Abstract
The rapid growth of the aging human population highlights the need for laboratory animal models to study the basic biologic processes of aging and susceptibility to disease, drugs, and environmental pollutants. Methods are needed to evaluate the health of aging animals over time, particularly methods for efficiently monitoring large research colonies. Here we describe an observational assessment method that scores appearance, posture, mobility, and muscle tone on a 5-point scale that can be completed in about 1 min. A score of 1 indicates no deterioration, whereas a score of 5 indicates severe deterioration. Tests were applied to male Brown Norway rats between 12 and 36 mo of age (n = 32). The rats were participating concurrently in experiments on the behavioral effects of intermittent exposure (approximately every 4 mo) to short-acting environmental chemicals. Results demonstrated that aging-related signs of deterioration did not appear before 18 mo of age. Assessment scores and variability then increased with age. Body weights increased until approximately 24 mo, then remained stable, but decreased after 31 mo for the few remaining rats. The incidence of death increased slightly from 20 to 28 mo of age and then rose sharply; median survival age was approximately 30 mo, with a maximum of 36 mo. The results indicate that our observational assessment method supports efficient monitoring of the health of aging rats and may be useful in studies on susceptibility to diseases, drugs, and toxicants during old age.
The population in the United States, and several other countries, is rapidly aging.17 By 2030, an estimated 20% of the population will be 65 y of age or older,5 and centenarians will no longer be a rarity.10,15 Aging is characterized by a general loss of function in multiple organ systems, an increase in the likelihood of injury or disease, prolonged recovery time, and increased susceptibility to the toxic effects of many drugs and environmental pollutants. Collectively these changes comprise what is often called ‘frailty.’6,26,32 Considerable interindividual variability in the signs of aging and frailty frequently is reported.14,16
To keep pace with this rapid demographic shift, animal models are needed to understand not only the basic biology of aging but also the diseases and vulnerabilities to chemicals, including drugs and environmental pollutants, that accompany aging. Considerable progress has already been made in aging research due to the use of a wide array of species, including yeast, nematodes, fruit flies, rodents, and nonhuman primates.13,23 By contrast, knowledge of chemical susceptibility due to aging has lagged.7
Laboratory investigators increasingly are refining their experimental designs to enhance the care and wellbeing of animals. In studies that may involve distress or pain in laboratory animals, investigators include humane endpoints as criteria for termination of the study for a particular animal.21,27 Longevity studies, in particular, are undergoing refined design, including increased planning for long-term animal health and welfare. Often in studies on aging, the changes that occur just prior to death may be overlooked when, in fact, these changes can introduce variability in the data and therefore uncertainty in their interpretation. To date, no single indicator has been identified that reliably predicts an organism's imminent demise.
We have begun to address some of the uncertainties regarding aging and susceptibility to environmental pollution by using Brown Norway rats, a common strain in aging research.22 We particularly were interested in developing a method that could be used to monitor the animals’ health and applied efficiently to a large number of aging animals. We developed our method by using rats that were assigned to experiments in which they periodically received (for example, every 4 mo) either a short-acting solvent or short-acting carbamate pesticide. Here we report a quick and efficient method to evaluate the health of aging rats. This method may have broad applicability in gerontologic, biomedical, and toxicologic research.
Materials and Methods
Animals.
Brown Norway rats (BN/BiRijN; n = 32) were ordered from the National Institute on Aging, NIH, and received from the contract laboratory (Harlan Laboratories, Indianapolis, IN). The rats ranged in age from 12 to 32 mo at the start of the assessment. The rats were drawn from a larger colony used in experiments on the behavioral effects of exposure to either a short-acting solvent (toluene) or a short-acting carbamate pesticide (carbaryl). Compounds were dissolved in corn oil and administered by oral gavage, after which effects on motor activity (ambulation and rearing) were assessed. These same rats continued to participate in longitudinal experiments, periodically receiving (for example, at 4-mo intervals) either compound (or vehicle) throughout their lifetime. No assessments were performed on the day of dosing or during the recovery period (which never exceeded 1 d). Rats were housed individually in clear solid polycarbonate cages (24 × 43 × 18 cm) on pine shavings in an SPF, AAALAC-accredited facility. Colony rooms in the facility were maintained at 21 ± 2 °C with humidity at 50% ± 20%. The daily 12:12-h light:dark cycle had lights on at 0600. Food (Purina 5001 Rodent Diet, PMI International, Brentwood, MO) and water were available ad libitum. All assessments occurred during the day in the colony room. All procedures were approved by the Institutional Animal Care and Use Committee of the National Health and Environmental Effects Research Laboratory (US Environmental Protection Agency, Research Triangle Park, NC).
