negative bacterial lipopolysaccharide or proinflammatory cytokines (IL-1 or TNF-α) (Stuehr and Marletta, 1985). We used this phenomenon for the in vitro experiments with a murine macrophage cell line RAW 264.7. We suggested that dithiaden (10−4 M and 5×10−5 M) caused a significant decrease in nitrite accumulation and also in iNOS protein expression in LPS-treated cells. However, only the 5×10−5 M concentration of dithiaden was non-toxic.
NO can be a double-edged sword. On one hand, NO is an important molecule involved in the regulation of many physiological and microbicidal processes. On the other hand, its overproduction is included in several chronic inflammatory diseases such as bronchitis, osteoarthritis or rheumatoid arthritis (McInnes et al., 1996). It is evident from our results, dithiaden does not directly scavenge nitric oxide generated in chemical system, this H1-antihistamine drug exerted significant inhibitory effects on the nitric oxide production by a murine macrophage cell line RAW 264.7. It can be concluded that this inhibition of nitric oxide production was caused by a decrease in iNOS expression.
We suggest that dithiaden by decreasing the nitric oxide production might contribute to the treatment of chronic inflammatory processes.
Aknowledgement
This study was conducted under the research plans AVOZ50040507 and AVOZ50040702 and supported by grants 1QS500040507 GA AS CR, GA CR 524/08/1753 and VEGA 2/7019/27.
REFERENCES
- Bakker RA, Schoonus SB, Smit MJ, Timmerman H, Leurs R. Histamine H(1)-receptor activation of nuclear factor-kappa B: roles for G beta gamma- and G alpha(q/11)-subunits in constitutive and agonist-mediated signaling. Mol Pharmacol. 2001;60:1133–1142. doi: 10.1124/mol.60.5.1133. [DOI] [PubMed] [Google Scholar]
- De Vos C. H1-receptor antagonists: effects on leukocytes, myth or reality? Clin Exp Allergy. 1999;29(3):60–63. [PubMed] [Google Scholar]
- Feelisch M, Kelm M. Biotransformation of organic nitrates to nitric oxide by vascular smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1991;180:286–293. doi: 10.1016/s0006-291x(05)81290-2. [DOI] [PubMed] [Google Scholar]
- Church MK. H(1)-antihistamines and inflammation. Clin Exp Allergy. 2001;31:1341–1343. doi: 10.1046/j.1365-2222.2001.01195.x. [DOI] [PubMed] [Google Scholar]
- Jancinova V, Drabikova K, Nosal R, Holomanova D. Extra- and intracellular formation of reactive oxygen species by human neutrophils in the presence of pheniramine, chlorpheniramine and brompheniramine. Neuro Endocrinol Lett. 2006;27(2):141–143. [PubMed] [Google Scholar]
- Kralova J, Ciz M, Nosal R, Drabikova K, Lojek A. Effect of H(1)-antihistamines on the oxidative burst of rat phagocytes. Inflamm Res. 2006;55(1):S15–16. doi: 10.1007/s00011-005-0020-6. [DOI] [PubMed] [Google Scholar]
- Kralova J, Nosal R, Drabikova K, Jancinova V, Denev P, Moravcova A, et al. Comparative investigations of the influence of H1-antihistamines on the generation of reactive oxygen species by phagocytes. Inflamm Res. 2008;57(1):S49–50. doi: 10.1007/s00011-007-0624-0. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- Lullmann H, Plosch H, Ziegler A. Ca replacement by cationic amphiphilic drugs from lipid monolayers. Biochem Pharmacol. 1980;29:2969–2974. doi: 10.1016/0006-2952(80)90046-5. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Leung BP, Field M, Wei XQ, Huang FP, Sturrock RD, Kinninmonth A, Weidner J, Mumford R, Liew FY. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996;184:1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini P, Corradin G, Corradin SB. Macrophage NO2– production as a sensitive and rapid assay for the quantitation of murine IFN-gamma. J Immunol Methods. 1991;139:107–114. doi: 10.1016/0022-1759(91)90357-l. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Nosal R, Drabikova K, Ciz M, Lojek A, Danihelova E. Effect of H1-antagonist Dithiaden on human PMN-leukocyte aggregation and chemiluminescence is stimulus-dependent. Inflamm Res. 2002;51:557–562. doi: 10.1007/pl00012427. [DOI] [PubMed] [Google Scholar]
- Nosal R, Drabikova K, Jancinova V, Petrikova M, Fabryova V. Antiplatelet and antiphagocyte activity of H1-antihistamines. Inflamm Res. 2005;54(1):S19–20. doi: 10.1007/s00011-004-0408-8. [DOI] [PubMed] [Google Scholar]
- Nosal R, Drabikova K, Jancinova V, Macickova T, Pecivova J, Holomanova D. On the pharmacology and toxicology of neutrophils. Neuro Endocrinol Lett. 2006;27(2):148–151. [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopoly-saccharide. Proc Natl Acad Sci U S A. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]