Abstract
Cefovecin sodium is a third-generation broad-spectrum cephalosporin antibiotic licensed for the treatment of skin infections in cats and dogs. The objective of our study was to assess whether its pharmacokinetic profile in squirrel monkey, rhesus macaques, and cynomolgus macaques was similar to that of dogs. Plasma levels were determined by using protein precipitation followed by liquid chromatography tandem mass spectrometry. After subcutaneous dosing at 8 mg/kg, the plasma terminal half-life of cefovecin was substantially shorter in the nonhuman primates (2.6 to 8.0 h) than in dogs (102 h). The total plasma exposure (AUC0-96h) was 10- to 40-fold lower in nonhuman primate species. In cynomolgus macaques, cefovecin showed a similar subcutaneous bioavailability (82% compared with 100%) and volume of distribution (0.16 compared with 0.12 L/kg) as compared to dogs; however, the plasma clearance of cefovecin was 20-fold higher. Cefovecin susceptibility testing and minimum inhibitory concentrations were not established for clinical isolates in nonhuman primates. However, if the minimum inhibitory concentrations of cefovecin for various nonhuman primates pathogens are in the same range as those observed for canine pathogens, our results suggest that cefovecin used at the same dosing regimen and frequency prescribed for the dogs will be ineffective and that increases in dose or frequency (or both) may be required.
Abbreviation: AUC, area under the plasma concentration–time curve
Cefovecin sodium (CAS no. 141195-77-9) is a recently approved, third-generation cephalosporin antibiotic licensed for use in dogs and cats in Europe and North America.7 It has a broad-spectrum of antimicrobial activity against gram-positive and gram-negative bacteria and is active against clinically relevant canine and feline pathogens associated with skin, soft tissue, and urinary tract infections.11 Cefovecin is administered as a single subcutaneous injection at 8 mg/kg once every 14 d.11 Published canine data indicate that the drug is fully bioavailable after subcutaneous administration, with a mean maximum concentration of 121 µg/mL, a mean elimination half-life of 133 h, and a clearance of 0.76 mL/h/kg after intravenous dosing.12 Cefovecin is highly bound to plasma proteins (96.0% to 98.7%).12 In dogs, it is not metabolized by the liver; the majority of the dose is eliminated in the urine, with some fraction excreted as unchanged drug in the bile.7
Cephalosporins are used widely as antibiotics and have well-established safety and efficacy profiles. The use of an injectable antibiotic with an extended duration of action would greatly benefit zoo and laboratory animal veterinarians working with nonhuman primates, because it would reduce the frequency of capture and restraint of animals for treatment, decrease stress to animals, and lessen the risk of injuries of animal handlers. Current antibiotic therapies available for nonhuman primates have impractical dosing regimens and can be difficult to administer. These challenges may contribute to treatment failure and potential development of drug resistance.1 A review of listservs and online correspondence (for example, Compmed, Industry Veterinarians) suggested the off-label use of cefovecin by primate veterinarians. Therefore, to assess the potential use of cefovecin in nonhuman primates, we determined its pharmacokinetic parameters in squirrel monkeys, rhesus macaques, and cynomolgus macaques and compared them with those in dogs. Investigation of antibiotic efficacy and the safety profile of cefovecin in nonhuman primates was outside the scope of this evaluation.
Materials and Methods
Animals.
Three 10-y-old male squirrel monkeys (Saimiri sciureus; Osage Research Primates, Kaiser, MO), three 4-y-old male rhesus (Macaca mulatta) macaques of Indian-origin (The Mannheimer Foundation, Homestead, FL), three 14- to 17-y-old male Mauritian-born cynomolgus (Macaca fascicularis) macaques, and two 7-y-old male beagle dogs (Canis familiaris; Marshall Farms Group, North Rose, NY) were used in this study.
Housing and care.
