Abstract
Pinworms are highly contagious parasites of laboratory rodents that often are treated with fenbendazole. To our knowledge, the effect of fenbendazole at therapeutic dosages on behavioral tests in mice has not been evaluated. Here we studied 6-wk-old male C57BL/6N mice. We compared the behavior of control mice (fed regular diet) with 3 groups of mice treated with dietary fenbendazole. Treatment groups were 4 wk of fenbendazole, 2 wk of fenbendazole followed by 2 wk of regular diet, and 2 wk of regular diet followed by 2 wk of fenbendazole. At the end of dietary treatment all groups were tested by open field for central, peripheral and vertical activity; elevated plus maze for anxiety; and rotarod for motor ability and then evaluated by clinical pathology and selected histopathology. Treated and control groups showed no differences in open field or elevated plus maze testing, histopathology, or clinical pathology. However mice treated for 4 wk with fenbendazole or 2 wk of fenbendazole followed by 2 wk regular diet stayed on the rotarod for shorter periods than did controls, and mice treated with 2 wk of regular diet followed by 2 wk fenbendazole showed a trend toward shorter rotarod times. In light of this study, we suggest that open field and elevated plus maze testing is unlikely to be affected by 4 wk fenbendazole treatment in male C57BL/6 mice; however, behavioral tests of motor ability such as rotarod tests may be affected during and for at least 2 wk after fenbendazole treatment.
Abbreviation: FF, fenbendazole diet only; RF, regular diet followed by fenbendazole diet; FR, fenbendazole diet followed by regular diet
Pinworms continue to beset modern rodent facilities,4,18,19,22 likely because the eggs of 2 species (Aspiculuris tetraptera and Syphacia muris) are environmentally persistent25 and resistant to common disinfectants8 while infections for all species can be difficult to detect due to intermittent shedding of eggs and reduction in parasite load with age.20,26 Although often thought to be merely nuisances2 (older reports of rectal prolapse11 did not rule out Helicobacter spp. or Citrobacter rodentium), pinworms can affect immunologic parameters,1,23 and their presence can inhibit interinstitutional transfer of mice for research collaborations.
Once pinworms are detected, attempts at eradication are common. Of the available anthelminthics, fenbendazole incorporated in the feed at 150 to 450 mg/kg, intermittently or continuously for as long as 8 wk, is a popular choice because of its ease of use and perceived safety, efficacy, and lack of interference with ongoing research.5,9,12,21 Fenbendazole is a benzimidazole derivative that is absorbed from the gastrointestinal tract and metabolized in the liver to its active form, fenbendazole sulfoxide.24 The drug is a microtubule polymerization inhibitor and binds to the structural protein β tubulin.15 Its efficacy as an anthelminthic results from a much greater affinity for helminth tubulin than for mammalian tubulin at 37 °C.15 Benzimidazole-resistant helminths have a greater proportion of low-affinity tubulin.16
Previous reports suggest that fenbendazole is not entirely lacking in research effects. For instance, fenbendazole inhibited tumor xenograft growth in immunodeficient mice when fed in combination with high vitamin levels,10 and the drug may have curtailed reproduction in Sprague–Dawley rats.13 Reported effects on the immune system range from no perceived effects7 to reduced activity in activated precursor B lymphocytes of BALB/cN mice.17 However, reports of effects on behavioral tests are scarce and limited to rats. For example, the offspring of fenbendazole-treated Sprague–Dawley rats demonstrated delayed righting reflex and reduced locomotor activity when a running wheel was first introduced.3 In contrast, no effects of fenbendazole on conditioned behavior (overall response rates, temporal patterning, interresponse time, drinking behavior) were detected in Sprague–Dawley rats.14 To our knowledge, effects of fenbendazole on behavior in mice have not been reported. Therefore we were surprised to receive anecdotal reports from a neuroscience laboratory at our institution of adverse effects on behavioral testing in vendor-supplied mice during a routine fenbendazole treatment.
