Abstract
The mouse is the most commonly used laboratory animal, accounting for up to 80% of all mammals used in research studies. Because rodents generally are group-housed, an efficient system of uniquely identifying individual animals for use in research studies, breeding, and proper colony management is required. Several temporary and permanent methods (for example, ear punching and toe clipping) are available for labeling research mice and other small animals, each with advantages and disadvantages. This report describes a new radiofrequency identification tagging method that uses 500-µm, light-activated microtransponders implanted subcutaneously into the ear or tail of mice. The preferred location for implanting is in the side of the tail, because implantation at this site was simple to perform and was associated with shorter implantation times (average, 53 versus 325 s) and a higher success rate (98% versus 50%) compared with the ear. The main benefits of using light-activated microtransponders over other identification methods, including other radiofrequency identification tags, is their small size, which minimizes stress to the animals during implantation and low cost due to their one-piece (monolithic) design. In addition, the implantation procedure uses a custom-designed 21-gauge needle injector and does not require anesthetization of the mice. We conclude that this method allows improved identification and management of laboratory mice.
Abbreviation: RFID, radiofrequency identification; ID, serial number
Laboratory mice play an important role in basic, biomedical, and mammalian research. Inbred and mutant mice are accepted universally as the primary model for analyzing and understanding inherited human disorders.17 Over 75 million laboratory mice are used worldwide annually, including 25 million in the United States. Much of this use is in biomedical research and pharmaceutical drug development; therefore mice play a key part in improving healthcare for people around the world. Mice are social animals, and group-housing, at recommended densities, generally is considered to reduce stress in rodents.6,9,16 However, this housing method requires an efficient system of uniquely identifying individual animals. Despite this need, our ability to identify and keep track of laboratory mice is limited. The most widely used methods for permanently identifying rodents—ear punching, ear tagging, and toe clipping—are relatively primitive. The number of possible identification numbers is limited with ear and toe clipping, thereby complicating breeding programs. In addition, these methods are potentially stressful for animals and do not foster ‘cradle-to-grave’ tracking. Compared with clipping methods, ear tags offer a greater number of identification codes and are simple to apply; however, they can cause irritation and are often subject to self-removal.2,19 Each of these methods may lead to errors associated with misreading by technicians, and, in some cases, lead to repetition of partial or full experiments, a situation that is both costly and labor-intensive.
Recently, radiofrequency identification transponders (RFID tags), which are implanted subcutaneously, have been used to label mice, given that these tags address several of the problems associated with nonelectronic methods. An RFID tag typically consists of electronic circuitry and a solenoid antenna enclosed in a glass capsule. The transponders transmit unique alphanumeric codes that are easily distinguished from each other by a dedicated reading device. The typical size of an RFID transponder capsule is 13 × 2 mm, although somewhat smaller RFID tags (6 × 1 mm) are available also. RFID tags are manufactured and distributed by several vendors.1,7,13 However, the current tags have not been cost-effective for routine identification of laboratory mice and, therefore, their use has primarily been limited to high-value animals used in specialized studies.
The purpose of the present report is to describe the use of a new type of RFID tag for identifying laboratory mice. It is based on a very small microtransponder that transmits a numerical serial code when activated by laser light. Microtransponders have been used previously in cell-based12 and multiplex DNA11 assays and as a means of tracking ants in behavioral studies.15 The main benefits of these devices over other RFID transponders is their small size, which minimizes stress to the animals during implantation, and low cost, due to their monolithic design. The microtransponder surface is silicon dioxide, similar to capsule-type implantable tags, a feature that is important for biocompatibility. The RFID tag is part of a system with a dedicated reader, software, and specialized injection device for implanting mice. These components combine to provide a system that simplifies identification individual mouse and tracking and, if widely adopted, can lead to improvements in animal welfare.
Materials and Methods
Mice.