Rhesus macaques, squirrel monkeys, and dogs were housed in compatible groups, whereas cynomolgus macaques were singly housed. Animals were fed commercially available laboratory animal feed from Harlan Teklad (Madison, WI) according to their metabolic requirements (rhesus and cynomolgus macaques were fed diet no. 2050J, squirrel monkeys no. 8794N, and dogs no. 2025). A variety of fresh fruit and vegetables was provided to the nonhuman primates on a daily basis as part of their enrichment program. Filtered city water was available ad libitum through the automatic distribution system. Housing standards and environmental conditions were maintained within the limits prescribed within the Guide to the Care and Use of Experimental Animals and the Guide for the Care and Use of Laboratory Animals.3,6 All studies were approved by the Merck Frosst Animal Care Committee and conducted in an AAALAC-accredited and CCAC-certified animal facility.
In vivo administration.
A single subcutaneous dose (8 mg/kg) of cefovecin (Convenia, Pfizer Animal Health, Kirkland, Quebec, Canada) was administered at 0.1 mL/kg to fasted animals. EDTA-treated plasma was collected at 0.25, 0.5, 1, 2, 4, 8, 24, 48, and 96 h after dosing. An intravenous dose (2 mg/kg; diluted to 2 mg/mL in saline) was administered to fasted cynomolgus monkeys at 1 mL/kg. EDTA-treated plasma was collected at 5 min and 0.5, 1, 2, 4, 8, 24, 48, and 96 h after dosing, and urine was collected for the intervals 0 to 4, 4 to 8, 8 to 24, 24 to 32, and 32 to 48 h after dosing. All samples were kept frozen at −70 °C until analysis.
Pharmacokinetic analysis.
Plasma drug levels were determined by using protein precipitation followed by liquid chromatography–tandem mass-spectrometry. All analyses were conducted by using a chromatography system (Transcend LX2 Parallel UHPLC System, ThermoFisher, Franklin, MA) coupled to a triple quadrupole mass spectrometer (API4000, Applied Biosystems, Foster City, CA). Standard solutions of cefovecin were prepared from a reconstituted aqueous stock solution (80 mg/mL). A 10-point standard curve ranged from 0.005 to 45 µg/mL, with 5 quality-control levels at 0.02, 0.05, 1.1, 17, 34 µg/mL prepared in blank plasma. Labetalol was used as internal standard for HPLC–mass spectrometry assays. Any samples exceeding the highest standard were diluted 1:3 with blank plasma prior to extraction. Noncompartmental pharmacokinetic calculations were performed by using WinNonLin (version 5.0.1, Pharsight, Mountain View, CA). The area under the plasma concentration–time curve (AUC) was determined by trapezoidal estimation with linear interpolation for increasing plasma concentrations and logarithmic interpolation for decreasing plasma concentrations. The first-order elimination rate constant (ke) was estimated by linear regression of the terminal points and was converted to a half-life by using the equation t1/2 = 0.693/ke.8 Volume of distribution was determined as Vdss = dose × (AUMC∞) / (AUC∞ × AUC∞), and clearance was estimated from CL = dose/AUC∞.8,14
In vitro assays.
Cryopreserved hepatocytes from beagle dogs and squirrel, cynomolgus, and rhesus monkeys were used to study the in vitro metabolic stability of cefovecin. Briefly, cells were thawed rapidly, washed, counted, and diluted to a final concentration of 1 × 106 cells/mL in Krebs–Henseleit buffer for each incubation.5 A final drug concentration of 0.5 µg/mL cefovecin was incubated (n = 4) for 0, 5, 15, 30, 60, and 90 min in the cell suspension at 37 °C in 95% air–5% CO2 with shaking at 400 rpm. The incubations were quenched at the specified time point with acetonitrile and analyzed by HPLC–mass spectrometry by using the same procedure as for pharmacokinetic studies. Positive controls of midazolam, phenacetin, bufuralol, diclofenac, and 7-hydroxycoumarin were used to assess the viability of the cells in light of the expected metabolic turnover.