Sentinel rats exposed to dirty bedding from all cages within a given room were tested regularly (monthly for the most prevalent pathogens and quarterly for all others) for rat parvovirus, rat minute virus, rat coronavirus, Kilham rat virus, Toolan H1 virus, Sendai virus, Mycoplasma pulmonis, pneumonia virus of mice, reovirus, mouse adenovirus, lymphocytic choriomeningitis virus, cilia-associated respiratory bacillus, hantavirus, rat Theiler virus, Encephalitozoon cuniculi, Helicobacter spp. (by fecal PCR), endoparasites (by test tape and fecal flotation), and ectoparasites (by pelt exam). All testing, including bacterial cultures of lung lavage fluids and colon swabs, has been negative for pathogenic organisms.
In addition, rats were monitored daily by the animal care staff during routine maintenance and by the attending veterinarian as needed. The present assessment method assisted those evaluations by providing more specific information about each animal's health status.
General approach.
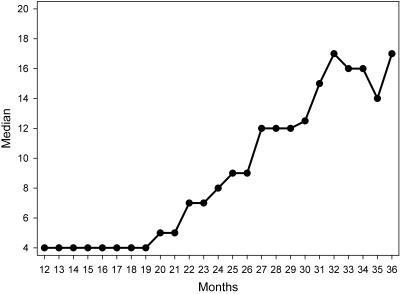
A hybrid design (that is, cross-sectional and longitudinal) was used, in which the assessments started with rats that ranged in age from 12 to 32 mo. Once assessments began, the rats were assessed periodically throughout their laboratory stay. As a consequence, not all rats were assessed at every age (Figure 1). The number of rats assessed through 19 mo represents a small sampling, owing to age differences at the start of these evaluations. Beyond 19 mo, the number of rats assessed at each age changed continuously, as more rats became available whereas others died or were euthanized. Figure 1 also shows that the number of rats declined steadily at the later ages (29 mo and older). Data were collected from every rat at least once each month.
Figure 1.
Total number of rats evaluated at each age (mo).
Assessor.
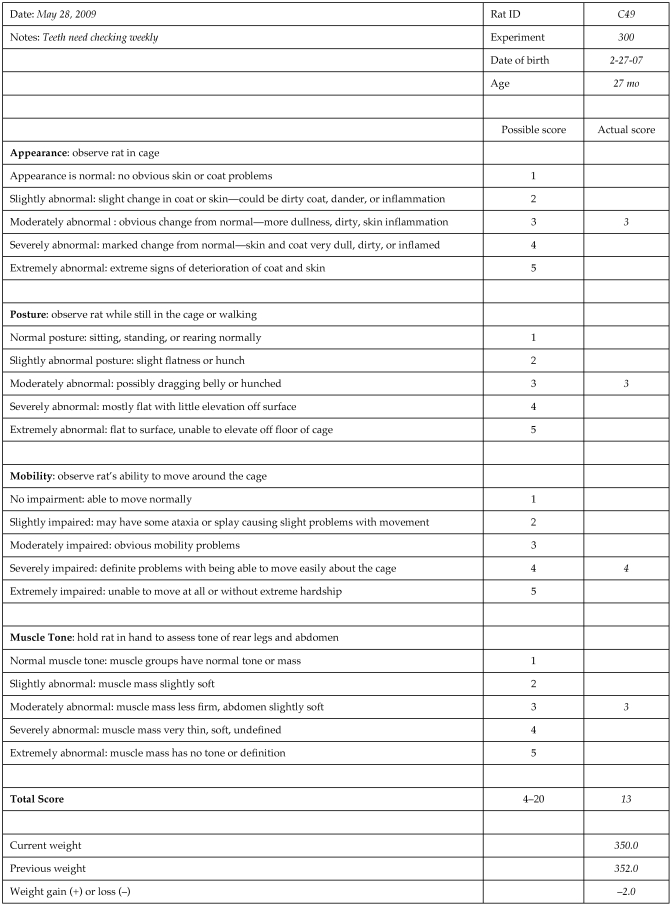
Assessments were based on a set of scoring criteria, which are summarized in an example of the scoring sheet (Figure 2). Even with these criteria, ratings are inherently subjective. To reduce variability and ensure consistency, the assessor needed to have a thorough understanding of the general characteristics of aging rats.1 The same assessor (PMP) conducted each evaluation. However, during initial development of the method and periodically thereafter, the same rats also were evaluated by a second assessor (DMK), with consistent results (data not shown).
Figure 2.