All mice were cared for in compliance with the Guide for the Care and Use of Laboratory Animals6 and conducted with approval from the IACUCs at Bristol-Myers Squibb Pharmaceutical Research Institute (Lawrenceville, NJ) and Rider University (Lawrenceville, NJ), as appropriate. Mice were maintained under conventional laboratory conditions with a 12:12-h light:dark cycle (lights on, 0600 to 1800), with food and water provided ad libitum. Mice were housed at Rider University in 16 × 25.4-cm cages with 4 mice per cage and 20 × 30-cm cages with 5 mice per cage at Bristol-Myers Squibb. BALB/cByJ and C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Unless specified otherwise, age- and strain-matched groups (8 to 10 mice each), 4 to 6 wk of age, were used for implantation studies. Mice were monitored for 10 min after implantation for changes in activity relative to control animals and grooming or attention to the implant site. A total of 175 mice were used: 92 BALB/cByJ, 35 C57BL/6J, and 48 B6.C57. Gender was not considered in the present study.
The microtransponders were preloaded into injectors and sterilized in an autoclave (Sterilmatic, MarketForge Industries, Everett, MA) at 118 °C for 20 min. Mice were restrained for tail implants in a flat-bottom restrainer (Sci-Ed Warehouse, Vernon Hills, IL), and the implantation site wiped with a gauze pad saturated with 70% alcohol. Mice for ear implants were anesthetized by brief CO2 exposure, during which an ear clamp, made by attaching a solid metal bar to the bottom section of a truncated, commercially available hair clip, was applied. Mice typically recovered within 1 min of removal from CO2 exposure. Mice that recovered before insertion of the microtransponder were not implanted and the procedure counted as unsuccessful.
Histology.
Animals were euthanized by CO2 narcosis at 4 mo of age. Ear tissue and tail segments (3 to 5 cm) were fixed in 10% neutral buffered formalin and tail segments decalcified. The microtransponders were removed, and tissues were embedded in paraffin and sectioned. Cross-sections (5 µm) were stained with hematoxylin and eosin. The extent of epidermal injury and inflammatory infiltration was scored as none, minimal (1- to 2-cell thickening, less than 25% infiltrating cells), mild (3- to 4-cell thickening, 25% to 50%), moderate (4- to 6-cell thickening, 50% to 70% infiltrating cells), and marked (greater than 6-cell thickening, greater than 70% infiltrating cells).
Microtransponders.
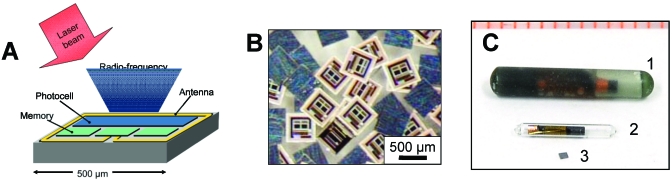
The microtransponders (also called p-Chips), are manufactured on silicon wafers in specialized foundries, by using processes similar to those used in the manufacturing of memory chips and computer processors. The wafers subsequently are thinned and diced into individual microtransponders, each with a dimension of 600 × 600 × 100 µm. The microtransponders (Figure 1) are composed of photocells, clock-signal extraction circuits, a logical state machine, a loop antenna, and a 50-bit memory in which the serial number for identification is stored. The photocells, when illuminated, provide power to the electronic circuits on the chip and activate the tag. The identification number, which is preprogrammed, is transmitted in the 1-MHz range through a variable magnetic field created close to the tag as a result of modulated current in the antenna corresponding to the electronic memory contents. The magnetic field is measured by a nearby coil/receiver in the ID reader and decoded to determine the serial number. The current microtransponder design uses 10 bits to encode the identification number, allowing 1024 unique serial numbers. The electronic memory contains an additional 40 unused bits, so that microtransponders can be manufactured with as many as 250 (1015) unique identification numbers.
Figure 1.
Microtransponders used for tagging small laboratory animals. (A) Schematic diagram of a microtransponder. Dimension shown is of the integrated antenna. (B) Photograph of microtransponders. The integrated circuit side and the back of the chips (dark gray squares) are shown. (C) Microchips suitable for tagging rodents, from (top) Avid Identification Systems, (middle) Lutronic, and (bottom) PharmaSeq. A millimeter scale is shown on the top of the picture.
The laser used in the wand operates in a pulsed-burst mode. This feature reduces the average output power by activating the high-power laser mode, which is used for ID decoding, only when the presence of a microtransponder is positively detected. This selectivity is accomplished by sending very short, periodic high-power laser pulses, during which returned RF signals are scanned to determine whether they match the specific pattern for a microtransponder. When a tag is detected, the high-power bursts of laser increase in frequency, culminating in an ID read that is reported by using application-specific software.