Plasma protein binding was determined by equilibrium dialysis. EDTA-treated plasma from beagle dogs, squirrel monkeys, and cynomolgus and rhesus macaques was used to measure protein binding in triplicate at cefovecin concentrations of 0.05, 0.5, 5, and 50 µg/mL. The plasma samples were dialyzed in against PBS and incubated for 5 h at 37 °C in 95% air–5% CO2. Samples were quenched with acetonitrile and quantified by HPLC–mass spectrometry by using the appropriate reference standard curves prepared in either buffer or plasma. A simple linear equation (y = mx + b) was derived from the unbound fraction (y) and cefovecin concentration (x) used in the plasma protein binding assay. The resultant slope (m) and intercept (b) estimates were used to predict free cefovecin concentrations from total concentrations in pharmacokinetic experiments.
Results
Pharmacokinetics.
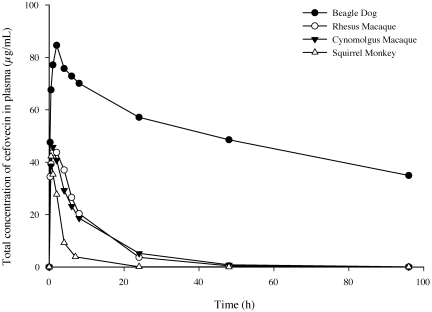
The total concentration–time profile following a single 8 mg/kg subcutaneous dose of cefovecin is shown in Figure 1. A summary of the calculated pharmacokinetic parameters is provided in Table 1, along with previously published results from dogs12 for comparison. Our results showed that peak cefovecin concentration occurred between 0.5 and 2 h, indicating rapid absorption; however, the maximal plasma concentration was approximately 2-fold lower in nonhuman primates than in dogs. Drug levels in monkeys decreased rapidly compared with those in dogs, with a calculated terminal half-life of 2.6 ± 0.1, 6.3 ± 1.8, 8.0 ± 0.6, and 102 h for squirrel monkeys, cynomolgus macaques, rhesus macaques, and dogs, respectively. The overall exposure (AUC0 to 96 h) was approximately 10-fold lower in cynomolgus and rhesus macaques and 40-fold less in squirrel monkeys when compared with dogs. Subcutaneous bioavailability in cynomolgus macaques was determined to be roughly 80%. Additional pharmacokinetic parameters in cynomolgus macaques were determined from intravenous dosing followed by plasma and urine collection (Table 1). Clearance was substantially higher in cynomolgus macaques than in dogs (15.0 compared with 0.76 mL/h/kg), whereas volume of distribution at steady state was roughly identical (0.16 compared with 0.12 L/kg). More than 80% of the cefovecin dose in macaques was recovered in urine within 48 h after dosing.
Figure 1.
Total mean (n = 2 to 3 animals per group) plasma concentration of cefovecin after subcutaneous administration of 8 mg/kg.
Table 1.
Pharmacokinetic parameters (mean ± 1 SD, where applicable) after subcutaneous and intravenous dosing of cefovecin
| Squirrel monkeys (n = 3) | Cynomolgus macaques (n = 3) | Rhesus macaques (n = 3) | Beagle dogs (n = 2) | Published data from beagle dogs (n = 12)a | |
| Subcutaneous dosing (8 mg/kg) | |||||
| Maximal plasma concentration (µg/mL) | 42 ± 9 | 46 ± 6 | 47 ± 8 | 85 | 121 ± 51 |
| Time (h) to maximal plasma concentration (range) | 0.5 (0.5) | 1 (1) | 2 (0.2–4) | 2 (2) | 6.2 ± 3.0 |
| AUC0 to 96 h (µg /mL/h) | 128 ± 38 | 468 ± 97 | 520 ± 70 | 4853 | not done |
| Terminal half-life (h) | 2.6 ± 0.1 | 6.3 ± 1.8 | 8.0 ± 0.6 | 102 | 133 ± 16 |
| Intravenous dosing (2 mg/kg) | |||||
| Terminal half life of intravenous dose (h) | 8.5 ± 1.2 | 136 ± 12 | |||
| Clearance (mL/h/kg) | 15 ± 5 | 0.76 ± 0.13 | |||
| Estimate of unbound clearance (mL/h/kg) | 400–700 | 36 | |||
| Volume of distribution (L/kg) | 0.16 ± 0.02 | 0.122 ± 0.011 | |||
| Bioavailability | 82 ± 19 | 99–107 |
From reference 12.