In the absence of published data, anecdotes regarding deleterious effects of treatments or concerns that treatments may interfere with results can cause research to be delayed or cancelled unnecessarily. Conversely, research that proceeds during treatment may be confounded. Therefore, we undertook this prospective controlled study to provide additional data on the effect of fenbendazole treatment in male C57BL/6N mice on 3 common behavioral tests (open field, elevated plus maze, and rotarod). C57BL/6N mice were selected because the C57BL strains are the most frequently used background strains for genetically manipulated mice.
Materials and Methods
Male C57BL/6N mice (age, 6 wk; n = 48; Harlan Teklad, Madison, WI) were randomly assigned on arrival from the vendor to 1 of 4 dietary groups of 12 mice, which received either regular diet (2018S, Harlan Teklad) or fenbendazole diet (2018S plus 150 mg/kg fenbendazole, Harlan Teklad). Control group mice were fed regular diet for 4 wk. Treatment groups were FF (4 wk of fenbendazole diet), FR (2 wk of fenbendazole diet followed by 2 wk of regular diet), and RF (2 wk of regular diet followed by 2 wk of fenbendazole diet). Mice were housed 2 per cage in individually ventilated racks (Allentown Caging, Allentown, PA), on autoclaved corncob bedding (Bed O'Cobs, The Andersons, Maumee, OH) with reverse-osmosis–treated hyperchlorinated water. Cages were sanitized every 2 wk by using aseptic procedures. The colony was routinely tested by sentinel surveillance and remained free of common mouse pathogens, including Sendai virus, pneumonia virus of mice, mouse hepatitis virus, mouse minute virus, mouse parvovirus types 1 and 2, Theiler mouse encephalomyelitis virus, reovirus, epizootic diarrhea of infant mice, lymphocytic choriomeningitis virus, ectromelia virus, murine adenovirus types 1 and 2, murine cytomegalovirus, Mycoplasma pulmonis, fur mites, and pinworms. Mice were weighed on arrival (day 0), after 2 wk on the diet (day 15), and just before euthanasia (day 30). After 4 wk of dietary treatment, mice were tested sequentially in 3 behavioral tests and then euthanized by carbon dioxide overdose. Blood was collected by cardiocentesis for clinical pathology, and liver, kidney, and head tissues were collected and preserved in 10% nonbuffered formalin for routine histopathology. Mice were examined prior to behavioral testing for physical abnormalities that could affect behavioral testing, such as fight wounds or whisker loss. All procedures were approved by the Johns Hopkins IACUC, and Johns Hopkins animal facilities are AAALAC-accredited.
Behavioral tests.
Mice were tested sequentially, first in the open field, then in the elevated plus maze, and finally on the rotarod. Mice were tested over a 3-d period, and all mice were tested in the same sequence. To eliminate tester variability, only one person conducted each test, and the tester was blinded to treatment group. Mice were tested in random order, and all mice were tested between the hours of 1800 and 2100 during the light cycle. The last-used diet was continued throughout the testing period.
Open field test.
Open field tests reflect multiple underlying traits, including locomotor activity, exploratory activity, olfaction and vision, as well as fear and anxiety,6 therefore this test is used frequently for initial evaluation of many mouse behavioral characteristics. The open field consisted of a square acrylic box incorporating an automated activity monitor (Cage Rack Flex-Field Photobeam Activity System, San Diego Instruments, San Diego, CA), which provides horizontal and vertical grids of 16 × 16 infrared beams. The total number of beam breaks in both horizontal and vertical planes over a period of 30 min was recorded and analyzed.
Elevated plus maze test.
The elevated plus maze test rests on the innate conflict between the tendency of mice to explore a novel environment and the aversive properties of a brightly-lit open area.6 This test is used widely as a model of anxiety-like behavior. Each mouse was placed in the center of a plus-shaped runway6 elevated 1 m above the floor and containing 2 dark enclosed arms and 2 open arms (San Diego Instruments). The numbers of entries into the dark and bright arms over a 5-min period were scored for each mouse.
Rotarod test.