Sample score sheet for observational assessment, with brief descriptions of the 4 measures. For each measure, the highest (most abnormal) score possible is 5. The total composite score possible for all measures combined is 20. In addition, body weight for the current and previous weeks is included, along with whether a weight gain or loss was present.
Scoring.
For each measure, the assessor assigned a score (rank), ranging from 1 (normal) to 5 (extremely impaired or abnormal; Figure 2). The maximal total score (sum of all 4 measures) was 20, with a score of 4 considered normal.
Frequency of assessment.
Older animals were assessed more frequently than were younger ones: animals 19 mo of age and younger were assessed once every 4 wk; rats 20 to 29 mo old were assessed every other week; and rats 30 mo of age and older were assessed weekly.
Body weight.
Body weights were collected weekly from all rats, regardless of age.
Mortality.
Animals occasionally either died spontaneously or were euthanized due to deteriorating health; the decision to euthanize a rat was made in consultation with the attending veterinarian (DMK). The criteria for euthanasia were based on clinical examination and assessment of the rat's level of pain or distress. These criteria included extreme lethargy, labored breathing, significant weight loss (10% to 20% from peak body weight) with reduced food intake, and paresis or paralysis. In our experience, weight loss is often, but not always, a good indicator of aging-related deterioration. For example, body weight may decrease precipitously due to incisor overgrowth from dental malocclusion or from a faulty water supply, resulting in dehydration. Body weight loss from these conditions will often correct itself when specifically addressed (for example, shortening the incisors and providing softened feed or a gel-based diet or correcting water supply problems). Rats were euthanized by carbon dioxide or sodium pentobarbital overdose.
Observational assessment.
Rats were assessed in the animal colony room using a noninvasive observational ranking method that provided information regarding the health of each animal. The observational assessment was based on common elements of behavioral screening batteries,11,29 and welfare concerns over aging animals including knowledge of humane endpoints and indicators of distress in aging rats.21,27 The evaluation consisted of 4 measures: appearance, posture, mobility, and muscle tone. Appearance, posture, and mobility were assessed prior to handling the rat, which was necessary to assess muscle tone.
The assessment began when the home cage was removed from the housing rack and placed on a flat surface. The rat was observed from above to assess appearance, posture, and mobility. After these measures were scored, the rat was removed from the cage to assess muscle tone. When the assessment was finished, the rat was returned to the home cage and housing rack.
Appearance.
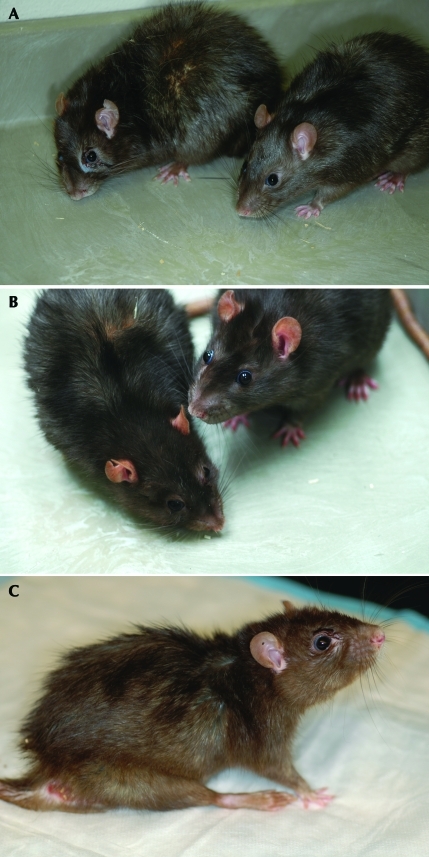
The assessor observed the hair coat, skin, tail, feet, legs, and facial features, including eyes and ears. Noted were any dermatitis, cysts, urinogenital or facial staining, or lacrimation, tumors, or unusual sounds (for example, wheezing, congestion, or vocalizations that may result from pain, distress, or illness; Figure 3).
Figure 3.
(A and B) Rats on the left show signs of facial staining, waxy yellowed skin, poor grooming, and changes in facial features possibly due to loss of facial muscle mass; rats on the right are included for comparison and show good posture, clear eyes, and a well-groomed glossy coat. (C) In addition to other signs of aging, this rat displays dermatitis at the base of the tail and extremely poor posture with rear leg splay and a dull coat. All of the rats in this figure are 24 to 25 mo of age.
Coat and skin.