Plasma protein binding and metabolic stability.
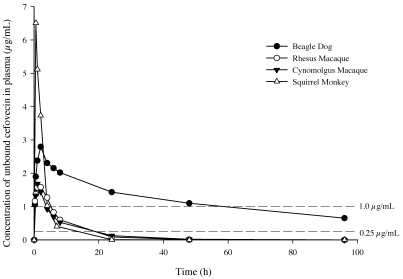
The plasma protein binding of cefovecin in all 4 species is presented in Table 2. Data are presented as the protein-bound fraction at 4 different cefovecin concentrations spanning 0.5 to 50 µg/mL. Protein binding was highest in dogs (97.7% to 99.2%) followed by cynomolgus macaques (96.2% to 97.9%), rhesus macaques (93.8% to 97.8%), and squirrel monkeys (83.6% to 92.1%). The binding of cefovecin to plasma proteins decreased with increasing drug concentration in all species, suggesting saturation of binding sites. A linear equation was derived from the protein binding results and was used to convert total concentration to free concentration over time (Figure 2). Protein binding also was used to estimate a range of unbound clearance (Table 1) by dividing the total clearance by the free fraction measured at cefovecin concentrations ranging from 0.5 to 50 µg/mL.
Table 2.
Plasma protein binding of cefovecin (%; mean ± 1 SD)
| Cefovecin dose (µg/mL) | Squirrel monkeys (n = 3) | Cynomolgus macaques (n = 3) | Rhesus macaques (n = 3) | Beagle dogs (n = 2) |
| 0.05 | 92.1 ± 0.9 | 97.7 ± 0.2 | 97.8 ± 0.1 | 98.7 ± 0.1 |
| 0.5 | 90.4 ± 0.4 | 97.9 ± 0.1 | 97.7 ± 0.1 | 99.2 ± 0.1 |
| 5 | 89.1 ± 0.1 | 97.4 ± 0.1 | 97.4 ± 0.1 | 98.9 ± 0.1 |
| 50 | 83.6 ± 1.2 | 96.2 ± 0.1 | 93.8 ± 1.0 | 97.7 ± 0.3 |
Figure 2.
Mean (n = 2 to 3 animals per group) plasma concentration of unbound cefovecin after subcutaneous administration of 8 mg/kg.
The metabolic turnover of cefovecin in hepatocytes was very low in all species (less than 10% conversion in 90 min), and the estimated intrinsic clearance was less than 0.5 µL/min per 1 × 106 cells. Similarly to published data for dogs,7 liver metabolism is, therefore, not a significant route of cefovecin elimination in nonhuman primates.
Discussion
Veterinarians have a much smaller pharmacopoeia than do human practitioners. In particular, the relatively limited usage and therefore low financial returns of veterinary products for nonhuman primates or other laboratory animal species often do not justify the costs associated with evaluation, safety assessment, and registration of drugs. Therefore off-label use of antibiotics or other veterinary products in nonhuman primates is very common. However, the scarcity of available published information requires veterinarians to exercise careful professional judgment to ensure desired efficacy and prevent any untoward effects. Ideally the pharmacokinetic parameters of the drug under consideration should be determined in the target species prior to any off-label use.
Because of its convenient dosing regimen in dogs, we evaluated the pharmacokinetics of cefovecin in 3 nonhuman primate species. The maximal plasma concentration of cefovecin after a single 8-mg/kg subcutaneous injection showed only a 2-fold difference between nonhuman primates and dogs (Figure 1). However, the terminal half-life was considerably shorter (12- to 40-fold) in nonhuman primates (Table 1), and exposures were much lower in nonhuman primates than in dogs. Similar to those in dogs, we observed high bioavailability (82%) and low distribution volume (0.16 L/kg) in cynomolgus macaques, but clearance was 20-fold higher (15 mL/h/kg) than that in dogs. Therefore we attributed the decreased plasma levels in nonhuman primates to an increased higher elimination rate rather than poor absorption.