ID reader.
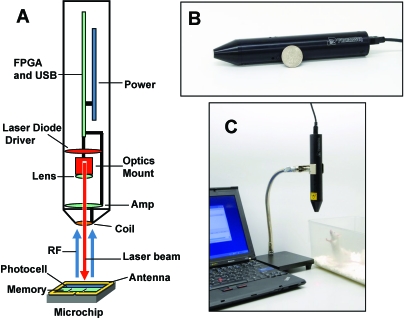
The ID reader (wand) is a Class 3R laser compliant,19 USB-powered device, connected to a standard PC, capable of reading the serial number (ID) of individual microtransponders (Figure 2). The wand dimensions are 17.5 cm in length and 2.8 cm in diameter. The wand contains a laser diode (HL6535, Opnext Hitachi, Fremont, CA) and programmable laser current driver, optical focusing module, USB 2.0 microcontroller, field programmable gate array, power regulators, and a radiofrequency receiver–coil assembly. The laser emits 60 mW (average) of optical power at 658 nm pulsed at 1 MHz and provides the data clock used by the microtransponder for synchronization of the transmitted ID data bits. The wand is attached to a flexible clamp and stand assembly (VWR Scientific, West Chester, PA; Figure 2 C) to free both of the operator's hands for animal handling.
Figure 2.
ID reader and workstation for reading IDs. (A) Design of the ID reader. (B) Size comparison: ID reader and a United States quarter. (C) ID reader as a part of work area for tagging mice. RF, radio frequency; FPGA, field-programmable gate array; USB, universal serial bus; Amp, amplifier.
Software processes the ID reads received from the wand using an error-checking decode algorithm with errors undetectable in 106 ID reads and stores them, along with a log of activities, in an MS SQL database. The software includes DLL (Microsoft, Redmond, WA) and LabView (National Instruments, Austin, TX) application programming interfaces and can export data directly to MS Excel (Microsoft).
Microtransponder injector.
An injector was designed and fabricated that would implant microtransponders permanently in mice, at a depth that is suitable for reading IDs. Because low cost and small size are key benefits of microtransponders over other RFID transponders, the injector design needed to maintain these features. The use of as small of a needle gauge as possible has the added benefit of minimizing stress to the animals. Different designs were fabricated in several gauges and tested for ease of use and subsequent readability of the chips. To reduce the number of animals used, injections initially were performed by using a thin piece of silicon rubber approximately 1 mm in thickness; the injector designs with acceptable performance then were tested on mice.
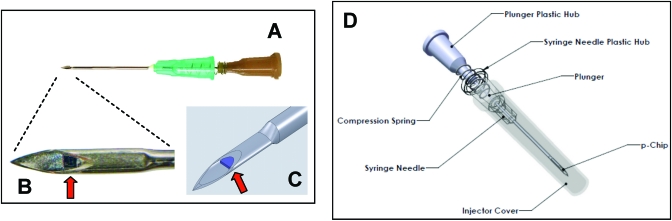
The final injector design uses a flattened 21-gauge injector needle and 27-gauge plunger assembly (Figure 3). Further refinements to the design include the incorporation of a 1.3-cm compression spring and a second crimp, 90° from the initial flattened side and not closer than 1.5 cm from the tip of the needle, to keep the plunger from sliding in the injector. To allow sterilization, a biocompatible adhesive able to withstand autoclave temperatures (121 °C) was identified and holds the microtransponder in place during storage and transport. The microtransponders themselves have excellent temperature stability and can be treated at up to 520 °C for 8 h and still have full radiofrequency activity (data not shown).
Figure 3.
Design of a flattened needle-based injector. (A) Photograph of the injector. (B) Photograph of the tip of a flattened needle (inner diameter, 815 µm) loaded with a microtransponder (width, 600 µm; arrow). (C) Concept drawing of the flattened needle with a microtransponder. (D) Schematic drawing of the covered, flattened needle injector loaded with a microtransponder (arrow).
Results
Microtransponder implantation.