The normal hair coat is flat with a glossy sheen. When animals age, piloerection or ‘staring coat,’ hyperkeratosis (scaling), dryness, inflammation, discoloration, and epidermal or subcutaneous masses may be present. Poor grooming can often be an indicator of systemic disease and can lead to dull coat, debris or dander accumulation, and dermatitis. The debris sometimes appears as a waxy yellowed coating around the hair follicles, especially on the back. When the hair starts to stand (or ‘stare’), these skin conditions can be seen easily. Because older rats may not be able to reach the upper back to clean, this area may show the signs of aging first.
Tail.
A healthy tail is free of fecal staining or accumulation around the perineum, inflammation, and discoloration.
Eyes, ears, and extremities.
Healthy eyes are clear with no evidence of corneal opacities, cataracts, or exophthalmos (bulging of the eye). Ears should be standing erect, and extremities are normal in size and conformation, with nails of normal length.
Teeth.
Misalignment of the front teeth or malocclusion may cause the rat to stop eating, thereby affecting its appearance. Although infrequent, broken teeth should be noted, because they can cause a rapid change in the appearance of an otherwise healthy rat.
Posture.
The healthy rat ordinarily stands (or walks) with its body elevated and with its back almost horizontal to the surface. Signs of worsening posture include arching of the back, a hunched position when sitting or walking, and dragging of the abdomen. Some rats may develop a posture with legs splayed to the sides and abdomen flat to the cage surface.
Mobility.
Mobility refers to the rat's ease of movement around the cage. Normal mobility includes walking with the legs extended under the body. The legs should be in line with the sides of the body and should not be extended such that the rat crawls or drags the hind limbs. During walking, there should be no evidence of stiff extension of the limbs, causing the rat to walk on the tips of its toes. There should be no exaggerated or splayed placement of the feet, causing excessive swaying (ataxia). With advancing age, it may become extremely difficult for a rat to move about its cage, resulting in crawling when in motion.
Muscle tone.
When muscles of the rear leg are palpated (held between a finger and thumb), they should be well-defined and firm but not rigid. As rats age, muscle mass in the leg may seem to disappear completely due to atrophy; as a result, the assessor may feel as though her or his finger and thumb are touching, with only skin in between. Abdominal muscles should be taut to the touch but can develop a flaccid, nonresponsive tone with age.
Leg muscle tone.
Leg muscle tone is determined by holding the rear leg muscle of the thigh between the thumb and index finger and palpating the area for tone, firmness, and mass.
Abdominal muscle tone.
Abdominal muscle tone is determined by holding the rat with its back cradled against the assessor's palm. The assessor's free hand applies gentle pressure to the abdominal muscles just below the ribcage. The first 3 fingers, when placed flat on the belly with tips just below the sternum, gives the assessor a good feel of the abdomen. The response of the abdomen should be quick, and the feel of the abdomen should be taut but flexible.
Data analysis.
Data are presented for the last assessment for each rat during a given month (and the only assessment for rats 19 mo or younger). For each measure, the frequency of a given score (1 to 5) was tabulated for all rats at each age and then expressed as a percentage of the total number of rats at that age. Relative frequencies are presented as histogram distributions. Changes in rank score with age were analyzed statistically by using the Kruskal–Wallis test (SAS Statistical Software, SAS Institute, Cary, NC) for each assessment measure. A P value of less than 0.05 was considered statistically significant. The last body weights collected each month from all rats in each age group were combined and are presented as mean ± SEM. Mortality was recorded and used to estimate median survival age.
Results
Results of the observational assessments are shown in Figures 4 through 6. The median total score was plotted as a function of age (Figure 4). Lower scores indicate a healthy condition, and higher scores indicate a deteriorating condition. Scores increased consistently from 20 to 31 mo of age, after which they remained high but variable.
Figure 4.
Age-related changes in median total score for rats 12 to 36 mo of age. Scores can range from 4 (no deterioration) to 20 (severe deterioration).