In vitro liver metabolism experiments indicated that the hepatic extraction of cefovecin is low in all 3 nonhuman primate species and that, as previously observed in dogs,12 liver metabolism does not play a significant role in its elimination. Samples were also analyzed by full-scan time-of-flight mass spectrometry to search for metabolites, but none were found (data not shown).
In dogs, cefovecin is eliminated mainly (70%) by renal excretion.7,12 Similarly, in cynomolgus monkeys, 80% of cefovecin was recovered in urine after intravenous dosing. Unbound clearance (total clearance/fraction unbound) in cynomolgus macaques ranged from 400 to 700 mL/h/kg, a value slightly higher than estimates of glomerular filtration rate for these animals (180 mL/h/kg),13 suggesting that either renal reabsorption does not occur in primates or that it is compensated by active secretion in the proximal tubule. This finding is in contrast to what was observed in dogs, in which reabsorption seems to dominate.12 Interestingly, exposure of total cefovecin in squirrel monkeys was lowest and exhibited the shortest half-life, which could partly be explained by an increased free fraction, which can enhance renal filtration.9 Except for those drugs that have a high renal extraction ratio and whose renal clearance is, therefore, limited by blood flow, clearance is regulated by the fraction unbound, regardless of whether filtration or filtration and secretion is occurring.8 If one assumes the subcutaneous bioavailability and route of elimination in squirrel monkeys are similar to those in cynomolgus macaques, then decreased protein binding would explain, in part, the reduced exposure and shorter elimination half-life in squirrel monkeys. However, the absence of intravenous data in squirrel monkeys precludes any definitive conclusions.
Cephalosporins are time-dependent antimicrobials, and successful therapy is achieved by maintaining the effective cephalosporin concentration above 90% of the minimum inhibitory concentration of the targeted pathogen.4 Protein binding is a key determinant of the efficacy of antibacterial agents2,9,15 and must be taken into account when determining target plasma concentrations. The binding of cefovecin to plasma proteins was lower in primates (Table 2), and the free fraction was as much as 12 times higher in squirrel monkeys and 2- to 3-fold higher in rhesus and cynomolgus monkeys than in dogs. Figure 2 shows a plot of unbound concentration over time that was calculated from the in vitro protein binding results and total plasma levels. In dogs, the 90% minimal inhibitory concentrations for the major clinically relevant canine pathogens are 0.25 for Staphylococcus intermedius and 1 µg/mL for Escherichia coli.10 In the absence of susceptibility data for nonhuman primate pathogens to cefovecin, these canine target concentration values were used to estimate thresholds for efficacious free concentrations. The concentration of unbound cefovecin remained above 1 µg/mL for at least 48 h and above 0.25 µg/mL for the duration of the sampling period (96 h). In contrast, the unbound concentrations of cefovecin decreased below 1.0 µg/mL in less than 6 h and below 0.25 µg/mL within 24 h after dosing in all 3 nonhuman primates (Figure 2).
In summary, the pharmacokinetics of cefovecin in nonhuman primate species after subcutaneous dosing showed a much shorter plasma terminal half-life and decreased exposure when compared with those in dogs. In absence of additional microbial susceptibility data for nonhuman primates pathogens, the current cefovecin dosing regimen prescribed for dogs (8 mg/kg SC every14 d) may not be suitable for the treatment of infections in the nonhuman primate species we evaluated. Our data indicate that either higher doses or more frequent administrations (or both) might be required; however, the optimal dosing regimen of cefovecin in individual nonhuman primates species should be established based on additional in vitro microbial susceptibility, pharmacokinetic, and safety studies.