Rotarod tests are used to measure motor coordination and balance. Mice must continuously walk forward to keep from falling off a rotating cylinder.6 Mice were tested for their ability to maintain balance on an accelerating rotating rod (rotarod; Rotamex 4/8, Columbus Instruments, Columbus, OH). The rotarod accelerated from 4 to 40 rpm over 5 min. The time elapsed before falling and the speed at that time were recorded for each mouse. Immediately before testing, mice were trained until their fall latency reached a plateau. For the experiments, the average of 3 consecutive runs per mouse was used for statistical analysis.
Clinical and anatomic pathology.
Histologic examination.
The liver, kidneys, salivary gland, and tongue were fixed in 10% nonbuffered formalin, and the dissected head was fixed and decalcified (Formical 4, Decal Chemical, Tallman, NY). Tissues were embedded in paraffin for sectioning and then stained with hematoxylin and eosin. The tissues were examined in a blinded fashion by a veterinary pathologist, who assigned a numerical score according to the number or severity of lesions present: 0, no significant lesions; 1, few or mild lesions only; and 2, numerous or moderate to severe lesions.
Hematologic and clinical chemistry analysis.
After carbon dioxide euthanasia, 500 to 600 μL blood was collected from each mouse by intracardiac aspiration with a 25-gauge needle and 1-mL syringe. Blood was placed in a 600-μL centrifuge tube coated with lithium heparin to prevent clotting. Total WBC count, segmented neutrophils, lymphocytes, RBC, hemoglobin, hematocrit, and platelets were analyzed by automated hemocytometer (Hemavet HV950FS, Drew Scientific, Oxford, CT). The remainder of the blood was centrifuged and the plasma drawn off and analyzed with an automated clinical chemistry analyzer (VeTACE, Alfa Wassermann, West Caldwell, NJ) for cholesterol, triglyceride, creatine kinase, ALT, AST, lactate dehydrogenase, amylase, ALP, glucose, total protein, total calcium, BUN, creatinine, albumin, high-density lipoprotein, and uric acid.
Statistical analysis.
The effects of fenbendazole in the open-field performance were evaluated by using 2-way repeated-measures ANOVA. The effects of fenbendazole in the elevated plus maze and rotarod performances were evaluated by using one-way ANOVA. Significant effects were explored further with post hoc comparisons. Data were analyzed and plotted by using SigmaPlot 2000 (SPSS, Chicago, IL). Statistics were performed by using SigmaStat 2.0 (SPSS), and values are presented as mean ± SEM; a P value of less than 0.05 was used to define significance. Animal weights were compared by using the Student t test.
Results
Behavioral tests.
Open field test.
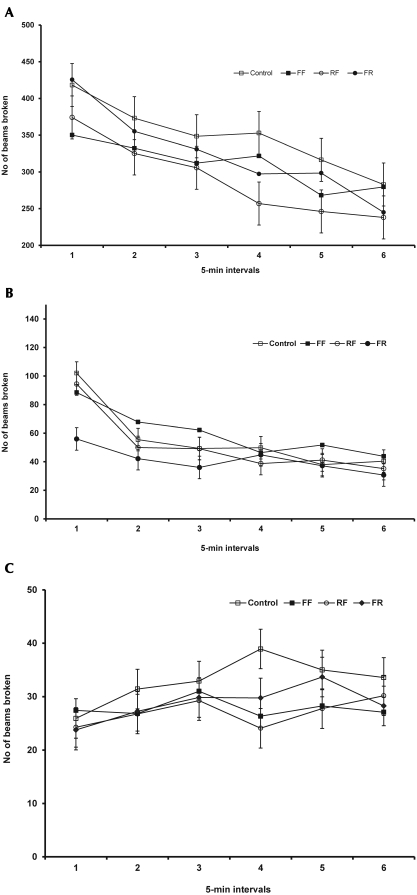
We conducted open field tests to assess the effects of fenbendazole on novelty-induced locomotor activity in mice. The drug had no significant effects on horizontal or vertical activities, specifically including absence of group effects on central activity (F3,287 = 0.932, P = 0.433; Figure 1 A), peripheral activity (F3,287 = 2.496, P = 0.072; Figure 1 B), or rearing (F3,287 = 0.677, P = 0.571; Figure 1 C). Two-way repeated-measures ANOVA during successive time intervals revealed a significant effect for all groups (including control) for central activity (F3,287 = 32.628, P < 0.001), peripheral activity (F3,287 = 21.387, P < 0.001), and rearing (F3,287 = 4.236, P < 0.001). Repeated-measures during successive time intervals are used to evaluate habituation to the open field. These data showed that mice showed reduced horizontal activity, but not vertical activity (rearing), over time.