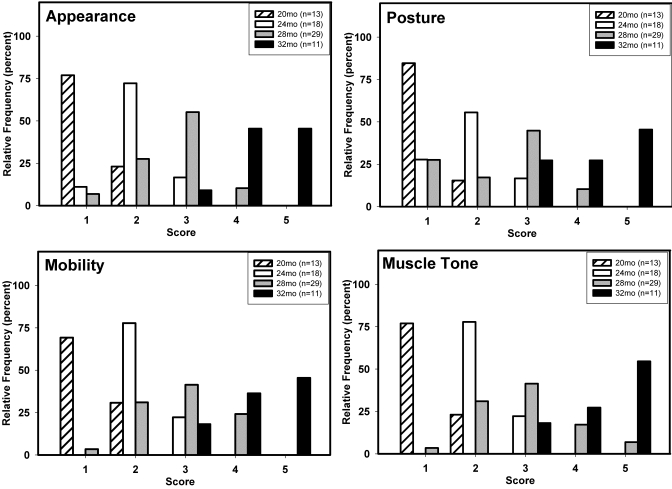
Frequency distributions for each measure were expressed as a percentage of the total number of rats assessed at each age (Figure 5). Representative ages (20, 24, 28, and 32 mo) were selected and shown to emphasize the major trends that accompanied aging. Data are not presented for rats younger than 20 mo, because signs of deterioration did not appear before this age, or for those older than 32 mo because of the steadily decreasing numbers of rats living beyond that age. For each measure, some signs of deteriorating health were evident in a few 20-mo-old rats (that is, they received a score of 2). At older ages, the distribution of scores shifted toward higher values, indicating aging-related deterioration in general health and condition. Statistical (Kruskal–Wallis) analysis indicated significant increases in scores with age for all 4 measures (all P < 0.0001). The consistency of the shifts in scores suggests that the collection of measures describes the aging phenotype. Variability in the distribution of scores also increased with age until 28 mo and decreased at 32 mo of age and thereafter (Figure 5).
Figure 5.
Age-related changes in score for each of the observational assessment measures (appearance, posture mobility, and muscle tone). Each panel shows frequency distributions of scores, expressed as a percentage of the total number of rats assessed at each of the designated ages. Sample sizes: 20 mo, n = 13; 24 mo, n = 18; 28 mo, n = 29; and 32 mo, n = 11.
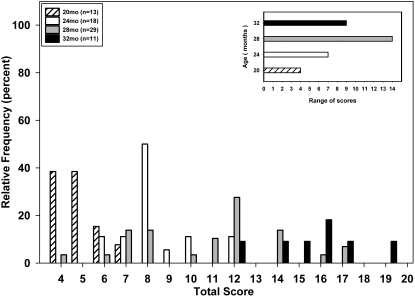
Figure 6 presents frequency distributions for total scores at each of the 4 age groups. The lowest possible total score was 4 (that is, the sum of 4 measures, each with a score of 1), and the maximum was 20. The shift with age toward higher scores was readily apparent. For example, the modal score increased from 4 to 5 (20 mo), 8 (24 mo), 12 (28 mo), and then 16 (32 mo). The insert in Figure 6 shows the increase in score variability with age; the range of scores increased from 4 (20 mo), to 7 (24 mo), and 14 (28 mo), and then decreased to 9 in the oldest (32 mo) rats.
Figure 6.
Age-related changes in total score. Each score is presented as a histogram and expressed as a percentage of the total number of rats at each of the designated ages. Sample sizes: 20 mo, n = 13; 24 mo, n = 18; 28 mo, n = 29; and 32 mo, n = 11; insert shows the range of scores at each age.
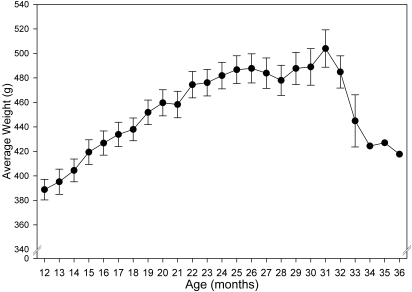
Body weight increased steadily between 12 and 24 mo before leveling off until 31 mo of age (Figure 7). Body weights then decreased beginning at 32 mo of age in the few remaining survivors.
Figure 7.
Age-related changes in body weight. Body weight (g; mean ± SEM) is presented for all rats at each month of life. The y axis is truncated for ease of data presentation.
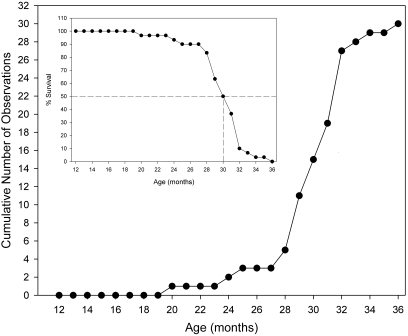
No deaths occurred prior to 20 mo of age. Thereafter, mortality data showed a 3-phase, sigmoid relationship with advancing age (Figure 8). In the first phase (between 20 and 28 mo of age), few deaths occurred. Between 28 and 32 mo of age (the second phase), mortality increased sharply through 32 mo of age. Finally, in the third phase (32 to 36 mo of age), the slope of the mortality function decreased, although few rats reached these oldest ages. The median survival age was approximately 30 mo.
Figure 8.
Age-related mortality and survival. Main figure shows cumulative number of rats that died at each age (mo). Insert shows the percentage of rats surviving at each age (mo); dotted lines indicate the age at which one-half of the rats survived.