We expected the microtransponder to be readable through the skin of the mouse, given that the ID can be read through clear or translucent (paper) materials (not shown). Initial studies aimed at determining the optimal location for insertion and reading of microtransponders were performed on BALB/c mice. The microtransponders were inserted in either an ear or the tail by using sterilized, preloaded injectors. Ease of insertion was evaluated according to the overall time required for implantation, the number of successful implantations among the total number of attempts, and observation of the mice's response to the procedure. Because we anticipated that the tail implantation procedure would be relatively rapid and pain-free, tail implantation studies were performed on unanesthetized mice. To aid implantation into ears, a special clamp was designed that gently held the ear in place, allowing the injector needle to slide between the top and bottom layers of skin. Mice were anesthetized for implantation into the ears.
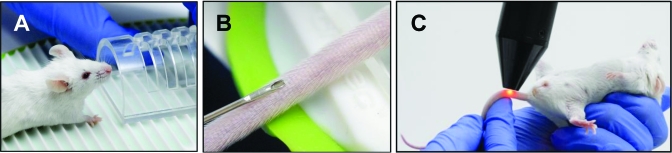
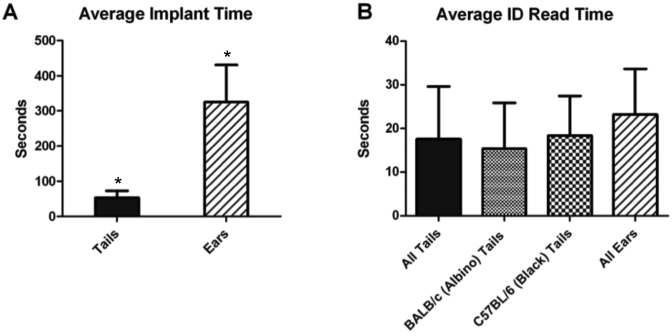
To implant microtransponders into tails, mice were placed in an acrylic restrainer that provides tail access (Figure 4 A). The microtransponders were implanted subcutaneously in the same location on all mice: above the prominent lateral vein, 1 to 1.5 cm from the base, on top of the line of cartilage (Figure 4 B). Implantation into the tail was relatively straightforward and took 53 ± 20 s, including the time to pick up and restrain the mouse (Figure 5). The tops of implanted microtransponders were an average of 0.35 mm below the surface of the tail, and the bottoms of the chips were a mean of 0.45 mm subcutaneous (Figure 6 A).
Figure 4.
Tagging procedure. (A) Mouse is placed in a rodent restrainer with tail access. (B) The microtransponder is implanted subcutaneously along the cartilage line between the lateral vein and midventral artery. (C) Reading the ID with the wand mounted on a stand allows the operator to have both hands free to manipulate the mouse.
Figure 5.
Time required for tagging mice. (A) Average time to implant a microtransponder, including the time to pick up the mouse, restrain it, and insert the microtransponder. The same operator implanted both tails and ears; two operators performed implantations into tails with similar efficiency (not shown). (B) Time (mean ± 1 SD) to read microtransponder implanted in the tails of BALB/c (albino; n = 8), C57BL/6 (black; n = 8), and BALB/c and C57BL/6 overall (all; n = 16) or ears of mice (n = 8). Data were analyzed by t test and ANOVA; *, P < 0.0001.
Figure 6.
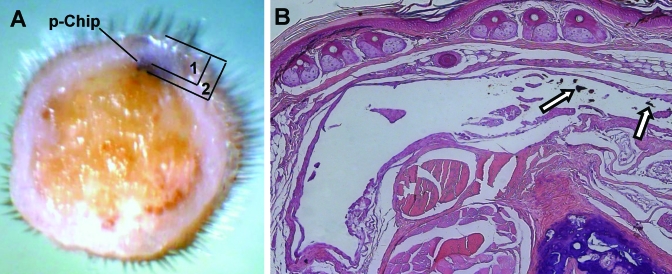
Photomicrographs of tail tissue. (A) Tail cross section overlaid with diagram indicating the chip location within the tail. The tail diameter (mean ± 1 SD; n = 7) was 3.66 ± 0.18 mm (indicated as 1), with the chip implanted 0.35 ± 0.05 mm from the top of the tail to the top of the chip (indicated as 2) and 0.45 ± 0.05 mm from the top of the tail to the bottom of the chip. Magnification, ×2. (B) Representative example of histology of tail tissue surrounding a microtransponder 3 mo after implantation. The arrows indicate debris of microtransponders. The arrowhead indicates the minimal epithelial response present in all 7 mice evaluated. Hematoxylin and eosin stain; magnification, ×10.