Figure 1.
Open field test behavior of male C57BL/6N mice did not differ between treatment and control groups. (A) Central activity. (B) Peripheral activity. (C) Vertical activity. Data are expressed as mean ± SEM (n = 12) per group. Data are reported as total number of infrared beams broken in a 5-min period.
Elevated plus maze test.
We used the elevated plus maze to assess the effects of fenbendazole treatment on anxiety in mice. We found no significant effects of the drug on the exploratory behavior. One-way ANOVA for the percentage of entries into open arms showed no significant effect of the drug between groups (F3, 47 = 0.110, P = 0.954; Figure 2 A). Likewise, one-way ANOVA for the percentage of time spent in open arms showed no significant effect of the drug between groups (F3, 47 = 1.658, P = 0.190; Figure 2 B).
Figure 2.
Elevated plus maze behavior of male C57BL/6N mice did not differ between treatment and control groups. (A) Percentage of entries into open arm. (B) Percentage of time spent in the open arm. Data are expressed as mean ± SEM (n = 12 per group). Data are reported as total number of infrared beams broken in a 5-min period.
Rotarod test.
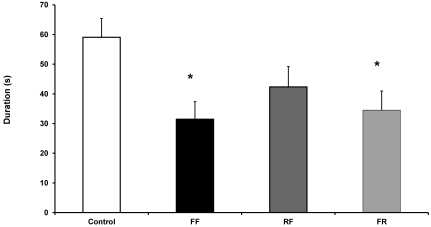
We used the rotarod test to analyze the effects of fenbendazole on motor coordination and balance skills. We found that mice in the FF (4 wk of fenbendazole-containing diet) and FR (2 wk of fenbendazole-containing diet followed by 2 wk of regular diet) groups stayed on the accelerating rotarod for significantly shorter periods than did controls (control compared with FF, P = 0.003; control compared with FR, P = 0.009; Figure 3). Mice in the RF group showed a trend for staying on the rod for shorter periods than controls (P = 0.070; Figure 3). Fenbendazole had a significant effect on time spent on the rotarod (one-way ANOVA, F3, 47 = 3.743, P = 0.018).
Figure 3.
Rotarod performance of male C57BL/6N mice expressed as time spent on the accelerating rotarod. Mice in the FF and FR groups, but not the RF group, stayed on the accelerating rotarod for significantly (P = 0.003, P = 0.009, and P = 0.07, respectively) shorter periods than did controls. Data are expressed as mean ± SEM (n = 12 per group).
Weight, physical examination, and histopathology.