Discussion
The confluence of increasing research into aging and increasing attention to the care and wellbeing of laboratory animals emphasizes the need for a simple, efficient test to evaluate the health and condition of aging animals. The method(s) must be capable of characterizing the deterioration that accompanies aging and efficient enough to evaluate animals in relatively large research colonies. We describe an observational assessment for rats that can be completed within 1 min by an experienced assessor. The assessment includes measures of appearance, posture, mobility, and muscle tone. Each measure is given a rank from 1 (normal) to 5 (severely affected). Changes in body weight are included also. Results were presented in which the observational assessment was used to evaluate aging-related changes in male Brown Norway rats.
A number of methods are available for assessing the clinical condition of laboratory rodents; these methods have been widely used in toxicologic, pharmacologic, and neurobiologic research. For example, observations of clinical condition have long been included in chronic toxicity studies.1,2 Many of the currently available assessment methods resemble that used in an early study in which several measures of laboratory mice used in drug experiments were scored systematically.11 In addition, several of these current methods are often used for chemical screening purposes,29 although they vary in the number of measures taken on each animal and the degree of interaction or manipulation of the animal.18 As useful as many of these methods are, their detailed characterization of behavior detracts from the efficiency needed for evaluating relatively large numbers of animals in a research colony. In our study, the condition of rats in their home cages was characterized simply by ranking their general appearance, posture, mobility, and body tone. In many regards, these are the common elements of most behavioral screening batteries.
Assessment scores for each measure increased with age. With few exceptions, rats that were 20 mo old were rated as normal (that is, most received a rank score of 1). Between 24 and 32 mo of age, scores increased for all measures, indicating progressive deterioration. The results of the ranking method in the present study are consistent with commonly reported observations of the clinical condition of rodents with advancing age. A major benefit of rank scores, however, is that the observations are quantified, thereby permitting statistical analysis of the data. In addition, the distribution of scores can be compared across ages. For example, it is commonly accepted that aging is accompanied by an increase in interindividual variability,14,16 although detailed analyses are relatively rare.4,24 The present results provide quantitative support for this commonly accepted principle, in that the range of scores increased until rats were 28 mo of age. The decrease in the range of scores at 32 mo of age was not unexpected and was probably due to the elimination of the rats that had deteriorated the most (and succumbed) prior to this assessment.
As rats and virtually all other organisms age, signs of deterioration become evident, the likelihood of disease increases, body weight often decreases in late stages, and death ultimately ensues. The present findings support these general trends. Important questions arise, however, regarding the possible redundancy of these endpoints and whether a ‘signature’ of aging-related deterioration can be identified that is sufficiently predictive to help decide when a research animal should be euthanized. The obvious goal for both experimenters and veterinarians would be to euthanize an animal before it died spontaneously. Many studies assessing body condition or other measurable parameters to predict imminent death often use a group of animals that is allowed to proceed to spontaneous death. We chose not to include such a group but instead euthanized all rats that developed a moribund condition. In addition, because these rats were part of an ongoing behavioral study, we did not perform any additional invasive procedures (for example, blood collection for determination of serum biomarkers). Future studies will attempt to correlate assessment scores with other clinical or postmortem findings that will allow us to more accurately predict impending death. However, according to the current results, a provisional threshold of concern for impending death of an individual rat seems to be a score of 18.
In the present study, mortality occurred in 3 phases, involving low, high, then again low incidence. This pattern of mortality has been obtained in numerous species including humans.3,8,30 From the middle (rapid) phase, the median survival time was estimated to be 30 mo, which is consistent with earlier reports on male Brown Norway rats.22,30 Body weight, in comparison, consistently increased up to 24 mo of age and was stable until 31 mo of age, after which body weight decreased in the dwindling few survivors. These results indicated that body weight was not an accurate predictor of mortality. In contrast, the observational assessments resulted in continuously increasing scores to a maximum that was obtained at 32 mo of age and then was sustained at even older ages in the few survivors. In addition, assessment scores increased consistently throughout the initial phase of low mortality and continued unabated through the second (rapid) mortality phase. Moreover, at the median survival age (30 mo) the median total score already exceeded half of the maximal score that was obtained in the oldest survivors. Assessment scores also increased during the phase when body weights had reached a plateau. Together, these results indicate that the age-related changes in assessment scores were not correlated with changes in body weight and that the scores increased well before marked mortality became apparent. Therefore, our current results provide strong evidence of the utility of our observational assessment method for providing a unique signature of aging-related deterioration that is more sensitive than are changes in body weight or probability of death.