Acknowledgments
The authors thank Stephanie Alleyn, Kathy Beauchesne, Joel Bosquet, Josianne Rozon, and Roberta Rasori (Merck In Vivo Science) for technical assistance. Thanks to Erin Mulrooney and France Landry (Merck DMPK) for help in preparing hepatocytes. The study was performed and funded by Merck Canada (Kirkland, Quebec, Canada).
References
- 1.Angulo FJ, Nunnery JA, Bair HD. 2004. Antimicrobial resistance in zoonotic enteric pathogens. Rev Sci Tech 23:485–496 [DOI] [PubMed] [Google Scholar]
- 2.Beer J, Wagner CC, Zeitlinger M. 2009. Protein binding of antimicrobials: methods for quantification and for investigation of its impact on bacterial killing. AAPS J 11:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canadian Council on Animal Care 1993. Guide to the care and use of experimental animals, vol 1, 2nd ed. Ottawa (Canada): Canadian Council on Animal Care [Google Scholar]
- 4.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 22:89–96 [DOI] [PubMed] [Google Scholar]
- 5.Houle R, Raoul J, Lévesque J-F, Pang KS, Nicoll-Griffith DA, Silva JM. 2003. Retention of transporter activities in cryopreserved, isolated rat hepatocytes. Drug Metab Dispos 31:447–451 [DOI] [PubMed] [Google Scholar]
- 6.Institute for Laboratory Animal Research 1985. Guide for the care and use of laboratory animals. Washington (DC): Department of Health and Human Services [Google Scholar]
- 7.Pfizer Animal Health [Internet]. 2008Convenia prescribing information. [Cited Feb 2010]. Available at: http://www.convenia.com/product.htm
- 8.Rowland M, Tozer TN. 1980. Clinical pharmacokinetics concepts and applications, p 57, 58, 82–85. Philadelphia (PA): Lea & Febiger [Google Scholar]
- 9.Schmidt S, Röck K, Sahre M, Burkhardt O, Brunner M, Lobmeyer MT, Derendorf H. 2008. Effect of protein binding on the pharmacological activity of highly bound antibiotics. Antimicrob Agents Chemother 52:3994–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Six R, Cherni J, Chesebrough R, Cleaver D, Lindeman CJ, Papp G, Skogerboe TL, Weigel DJ, Boucher JF, Stegemann MR. 2008. Efficacy and safety of cefovecin in treating bacterial folliculitis, abscesses, or infected wounds in dogs. J Am Vet Med Assoc 233:433–439 [DOI] [PubMed] [Google Scholar]
- 11.Stegemann MR, Passmore CA, Sherington J, Lindeman CJ, Papp G, Weigel DJ, Skogerboe TL. 2006. Antimicrobial activity and spectrum of cefovecin, a new extended- spectrum cephalosporin, against pathogens collected from dogs and cats in Europe and North America. Antimicrob Agents Chemother 50:2286–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stegemann MR, Sherington J, Blanchflower S. 2006. Pharmacokinetics and pharmacodynamics of cefovecin in dogs. J Vet Pharmacol Ther 29:501–511 [DOI] [PubMed] [Google Scholar]
- 13.Tahara H, Kusuhara H, Chida M, Fuse E, Sugiyama Y. 2006. Is the monkey an appropriate animal model to examine drug-drug interactions involving renal clearance? Effect of probenecid on the renal elimination of H2 receptor antagonists. J Pharmacol Exp Ther 316:1187–1194 [DOI] [PubMed] [Google Scholar]
- 14.Toutain PL, Bousquet-Mélou A. 2004. Volumes of distribution. J Vet Pharmacol Ther 27:441–453 [DOI] [PubMed] [Google Scholar]
- 15.Zeitlinger MA, Sauermann R, Traunmuller F, Georgopoulos A, Muller M, Joukhadar C. 2004. Impact of plasma protein binding on antimicrobial activity using time-killing curves. J Antimicrob Chemother 54:876–880 [DOI] [PubMed] [Google Scholar]