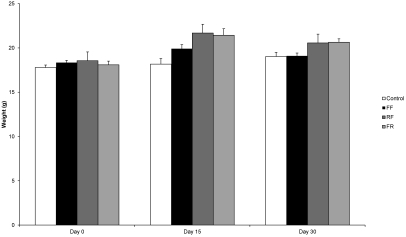
All mice remained physically normal, with intact whiskers and absence of fight wounds. Mice in the FF, FR, and RF groups were significantly (P = 0.04, P = 0.004, and P = 0.003, respectively) heavier than were controls at day 15, and the RF and FR groups were significantly (P = 0.02 and P = 0.01, respectively) heavier than were controls at day 30. Although significant, numeric differences in weight were small (Figure 4). The liver, kidney, and middle and inner ear tissues were examined histologically. Mild hepatic anisocytosis (variation in cell size) was present in 9 of the control mice, 9 FF mice, 7 FR mice, and 10 RF mice (n = 12 per group). Mild centrilobular hypertrophy (slightly larger relative size of centrilobular hepatocytes) was identified in 3 control mice, 2 FF mice, 2 FR mice, and 4 RF mice. These liver changes did not correlate with increased liver enzyme activities or other findings and could not be attributed to treatment effect. Mild, focal infiltrates of few inflammatory cells in the liver or gall bladder (or both) were identified in 0 to 2 mice in each group. Decalcified head sections were examined from all mice. None had otitis media or otitis interna that might be expected to affect behavioral results. Sporadic findings in the head and neck region of 0 to 2 mice per group included: focal retinal degeneration; mild otitis externa; mild, focal inflammation of the submandibular salivary gland; mild accumulations of proteinaceous material in the middle ear, and a single case of neoplasia of the submandibular salivary gland. In summary, histologic changes were very mild or sporadic and could not be attributed to treatment effect. ANOVA indicated no significant difference in lesion score between treatment groups (P = 0.8126).
Figure 4.
Weights of male C56BL/6N mice were significantly greater in FF, FR, and RF groups at day 15 (P = 0.04, P = 0.004, P = 0.003, respectively) and in RF and FR groups at day 30 (P = 0.02, P = 0.01, respectively) compared with those of controls. Data are expressed as mean ± SEM (n = 12 per group).
Hematology and clinical chemistry.
All results were within normal limits. Analysis of variance showed no significant differences between treatment groups (P = 0.9995).
Discussion
This study showed no significant effects of fenbendazole treatment on open field or elevated plus behavioral tests in young male C57BL/6N mice compared with controls. However, mice treated with 4 wk of fenbendazole diet (FF mice) or 2 wk of fenbendazole diet followed by 2 wk regular diet (FR mice) showed significantly reduced ability to stay on the accelerating rotarod, and mice on 2 wk of regular diet followed by 2 wk of fenbendazole diet (FR mice) showed a trend toward shorter rotarod endurance.
This study was initiated because of anecdotal reports that fenbendazole treatment had affected behavioral testing in a neuroscience laboratory at our institution. We tested open field, rotarod, and elevated plus maze in the current study because these tests are used frequently in neuroscience laboratories. Spontaneous activity in the open field is a standardized measure of motor function, rotarod testing is rigorous test of motor coordination and balance, and the elevated plus maze is a test of anxiety.6 Reduced open field activity in concert with reduced rotarod persistence would have indicated a problem with motor function. Reduced open field activity (particularly entries into the central open area) together with reduced entries into the open arms of the elevated plus maze would have revealed an increase in anxiety. Our finding that only rotarod ability was affected, primarily in the FF and FR treatment groups, suggests that fenbendazole has no effect on general motor activity but exerts a subtle effect on motor coordination and balance that takes more than 2 wk to develop and persists at least 2 wk after treatment cessation. The only prior published evidence for an effect of fenbendazole on behavioral tests in rodents was reduced motor ability in the offspring of fenbendazole-treated Sprague–Dawley rats.3 The authors of that study speculated that the reduced running wheel activity may have been due to a difference in reaction to novelty because the effect occurred only during initial introduction of the running wheel. Mice in our study were acclimated to the rotarod before testing, and results were averaged over 3 trials, suggesting that the effect in our mice is not related to novelty.
Another potential cause for decreased motor activity is disorientation due to systemic toxicity; however, liver and kidney function tests and hepatic and renal histopathology were all within normal limits and revealed no significant differences between test and control groups. After initial behavioral results became known, we conducted histopathology of ear tissues to discover whether middle ear abnormalities were responsible for loss of balance. However, the minor accumulations of proteinaceous material noted in the middle ears of several mice were unlikely to have been responsible for reduced rotarod ability.