The Brown Norway rat is a common strain in laboratory aging studies, along with the Fischer 344 strain and the Brown Norway–F344 hybrid cross.22 Brown Norway rats, like Fischer 344 rats, grow to moderate size (approximately 500 g) and are considerably smaller than many of the outbred stocks of rat (for example, Sprague–Dawley, Long Evans, Wistar), which often approach or surpass 1000 g. Brown Norway rats have the added advantage of developing few of the pathologies of old age that are found in Fischer 344 rats, Brown Norway–F344 hybrids, and many of the outbred stocks.22 In the present report, mean body weight reached a maximum of approximately 500 g, which is similar to that reported previously.22,30 In addition, the very old Brown Norway rats showed decreases in body weight, also consistent with previous reports.19,30
Assessments were made in rats that periodically received either a short-acting pesticide (carbaryl) or an organic solvent (toluene). Each chemical has different effects on behavior, yet the chemicals are similar in their brief duration of action.9,20 Moreover, each chemical is cleared rapidly from the body in a matter of a few hours.25,28 Given the rats’ history, we cannot dissociate effects of aging due to normal events from those influenced by toxicant exposure. Our results, therefore, cannot be considered normative data on signs of aging in male Brown Norway rats; instead, they show how scores change with age and that they change in ways that cannot be reduced to changes in body weight or likelihood of survival. A study is underway using rats that have no history of toxicant exposure, and so far (a maximum of 17 mo of age), their data resemble those presented in this report.
Assessments used a hybrid design that was initially cross-sectional (as rats arrived for their assigned experiments) and then longitudinal, with the frequency of assessment increasing with age until the rats either died or were euthanized. Such hybrid designs are common in aging research.3,31 Although a longitudinal assessment with all rats starting at the same age would be instructive, the similarity of results for body weight and survival with published accounts30 attests to the robust nature of the results obtained with the current design.
Laboratory animal models will become increasingly important as interest grows in better understanding the aging process in humans. The Brown Norway rat has been used extensively as an animal model in aging research.22,30 The relatively simple measures described in the current report appear to be sufficient for a general characterization of aging, and their efficiency recommends application in monitoring the health of aging animal colonies. These measures are key elements in an animal model of the frailty phenotype that accompanies old age in humans6,32 and offer a parallel to the ‘activities of daily living’ that are widely used to chart the course of functional deterioration in aging humans.5,12,33
In summary, this report presents an efficient observational assessment method for evaluating aging laboratory rats. The method comprises tests that collectively describe the trajectory of aging and changes in variability between rats. The method may be beneficial for evaluating the health of rats in aging colonies and for studying the effects of toxic compounds and the diseases of aging.
Acknowledgments
We thank Drs Andrew Geller, Mary Gilbert, Christopher Gordon, Ginger Moser, and Stephanie Padilla for comments on an earlier version of the manuscript.
This manuscript has been reviewed by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- 1.Arnold DL, Emerson JL, Hollander CF, Knook DL, Kroes R, McMartin DN, Oehme FW, Olsen P, Sugar J, van Bezooijen CFA. 1984. Age-associated (geriatric) pathology: its impact on long-term toxicity studies, p 51–107. : Grice HC. Current issues in toxicology. New York (NY): Springer–Verlag [Google Scholar]
- 2.Barnes JM, Denz FA. 1954. Experimental methods in determining chronic toxicity: a critical review. Pharmacol Rev 6:191–242 [PubMed] [Google Scholar]
- 3.Brooks A, Lithgow GJ, Johnson TE. 1994. Mortality rates in a genetically heterogeneous population of Caenorhabditis elegans. Science 263:668–671 [DOI] [PubMed] [Google Scholar]
- 4.Dannefer D, Sell RR. 1988. Age structure, the life course, and ‘aged heterogeneity’: prospect for research and theory. Compr Gerontol B 2:1–10 [PubMed] [Google Scholar]
- 5.Federal Interagency Forum on Aging-Related Statistics 2008. Older Americans: key indicators of well-being. Washington (DC): US Governmental Printing Office [Google Scholar]