Implanting into a mouse ear required a higher skill level to perform than did tail implantation. The entire process required several minutes (5.4 ± 1.7 min) per mouse to complete, including the time to anesthetize the mouse (Figure 5). Even with the clamp, care had to be taken to ensure that the needle did not pierce the ear, causing the microtransponder to fall out. We found that approximately 50% of the ear implants were successful. Once implanted in either a tail or ear, the microtransponders appeared to be imperceptible to the animals; there were no observed instances of excessive grooming or scratching of the implanted areas.
Reading IDs of tagged mice.
Reading the microtransponders was rapid and required minimal manipulation of the mice. To read microtransponders in tails, the mice were scruffed by using the 2-hand method and the tail held directly under the tip of the wand (Figure 4 C). Reading microtransponders inserted in tails took 15 ± 10 s compared with 23 ± 10 s for ear implants (Figure 5). Holding the mice to read microtransponders inserted into the ear was somewhat more difficult, particularly for technicians with larger hands, because the implanted ear tended to fold back onto itself, covering the microtransponder, when the mouse was scruffed. This problem was overcome by pushing the ear forward a little when the mouse was scruffed so that the ear would rest on the handler's thumb during reads so that overall the time to read ear chips did not differ significantly from that to read those in tails (Figure 5).
Because different strains are important in different types of research, we needed to determine the viability of microtransponder tagging in not only one of the most commonly used strains for genetically engineered mice (that is, BALB/c mice) but also in one in which ID reading was anticipated to be particularly difficult—C57BL/6 mice, which have black hair and skin pigmentation. Read times for black mice (C57BL/6) did not differ significantly from those for albino (BALB/c) mice (Figure 5). All of the microtransponders implanted in C57Bl/6 mice have remained readable for the 7 mo that have elapsed since implantation. Testing of the overall performance of the microtransponder system gives a success rate of implantation and reading of microtransponders in the tails of mice of 98%. Overall, 175 mice were tagged.
Biocompatibility studies.
After microtransponder implantation, mice were inspected visually weekly for any signs of inflammation or other adverse reactions. None of the mice implanted with microtransponders displayed any gross pathologic changes. Histologic examination of tail tissue surrounding the microtransponders 3 mo after implantation showed minimal epidermal injury and inflammatory infiltration in all 7 mice evaluated (Figure 6). Minimal (7 of 8 mice) to mild (1of 8 mice) inflammation was present in ear tissue surrounding the microtransponder implants 5 mo after implantation (not shown). An additional 36 mice are continuing to be followed for a period of at least 1 y to monitor the biocompatibility and long-term stability of the microtransponders.
An important consideration in working with genetically modified mice is the need to identify individual mice at the time they are genotyped which, if tail biopsy is used, is recommended to be done before day 17 to 21, depending on the strain.3,5 Initial studies were performed to assess the ease of tagging young mice with microtransponders at 15 d (5 mice), 17 d (7 mice), and 21 d (7 mice) after birth. After implanting, the microtransponders could be read in 18 of 19 mice. Postmortem examination of the one microtransponder that could not be read revealed that it inadvertently was implanted with the circuit side facing down.
Discussion
Transgenic, knockout, and other genetically modified rodent colonies play an important role in many advanced biomedical research programs, and as such, the background strain in which the genetic change is incorporated on often plays a critical role in the phenotypic expression. Given the importance to researchers of various mouse strains, which can display a variety of colors and pigmentation, it was important to demonstrate the viability of microtransponder tagging in different strains of mice. We have found that the ability to read microtransponders implanted in black (C57BL/6) mice as compared with albino (BALB/c) mice does not differ. This alleviates any concern that pigmentation in the tails would interfere with light penetration and subsequent reading of the microtransponders, demonstrating the general utility of this approach. We have also found that inserting in the same spot on all mice makes it easier to locate the microtransponders after implantation, and this location has the benefit of having lighter pigmentation on dark mice as well as not interfering with access to the tail veins.
We expended considerable effort in developing procedures and designing a suitable injector so that microtransponders could be implanted reliably and efficiently. Microtransponders are preloaded in injectors, enhancing the ease-of-use, speed, and safety. The implantation method uses a subcutaneous injection technique commonly used by animal technicians, and our experience has shown that mastery of the technique can be attained with less than 1 h of training. In addition, the laser pulse feature on the wand, designed to enhance laser safety, makes finding the implanted microtransponder uncomplicated. A user simply has to look for an increase in the ‘flicker’ from the laser to locate the microtransponder for ID reading.