Other causes, such as weakness due to inappetance, also were discounted because mice in treated groups were at least as heavy as controls; in fact RF and FR groups were significantly heavier at day 30, although actual weight differences were small and most likely attributable to differences in dietary hardness—the regular feed, but not the fenbendazole feed, was autoclaved. The lack of anorexia among our mice was consistent with a study in rats,27 which showed no difference in food intake or body weight in rats fed diets with and without added fenbendazole.
We selected 6-wk-old male C57BL/6N mice for this study because C57BL strains are the most common background strains used for genetic mouse models. Male mice are used frequently for behavioral testing because of the potentially confounding effect of the female reproductive cycle. Additional studies are needed to determine whether the effect is present in female C57BL/6 mice and in other common background strains.
The fenbendazole dosage (150 ppm) and treatment periods (4 wk or 2 wk on–2 wk off) were selected in light of common treatment paradigms: fenbendazole is often used continuously for 4 wk or more or cycled for 2 wk on and 2 wk off for several treatment cycles.21 Further studies are needed to determine whether longer treatment duration exacerbates the effect on rotarod performance and to determine whether the effect persists permanently or disappears with a washout period longer than 2 wk.
This study showed a significantly reduced ability of fenbendazole-treated male C57BL/6N mice to persist on an accelerating rotarod compared with mice on regular diet. The effect was significant in mice treated for 4 wk or for 2 wk then returned to regular diet for 2 wk prior to testing but not in mice treated for 2 wk and then tested immediately, suggesting that the effect took more than 2 wk to develop but persisted at least 2 wk after treatment cessation. Because open field tests were not affected, motor effects were subtle. Additional tests of motor ability, such as grip strength and balance beam testing, are needed to further describe the effect. The cause of the rotarod effects is unknown and deserves further study. Whether fenbendazole's inhibitory effect on microtubule polymerization is responsible for the subtle motor coordination effects we observed despite the drug's low affinity for mammalian tubulin15 remains to be evaluated.
Given our results, we suggest that, for male C57BL/6 mice, fenbendazole is not a factor in open field and elevated plus maze testing but should be used with caution during tests of motor function, such as rotarod, because treatment may confound research results.
Acknowledgments
The authors thank Dr Myriam Dumas Hahn (Department of Pathology, Fluminense Federal University) for support of João P L Daher's training program, Ms Nadine Forbes for technical assistance, Dr Robert J Adams for departmental support, and Harlan Teklad for donation of the mice. This work was supported in part by Johns Hopkins Research Animal Resources, NIH grants 5R01MH083728-02 (MVP) and NINDS NS38377 (TMD), and the Herbert Friedberg Postdoctoral Fellowship in Parkinson Disease (BSG).
Harlan Teklad had no input into this study apart from donation of the mice.
References
- 1.Agersborg SS, Garza KM, Tung KS. 2001. Intestinal parasitism terminates self tolerance and enhances neonatal induction of autoimmune disease and memory. Eur J Immunol 31:851–859 [DOI] [PubMed] [Google Scholar]
- 2.Baker D. 2007. Flynn's parasites of laboratory animals. Ames (IA): American College of Laboratory Animal Medicine and Blackwell Publishing [Google Scholar]
- 3.Barron S, Baseheart BJ, Segar TM, Deveraux T, Willford JA. 2000. The behavioral teratogenic potential of fenbendazole: a medication for pinworm infestation. Neurotoxicol Teratol 22:871–877 [DOI] [PubMed] [Google Scholar]