- 6.Fried LP, Tangen C, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. 2001. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 7.Geller AM, Zenick H. 2005. Aging and the environment: a research framework. Environ Health Perspect 113:1257–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gompertz B. 1825. On the nature of the function expressive of the law of human mortality and a new mode of determining the value of life contingencies. Philos Trans R Soc London 115:513–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon CJ, Samsam TE, Oshiro WM, Bushnell PJ. 2007. Cardiovascular effects of oral toluene exposure in the rat monitored by radiotelemetry. Neurotoxicol Teratol 29:228–235 [DOI] [PubMed] [Google Scholar]
- 10.Hadley EC, Lakatta EG, Morrison-Bogorad M, Warner HR, Hodges RJ. 2005. The future of aging therapies. Cell 120:557–567 [DOI] [PubMed] [Google Scholar]
- 11.Irwin S. 1968. Comprehensive observational assessment: 1a. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacology (Berl) 13:222–257 [DOI] [PubMed] [Google Scholar]
- 12.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. 1963. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. J Am Med Assoc 185:914–919 [DOI] [PubMed] [Google Scholar]
- 13.Kim SK. 2007. Common aging pathways in worms, flies, mice and humans. J Exp Biol 210:1607–1612 [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood TBL. 2005. Understanding the odd science of aging. Cell 120:437–447 [DOI] [PubMed] [Google Scholar]
- 15.Lescai F, Marchegiani F, Franceschi C. 2009. PON1 is a longevity gene: results of a meta-analysis. Aging Res Rev 8:277–284 [DOI] [PubMed] [Google Scholar]
- 16.Lupien SJ, Wan N. 2004. Successful ageing: from cell to self. Philos Trans R Soc Lond B Biol Sci 359:1413–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz W, Sanderson W, Scherbov S. 2008. The coming acceleration of global population ageing. Nature 451:716–719 [DOI] [PubMed] [Google Scholar]
- 18.MacPhail RC, Tilson HA. 1995. Behavioral screening tests: past, present, and future, p 231–238. : Chang LW, Slikker W., Jr Neurotoxicology: approaches and methods. New York (NY): Academic Press [Google Scholar]
- 19.Matsumoto AM, Marck BT, Gruenewald DA, Wolden-Hanson T, Naai MA. 2000. Aging and the neuroendocrine regulation of reproduction and body weight. Exp Gerontol 35:1251–1265 [DOI] [PubMed] [Google Scholar]
- 20.McDaniel KL, Padilla S, Marshall RS, Phillips PM, Podhorniak L, Qian Y, Moser VC. 2007. Comparison of acute neurobehavioral and cholinesterase inhibitory effects of N-methylcarbamates in rat. Toxicol Sci 98:552–560 [DOI] [PubMed] [Google Scholar]
- 21.Morton DB. 1999. Humane endpoints in animal experimentation for biomedical research: ethical, legal, and practical aspects, p 5–12. : Hendriksen CFM, Morton DB. Humane endpoints in animal experimentation for biomedical research. London (UK): Royal Society of Medicine Press [Google Scholar]
- 22.Nadon NL. 2006. Gerontology and age-associated lesions, p 761–772. : Suckow MA, Weisbroth SH, Franklin CL. The laboratory rat, 2nd ed. New York (NY): Elsevier Academic Press [Google Scholar]
- 23.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. 2009. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med 15:331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nebert DW. 2005. Interindividual susceptibility to environmental toxicants—a current assessment. Toxicol Appl Pharmacol 207:S34–S42 [DOI] [PubMed] [Google Scholar]
- 25.Padilla S, Marshall RS, Hunter DL, Lowit A. 2007. Time course of cholinesterase inhibition in adult rats treated acutely with carbaryl, carbofuran, formetanate, methomyl, methiocarb, oxamyl, or propoxur. Toxicol Appl Pharmacol 219:202–209 [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Mitnitski AB, MacKnight C. 2002. Some mathematical models of frailty and their clinical implications. Rev Clin Gerontol 12:109–117 [Google Scholar]
- 27.Stokes WS. 2002. Humane endpoints for laboratory animals used in regulatory testing. ILAR J 43suppl:S31–S38 [PubMed] [Google Scholar]
- 28.Sullivan MJ, Conolly RB. 1988. Comparison of blood toluene levels after inhalation and oral administration. Environ Res 45:64–70 [DOI] [PubMed] [Google Scholar]
- 29.Tilson HA, Moser VC. 1992. Comparison of screening approaches. Neurotoxicology 13:1–13 [PubMed] [Google Scholar]
- 30.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. 1999. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci 54:B492–B501 [DOI] [PubMed] [Google Scholar]
- 31.Tzankoff SP, Norris AH. 1978. Longitudinal changes in basal metabolism in man. J Appl Physiol 45:536–539 [DOI] [PubMed] [Google Scholar]
- 32.Walston J, Fedarko N, Yang H, Leng S, Beamer B, Espinoza S, Lipton A, Zheng H, Becker K. 2008. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci 63:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiener JM, Hanley RJ. [Internet]. 1989 Measuring the activities of daily living among the elderly: a guide to national surveys. [Cited 12 Aug 2009]. Available at http://aspe.hhs.gov/daltcp/reports/guide.htm