An important concern is the long-term reliability of implanted microtransponders. It is desirable for the microtransponder to remain and function in the body of the animal for its lifetime (as long as 2 y). Over the course of the study, we implanted 175 mice with microtransponders, and the overall success rate of implanting and reading microtransponders in the tails of these mice was 98% over the 6 mo of the current study. This value includes data prior to incorporation of all the injector refinements, such as improved methods for loading the injectors to prevent upside down implantation, which are expected to improve the success rate even further. Nevertheless a larger population (on the scale of thousands of mice) needs to be implanted and tested over a longer period of time (up to 2 y). Also more pups of different ages and strains will be tagged in the future to determine the youngest age to recommend for microtransponder implantation.
The maintenance of animal health is a critical issue in colony management.6 Due to their novel light-activated feature, the microtransponders currently have to be brought within a few millimeters of the wand, raising the possibility of cross-contamination of mice. Although improvements to increase the read distance are ongoing, cross-contamination will always be a concern with any instrument used on multiple animals within a facility. Regarding the wand, this potential can be minimized in several ways. The electrical and optical components of the wand are sealed within an impermeable shell, allowing the outside to be wiped with an alcohol or compatible disinfecting solution. In addition, disposable plastic sleeves that do not interfere with reading ability can be placed over the wand to prevent cross-contamination.
Several problems have been identified in association with traditional RFID microchips.4 One study14 that monitored implanted mice for 2 y found that 1) implants cause little tissue reaction, 2) 2% of the mice lose their chips, and 3) 2.8% of chips fail. Tumors associated with the use of RFID tags have been reported to occur at low frequency in long-term rodent carcinogenicity and toxicity studies.10,18 Therefore, we initiated biocompatibility studies with uncoated microtransponders. In this regard, 4 different strains of mice at different facilities have been tagged with microtransponders, some for more than 7 mo, without any indication of gross biocompatibility issues. Histologic examination performed at 3 and 5 mo indicated that most mice had minimal reaction to the microtransponders. In addition, long-term biocompatibility studies have recently been initiated with uncoated and poly(p-xylylene) polymer-coated microtransponders. Poly(p-xylylene) polymers often are used to provide barrier protection in implanted devices.8 This modification is expected to reduce tissue reaction even further, and to date, no adverse effects have been noted. However, long-term studies, which are ongoing, are necessary to fully validate biocompatibility.
The use of electronic RFID devices, in general, can dramatically reduce or even eliminate misreading errors. However, these devices have not gained widespread use, primarily due to cost and handling concerns. The light-activated microtransponders presented here offer a refinement of identification and tracking methods for laboratory mice that can be applied colony-wide. This device likely will appeal to diverse biomedical researchers and animal colony managers and has the potential to greatly increase the use of RFID tags in laboratory mice, ultimately leading to improved animal welfare and aiding in research with mice and preclinical studies.
Acknowledgments
We thank Joseph Nolfo, David Shuster, and Gary Reitnauer (Bristol-Myers Squibb) for assistance with microtranponder implantation experiments. We also thank Jonathan Karp (Rider University) for his assistance with the mouse colony and Richard G Morris (PharmaSeq) for insightful discussions regarding the experiment setup and reading of the manuscript. This work was supported by a grant from the NIH (GM087834 to WM).
The authors declare that they have direct equity ownership in a company having a direct commercial interest in the subject matter discussed in the MS. They also declare that they have patents or patents pending.