- 4.Clifford CB, Watson J. 2008. Old enemies, still with us after all these years. ILAR J 49:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coghlan LG, Lee DR, Psencik B, Weiss D. 1993. Practical and effective eradication of pinworms (Syphacia muris) in rats by use of fenbendazole. Lab Anim Sci 43:481–487 [PubMed] [Google Scholar]
- 6.Crawley J. 2007. What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York (NY): Wiley-Liss [Google Scholar]
- 7.Cray C, Villar D, Zaias J, Altman NH. 2008. Effects of fenbendazole on routine immune response parameters of BALB/c mice. J Am Assoc Lab Anim Sci 47:32–36 [PMC free article] [PubMed] [Google Scholar]
- 8.Dix J, Astill J, Whelan G. 2004. Assessment of methods of destruction of Syphacia muris eggs. Lab Anim 38:11–16 [DOI] [PubMed] [Google Scholar]
- 9.Duwel D. 1977. Fenbendazole II. Biological properties and activity. Pesticide Science 8:550–555 [Google Scholar]
- 10.Gao P, Dang CV, Watson J. 2008. Unexpected antitumorigenic effect of fenbendazole when combined with supplementary vitamins. J Am Assoc Lab Anim Sci 47:37–40 [PMC free article] [PubMed] [Google Scholar]
- 11.Hoag WG. 1961. Oxyuriasis in laboratory mouse colonies. Am J Vet Res 22:150–153 [PubMed] [Google Scholar]
- 12.Huerkamp MJ, Benjamin KA, Webb SK, Pullium JK. 2004. Long-term results of dietary fenbendazole to eradicate Syphacia muris from rat colonies. Contemp Top Lab Anim Sci 43:35–36 [PubMed] [Google Scholar]
- 13.Johnston NA, Bieszczak JR, Verhulst S, Disney KE, Montgomery KE, Toth LA. 2006. Fenbendazole treatment and litter size in rats. J Am Assoc Lab Anim Sci 45:35–39 [PubMed] [Google Scholar]
- 14.Keen R, Macinnis M, Guilhardi P, Chamberland K, Church R. 2005. The lack of behavioral effects of fenbendazole: a medication for pinworm infection. Contemp Top Lab Anim Sci 44:17–23 [PubMed] [Google Scholar]
- 15.Lacey E. 1990. Mode of action of benzimidazoles. Parasitol Today 6:112–115 [DOI] [PubMed] [Google Scholar]
- 16.Lacey E, Gill J. 1994. Biochemistry of benzimidazole resistance. Acta Trop 56:245–262 [DOI] [PubMed] [Google Scholar]
- 17.Landin AM, Frasca D, Zaias J, Van der Put E, Riley RL, Altman NH, Blomberg BB. 2009. Effects of fenbendazole on the murine humoral immune system. J Am Assoc Lab Anim Sci 48:251–257 [PMC free article] [PubMed] [Google Scholar]
- 18.Livingston RS, Riley LK. 2003. Diagnostic testing of mouse and rat colonies for infectious agents. Lab Anim (NY) 32:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perec-Matysiak A, Okulewicz A, Hildebrand J, Zalesny G. 2006. Helminth parasites of laboratory mice and rats. Wiad Parazytol 52:99–102 [PubMed] [Google Scholar]
- 20.Phillipson RF. 1974. Intermittent egg release by Aspiculuris tetraptera in mice. Parasitology 69:207–213 [DOI] [PubMed] [Google Scholar]
- 21.Pritchett KR, Johnston NA. 2002. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp Top Lab Anim Sci 41:36–46 [PubMed] [Google Scholar]
- 22.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173 [DOI] [PubMed] [Google Scholar]
- 23.Sato Y, Ooi HK, Nonaka N, Oku Y, Kamiya M. 1995. Antibody production in Syphacia obvelata infected mice. J Parasitol 81:559–562 [PubMed] [Google Scholar]
- 24.Short CR, Barker SA, Flory W. 1988. Comparative drug metabolism and disposition in minor species. Vet Hum Toxicol 30Suppl 1:2–8 [PubMed] [Google Scholar]
- 25.Taffs LF. 1976. Pinworm infections in laboratory rodents: a review. Lab Anim 10:1–13 [DOI] [PubMed] [Google Scholar]
- 26.van der Gulden WJ. 1967. Diurnal rhythm in egg production by Syphacia muris. Exp Parasitol 21:344–347 [DOI] [PubMed] [Google Scholar]
- 27.Vento PJ, Swartz ME, Martin LB, Daniels D. 2008. Food intake in laboratory rats provided standard and fenbendazole-supplemented diets. J Am Assoc Lab Anim Sci 47:46–50 [PMC free article] [PubMed] [Google Scholar]