References
- 1.Avid [Internet]. AVID specialized applications. [Cited 24 Sep 2010]. Available at: http://www.avidid.com/special/index.html [Google Scholar]
- 2.Baron BW, Langan G, Huo D, Baron JM, Montag A. 2005. Squamous cell carcinomas of the skin at ear tag sites in aged FVB/N mice. Comp Med 55:231–235 [PubMed] [Google Scholar]
- 3.Castelhano-Carlos MJ, Sousa N, Ohl F, Baumans V. 2010. Identification methods in newborn C57BL/6 mice: a developmental and behavioural evaluation. Lab Anim 44:88–103 [DOI] [PubMed] [Google Scholar]
- 4.Cover CE, Keenan CM, Bettinger GE. 1989. Ear-tag–induced Staphylococcus infection in mice. Lab Anim 23:229–233 [DOI] [PubMed] [Google Scholar]
- 5.Hankenson FC, Garzel LM, Fischer DD, Nolan B, Hankenson KD. 2008. Evaluation of tail biopsy collection in laboratory mice (Mus musculus): vertabral ossification, DNA quantity, and acute behavioral responses. J Am Assoc Lab Anim Sci 47:10–18 [PMC free article] [PubMed] [Google Scholar]
- 6.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 7.Kent. [Internet] RFID transponder system. [Cited 24 Sep 2010]. Available at: http://www.kentscientific.com/products/productView.asp?ProductId%3D6239
- 8.Kramer P, Sharma AK, Hennecke EE, Yasuda H. 1984. Polymerization of para-xylylene derivatives (parylene polymerization). I. Deposition kinetics for parylene N and parylene C. J Polymer Sci 22:475–491 [Google Scholar]
- 9.Laber K, Veatch LM, Lopez MF, Mulligan JK, Lathers DMR. 2008. Effects of housing density on weight gain, immune function, behavior, and plasma corticosterone concentrations in BALB/c and C57BL/6 mice. J Am Assoc Lab Anim Sci 47:16–23 [PMC free article] [PubMed] [Google Scholar]
- 10.Le Calvez S, Perron-Lepage MF, Burnett R. 2006. Subcutaneous microchip-associated tumours in B6C3F1 mice: a retrospective study to attempt to determine their histogenesis. Exp Toxicol Pathol 57:255–265 [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Flint J, Azaro M, Coradetti T, Kopacka W, Streck D, Wang Z, Dermody J, Mandecki W. 2007. Microtransponder-based multiplex assay for genotyping cystic fibrosis. Clin Chem 53:1372–1376 [DOI] [PubMed] [Google Scholar]
- 12.Mandecki W, Ardelt B, Coradetti T, Davidowitz H, Flint J, Huang Z, Kopacka W, Lin X, Wang Z, Darzynkiewicz Z. 2006. Microtransponders, the miniature RFID electronic chips, as platforms for cell growth in cytotoxicity assays. Cytometry A 69:1097–1105 [DOI] [PubMed] [Google Scholar]
- 13.Lutronic International [Internet]. Nonatec transponder. [Cited 24 Sep 2010]. Available at: http://www.nonatec.net/pdf/fiche_Transpondeur2.pdf [Google Scholar]
- 14.Rao GN, Edmondson J. 1990. Tissue reaction to an implantable identification device in mice. Toxicol Pathol 18:412–416 [DOI] [PubMed] [Google Scholar]
- 15.Robinson EJH, Thomas O, Richardson TO, Sendova-Franks AB, Feinerman O, Franks NR. 2009. Radio-tagging reveals the roles of corpulence, experience, and social information in ant decision-making. Behav Ecol Sociobiol 63:627–636 [Google Scholar]
- 16.Robinson V, Morton DB, Anderson D, Carver JFA, Francis RJ, Hubrecht R, Jenkins E, Mathers KE, Raymond R, Rosewell I, Wallace J, Wells DJ. 2003. Refinement and reduction in production of genetically modified mice. Lab Anim 37:S1–S50 [PubMed] [Google Scholar]
- 17.Silver LM. 1995. Mouse genetics: concepts and applications. New York (NY): Oxford University Press [Google Scholar]
- 18.Tillmann T, Kamino K, Dasedbrock C, Ernst H, Kohler M, Morawietz G, Campo E, Cardesa A, Tomatis L, Mohr U. 1997. Subcutaneous soft tissue tumours at the site of implanted microchips in mice. Exp Toxicol Pathol 49:197–200 [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services [Internet]. Compliance guide for laser products. [Cited 24 Sep 2010]. Available at: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM095304.pdf [Google Scholar]
- 20.Waalkes MP, Rehm S, Kasprzak KS, Issaq HJ. 1987. Inflammatory, proliferative, and neoplastic lesions at the site of metallic identification ear tags in Wistar (Crl:[WI]BR) rats. Cancer Res 47:2445–2450 [PubMed] [Google Scholar]